Abstract
The mechanisms preventing efficient remyelination in the adult mammalian central nervous system after demyelinating inflammatory diseases, such as multiple sclerosis, are largely unknown. Partial remyelination occurs in early disease stages, but repair capacity diminishes over time and with disease progression. We describe a potent candidate for the negative regulation of oligodendroglial differentiation that may underlie failure to remyelinate. The p57kip2 gene is dynamically regulated in the spinal cord during MOG-induced experimental autoimmune encephalomyelitis. Transient down-regulation indicated that it is a negative regulator of post-mitotic oligodendroglial differentiation. We then applied short hairpin RNA-mediated gene suppression to cultured oligodendroglial precursor cells and demonstrated that down-regulation of p57kip2 accelerates morphological maturation and promotes myelin expression. We also provide evidence that p57kip2 interacts with LIMK-1, implying that p57kip2 affects cytoskeletal dynamics during oligodendroglial maturation. These data suggest that sustained down-regulation of p57kip2 is important for oligodendroglial maturation and open perspectives for future therapeutic approaches to overcome the endogenous remyelination blockade in multiple sclerosis.
Keywords: differentiation, intrinsic inhibitor, multiple sclerosis, oligodendrocyte, remyelination
Multiple Sclerosis (MS) is the most common inflammatory demyelinating disease of the human central nervous system (CNS), featuring gradual degeneration and loss of previously established functional myelin sheaths and, eventually, of oligodendrocytes. As a consequence, saltatory nerve conduction is impaired, and axons are damaged (1, 2). The disease course can be highly variable, and most patients first experience recurrent and reversible neurological symptoms (relapsing-remitting MS) before a transition to secondary progressive MS occurs (3).
Oligodendrocytes, as the myelin-producing cells of the CNS, are key target cells in MS. Immune-mediated attack on the myelin sheaths leads to functionally impaired glial cells and may induce oligodendroglial death (4). Despite a generally limited regeneration capacity of the adult CNS, remyelination does occur, particularly in early disease stages, and can lead to functional improvement most likely resulting from activation of resident oligodendrocyte precursor cells (OPCs) that can differentiate into functional myelinating cells (5). Nevertheless, compared with peripheral nervous system (PNS) lesions in which remyelination of nerve fibers is widely observed, remyelination of CNS lesions is insufficient and rarely leads to a complete clinical remission. This insufficiency may result from the limited size of the OPC pool in the CNS as well as incomplete OPC activation and/or differentiation.
Several lines of evidence suggest that deficient remyelination may be a result of blocked cellular differentiation (6, 7); the transcriptional regulators, Hes1, Hes5, Id2, and Id4, the Notch signaling pathway, and the transmembrane protein LINGO-1 all negatively influence oligodendroglial differentiation (8–14). However, the expression of inhibitory factors might be stage specific, with the differentiation process regulated differently for early progenitors versus adult resident precursor cells. This circumstance suggests that remyelination in adults is a process different from myelination during development, reflecting differences between perinatal and adult OPCs (15). In addition, normal developmental processes do not occur in the background of an inflammatory disease, which further highlights the need to explore remyelination in adult MS-related model systems.
In this study, we demonstrate that the p57kip2 gene is regulated during the course of MOG-induced experimental autoimmune encephalomyelitis (EAE), a model that shares many pathological features with MS, including partial remyelination (16, 17). Its observed transient down-regulation before disease remission suggests it to be an intrinsic inhibitor of oligodendroglial (re)differentiation. As a member of the cip/kip family, p57kip2 was originally described as a cyclin-dependent kinase inhibitor (CDKI), but a number of other cellular processes were recently shown to depend on cip/kip proteins (18). For instance, long-term suppression of p57kip2 in Schwann cells, the myelinating glial cells of the PNS, efficiently induces their differentiation in culture, even in the absence of axons (19). We therefore investigated the impact of down-regulated p57kip2 levels on oligodendroglial maturation and report here that this results in acceleration of cellular differentiation in vitro. These findings may influence our understanding of naturally occurring remyelination, and down-regulation of the p57kip2 gene could provide the basis for remyelination therapies.
Results
Dynamic Regulation of p57kip2 Expression in the Diseased Spinal Cord.
