Abstract
Spatial vision in different organisms is mediated by 2 classes of photoreceptors: microvillar and ciliary. Recently, additional photosensitive cells implicated in nonvisual light-dependent functions have been identified in the mammalian retina. A previously undescribed photopigment, melanopsin, underlies these photoresponses, and it has been proposed that its transduction mechanisms may be akin to the lipid-signaling scheme of invertebrate microvillar receptors, rather than the cyclic-nucleotide cascade of vertebrates. Melanopsin has an ancient origin in deuterostomia, and expresses in 2 morphologically distinct classes of cells in the neural tube of Amphioxus, the most basal extant chordate: pigmented ocelli, and Joseph cells. However, to our knowledge, their physiology and alleged photosensitivity had never been investigated. We dissociated both types of cells, and conclusively demonstrated by patch-electrode recoding that they are primary photoreceptors; their receptor potential is depolarizing, accompanied by an increase in membrane conductance. The action spectrum peaks in the blue region, ≈470 nm, similar to the absorption of melanopsin in vitro. The light-dependent conductance rectifies inwardly; Na and Ca are differentially implicated in the 2 cell types. Fluorescence Ca imaging reveals that photostimulation rapidly mobilizes calcium from internal stores. Intracellular 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate severely impairs the photoresponse, indicating that light-evoked Ca elevation is an important event in photoexcitation. These observations support the notion that the lineage of microvillar photoreceptors and its associated light-signaling pathway also evolved in the chordates. Thus, Joseph cells and pigmented ocelli of the Amphioxus may represent a link between ancestral rhabdomeric-like light sensors present in prebilaterians and the circadian photoreceptors of higher vertebrates.
Keywords: calcium, phototransduction, vision evolution, melanopsin
In metazoa, 2 main lineages of photoreceptor cells underlie spatial vision, microvillar and ciliary, and differ profoundly in terms of morphology of the light-sensing organelle, physiology of the photo-response, and biochemistry of the underlying signaling cascade. Spatial vision is not the only function that depends on the ability to detect light. Illumination also regulates the photo-entrainment of circadian rhythms (1), the pupillary reflex (2), and hormone secretion (3). These ancillary functions require no spatial resolution, only the determination of average level of light; nonetheless, it was long assumed that they relied on the same photoreceptors that process spatial visual cues. Thus, it came as a surprise that such functions are not impaired after the loss of functional rods and cones both in human patients (4) and in retinal-degeneration mice mutants (5–8). Therefore, the notion that alternative light sensors were involved became inescapable.
The location of the enigmatic new light sensors was circumscribed to the eye (9, 10), and subsequently, pin-pointed to a selected subpopulation of retinal ganglion cells (11), dubbed intrinsically photosensitive retinal ganglion cells (ipRGCs). The photopigment implicated in nonspatial vision was identified as melanopsin (12); as one would expect, it expresses, among others, in some retinal ganglion cells (11, 13–15), and its involvement in circadian photoreception was confirmed by the disruption of photoentrainment in knockout mice (6). The question naturally arose as to the scheme used by melanopsin to transduce light. Surprisingly, several clues point to conjecture that melanopsin may convey the light signal via a scheme akin to that of the microvillar photoreceptors of invertebrate eyes: not only is the primary sequence of melanopsin reminiscent of that of some invertebrate rhodopsins (12), but, like in microvillar photoreceptors, photostimulation of ipRGCs induces a depolarization of the membrane potential (11). Some studies employing heterologous expression of melanopsin support the proposition of signaling via PLC (16, 17), but in others, the results were diverse (18, 19). An important caveat with such an approach is that G protein-mediated cascades can be promiscuous, and implanted receptors often signal through pathways that differ from those of native cells. A case in point is mammalian rhodopsin mediating light responses in Xenopus oocytes by stimulating the native phosphoinositoid cascade (20). Therefore, it is crucial to conduct functional studies in native melanopsin-expressing cells. A few reports on mice ipRGCs documented phenomena consistent with a rhabdomeric-like light-signaling pathway. For example, in a coneless/rodless background, a subset of retinal ganglion cells produce a light-stimulated increase in cytosolic calcium (14, 21) that is suppressed by the IP3 receptor antagonist 2-APB (22). Also, the electrical photoresponse is disrupted by inhibitors of Gq and phospho-lipase C (PLC) (23). However, much remains to be elucidated, and the extreme scarcity of ipRGCs (≈1% of the retinal ganglion cell population) makes such physiological measurements painstaking.
