Abstract
The serine–threonine kinase LKB1 regulates cell polarity from Caenorhabditis elegans to man. Loss of lkb1 leads to a cancer predisposition, known as Peutz–Jeghers Syndrome. Biochemical analysis indicates that LKB1 can phosphorylate and activate a family of AMPK- like kinases, however, the precise contribution of these kinases to the establishment and maintenance of cell polarity is still unclear. Recent studies propose that LKB1 acts primarily through the AMP kinase to establish and/or maintain cell polarity. To determine whether this simple model of how LKB1 regulates cell polarity has relevance to complex tissues, we examined lkb1 mutants in the Drosophila eye. We show that adherens junctions expand and apical, junctional, and basolateral domains mix in lkb1 mutants. Surprisingly, we find LKB1 does not act primarily through AMPK to regulate cell polarity in the retina. Unlike lkb1 mutants, ampk retinas do not show elongated rhabdomeres or expansion of apical and junctional markers into the basolateral domain. In addition, nutrient deprivation does not reveal a more dramatic polarity phenotype in lkb1 photoreceptors. These data suggest that AMPK is not the primary target of LKB1 during eye development. Instead, we find that a number of other AMPK-like kinase, such as SIK, NUAK, Par-1, KP78a, and KP78b show phenotypes similar to weak lkb1 loss of function in the eye. These data suggest that in complex tissues, LKB1 acts on an array of targets to regulate cell polarity.
Keywords: AMPK, SIK, NUAK, Par-1, KP78
Mutations in lkb1 result in Peutz–Jeghers syndrome (PJS), a disease characterized by benign gastrointestinal hamartomatous polyps. PJS patients are predisposed to develop malignant cancers of epithelial tissue origin throughout their lifetime. LKB1 (Par-4/XEEK1/STK11) is a serine/threonine kinase (1), and most of the identified mutations in PJS patients have inactivating mutations in the kinase domain (2).
LKB1 (Par-4) is essential for the correct distribution of polarity determinants during Caenorhabditis elegans (3, 4) and Drosophila (5) development. In mice, loss of LKB1 leads to embryonic lethality and neural tube defects (6), and lbk1 heterozygous mice exhibit intestinal polyps (7). In mammalian cells, overexpression of LKB1 can induce polarization of membranes in the absence of cell contacts (8). It is thought that LKB1's role in cancer may be linked regulation of cell polarity.
In Drosophila epithelia, membranes are subdivided into 3 domains: the subapical region (SAR), the zonula adherens (ZA), and the septate junctions (SJ) (9). The SAR is located apical to the ZA and comprises 2 essential complexes: Crumbs (Crb)/Stardust (Sdt)/PatJ and Bazooka (Baz; Par3)/Par6/aPKC. These complexes interact to regulate ZA formation (9). ZA formation also depends on E-cadherin and Armadillo (Arm; β-catenin), which join the plasma membrane to the intracellular Actin cytoskeleton and mediate adhesive contacts between cells. Basal to the ZA is the SJ, composed of the Scribble/Lgl/Dlg complex, which regulate the Crb and Baz complexes (10, 11). The SJ functions as a barrier to paracellular diffusion (10).
LKB1 has been extensively examined in Drosophila (5, 12, 13). In lkb1 embryos and larval wing discs, apical and basolateral markers are mislocalized (13). Notably, in follicle cells, severe defects in epithelial polarity were observed in large lkb1 clones but not in smaller clones induced during later cell divisions (5). Polarity defects become fully penetrant under glucose starvation, suggesting a link between cell polarity and energy levels (12). LKB1 can phosphorylate and activate AMP kinase (AMPK) and the AMPK-like family of proteins (1). AMPK regulates tight junctions (14, 15) and ampkα−/− mutants phenocopy lkb1 polarity defects in embryos and follicle cells. Significantly, lkb1 mutants can be rescued by the expression of a phosphomimetic version of AMPKα (AMPKαT184D) (12, 13). These data have led to a model whereby LKB1 regulates polarity establishment via AMPK.
There may be tissue-specific differences in how LKB1 regulates polarity. In lkb1 follicle cells, aPKC and Arm become diffuse or ectopically localized along lateral membranes (5). In low-energy conditions, polarity defects worsen. Dystroglycan extends laterally and occasionally mislocalizes to the apical domain and F-actin accumulates apically. aPKC, Coracle, Crb, Dlg, and E-Cadherin are lost, but Baz is not affected (12). In contrast, in lkb1 embryos, aPKC, Baz, Arm, and Dlg lose their apical localization and become more basal (13). Although Par-1 appears to be a critical direct target of LKB1 in some tissues (1, 16), polarity establishment in the embryo is independent of Par-1 (13).