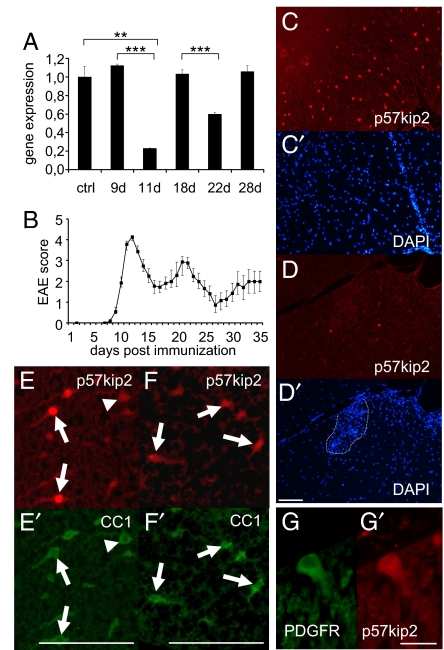
We performed quantitative RT-PCR analysis of spinal cord RNA to determine changes in p57kip2 expression during the course of EAE induced by immunization of DA rats with MOG protein. Most prominent was a strong down-regulation of p57kip2 expression during the first bout (at 11 days) and a moderate down-regulation during the second bout (at 22 days), indicating that lowered p57kip2 expression levels correlate with the onset of remission (Fig. 1 A and B). Beyond 28 days, no further regulation of p57kip2 expression was observed. Immunostaining revealed many p57kip2 expressing cells throughout the healthy spinal cord (Fig. 1C). EAE-related inflammation resulted in decreased transcript levels reflected by a lower number of p57kip2-positive cells (shown for 11 days after MOG immunization in Fig. 1D). Interestingly, we observed that the reduction of the p57kip2 signal was overall and not dependent on direct contact with infiltrating immune cells (dashed line in Fig. 1D′). In the white matter of healthy adult spinal cord (Fig. 1 E and E′) and at the end of the second bout (24 days after MOG immunization; Fig. 1 F and F′), p57kip2-positive cells were mostly CC1-positive oligodendrocytes. A lower number of p57kip2-positive cells expressed the oligodendrocyte precursor marker platelet-derived growth factor receptor-α (PDGFR-α; Fig. 1 G and G′). We detected no strong p57kip2 signals in infiltrating immune cells (Fig. 1D′) and no overlap with GFAP signals, indicating that astrocytes did not contribute to the p57kip2 signals. Of note, in the healthy spinal cord the majority (>90%) of p57kip2-expressing cells displayed strong nuclear as well as perinuclear p57kip2 signals (Fig. 1E, arrows), whereas a lower number of cells showed only cytoplasmic/perinuclear signals of weaker intensity (Fig. 1E, arrowhead). This latter subpopulation was substantially increased in the diseased spinal cord (Fig. 1 F and F′), indicating that p57kip2 down-regulation might be accompanied by protein relocation. The observation that p57kip2 down-regulation fades over time can therefore be interpreted as this gene acting as oligodendroglial inhibitor in (advanced) pathophysiological situations.
Fig. 1.
Regulation of p57kip2 expression in MOG-EAE spinal cords. (A) Quantitative RT-PCR analysis of p57kip2 expression in the diseased spinal cord. Down-regulation is observed at the first and, to a lesser degree, at the second bout, as revealed by the peak EAE scores near days 11 and 22 shown in B [One of 3 independent measurements is shown; GAPDH expression was used as reference and data are mean values ± SEM (t test: ∗∗, P < 0.01, ∗∗∗, P < 0.001)]. (C–D′) Anti-p57kip2 immunostainings and DAPI counter stains of healthy spinal cord (C and C′) and 11-day MOG-EAE spinal cord (D and D′) sections; dotted line in D′ marks infiltrating immune cells. Double immunostainings for p57kip2 and CC1 (E and E′) and for p57kip2 and PDGFR-α (G and G′) indicating that within the healthy spinal cord white matter, p57kip2-expressing cells are oligodendrocytes and oligodendroglial precursor cells. Note that in the healthy tissue the majority of cells feature strong nuclear p57kip2 expression (arrows), whereas few cells show low cytoplasmic/perinuclear expression levels (arrowhead). At the end of the second bout (24 days), CC1-positive cells were found to express p57kip2 again (F and F′). [Scale bars: 100 μm (D′, E′, and F′); 20 μm (G′).]
Regulation of p57kip2 Expression in Cultured Oligodendroglial Cells.