Some of those difficulties could be obviated by studying organisms in which melanopsin-expressing cells were either not as dramatically under-represented, and/or they could be readily identified. This identification could be accomplished with a transgenic mouse, but alternative species could bring additional benefits, like the opportunity to examine broader evolutionary implications and significance of this light-detecting system, whose origin, nature, and function(s) remain poorly understood. Amphioxus (Branchiostoma), a primitive chordate of great importance in evolutionary studies, constitutes an appealing organism to address such questions. Amphioxus had long been considered a sister group to the vertebrates, but has recently been shown by molecular phylogeny to be the most basal chordate, i.e., the most ancient living ancestor of vertebrates (24); this puts it in a privileged position to investigate the origin of different light-signaling systems. This organism has been the subject of many investigations in development and morphology, and its genome has recently been sequenced (25). However, little is known of its neurobiology and sensory physiology. In the neural tube, a single structure has been tentatively identified as a primitive eye, and is comprised of ciliary cells (26). Another cluster of ciliated cells in the lamellar body of the larva may also subserve light-sensing functions, but these cells seem to disperse or disappear during subsequent developmental stages (27). Two additional classes of cells, distributed in the rostral portion of the neural tube, have been proposed to be photoreceptors: (i) pigmented ocelli (also referred to as dorsal ocelli or organs of Hesse), formed by a dark cell partly engulfing a separate, clear cell; and (ii) Joseph cells (26). Interestingly, in both cases electron microscopy reveals the presence of microvilli, reminiscent of the rhabdomeric photoreceptors of invertebrates (28–31). More recently, Amphioxus melanopsin was molecularly identified from cDNA (32); its distribution appears to coincide with that of the 2 classes of putative microvillar photoreceptors. This molecule may form a functional photopigment, because in Joseph cells, the presence of retinal has been detected by a fluorescence assay (30). However, no physiological investigation has provided any direct evidence for the notion that these 2 classes of cells are indeed light-sensitive. In this report. we present an electrophysiological characterization of Joseph cells and pigmented ocelli, and provide unequivocal evidence that they are bona fide primary photoreceptors. Also, we show that key aspects of the light signaling pathway parallel that of rhabdomeric photoreceptors. Portions of this work have been presented in preliminary form (33).
Results
Preparation.
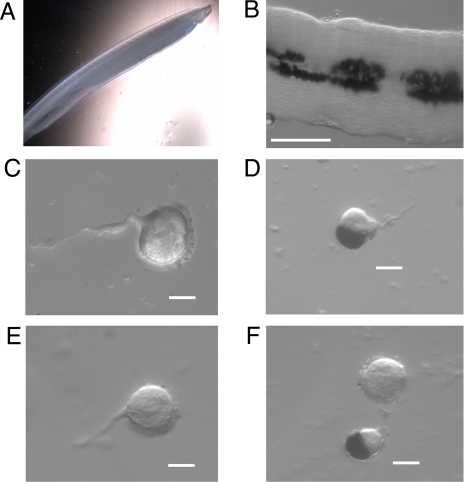
All measurements were conducted in isolated cells, an especially valuable approach to characterize putative previously undescribed photoreceptors, because it provides the utmost assurance that all light responses examined arise from the primary photosensitivity of the cell under study, rather than from cell–cell interactions. In this regard, it should be pointed out that, in the mammalian retina, there are indications that ipRGCs are electrically coupled to other neurons (14), a situation likely to distort the characterization of their light response. The steps to obtain dissociated cells are illustrated in Fig. 1. Fig. 1 A and B shows an intact specimen of Branchiostoma floridae and an excised piece of the neural tube. Two of the classes of isolated cells that are routinely obtained after enzymatic dissociation are also illustrated, and correspond to the description of Joseph cells (exhibiting a microvilli-covered region) (Fig. 1 C and E) and pigmented ocelli (with their distinctive darkly colored accessory cell) (Fig. 1 D and F). Pigmented ocelli tend to be significantly smaller than Joseph cells (≈10–12 vs. ≈15–18 μm), and their microvilli abut the cup-shaped accessory cell, and therefore, are not visible. A short stretch of axon is often seen to emanate from both types of cells.
Fig. 1.
Isolation of cells from the neural tube. (A) Intact specimen of Amphioxus, B. floridae. (B) A piece of neural tube excised from the animal after a dorsal incision. (Calibration bar, 200 μm.) (C and E) Examples of enzymatically isolated Joseph cells. (D and F) Dissociated pigmented ocelli (a Joseph cell also appears in F). (C–F, calibration bars, 10 μm.)
Basic Electrical Properties.
Whole-cell patch-electrode recording was used to measure either the membrane potential, or the membrane current under voltage clamp. In the case of pigmented ocelli, the recording electrode was placed in the translucent region of the complex (i.e., the microvilli-bearing cell). Current-clamp recordings showed that the average resting potential is very similar for Joseph cells and for pigmented ocelli (−56.3 ± 2.2 mV, n = 5; and −56.1 ± 3.2 mV, n = 4). Depolarizing current steps were used to determine that average resting input resistance was 344 ± 41 and 714 ± 234 MΩ, respectively; the membrane capacitance was 114 ± 22.1 pF for Joseph cells and 136 ± 13.3 pF for pigmented ocelli (n = 4 in each case). Assuming the standard value for specific membrane capacitance (≈1 μF × cm−2), the obtained capacitance values are in accordance with the notion that 25–40% of the cell surface is covered with microvilli (≈2 μm in length, 0.2 μm diameter) (28, 30). In both cell types, larger-amplitude current steps evoked action potentials; voltage-clamp recordings show depolarization-activated inward and outward currents with a threshold comparable with that of the action potentials.