The Drosophila retina arises from the eye imaginal disc, a columnar epithelium that undergoes a dramatic remodeling of tissue structure during pupal development. Cells undergo a 90° rotation that turns the apices of the photoreceptor cells (PRCs) toward each other; a process that depends on the adherens junction (AJ) (17). Between 37% and 55% pupal development (pd), the PRC apical surfaces expand, dramatically increasing in depth perpendicular to the plane of the epithelium. At ∼37% pd, PRCs apical surfaces begin to differentiate into rhabdomeres (16).
Here, we show that LKB1 regulates apical–basal polarity in the Drosophila eye. Loss of LKB1 does not affect the establishment of polarity but, rather, the dramatic remodeling of polarity that occurs in the pupal retina. LKB1 also is needed to restrict the length and placement of AJs. The effects of loss of LKB1 are independent of nutritional status, and loss of ampkα in PRCs does not lead to polarity defects in the retina, irrespective of nutrient conditions. Instead, we found that Par-1 and the hitherto uncharacterized Drosophila AMPK-like kinases NUAK, SIK, KP78a, and KP78b contribute to epithelial polarity in the retina and that loss of these genes yields phenotypes similar to (although weaker than) lkb1.
Thus, in contrast to recent studies that have proposed that AMPK is the major effector downstream of LKB1, we find that in the more elaborately polarized pupal retina, LKB1 acts on diverse targets to regulate polarity and morphogenesis.
Results
Mutation of lkb1 Leads to the Disruption of Pupal Photoreceptor Development.
To examine the role of LKB1 in eye development, we used 2 different lkb1 alleles. lkb14A4-2 has a deletion removing the untranslated region, the start codon and the start of the ORF, and lkb14B1-11 contains a nonsense mutation at amino acid 98, disrupting the coding region for the kinase domain (5). We used the FLP/FRT system to analyze homozygous lkb14A4-2 and lkb14B1-11 tissue. FLP was driven by the eyeless promoter and a Minute mutation was included on the wild-type chromosome to slow proliferation of wild-type cells, so the eye was largely composed of lkb1 tissue.
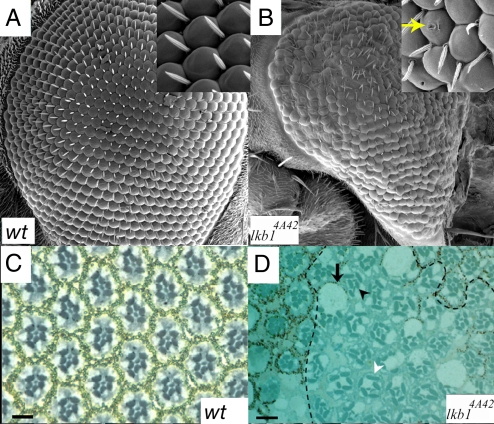
lkb14A4-2 and lkb14B1-11 mutant retinas were smaller than wild type, suggesting possible defects in growth and/or apoptosis (Fig. 1). Bristle and ommatidia organization was disrupted (Fig. 1B). We also observed pitting in lkb1 mutant ommatidia (Fig. 1B Inset). Pitting indicates defects in the lens material secreted by cone cells, and suggests defects in cone cells structure or function. Mutant retinas occasionally had small black spots in the center of the eye, suggesting cell death (18). lkb14B1-11 eyes were intermediate in size between lkb14A4-2 and wild-type retinas, and defects in ommatidial and bristle organization were less severe than lkb14A4-2 retinas [compare Fig. 1D and supporting information (SI) Fig. S1A], suggesting that lkb14B1-11 possesses residual function. However, apart from the differences in severity between the alleles, all phenotypes described here were observed in both alleles.
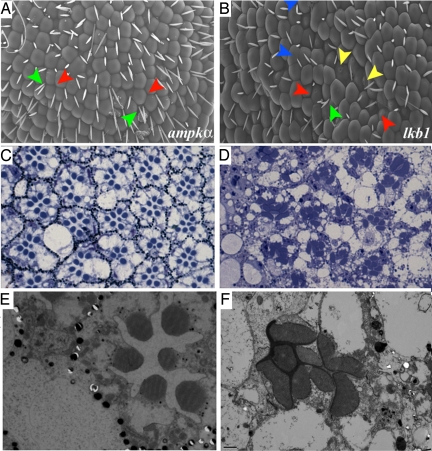
Fig. 1.
Mutation of lkb1 disrupts eye development. (A) SEMs of a wild-type eye show a regular array of ommatidia and bristles. (B) SEMs of the lkb14A4-2 eye; defects include fused ommatidia, missing and excess bristles, and disorganized bristles. The eye is also smaller, rougher, and misshapen with “pitting” of the surface (Inset). (C) Light micrograph of a 1-μm cross section through a wild-type retina reveals a stereotypical arrangement of photoreceptors and ommatidia. (D) lkb1 clones are identified by the lack of pigment and are contained within dashed lines. Loss of lkb1 leads to a loss of photoreceptors (black arrowhead), misshapen rhabdomeres (white arrowhead), and enlarged cell bodies (black arrow). (Scale bars, 10 μm.)