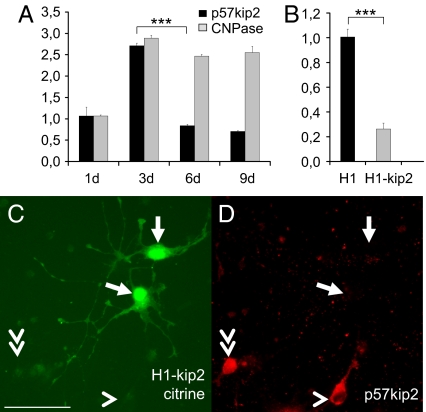
As a next step, we investigated expression and regulation of p57kip2 in cultured primary oligodendroglial cells derived from newborn rat cortices. Oligodendrocyte precursor cells (OPCs) were induced to differentiate in culture by means of growth factor withdrawal and then analyzed for gene and protein expression. Determination of gene expression levels by means of real-time quantitative RT-PCR demonstrated an initial increase of p57kip2 expression concomitant with cell cycle exit and differentiation onset followed by decreasing transcript levels during the course of cellular maturation (Fig. 2A). This process was accompanied by a moderate induction of CNPase- (Fig. 2A) as well as a strong induction of MBP expression. A similar biphasic expression profile has already been described for cultured OPCs derived from P7 optical nerves (20).
Fig. 2.
p57kip2 regulation in cultured oligodendroglial cells. (A) Quantitative RT-PCR analysis revealed an initial gene induction followed by down-regulation of p57kip2 expression during the oligodendrocyte differentiation process which is accompanied by induction of CNPase expression (d, days in culture). (B) This analysis also confirmed p57kip2 down-regulation in suppressed and sorted oligodendroglial cells [One representative experiment of 5 is shown; GAPDH expression was used as reference and data are mean values ± SEM (t test: ∗∗∗, P < 0.001); H1, control transfected cells; H1-kip2, p57kip2-suppressed cells]. (C and D) Anti-p57kip2 immunostaining (D) demonstrated that 6 days following transfection, p57kip2-suppressed-OPCs (C; marked by expression of citrine) were still devoid of p57kip2 expression. The arrows point to transfected cells, the arrowhead marks a cell with p57kip2 signals outside the nucleus, and the double arrowhead points to a cell with strong nuclear p57kip2 expression. [Scale bar, 50 μm (C).]
Following the observation that p57kip2 is down-regulated in both the remyelinating spinal cord as well as in differentiating OPCs, we determined to what degree p57kip2 gene suppression affects the cellular differentiation process. To this end we applied RNA interference to decrease this gene's activity in cultured oligodendroglial cells. However, silencing RNA (siRNA) dependent approaches led only to transient suppression of p57kip2 expression. As an alternative, we used a small hairpin RNA (shRNA) cassette (19), which allows long-term p57kip2 suppression and was previously shown to be specific for p57kip2 and free from off-target effects, such as an IFN response induction (19). By using this approach, oligodendroglial p57kip2 expression could be reduced for up to 9 days as shown by quantitative RT-PCR of transfected and sorted OPCs 2 days following transfection (Fig. 2B) as well as by anti-p57kip2 immunostaining 6 days following transfection (Fig. 2 C and D). Note that among cultured OPCs, cells with strong nuclear p57kip2 expression (double arrowhead) as well as cells with perinuclear and cytosolic expression only (arrowhead) were detected.
Reduced p57kip2 Levels Lead to Accelerated Oligodendroglial Differentiation.
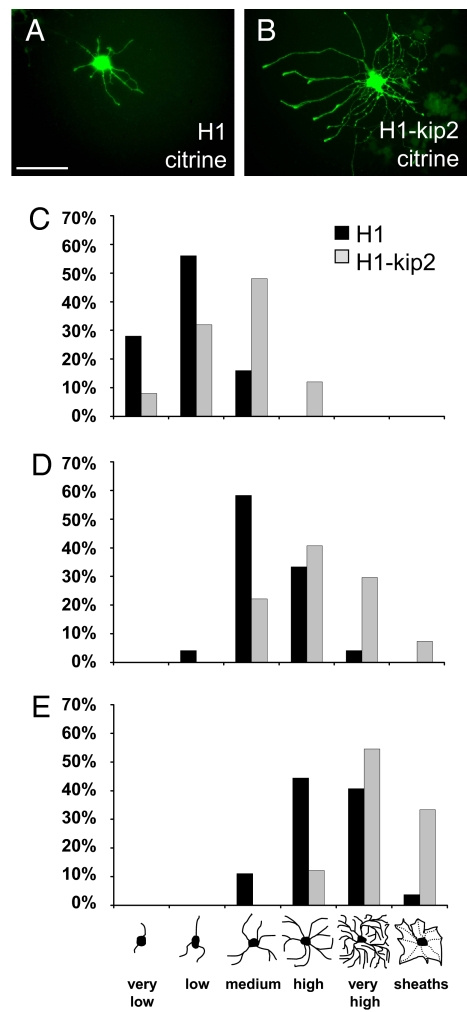