Light-Evoked Responses.
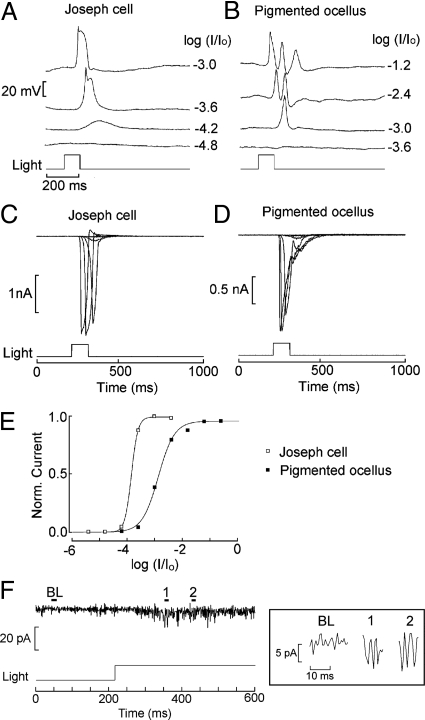
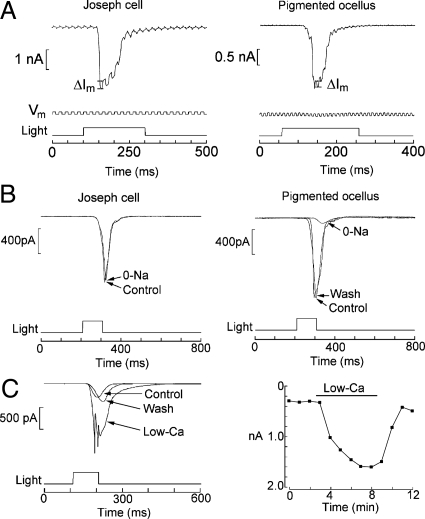
The photosensitivity of Joseph and Hesse cells has been hypothesized on morphological grounds alone, without any supporting physiological measurements. Fig. 2 A and B shows that, in dissociated cells of both types, light stimulation induces a depolarization of membrane potential, the first conclusive proof that both are primary photoreceptors. The receptor potential is graded with light intensity, and at higher intensities regenerative responses appear. To eliminate voltage-dependent mechanisms, the light response was examined under voltage clamp. Fig. 2 C and D shows inward photocurrents recorded at −50 mV; light intensity was increased in 0.6 log steps. In Fig. 2E, the normalized peak photocurrent was fitted by sigmoid curves; half-saturating intensity was obtained with 5.1 × 1011 photons × s−1cm−2 for the Joseph cell, and 4.8 × 1012 photons × s−1cm−2 for the pigmented ocellus. The lower light sensitivity of pigmented ocelli vs. Joseph cells (average difference 0.98 log) may reflect either an intrinsic difference in transduction gain, or light screening by the pigmented accessory cell. The asymptotic amplitude, measured in artificial seawater (ASW) at a holding potential of −50 mV, averaged 1.52 ± 0.31 nA for pigmented ocelli (n = 8), and 1.75 ± 0.29 nA for Joseph cells (n = 4). The photoresponse is extraordinarily rapid. For a just-saturating light flash the latency was 31.4 ± 5.4 (n = 8) and 36.3 ± 9.8 ms (n = 4) in Hesse and Joseph cells, respectively. The full-width at half-maximal amplitude was 47 ± 16 ms in pigmented ocelli and 30.8 ± 7.6 ms in Joseph cells. Sustained, dim illumination caused small-amplitude, rapid fluctuations (Fig. 2F); stretches of recording that contained nonoverlapping waves show stereotyped current excursions with an approximately constant amplitude of 6–8 pA, and a duration of ≈3 ms (Fig. 2F Inset). These waves are likely to represent single-photon responses, but accurate counting of their frequency as a function of light intensity was not possible, owing to the poor signal-to-noise ratio, which could not be improved by low-pass filtering because of their rapid time course.
Fig. 2.
Light responses in isolated microvillar cells. (A) Depolarizing receptor potentials in a Joseph cell in current clamp, in response to 100-ms flashes of increasing intensity. Effective unattenuated light 3.53 × 1015 photons × s−1cm−2. (B) Similar recording in a pigmented ocellus. Notice the higher threshold. (C and D) Inward photocurrents measured under voltage clamp in the 2 cell types. Holding potential −50 mV. (E) Superimposed, normalized plots of peak amplitude of the photocurrent as a function of light attenuation. (F) Fluctuations induced in a pigmented ocellus by dim continuous illumination (−3.6 log attenuation, ≈8.9 × 1011 photons × s−1cm−2). (Inset) Blow-up of baseline period and 2 intervals containing nonoverlapping, seemingly stereotyped light-induced waves; corresponding times are marked on the full trace.
Adaptation.