To analyze phenotypes at a higher resolution, we examined 1-μm sections. Sections revealed severe disruption of photoreceptor morphology with ommatidia containing extra or missing photoreceptor cells (PRCs). R7 was frequently lost (Fig. 1D). PRCs were frequently enlarged, and rhabdomeres were elongated (white arrowhead). These phenotypes are reminiscent of crb−/− clones in the retina (19, 20). The lkb1−/− phenotype could be rescued by transgenic expression of LKB1 (Fig. S1C), confirming that these defects are due to loss of LKB1.
These phenotypes could be due to misspecification of PRCs, defects in epithelial polarity, and/or cell death. To distinguish between these possibilities, we looked earlier in development. We found in larval discs that cell fate was unaffected in lkb1 mutant clones. Spalt was used to mark the R3 and R4 PRCs (21), Boss to highlight the R8 (22), Prospero to mark the R7 PRCs (23), Bar to mark PRCs 1 and 6 (24), and Rough to mark R2 and R5 (25). The full complement of correctly specified PRCs are present in larval lkb1−/− clones (Fig. S2). Bar staining in lkb1 clones revealed mild defects in PCP patterning (see also Fig. S1B), consistent with a weak link between PCP and cell polarity (26). lkb1−/− larval tissue also maintains correct polarity, as assessed by staining of aPKC, Arm, and phalloidin (Fig. 2A).
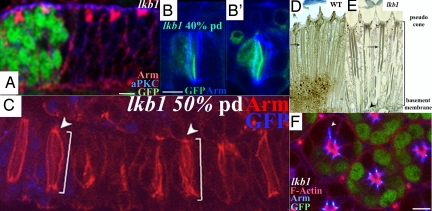
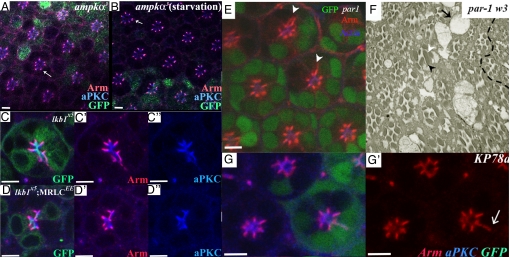
Fig. 2.
lkb1 affects polarity at pupal stages and PRCs extend properly. (A, B, and F) lkb14B1-11. (C and D) lkb14A42. (A) Epithelial polarity is maintained in lkb1 third-instar clones; aPKC (blue) and Arm (red) are correctly localized in lkb1 tissue (marked by loss of GFP). (B) Junctional membranes in 40% pd lkb1 PRCs do not fragment, as shown by continuous Arm (blue) staining. (C) At 50% pd, PRCs undergo normal proximodistal extension (white scale bar), and rhabdomere feet remain attached to the basement membrane (white arrowhead). (D and E) Adult longtitudinal sections; lkb1 rhabdomeres (E) show breaks throughout the proximal–distal length of the rhabdomere, but PRCs extend normally to the basement membrane (black arrowhead). lkb1 rhabdomeres show “waviness” of the lateral membranes (arrow in E) compared with wild type (arrow in D). (F) Confocal section although a 40% pd, lkb1 mosaic eye, showing a wild-type ommatidia (green) alongside a mutant ommatida. Arm staining (blue) extends in cells lacking lkb1. (Scale bars, 5 μm.)
During pupal development, the apical surfaces of cells in the eye disc are remodeled and rotate 90°, and apical surfaces converge at the center of the ommatidium as early as 10% pd. Developing ommatidia mutant for LKB1 lacked the regular size and shape seen in wild-type ommatidia. Members of the SAR complex (Crb, Std, and Patj) and members of the Par complex (aPKC, Baz, and Par-6) have similar defects in rhabdomere formation, resulting in mutant rhabdomeres that are often elongated, split, bulky, or fused (19, 20, 27, 28).
Because lkb1 adult PRC morphology resembled crb mutants, we tested whether PRC development was similarly affected. crb−/− pupal PRCs show fragmented ZA as early as 43% pd and fail to extend rhabdomeres (20, 29). Side views of the ZA (marked by Arm) show that lkb1 rhabdomeres are not fragmented from 40% pd to eclosion (Fig. 2 B–E). Instead, there is a marked waviness of the lateral membranes (Fig. 2 C and E) compared with wild type (arrow in Fig. 2D), suggesting a weakening of structural integrity. Z-sections reveal that lkb1 ZAs properly extend all of the way to the retinal floor in a manner identical to wild type (Fig. S3). Thus, defects observed in lkb1 mutant PRCs are not due to fragmentation of junctions or a failure of extension.