A number of different pathways are known to promote oligodendroglial differentiation featuring different patterns of gene expression (21, 22). Growth factor withdrawal results in immediate cell cycle exit and differentiation onset, whereas environmental cues such as retinoic acid or thyroid hormone appear to depend on an intracellular molecular clock (23, 24). To determine to what degree decreasing levels of p57kip2 affect differentiation kinetics, we used mitogen withdrawal to study maturation of post-mitotic cells and determined the distribution of cellular morphologies of control-transfected and p57kip2-suppressed oligodendroglial cells (Fig. 3). For visualization, OPCs were co-transfected with a citrine expression vector as described previously (19). Differentiation of cultured OPCs is not synchronized and thus is seen as a heterogeneous population of cells with various degrees of maturation, featuring increasing numbers of processes and secondary branches. In our analysis, we distinguished 6 different morphologies (see bar at the bottom of Fig. 3E) from a “very low” number of processes in progenitor cells to multiple process-bearing cells (“low,” “medium,” and “high”) to mature cells with a “very high” degree of arborization or a flattened appearance (“sheaths”). Twenty-four hours after transfection, PDGF-AA and bFGF were withdrawn from the culture, and OPCs were exposed to low serum containing medium stimulating differentiation. After 24 h in this differentiation-promoting medium, p57kip2-suppressed cells appeared to be slightly advanced in their morphological maturation. However, at later time points (3, 6, and 9 days post-differentiation onset), a strong acceleration of morphological maturation was observed in p57kip2 suppressed cells (gray bars) compared with control transfected cells (black bars; Fig. 3 C–E). This maturation-promoting effect was also observed when additional myelin-enhancing stimuli were provided such as thyroid hormones and/or ciliary neurotrophic factor (CNTF) (12, 20, 25) or in presence of growth factors, indicating that this differentiation-promoting effect is a specific consequence of lowered p57kip2 levels.
Fig. 3.
p57kip2 suppression accelerates morphological maturation of cultured oligodendroglial cells. (A and B) Representative citrine-positive control-transfected (A) and p57kip2-suppressed OPCs (B) 3 days following induction of differentiation, is shown. The control transfected cell (H1) features a medium, whereas the p57kip2-suppressed cell (H1-kip2) features a high degree of morphological maturation. (C–E) Determination of OPC morphology distribution of control-transfected (H1; black bars) versus p57kip2-suppressed (H1-kip2, gray bars) cells at 3 (C), 6 (D), and 9 days (E) post-differentiation onset. At all time points, p57kip2-suppressed cells were morphologically advanced as compared with control-transfected cells. Six different morphologies were distinguished; 1 representative experiment of 7 is shown. [Scale bar, 50 μm (A).]
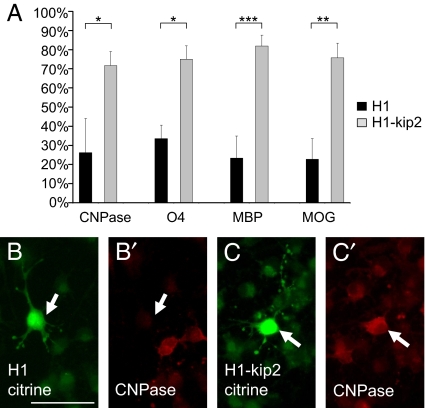
Oligodendroglial maturation is also reflected by the induction and expression of specific marker proteins. We determined whether the pattern of marker expression was altered on p57kip2 suppression (Fig. 4). Following transfection and subsequent induction of cellular differentiation, control and p57kip2-suppressed cells were fixed at various time points and subjected to immunofluorescent staining with antibodies directed against the early marker O4 as well the myelin proteins CNPase (2′,3′-cyclic nucleotide 3′-phospho-diesterase), MBP (myelin basic protein), and MOG (myelin oligodendrocyte glycoprotein). We demonstrated that down-regulation of p57kip2 leads to a significant induction of oligodendrocyte markers at all time points investigated and shown for CNPase, O4, and MBP 2 days and MOG 4 days following initiation of cell differentiation (Fig. 4). Similar to the morphological analysis presented above, this marker induction was observed under a variety of culture conditions stimulating OPC differentiation.