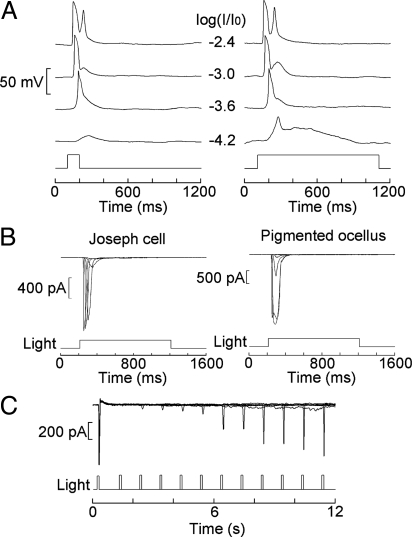
A striking feature of the light response is its transient nature and rapid desensitization. In Fig. 3A, on increasing light duration from 100 ms to 1 s, the receptor potential of Joseph cells returns to baseline with the same time course as that of a response to a brief flash. Only at the dimmest light intensity, a more prolonged “hump” is discernible. Fig. 3B shows photocurrents evoked by 1-s light steps of increasing intensity in the 2 cell types: no hint of a sustained response could be detected (n = 3 for each). Despite such profound light-induced loss of responsiveness, the photocurrent is rapidly restored if a brief period of dark adaptation is interposed. The time course of recovery was quantified by using a double-flash protocol, varying systematically the temporal interval between the paired near-saturating light stimuli. Fig. 3C shows that the response of a Joseph cell recovered with a time constant of ≈9 s, so within 15–20 s the asymptotic amplitude of the dark-adapted photocurrent could be fully regained; similar results were obtained in 2 Joseph cells and 1 pigmented ocellus.
Fig. 3.
Rapid desensitization and recovery of the light response. (A) Receptor potentials elicited in a Joseph cell by brief flashes (100 ms) vs. longer light steps (1 s) of increasing intensity. Except for the lowest light intensity, there is no discernible difference in the time course of the response pairs. (B) Under voltage clamp, the photocurrent evoked by a 1-s light step returns to baseline for both Joseph cells and pigmented ocelli (light attenuation −4.2 to −1.2 log). (C) Double-flash experiment; paired stimuli separated by a variable interval (1–11 s) were presented every minute to a Joseph cell. The photocurrent recovered from the desensitization induced by the first flash with a time constant of ≈9 s. Light intensity, 3.53 × 1012 photons × s−1cm−2.
Spectral Sensitivity.
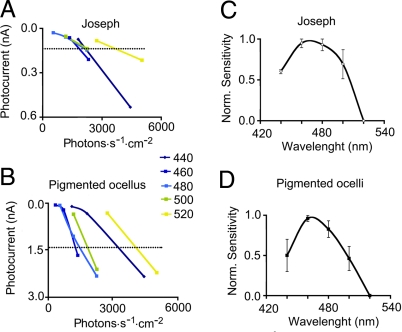
The presence of melanopsin in cells scattered along the neural tube of Branchiostoma belcheri had been described (32); in vitro, the absorption of the reconstituted photopigment after heterologous expression peaks in the blue region (λmax ≈ 480 nm). We measured the wavelength-dependency of the photocurrent in isolated Joseph cells and pigmented ocelli. To obtain the action spectrum under voltage clamp, narrow-band flashes of light were applied between 440 and 520 nm, modulating their intensity at 0.3 log increments. The intensity-response relation obtained at each wavelength was then interpolated to estimate the effective photon flux required to elicit a criterion response in the linear range. Some of these recordings were performed with the perforated-patch technique (using Nystatin), to optimize response stability during the lengthy procedure. Fig. 4 A and B illustrates the results for individual representative cells of each type; the dashed horizontal line indicates the criterion response amplitude. Fig. 4 C and D shows average action spectra, obtained by normalizing data with respect to the peak sensitivity for each cell; the 2 curves peak ≈470 nm, in good agreement with the properties of B. belcheri melanopsin (32), and with the spectral sensitivity of melanopsin-mediated functions in intact higher organisms. The fact that the photoresponse survives extensive light irradiation (provided that a brief dark-adaptation period is interposed) is also in accordance with the thermal stability of heterologously expressed melanopsin (17, 18, 32).
Fig. 4.
Action spectra of the light response. The photocurrent elicited by monochomatic flashes (440–520 nm; 10 nm bandwidth) was recorded under voltage clamp at −50 mV, varying stimulus intensity at 0.3 log increments. (A and B) Intensity-response relation at each wavelength for a Joseph cell and a pigmented ocellus, respectively. A criterion response amplitude in the linear range was set (dashed lines), and the average normalized number of photons required to elicit it was calculated from the interpolated curves. (C and D) Pooled spectral sensitivity curves (n = 3–5 per point), plotting [1− (X − Xmin)/(Xmax − Xmin)] for each wavelength (where X represents effective photon flux).
Ionic Basis of the Photocurrent.
To investigate the mechanisms underlying the light response, the changes in membrane conductance were examined by applying repetitive voltage steps superimposed on the holding potential. Fig. 5A shows that, in both cell types, the delivery of a flash augmented the size of the membrane current perturbations during the photocurrent, indicating an increase in membrane conductance (Joseph cells, n = 3; pigmented ocelli, n = 2). The light-induced conductance increase was graded with stimulus intensity. Thus, light opens ion channels in both cell types, as it does in invertebrate photoreceptors.