Lkb1 Mutants Lose Polarity at Pupal Stages.
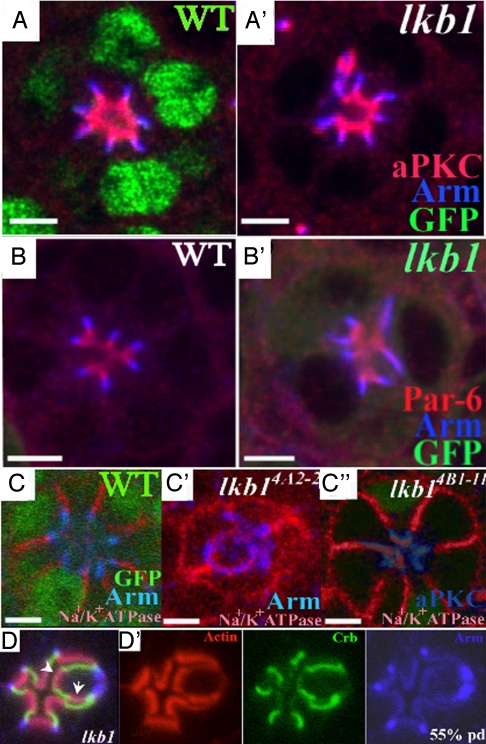
Analysis of polarity markers revealed dramatic defects in lkb1 mutant pupal PRCs. Apical markers such as aPKC and Par6 spread basal to their normal domain at 43% pd (Fig. 3 A and B). The stalk domain was similarly affected in lkb1 mutant PRCs, as assessed by the stalk domain components, PatJ, Std, and Crb (Fig. S4) (19). The AJ marker Arm normally localizes just basal to the stalk membrane. In lkb1 PRCs, Arm frequently expands, occasionally overlapping with stalk membrane markers but mostly spreading toward the basolateral membrane (Fig. 2F arrowhead, Fig. 3 A–D, and Fig. S5). In controls, there is clear separation of junctional and basolateral domains (Fig. 3C), but in lkb1 PRCs, there is significant overlap of Arm and the basolateral marker Na+/K+–ATPase (Fig. 3C′) (30, 31), suggesting that PRCs have lost distinct lateral membrane identity. Extra membrane domains are also sometimes observed, e.g., 3 subapical domains, 2 apical domains (white arrowheads in Fig. 3D). These data suggest that LKB1 has a crucial role in developing or maintaining distinct membrane domains in the pupal retina.
Fig. 3.
lkb1 loss of results in polarity defects in the pupal retina. GFP (green) marks wild-type tissue in A and C and mutant tissue in B. lkb14B1-11 (A–C) and lkb14A42 (D and E). Apical markers aPKC and Par-6 (red) show expansion into the basolateral domain in lkb1 mutant clones. (B′) The junctional marker Arm (blue) also shows aberrant expansion into the basolateral domain. (C′) The basolateral marker Na+/K+ ATPase (red) also mislocalizes to the apical membrane in lkb14B1-11 mosaic PRCs. (C″) lkb14A42 mosaic retinas show a more severe phenotype, where Na+/K+ ATPase (red) can be found in a ring like structures overlapping Arm (blue). (D′) Extra membrane domains are sometimes observed, e.g., 3 subapical domains, 2 apical domains (white arrowheads). (Scale bars, 5 μm.)
Expression of the pan caspase inhibitor, p35, did not ameliorate the polarity defects (Fig. S4G), suggesting that defects are not due to cell death. Taken together, these data provide evidence that LKB1 is involved in the global organization of distinct membrane domains during pupal development.
AJ Defects in LKB1 Mutants.
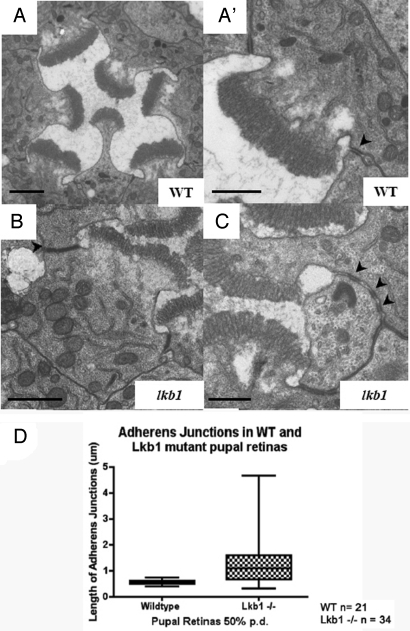
In wild-type retinas, AJs are consistently regular in size and placement. Ultrathin (70 nm) sections of retinas at 50% pd show expanded AJs in lkb1 PRCs compared with controls (Fig. 4B), and some cells appear to have >2 AJs (Fig. 4C). Some AJs are clearly separate from each other, e.g., where AJs appear at 4 corners of a single cell; in other cases, many smaller junctions appear next to each other, which may be a product of a single AJ that has fragmented (Fig. 4C). AJs normally uniformly occupy an apicolateral position. In lkb1 retinas, as well as exhibiting lateral expansion, ectopic junctions also occasionally appear on the lateral and basal membranes.