Fig. 4.
p57kip2 suppression stimulates oligodendroglial marker expression. (A) Two days following differentiation induction, significantly more p57kip2-suppressed cells (H1-kip2; gray bars) expressed the early oligodendroglial marker O4 and the myelin proteins MBP and CNPase compared with control-transfected (H1; black bars). Similarly, 4 days post-differentiation onset, the number of MOG expressing cells was increased [One representative experiment of 9 shown; data are mean values ± SEM (t test: ∗, P < 0.05, ∗∗, P < 0.01, ∗∗∗, P < 0.001)]. (B and B′) Representative examples of CNPase-negative control-transfected (H1) and (C and C′) CNPase-positive p57kip2-suppressed (H1-kip2) cells are shown. Arrows point to transfected and citrine positive cells. [Scale bar, 50 μm (B).]
p57kip2 Overexpression Can Induce LIMK-1 Nuclear Translocation.
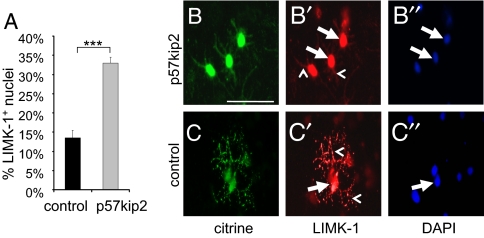
It has previously been shown that the p57kip2 protein can directly interact with LIM domain containing binding partners such as LIMK-1 (26), and we gathered preliminary evidence that in Schwann cells such specific protein/protein contacts can occur (27). Given that cytosolic LIMK-1, through phosphorylation of cofilin, can affect actin filament stability, hence, cell shape and motility, such an interaction might be part of p57kip2's mode of action in inhibiting oligodendroglial differentiation. We therefore investigated the subcellular localization of LIMK-1 in cultured OPCs. In addition, we evaluated the impact of p57kip2 overexpression on an existing subcellular LIMK-1 distribution (Fig. 5). OPCs were either co-transfected with a p57kip2-overexpressing or an empty control vector together with the citrine expression vector (19). Differentiation promoting medium was added and, after 48 h cells, were scored for LIMK-1 subcellular localization by means of immunofluorescence staining. In nontransfected and control-transfected cells, 2 subpopulations of LIMK-1 expressing OPCs were observed. In the majority of cells, signals could be detected in cellular processes and soma (including cell nucleus; Fig. 5 C and C′), whereas a minority of cells displayed strong nuclear signals and reduced LIMK-1 in processes (Fig. 5 B and B′). Following p57kip2 overexpression, a significant increase in the number of cells with nuclear signals could be observed (Fig. 5A), indicating that in oligodendroglial cells p57kip2 can translocate LIMK-1.
Fig. 5.
p57kip2 overexpression leads to nuclear accumulation of LIMK-1. (A) Quantitative determination of the degree of nuclear LIMK-1-expressing oligodendrocytes in control-transfected (black bar) versus p57kip2-overexpressing OPCs (gray bar) [One representative experiment of 2 shown; data are mean values ± SEM (t test: ∗∗∗, P < 0.001)]. (B and C) Anti-LIMK-1 immunostainings of cultured oligodendroglial cells 2 days following differentiation onset are shown. (C) Most of the control-transfected cells showed a widespread LIMK-1 expression. (B) On p57kip2 overexpression, more cells showed strong nuclear and reduced or absent LIMK-1 signals in processes. Arrows mark nuclear and arrowheads mark localization in processes. [Scale bar, 50 μm (B).]
Discussion
We demonstrate that expression of the p57kip2 gene is transiently down-regulated under inflammatory, demyelinating, pathophysiological conditions before the onset of disease remission. Mimicking down-regulation by means of long-term shRNA dependent suppression demonstrated that differentiation of oligodendroglial cells is accelerated in response to lowered p57kip2 levels. Our previous studies revealed a similar function in the related Schwann cell lineage (19), and together these data strongly suggest that p57kip2 encodes an intrinsic inhibitor of myelinating glial cell differentiation.
Whereas cultured Schwann cells are not able to differentiate spontaneously, at least when p57kip2 levels are unchanged, they readily do so in the lesioned or diseased PNS, which contributes to successful nerve repair (28). Cultured OPCs can differentiate but in vivo their capacities to adapt and to de- and redifferentiate are highly limited, resulting in conduction deficits and subsequent axonal degeneration (1, 2). Apart from inhibitory influences from the surrounding tissue, intrinsic blockades such as that provided by p57kip2 may also account for these differences between glial cell types (6, 7) and the ability to control inhibitor expression levels may dictate whether remyelination and repair can occur. Considering that substantial regulation by p57kip2 is only seen in early disease stages, it could be responsible for the limited regeneration capacity of the inflamed CNS seen later in disease. These data all suggest that disease-stage specific regulation of p57kip2 could constitute a promising approach for future remyelination therapies.