Fig. 5.
Properties of the photoresponse. (A) Light induced membrane conductance changes. Photoreceptors of either type were voltage clamped at −50 mV, with a rectangular perturbation of the holding potential (10 mV; 50–100 Hz; 0.5 duty-cycle). Light stimulation caused the concomitant perturbations in membrane current to increase during the photocurrent. (B) Role of sodium in the photocurrent. In Joseph cells, Na removal (substituted by Tris) produced no significant change in current amplitude (Left). By contrast, in pigmented ocelli the light response was reversibly reduced (Right). (C) Effects of lowering extracellular Ca2+ from 10 mM to 0.5 mM (replaced with Mg2+) in a pigmented ocellus stimulated by repetitive flashes of constant intensity (1 per minute). The photocurrent reversibly increased (Left). Time course of the effects of the solution change (Right). Light intensities: Pigmented ocelli, −1.2 log, corresponding to 2.2 × 1014 photons × s−1× cm−2; Joseph cells, −2.4 log, equivalent to 14 × 1012 photons × s−1cm−2.
The photocurrent exhibits a strong inward rectification, and remains inward even with large depolarizations, indicating a dominant selectivity for ions with a very positive equilibrium potential. After iso-osmotic replacement of Na (ENa = +73 mV, under these ionic conditions) with Tris, the photocurrent of Joseph cells only suffered a marginal reduction in amplitude, if any (Fig. 5B Left). By contrast, in pigmented ocelli, it was reversibly depressed by 89 ± 0.11% (Fig. 5B Right; n = 3). The same outcome was obtained by rapid, local superfusion by using a puffer pipette, which only entails a brief exposure to 0-Na; therefore, it is unlikely that the depression of the light response may be due to desensitization of the transduction process, rather than ion conduction (e.g., if Na removal promoted Ca loading by reverse operation of a Na/Ca exchanger). Thus, it appears that sodium ions are major contributors to the photocurrent in pigmented ocelli, whereas they seemingly have a minor role in Joseph cells. We then assessed manipulations of extracellular calcium. In pigmented ocelli, lowering Ca to 0.5 mM by equimolar replacement with Mg increased the photocurrent (Fig. 5C); the effect argues against a Ca contribution to the light-evoked current and is compatible instead with blockage of the permeation pathway, as it occurs in other photoreceptors. In Joseph cells, Ca reduction decreased the photocurrent, but the effect was often irreversible, hinting at potential detrimental effects of low calcium exposure. Although further experiments will be required to fully clarify this issue, one can tentatively conclude that the light-activated conductance is cationic in both cell types, with Na playing the most prominent role in pigmented ocelli.
Signaling Mechanisms.
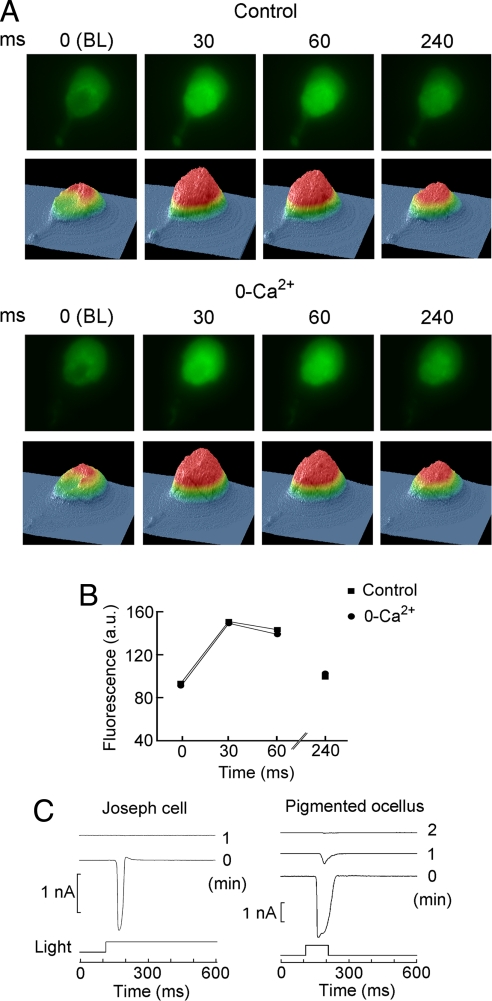
It has been proposed that melanopsin may transduce light via the phosphoinosidite pathway, and a necessary consequence of phosphatidylinositol bisphosphate (PIP2) hydrolysis by PLC is the production of inositol trisphosphate (IP3) and activation of IP3 receptors, causing the release of Ca ions from intracellular stores. To ascertain whether light-induced mobilization of calcium occurs in Amphioxus microvillar photoreceptors, cells loaded with Fluo-3 were imaged synchronizing frame acquisition with the activation of the epi-illumination beam. Fig. 6A Upper shows a sequence of fluorescence images (λ > 520 nm) of a Joseph cell; beneath each image, a surface plot indicates the distribution of the intensity of the emitted light. The exposure time (<30 ms) was briefer than the latency of electrophysiological response, so that the first frame represents basal fluorescence. A conspicuous increase in fluorescence occurs in subsequent images, peaking in the second and subsequently decaying; repetition of the sequence after 5-min recovery in the dark indicated that the decay is not due to bleaching of the dye. To determine the source of the calcium increase, the same protocol was applied after extensive superfusion with Ca-free medium (Fig. 6A Lower). The light-induced increase in Ca fluorescence was similar, indicating that light stimulation causes the release of calcium from intracellular stores. The time course of the optical signal in the 2 conditions is plotted in Fig. 6B, taking the spatially integrated light intensity of each frame. Pigmented ocelli behave in the same manner. The functional relevance of calcium mobilization for visual excitation was addressed by examining the effect of including a high concentration (6 mM) of the rapid Ca chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) in the electrode-filling solution. Buffering intracellular calcium changes essentially obliterated the photocurrent within 1–2 min after attaining the whole-cell configuration, which is in the range of the estimated time of dialysis (Fig. 6C).