Fig. 4.
Adherens junctions are longer, more numerous, and mislocalized in lkb1 photoreceptor cells. (A and A′) Ultrathin sections (70 nm) of a wild-type ommatidium at 50% pd. AJ in wild-type PRCs occupy an apicolateral position in the cell, and each cell has 2 AJs (black arrowhead) of uniform length (0.5 μm). (B–C) lkb1 AJs are frequently longer (white arrowhead in B) and sometimes disjointed (black arrowheads in C). (Scale bars, 1 μm in A and B and 0.5 μm in A′ and C.) (D) Box-plot analysis of lkb1 AJ length in PRCs in 50% pd pupal retinas. The average length of AJs in lkb1 PRC increased to 1.28 μm. AJs also exhibit an increased range in length. Smaller junction lengths may indicate fragmented AJs.
Quantitation revealed that the average length of wild-type AJs was very consistent [0.55 ± 0.10 μm; in contrast lkb1 AJs were longer and variable in length (1.28 ± 0.83 μm; P < 0.0001)] (Fig. 4D). The shorter junctions are possibly a result of junction breakdown, because they often display as a string of “mini” junctions along the apicolateral membrane (Fig. 4C). These data suggest that LKB1 is required for the proper localization of AJs at the apicolateral membrane, for AJ integrity, and for the restriction of AJ length.
ampkα Loss-of-Function Clones Do Not Phenocopy lkb1 in the Retina.
LKB1 has been proposed to regulate polarity primarily via regulation of AMPK in embryos, wing discs, and follicle cells. Surprisingly, examination of adult ampkα−/− eyes revealed significant differences from lkb1−/− eyes (Fig. 5). ampkα eyes do not exhibit any pitting (Fig. 5A), and rhabodmeres are not elongated (Fig. 5C) In addition, the normal termination of axons is largely intact in ampkα, whereas in lkb1 mutants, the lamina appears to be fused with the medulla (Fig. S6).
Fig. 5.
lkb1 and ampkα mutant adult eyes. (A–F) SEMs of adult eyes. ampkα and lkb1 mutant eyes are “rough” (A and B). lkb1 mutant ommatidia show pitting of the surface (yellow arrowhead in B) and rhabdomere fusion (blue arrowhead in B) that is not observed in ampkα mutant eyes (A). Bristles missing between ommatidia (red arrows in A and B) or duplicated (green arrows in A and B) are frequently observed. (C and D) Light micrographs of ampkα (C) and lkb1 (D) mutant eyes. Both ampkα and lkb1 mutant eyes show enlarged cell bodies (C and D), whereas only lkb1 shows elongation of rhabdomeres (D compared with C). (E–F) TEM of ampkα (E) and lkb1 (F) mutants. R7 is sometimes absent in sections of ampkα mutants (E), whereas the rhabdomere membrane is often enlarged in lkb1 mutants (F).
ampkα3 mutant clones at 43% pd displayed no expansion of Arm under normal conditions (Fig. 6A) or under conditions of energy deprivation (Fig. 6B). Quantitative analysis of Arm length in control photoreceptors under energy deprivation (0.5 ± 0.2 μm) was similar to ampk mutants (0.5 ± 0.1 μm). In addition, expression of MRLCEE did not rescue the disruption of PRC in lkb1 clones (Fig. 6 C and D). Thus, unlike in embryos and follicles cells; in the pupal retina, LKB1 does not appear to function primarily through activation of AMPK, and MRLC but, instead, acts on other targets to regulate epithelial polarity.
Fig. 6.
ampk−/− does not alter Arm localization, whereas KP78a loss phenocopies lkb1. GFP (green) marks the wild-type tissue in A, B, and E, MARCM clones in C and D, and flp-out clones in G and H. ampkα3 mutant clones do not phenocopy lkb1 clones raised on normal food (A) or starvation food (B). ampkα3 mutant PRCs show discrete Arm (red) localization (arrows in A and B). Expression of MRLCEE in lkb1 clones does not rescue lkb1 polarity defects (D). (C) MARCM lkb1X5 clones show basolateral spreading of aPKC (blue) and Arm (red). (D) MARCM lkb1X5 clones expressing MRLCEE do not show a rescue of the basolateral spreading of apical (aPKC, blue) and junctional markers (Arm, red). (E) par1w3 clones show expansion of Arm (red) toward the basolateral domain of PRCs (arrowheads). (F) par1w3 clones (lack of pigment cells) show elongated rhadomeres (arrowhead) and enlarged cells bodies (arrow); phenotypes also characteristic of lkb1 loss of function in the eye. (G) Expression of KP78a RNAi results in basolateral spreading of apical (aPKC, blue) and junctional markers (Arm, red). (Scale bars, 5 μm.)