In a recent study on cultured P7-P8 optical nerve oligodendrocytes, it was shown that the induction of p57kip2 is a component of the intracellular timer mechanism that controls the differentiation onset in the presence of mitogens and on specific differentiation cues (20). We here demonstrate that the secondary decrease in p57kip2 expression is functionally coupled to the maturation process and does not only constitute the downside of the peak expression. It therefore appears that p57kip2 controls oligodendrocyte differentiation at various levels and via several pathways. The extent of up- and down-regulation is probably dependent on the OPC source and differentiation state [e.g., whether or not additional selection markers such as O4 (20) were used]. Such differences might even be more widespread among the different oligodendroglial lineages (29) and could include contributions from p21cip1 and p27kip1 (30, 31). However, precursor cell cycle control in the adult CNS appears not to depend on p57kip2 induction, because up-regulation was clearly not detected during the course of MOG-induced EAE in our studies. It is therefore possible that such a (p57kip2-dependent) timer function does not act on adult OPC differentiation and that adult remyelination is primarily regulated by epigenetic mechanisms (32).
In contrast to our data, Dugas and colleagues (20) reported a negative effect on OPC differentiation of siRNA-dependent suppression in presence of growth factors, an effect which was substantially reduced on withdrawal of growth factors. Under these conditions, combined suppression of p57kip2 and p27kip1 appeared to have a much larger effect, supporting the idea that several CDKIs act together. Our findings, on the other hand, show that p57kip2 suppression promotes maturation (Figs. 3 and 4). Although it cannot be excluded that OPCs from different sources respond differently to RNA interference, this difference could be simply technical because we were neither able to stably suppress p57kip2 expression by using siRNAs in primary Schwann cells (19) nor in OPCs, and instead used shRNA-encoding constructs. However, for both procedures, we used identical interference sequences recognizing all known cDNA sequences of the rat p57kip2 gene. This DNA approach was previously shown to be specific for p57kip2 and free from off-target effects (19) and we verified that suppression was achieved and also maintained (Fig. 2). It thus appears that a sustained down-regulation is imperative for an effect on OPC differentiation and that the vector based shRNA approach is highly efficient.
Because we found that long-term p57kip2 suppression accelerates differentiation parameters under a number of different culture conditions, including that used for P7-P8 optical nerve cells (20), it is unlikely that functional differences were because of different culture media compositions.
A general assumption is that mature oligodendrocytes are not able to de- and redifferentiate and that therefore remyelination must be a consequence of resident precursor cell activation. It was therefore surprising to see that almost all oligodendroglial cells within the spinal cord clearly down-regulated p57kip2, independent on whether they were mature or precursor cells or whether they were close to immune infiltrates. In light of this strong overall regulation in early phases of MOG-induced EAE, one could speculate that in such situations the majority of cells at least attempt to attain a cellular state during which redifferentiation and repair is facilitated. Whether the observed overall down-regulation of p57kip2 in early phases allows both cell types (resident precursor cells as well as CC1-positive oligodendrocytes) to adapt, at least partially, with precursor cells being more efficient or successful in executing differentiation, is currently unknown and awaits functional experiments in vivo.
Down-regulation of p57kip2 in vivo appears to be a consequence of inflammation. Nevertheless, our data suggest that either the signaling molecules controlling p57kip2 expression differ at different stages of disease or that the oligodendroglial capacity to respond diminishes over time. Either mechanism could be responsible for the observation that less regulation can be seen in later attacks and during progression to more chronic stages. It is also not known what signals account for this overall p57kip2 regulation during inflammation. The identification of such presumably diffusing regulatory factors could promote the development of p57kip2-based remyelination therapies, and thus provide an alternative approach to the currently available in vivo gene suppression approaches (33).
Our overexpression studies indicate that p57kip2 interacts with LIMK-1. It is therefore conceivable that subtle changes of p57kip2 levels, such as on down-regulation in vivo or in cultured cells, could regulate the subcellular distribution of LIMK-1 affecting the equilibrium of enzymes and regulators that control cytoskeletal dynamics (34). Down-regulation of p57kip2 could thus moderately increase cytosolic levels of LIMK-1, leading to increased cofilin phosphorylation and inactivation and thus promote actin filament growth and stabilization, resulting in the observed oligodendroglial morphological changes. It is tempting to speculate that such cell specific regulations of ubiquitously expressed enzymes might be involved in cell differentiation and regeneration as they occur in demyelinating diseases. Whether or not p57kip2 is the only means to control LIMK-1 activity remains to be addressed by future experiments. In addition, future experimental approaches will also be needed to understand how down-regulation of a single gene can affect multiple cellular parameters. These studies will reveal the extent of induced alterations in gene expression, whether or not they are secondary to morphological events and whether interactions with related LIM proteins account for these additional aspects of glial differentiation.