Fig. 6.
Role of calcium in the light response. (A) Light stimulation elevates cytosolic calcium in a Joseph cell loaded with Fluo-3. (Upper) Sequence of fluorescence images (excitation peak 496 nm, emission >520 nm) acquired every 30 ms after activating the epi-fluorescence beam; corresponding color-coded surface plots are shown beneath each image. The first frame represents basal fluorescence; the increase in fluorescence after the second frame reports a light-induced increase in intracellular calcium. (Lower) The same procedure was repeated in the same cell after superfusing the recording chamber with nominally Ca-free ASW. A similar increase in fluorescence was observed, indicating that photostimulation causes the release of calcium from intracellular stores. (B) Integrated fluorescence plotted as a function of time elapsed after the opening of the shutter. Peak emission is attained in the second frame, which integrated light during the interval 30–58 ms. (C) Importance of calcium elevation for the photoresponse. (Left) A Joseph cell was internally dialyzed via the patch electrode with 6 mM BAPTA. A repetitive flash was delivered every minute (light attenuation −2.4 log, equivalent to 14 × 1012 photons × s−1cm−2), starting immediately after rupturing the patch to attain the whole-cell configuration. The photocurrent was rapidly abolished. (Right) Similar experiment conducted in a pigmented ocellus.
Discussion
In Amphioxus, at least 4 cell types have been postulated to function as photoreceptors, on the basis of their morphological characteristics (26). Several opsins have been cloned from B. belcheri cDNA, and classified as Go-coupled rhodopsins, Gt-coupled rhodopsins, peropsin, and melanopsin (32, 34); the pattern of expression of the latter coincides with the location of Joseph cells and pigmented ocelli, but to our knowledge, no functional study had ever addressed the alleged role of these cells as light-sensors. The present results conclusively establish that both classes of microvillar cells are indeed primary photoreceptors, with a spectral sensitivity that agrees well with the absorption spectrum of melanopsin (32).
In both cell types, the receptor potential is depolarizing, and arises from an increase in membrane conductance, like in rhabdomeric photoreceptors of invertebrates. Ion-substitution experiments suggest differences in selectivity across the 2 classes of photoreceptors. Detailed analysis of selective permeation would require examining the ionic dependency of the reversal potential, but the strong inward rectification of the photocurrent did not allow such measurements. Nonetheless, the differential role for Na and Ca is reminiscent of light-dependent ion channels in various invertebrate rhabdomeric photoreceptors (35, 36). Membrane current fluctuations elicited by dim light were rapid and only a few picoamperes in amplitude; on the assumption that these waves represent quantal responses, the transduction gain would appear to be only severalfold greater than in mouse ipRGCs (37); the much larger overall light sensitivity of Amphioxus (2–3 orders of magnitude) may, thus, be due chiefly to higher expression levels of melanopsin. As for the signaling cascade that activates the light-dependent conductance, there appear to be obvious similarities to the PLC pathway described in rhabdomeric visual cells of arthropods. Immunohistochemical data have shown the presence of a Gq in pigmented ocelli and Joseph cells (32), whereas our observations of light-induced Ca release from internal stores is consistent with an IP3-dependent mechanism. The striking sensitivity of the photoresponse to buffering cytosolic calcium points to a prominent role for light-induced Ca elevation in the regulation of the excitatory process. Considering that the Ca elevation is rapid (with a latency that, to a first approximation, is similar to that of the photocurrent), it is tempting to speculate that Ca may even be implicated in channel gating, but any conclusion is premature until more information becomes available on different aspects of the transduction process.