LKB1 phosphorylates and activates a number of AMPK-like kinases, including Par1. Indeed, Par-1 and LKB1 were first identified in a C. elegans screen for genes required for the formation of the anterior–posterior axis during embryogenesis (3, 32, 33). lkb1 and par-1 mutants show similar phenotypes in oocytes: defective polarization of the cytoskeleton and mislocalization of polarized mRNAs and proteins (5). We examined par-1−/− PRCs and saw basolateral expansion of Arm (ref. 34 and Fig. 6E) and enlargement of cell bodies (Fig. 6F, arrow) and elongation of rhabodmeres (Fig. 6F, arrowhead) as seen in lkb1 mutant PRCs (Fig. 1D).
However, loss of Par1 only weakly phenocopies loss of LKB1, with increases in Arm length similar to the weaker LKB1 allele, lkb14b1-11 (par1 = 1.4 ± .5 μm; lkb14b1-11 = 1.7 ± 0.5 μm, compared with control Arm length of 0.9 ± 0.2 μm, all under normal energy conditions). The defects in Arm localization in the stronger LKB1 allele are so dramatic that they are difficult to quantitate reliably and are frequently lost from the developing retina. Because loss of Par1 does not fully phenocopy LKB1 loss, we wondered whether other AMPK-like kinases regulate polarity in the eye. There are no available null alleles to these genes, and their functions have not yet been studied in Drosophila. We therefore used RNAi to knock down expression of all AMPK-like kinases in the retina. Expression of RNAi to 4 other AMPK-like kinases led to defects in PR development [CG15072 (similar to mammalian SIK and QSK), CG11871 (homologous to mammalian NUAK), CG6715 (KP78a) and CG17216 (KP78b)]. We observed basolateral spreading of Arm (Fig. 6G and Fig. S5), similar to that seen in lkb4B1–11 mutant retinas, although less strongly than in lkb14A42 mutants.
Quantitation revealed significant extension of Arm staining in these mutants. In controls, Arm length is 0.9 ± 0.2 μm, whereas in the weaker lkb14B1–11 mutants, Arm length increases to 1.7 ± 0.5 μm. Similar increases in Arm length are seen in retinas expressing several of the AMPK-like kinases of RNAi (CG16334 = 1.6 ± 0.6 μm; CG39866 = 1.8 ± 0.7 μm, KP78a = 1.7 ± 0.5 μm, and for KP78b = 1.5 ± 0.5 μm). Interestingly the percentage of Arm length relative to the length of the lateral membrane in lkb1 mutants is significantly shorter, compared with the other lines, consistent with the stronger effect of LKB1 on polarity. In controls, Arm marks 17 ± 3.7% of the lateral membrane, whereas in lkb1 mutants, Arm occupies 38.5 ± 10.7%. Arm length compared with lateral membrane length is less dramatic in the AMPK-like kinase RNAi lines (CG16334 = 28.6 ± 11.8%; CG39866 = 30.7 ± 12%; KP78a = 33 ± 9.2%; KP78b = 29.4 ± 10%). Together, these data indicate that the regulation of epithelial polarity downstream of LKB1 in the pupal retina is more complex than in embryos or in follicle cells and does not work solely through AMPK or Par1.
Discussion
LKB1 is an important regulator of cell polarity in many systems (3–6, 8, 12, 13), yet how LKB1 regulates polarity is still unclear. The only well-defined targets thus far in Drosophila are AMPK and Par-1. We show here that LKB1 is essential during the remodeling of polarity in the fly eye. Apical markers such as aPKC and junctional markers such as Arm lose their normally discrete localization and spread basolaterally. Basolateral markers such as Na+/K+ ATPase, extend aberrantly toward the apical membranes. AJs expand, and components of the basolateral membrane mix with apical and junctional markers.
Although recent studies suggested that the major downstream target of LKB1 in the wing and embryo is AMPK, acting through MRLC. (12, 13), examination of ampkα3 eyes did not reveal defects similar lkb1 eyes. Furthermore, the expression of activated MRLC did not rescue lkb1 defects in PRCs, unlike the wing. Thus, polarity establishment and maintenance in the Drosophila eye involves a different set of targets than in the embryo or follicle cells.