In summary, we have shown that a sustained reduction of p57kip2 expression levels in oligodendroglial cells facilitates and promotes their differentiation. This observation indicates that p57kip2 is a regulator of (re)myelination at the interface between morphogenesis and gene expression. This outcome is of particular interest regarding our still-limited understanding of (re)myelination mechanisms and will be important in defining novel strategies to promote endogenous remyelination and CNS repair. In this regard, it will be imperative to determine to what degree p57kip2 expression directly affects (re)myelination in vivo. Because p57kip2 knockout mice suffer from a number of tissue defects and die around birth (35), such functional studies can only been carried out once suitable mouse mutants have been generated.
Materials and Methods
MOG-Induced Experimental Autoimmune Encephalomyelitis (MOG-EAE).
All rat experiments were performed in accordance with institutional guidelines. MOG-EAE was induced in 10- to 14-week-old female DA (RT1av1) rats (Harlan) by active immunization with the recombinant MOG protein corresponding to the N-terminal sequence of rat MOG (amino acids 1–125) in complete Freund's adjuvans, as described previously (36). The clinical status of the animals was scored as follows: 0 = no clinical signs; 1 = loss of tail tone; 2 = complete tail paresis; 3 = hind limb weakness; 4 = complete hind limb paraplegia; 5 = tetraparesis; 6 = moribund state; and 7 = death. Animals were followed for a maximum of 35 days and developed a multiphasic disease course with 2 episodes reaching their peak at days 11–12 and 21–22, respectively (see Fig. 1B). For isolation of total RNA, animals were killed at days 9, 11, 18, 22, and 28 after immunization (n = 3–4 per time point), corresponding to onset and peak of the first (days 9, 11) and second episode (days 18, 22), and the time point of remission from the second episode (day 28), respectively. As controls, naïve nonimmunized DA rats (n = 3) were used.
Oligodendroglial Cell Culture.
Purification and culturing of OPCs was performed according to (37). Briefly, dissociated P1 rat cortices were cultured on polyD-lysine (PDL)-coated cell culture flasks in DMEM substituted with 10% FCS and 4 mM L-glutamine. After 10 days, flasks were shaken at 250 rev/min for 2 h to deplete from microglial contamination. Then flasks were shaken for another 20 h in which OPCs were dislodged from the underlying astrocyte-layer and replated on PDL-coated culture dishes or glass cover slips in high glucose DMEM-Sato-based medium containing bovine 5 μg/mL insulin, 50 μg/mL human transferrin, 100 μg/mL BSA, 6.2 μg/mL progesterone, 16 μg/mL putrescine, 5 ng/mL sodium selenite, and 4 mM L-glutamine (all Sigma). Anti-A2B5 staining revealed that at this point the cultures consisted of 98% oligodendroglial cells. OPCs were either kept in proliferation medium (Sato medium with 10 ng/mL bFGF and 10 ng/mL PDGF-AA; R&D Systems and Peprotech), whereas differentiation was initiated by Sato medium which was depleted from growth factors and supplemented with either 0,5% FCS, 10 ng/mL CNTF (Chemicon) or 400 ng/mL T3/T4 thyroid hormones (Sigma). Generation and transfection of pSUPER based suppression vectors (OligoEngine) or pIRES2EGFP based expression vectors (BD Biosciences) were described previously (19). Isolation of citrine positive OPCs was done by fluorescence activated cell sorting (FACS Aria, BD Biosciences).
Immunostaining.
Immunostaining on paraffin sections from paraformaldehyde-perfused rat spinal cords or paraformaldehyde-fixed cultured cells was performed as described previously (19). Primary antibodies were diluted as follows: rabbit anti-p57kip2 antibody (1/200; Sigma-Aldrich), mouse anti-APC/CC1 (1/1,000; Calbiochem), mouse anti-O4-, mouse anti-PDGFR-α-, mouse anti-A2B5 antibodies (1/100, 1/300, and 1/200, respectively; all Chemicon), mouse anti-MOG antibody (1/1,000; B. Hemmer), mouse anti-MBP- and mouse anti-CNPase antibodies (1/1,000 and 1/500, respectively; both Sternberger Monoclonals) and rabbit anti-LIMK-1 (1/500; BD Biosciences). Alexa Fluor 488-, Alexa Fluor 594-, or horseradish peroxidase-conjugated antibodies (1/500; all Molecular Probes) were used for signal visualization. Nuclei were stained with DAPI (Roche).