On the basis of genetic markers, it has been proposed that ipRGCs of mammals may be the remnants of an ancestral rhabdomeric photoreceptor (38); such view implies that microvillar light-sensors, a lineage dating back to prebilaterians, are represented in vertebrate taxa, contrary to a long-held assumption. Melanopsin-expressing cells of Amphioxus, the most basal extant chordate, bridge this evolutionary gap (39); it is noteworthy that this organism has seemingly suffered minimal evolutionary rearrangement of its genome (25), and is probably close to its ancestral condition (40). It has been proposed, initially by Darwin, that the earliest “proto-eye” ever to appear may have consisted of a photoreceptor associated with a screening pigmented cell to confer directional photosensitivity (41). Molecular phylogeny suggests that such early light-sensitive cell in the proto-eye was probably a microvillar photoreceptor or a precursor thereof (42), an arrangement strikingly resembling the extant pigmented ocelli of Amphioxus.
If the light-sensitivity of ciliary cells in the central eye and the lamellar body of Amphioxus will also be functionally confirmed, in this organism, the 2 fundamental lineages of photoreceptors coexist. Such scenario has been extensively documented in the double retina of some marine bivalve mollusks (43), but it was regarded as an oddity. However, the light responsiveness of microvillar-derived ipRGCs in the mammalian retina, alongside with that of rods and cones, indicates that such situation may not be uncommon. In fact, molecular clues point to additional taxa, like Polychaeta, where the expression of opsins associated with both the ciliary and the microvillar signaling cascades has been reported (44). The presence of photoresponses with opposite polarities may also turn out to be of wider generality than previously surmised.
Materials and Methods
Cell Isolation.
Amphioxus (B. floridae) were obtained from Gulf Specimens Marine Laboratories, and maintained in a seawater aquarium on a diet of marine phytoplankton, and a 12-h light/dark cycle. Specimens were anesthetized by hypothermia, the rostral end was cut and pinned to a Sylgard-coated chamber, and the neural tube was excised. The tissue was then incubated with Pronase (750 units/mL, 50 min at 22 °C; Boehringer), followed by extensive washing in ASW supplemented with 4% FCS, and mechanical trituration with a fine-bore fire-polished Pasteur pipette. The resulting suspension was plated into a perfusion chamber mounted on the stage of an inverted microscope (Zeiss Axiovert). The coverslip bottom of the chamber was pretreated with Concanavalin-A to promote cell adhesion. Dissociated cells remain physiologically viable for several hours.
Electrophysiological Recording.
Patch pipettes were fabricated from borosilicate glass, fire-polished, and filled with an intracellular solution (standard composition, 100 mM KCl/200 K-Aspartate or K-glutamate 5 MgCl2/5 Na2ATP/20 NaCl/1 EGTA/300 Sucrose/10 Hepes/0.2 GTP, pH 7.3). Electrode resistance in ASW is 2–4 MΩ; series resistance was compensated electronically (maximum residual error <2 mV). Data were digitized with an analog-digital interface (Data Translation), which served also to generate stimuli under the control of software developed in-house. Extracellular ions were exchanged by a system of reservoirs and multiport valves, perfusing the entire flow-chamber. Alternatively, for rapid application, “puffer” pipettes were lowered to a preset target position near the cell by a programmable positioner (Eppendorf). Pressure-ejection was controlled by solenoid-activated valves.
Imaging.
The fluorescent calcium indicator Fluo-3 was loaded into cells by incubating the neural tube with the acetylmethoxyester form of the dye (5 μM + 0.05% Pluronic F-127, 2 h) before dissociation. A cooled CCD camera (Roper Cool Snap HQ) was synchronized with the opening of the shutter of the epi-illumination beam; frames were acquired every 30 ms. Excitation and emission wavelenghts were λmax = 496 nm and λem >520 nm (Chroma).
Light Stimulation.
Broad-band light stimuli were generated by a tungsten-halogen light source (Oriel); IR was removed by a heat-absorbing filter (λ > 800 nm). Solenoid-driven shutters (Uniblitz), calibrated neutral density filters (Melles-Griot), and interference filters (Omega Optical) controlled the duration, intensity, and wavelength of stimulation. A pin-hole restricted the illuminated region to a focused spot (≈150 μm). Light was measured with a radiometer (UDT), and converted to effective photon flux at 500 nm by matching the effects of monochromatic vs. broad-band light in an in vivo calibration (44); the unattenuated beam intensity was 3.53 × 1015 photons × s−1cm−2. For spectral sensitivity measurements, light through each narrowband filter was individually calibrated. During experimental manipulations, the cells were illuminated at λ > 780 nm, and viewed with the aid of a CCD camera (Sony).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Devlin PF, Kay SA. Circadian photoreception. Annu Rev Physiol. 2001;63:677–694. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- 2.Kardon R. Pupillary light reflex. Curr Opin Ophthalmol. 1995;6:20–26. doi: 10.1097/00055735-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hazlerigg D, Loudon A. New insights into ancient seasonal life timers. Curr Biol. 2008;18:R795–R804. doi: 10.1016/j.cub.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Klerman EB, et al. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J Biol Rhythm. 2002;17:548–555. doi: 10.1177/0748730402238237. [DOI] [PubMed] [Google Scholar]