We found that loss of function of a number of AMPK-related kinases (SIK, NUAK, KP78a, KP78b, and Par-1) partially phenocopy lkb1 in the pupal retina. In agreement, par-1 RNAi in the embryo has been shown to lead to the basal expansion of apical and junctional markers and the mislocalization of basolateral markers (35). We were unable to rescue the effects of loss of LKB1 with overexpressed Par-1, or a phosphomimetic version of Par-1. Given that we see weak phenocopies of the lkb1 phenotype with loss of Par-1, SIK, NUAK, KP78a, and KP78b, we speculate that the effects of loss of LKB1 are due to a loss of regulation of a suite of AMPK-like kinases. We note that because RNAi knockdowns are not nulls, our data do not exclude a role for the AMPK-like kinases that had no phenotypic effect on the eye in our assays. Generation of null alleles of all of the AMPK-like kinases will be necessary to fully define the contribution of each kinase to polarity development in the eye.
Taken together, these data argue against the simple model that LKB1 regulates polarity solely through AMPK. Interestingly, in mammals, there are tissues where LKB1 signals through AMPK and other tissues where LKB1 does not affect AMPK activity (36, 37). Thus, tissue-specific modulation of LKB1 function may be a general theme.
Why is the regulation of polarity, downstream of LKB1 more complex in the pupal retina? The embryo and follicle cells are systems in which epithelial polarization is being established (38, 39), whereas pupal PRCs undergo a 90° remodeling of already established polarized membranes (17, 40). We speculate that this remodeling process requires additional mechanisms for its precise regulation and may be why LKB1 acts on additional downstream targets to regulate polarity in the eye.
It is possible that lack-of-polarity defects in the lkb1 larval disc may be due to perdurance of LKB1 protein. However, we favor a model in which LKB1 acts specifically at the pupal stage, because a number of polarity genes such as crb, par-1, and sdt show polarity defects specifically at pupal stages, when dramatic reorganization of polarity is occurring (20, 27, 34). It is unlikely that these diverse proteins all persist for exactly the same length of time and, instead, suggests a crucial role in the remodeling of the polarity that occurs at this time.
Together, these data suggest that LKB1 can regulate a diverse suite of targets, the regulation of which occurs in a developmental or tissue-specific manner and that more complex tissues, such as the pupal retina, require a more extensive set of targets to develop elaborate cellular polarity.
Materials and Methods
lkb14A4-2 and lkb14B1-11 lkb1X5, ampkα3, UAS-MRLCEE, UAS-RNAi KP78a (line 47658, VDRC), KP78b (line 51995, VDRC), Sik3 (line 39866, VDRC), and NuAk (line 16334, VDRC) were used. Clonal analysis was performed by using the FRT/Flp technique using eyFLP or hsFLP with lkb1 FRT 82b/FRT 82b M+ UbiGFP or hsFLP with ampkα3 FRT 101/FRT 101 UbiGFP. hsFLP clones were induced by heat-shocking the larvae for 1 h at 37 °C. Adult mutant eye clones were generated according to the EGUF/hid method. RNAi lines were crossed to y w hs FLP122;tub>y+>Gal4 UAS-GFP. Flip-out clones were induced by heat-shocking. The MARCM technique was used to examine lkb1 clones expressing MRLCEE or Par-1 by using eyFLP, UAS-mCD8::GFP; tubGal80 FRT 82b, tubGal4, UAS-MRLCEE or UAS-Par-1, lkb1 FRT 82b. Standard techniques were used for imaging retinas at the light and EM level (details in SI Materials and Methods).
Supplementary Material
Acknowledgments.
We gratefully acknowledge antibodies and fly stocks from R. Barrio (Centro de Biologia Molecular bioGUNE), C. Doe (University of Oregon), J. Knoblich (Institute of Molecular Biotechnology), E. Knust (Max Planck Institute, Dresden, Germany), F. Pichaud (University College London), A. Wodarz (University of Göttingen), and the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA. This work was funded through Canadian Institutes of Health Research Grant FRN 79406 (to H.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812469106/DCSupplemental.