- 5.Foster RG, et al. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol Sensory Neural Behav Physiol. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 6.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 7.Lucas RJ, et al. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 8.Mrosovsky N, et al. Persistence of masking responses to light in mice lacking rods and cones. J Biol Rhythm. 2001;16:585–588. doi: 10.1177/074873001129002277. [DOI] [PubMed] [Google Scholar]
- 9.Nelson RJ, Zucker I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp Biochem Physiol. 1981;69A:145–148. [Google Scholar]
- 10.Provencio I, Wong S, Lederman AB, Argamaso SM, Foster RG. Visual and circadian responses to light in aged retinally degenerate mice. Vis Res. 1994;34:1799–1806. doi: 10.1016/0042-6989(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 11.Berson D, Dunn F, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 12.Provencio I, Jiang G, De Grip W, Hayes W, Rollag M. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 15.Semo M, et al. Melanopsin retinal ganglion cells and the maintenance of circadian and pupillary responses to light in aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;17:1793–1801. doi: 10.1046/j.1460-9568.2003.02616.x. [DOI] [PubMed] [Google Scholar]
- 16.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 17.Panda S, et al. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 18.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 19.Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 20.Khorana HG, Knox BE, Nasi E, Swanson R. Expression of a bovine rhodopsin gene in Xenopus oocytes: Demonstration of light-dependent ionic currents. Proc Natl Acad Sci USA. 1988;85:7917–7921. doi: 10.1073/pnas.85.21.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwick AT, et al. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci. 2007;27:13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekaran S, et al. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J Neurosci. 2007;27:3981–3986. doi: 10.1523/JNEUROSCI.4716-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham DM, et al. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99:2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- 24.Schubert M, Escriva H, Neto J-X, Laudet V. Amphioxus and tunicates as evolutionary model systems. Trends Ecol Evol. 2006;21:269–277. doi: 10.1016/j.tree.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Putnam NH, et al. The amphioxus genome and the evolution of chordate karyotype. Nature. 2008;453:1064–1072. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- 26.Lacalli TC. Sensory systems in amphioxus: A window on the ancestral chordate condition. Brain Behav Evol. 2004;64:148–162. doi: 10.1159/000079744. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz S, Anandon R. The fine structure of lamellate cells in the brain of amphioxus (Branchiostoma lanceolatum, Cephalochordata) Cell Tissue Res. 1991a;263:597–600. doi: 10.1007/BF00327295. [DOI] [PubMed] [Google Scholar]
- 28.Eakin RM, Westfall JA. Fine structure of photoreceptors in Amphioxus. J Ultra Res. 1962;6:531–539. doi: 10.1016/s0022-5320(62)80007-0. [DOI] [PubMed] [Google Scholar]
- 29.Nakao T. On the fine structure of the amphioxus photoreceptor. Tohoku J Exp Med. 1964;82:349–363. doi: 10.1620/tjem.82.349. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Yoshida M. Morphological and histochemical studies on Joseph cells of amphioxus, Branchiostoma belcheri Gray. Exp Biol. 1986;46:67–73. [PubMed] [Google Scholar]
- 31.Ruiz S, Anandon R. Some considerations on the fine structure of rhabdomeric photoreceptors in the amphioxus, Branchiostoma lanceolatum (Cephalochordata) J Hirnforsch. 1991b;32:159–164. [PubMed] [Google Scholar]
- 32.Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate Melanopsin: Evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 33.Gomez M, Angueyra JM, Nasi E. Examining the ancient phototransduction mechanisms of a primitive chordate. J Gen Physiol. 2008;132:4a. [Google Scholar]
- 34.Koyanagi M, Terakita A, Kubokawa K, Shichida Y. Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores. FEBS Lett. 2002;531:525–528. doi: 10.1016/s0014-5793(02)03616-5. [DOI] [PubMed] [Google Scholar]
- 35.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 36.Gomez M, Nasi E. Ion permeation through light-activated channels in rhabdomeric photoreceptors: Role of divalent cations. J Gen Physiol. 1996;107:715–730. doi: 10.1085/jgp.107.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Do MTH, et al. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 2003;47:563–571. [PubMed] [Google Scholar]
- 39.Koyanagi M, Terakita A. Gq-coupled Rhodopsin Subfamily Composed of Invertebrate Visual Pigment and Melanopsin. Photochem Photobiol. 2008;84:1024–1030. doi: 10.1111/j.1751-1097.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 40.Lacalli TC. New perspectives on the evolution of ptotochordate sensory and locomotory systems, and the evolution of brains and heads. Phil Trans Roy Soc Lond B. 2001;356:1565–1572. doi: 10.1098/rstb.2001.0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gehring WJ, Ikeo K. Pax 6: Mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 42.Arendt D, Wittbrodt J. Reconstructing the eyes of urbilateria. Phil Trans Roy Soc Lond B. 2001;356:1545–1563. doi: 10.1098/rstb.2001.0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasi E, Gomez M, Payne R. Phototransduction mechanisms in microvillar and ciliary photoreceptors of invertebrates. In: Hoff AJ, Stavenga DG, de Grip WJ, Pugh EN, editors. Molecular Mechanisms in Visual Transduction. Handbook of Biological Physics. Vol 3. Amsterdam: Elsevier; 2000. [Google Scholar]
- 44.Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]