References
- 1.Lizcano JM, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marignani PA. LKB1, the multitasking tumour suppressor kinase. J Clin Pathol. 2005;58:15–19. doi: 10.1136/jcp.2003.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts JL, Morton DG, Bestman J, Kemphues KJ. The C. elegans par-4 gene encodes a putative serine–threonine kinase required for establishing embryonic asymmetry. Development. 2000;127:1467–1475. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- 4.Morton DG, Roos JM, Kemphues KJ. par-4, a gene required for cytoplasmic localization and determination of specific cell types in Caenorhabditis elegans embryogenesis. Genetics. 1992;130:771–790. doi: 10.1093/genetics/130.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior–posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 6.Ylikorkala A, et al. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science. 2001;293:1323–1326. doi: 10.1126/science.1062074. [DOI] [PubMed] [Google Scholar]
- 7.Jishage K, et al. Role of Lkb1, the causative gene of Peutz–Jegher's syndrome, in embryogenesis and polyposis. Proc Natl Acad Sci USA. 2002;99:8903–8908. doi: 10.1073/pnas.122254599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 9.Muller HA, Wieschaus E. Armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134:149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 11.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 12.Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–392. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Lee JH, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 14.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci USA. 2007;104:819–822. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci USA. 2006;103:17272–17277. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JW, Imai Y, Lu B. Activation of PAR-1 kinase and stimulation of tau phosphorylation by diverse signals require the tumor suppressor protein LKB1. J Neurosci. 2007;27:574–581. doi: 10.1523/JNEUROSCI.5094-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longley RL, Jr, Ready DF. Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev Biol. 1995;171:415–433. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- 18.Kinghorn KJ, et al. Neuroserpin binds Abeta and is a neuroprotective component of amyloid plaques in Alzheimer disease. J Biol Chem. 2006;281:29268–29277. doi: 10.1074/jbc.M600690200. [DOI] [PubMed] [Google Scholar]
- 19.Hong Y, Ackerman L, Jan LY, Jan YN. Distinct roles of Bazooka and Stardust in the specification of Drosophila photoreceptor membrane architecture. Proc Natl Acad Sci USA. 2003;100:12712–12717. doi: 10.1073/pnas.2135347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellikka M, et al. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 21.Mollereau B, et al. Two-step process for photoreceptor formation in Drosophila. Nature. 2001;412:911–913. doi: 10.1038/35091076. [DOI] [PubMed] [Google Scholar]
- 22.Kramer H, Cagan RL, Zipursky SL. Interaction of bride of sevenless membrane-bound ligand and the sevenless tyrosine-kinase receptor. Nature. 1991;352:207–212. doi: 10.1038/352207a0. [DOI] [PubMed] [Google Scholar]
- 23.Kauffmann RC, Li S, Gallagher PA, Zhang J, Carthew RW. Ras1 signaling and transcriptional competence in the R7 cell of Drosophila. Genes Dev. 1996;10:2167–2178. doi: 10.1101/gad.10.17.2167. [DOI] [PubMed] [Google Scholar]
- 24.Higashijima S, Michiue T, Emori Y, Saigo K. Subtype determination of Drosophila embryonic external sensory organs by redundant homeo box genes BarH1 and BarH2. Genes Dev. 1992;6:1005–1018. doi: 10.1101/gad.6.6.1005. [DOI] [PubMed] [Google Scholar]
- 25.Kimmel BE, Heberlein U, Rubin GM. The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev. 1990;4:712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- 26.Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Nam SC, Choi KW. Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development. 2003;130:4363–4372. doi: 10.1242/dev.00648. [DOI] [PubMed] [Google Scholar]
- 28.Richard M, Grawe F, Knust E. DPATJ plays a role in retinal morphogenesis and protects against light-dependent degeneration of photoreceptor cells in the Drosophila eye. Dev Dyn. 2006;235:895–907. doi: 10.1002/dvdy.20595. [DOI] [PubMed] [Google Scholar]
- 29.Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- 30.Sun B, Wang W, Salvaterra PM. Functional analysis and tissue-specific expression of Drosophila Na+,K+-ATPase subunits. J Neurochem. 1998;71:142–151. doi: 10.1046/j.1471-4159.1998.71010142.x. [DOI] [PubMed] [Google Scholar]
- 31.Lebovitz RM, Takeyasu K, Fambrough DM. Molecular characterization and expression of the (Na+ + K+)-ATPase alpha-subunit in Drosophila melanogaster. EMBO J. 1989;8:193–202. doi: 10.1002/j.1460-2075.1989.tb03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81:611–620. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- 33.Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 34.Nam SC, Mukhopadhyay B, Choi KW. Antagonistic functions of Par-1 kinase and protein phosphatase 2A are required for localization of Bazooka and photoreceptor morphogenesis in Drosophila. Dev Biol. 2007;306:624–635. doi: 10.1016/j.ydbio.2007.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayraktar J, Zygmunt D, Carthew RW. Par-1 kinase establishes cell polarity and functions in Notch signaling in the Drosophila embryo. J Cell Sci. 2006;119:711–721. doi: 10.1242/jcs.02789. [DOI] [PubMed] [Google Scholar]
- 36.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes AP, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 38.Tanentzapf G, Smith C, McGlade J, Tepass U. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J Cell Biol. 2000;151:891–904. doi: 10.1083/jcb.151.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tepass U. Epithelial differentiation in Drosophila. BioEssays. 1997;19:673–682. doi: 10.1002/bies.950190807. [DOI] [PubMed] [Google Scholar]
- 40.Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.