RNA Preparation, cDNA Synthesis, and Quantitative RT-PCR.
For purification of total RNA from spinal cord and cultured cells, we used the TRIzol reagent (Invitrogen) and the RNeasy procedure (Qiagen), respectively, following the protocols of the suppliers. Isolated RNA was reverse transcribed by using the high capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative determination of gene expression levels was performed on an ABI 7000 sequence detection system by using Power SybrGreen universal master mix (Applied Biosystems). Primer sequences were determined by means of PrimerExpress 2.0 software (Applied Biosystems) and subsequently tested for the generation of specific amplicons (for sequences see ref. 19). GAPDH and ODC were used as reference genes, and relative gene expression levels were determined according to the manufacturer's ΔΔCt method (Applied Biosystems). Each sample was measured in quadruplicate; data are shown as mean values ± SEM.
Acknowledgments.
We thank B. Blomenkamp and S. Hamm for technical assistance, Dr. B. Hemmer (Technische Universität München, Klinikum rechts der Isar) for providing the MOG antibody, Dr. J. Relvas and Dr. U. Suter for helpful discussions, and Dr. S. Reingold for critical review of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB590) and from the Research Commission of the Medical Faculty and the Research Promotion Fund (both Heinrich-Heine University, Düsseldorf, Germany).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123:1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- 2.Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 3.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 4.Hisahara S, Okano H, Miura M. Caspase-mediated oligodendrocyte cell death in the pathogenesis of autoimmune demyelination. Neurosci Res. 2003;46:387–397. doi: 10.1016/s0168-0102(03)00127-5. [DOI] [PubMed] [Google Scholar]
- 5.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhlmann T, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 7.Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gokhan S, et al. Combinatorial profiles of oligodendrocyte-selective classes of transcriptional regulators differentially modulate myelin basic protein gene expression. J Neurosci. 2005;25:8311–8321. doi: 10.1523/JNEUROSCI.1850-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K. Inhibition of Notch signaling enhances tissue repair in an animal model of multiple sclerosis. J Neuroimmunol. 2005;170:3–10. doi: 10.1016/j.jneuroim.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T, Raff M. The Id4 HLH protein and the timing of oligodendrocyte differentiation. EMBO J. 2000;19:1998–2007. doi: 10.1093/emboj/19.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu A, et al. A molecular insight of Hes5-dependent inhibition of myelin gene expression: Old partners and new players. EMBO J. 2006;25:4833–4842. doi: 10.1038/sj.emboj.7601352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi S, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Sdrulla A, Johnson JE, Yokota Y, Barres BA. A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron. 2001;29:603–614. doi: 10.1016/s0896-6273(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Liu Y, Levine EM, Rao MS. Hes1 but not Hes5 regulates an astrocyte versus oligodendrocyte fate choice in glial restricted precursors. Dev Dyn. 2003;226:675–689. doi: 10.1002/dvdy.10278. [DOI] [PubMed] [Google Scholar]
- 15.Engel U, Wolswijk G. Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells derived from adult rat spinal cord: In vitro characteristics and response to PDGF, bFGF, and NT-3. Glia. 1996;16:16–26. doi: 10.1002/(SICI)1098-1136(199601)16:1<16::AID-GLIA3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Storch MK, et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–694. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds R, et al. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- 18.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: Cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Heinen A, et al. The cyclin-dependent kinase inhibitor p57kip2 is a negative regulator of Schwann cell differentiation and in vitro myelination. Proc Natl Acad Sci USA. 2008;105:8748–8753. doi: 10.1073/pnas.0802659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dugas JC, Ibrahim A, Barres BA. A crucial role for p57(Kip2) in the intracellular timer that controls oligodendrocyte differentiation. J Neurosci. 2007;27:6185–6196. doi: 10.1523/JNEUROSCI.0628-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billon N, et al. Roles for p53 and p73 during oligodendrocyte development. Development. 2004;131:1211–1220. doi: 10.1242/dev.01035. [DOI] [PubMed] [Google Scholar]
- 22.Tokumoto YM, Tang DG, Raff MC. Two molecularly distinct intracellular pathways to oligodendrocyte differentiation: Role of a p53 family protein. EMBO J. 2001;20:5261–5268. doi: 10.1093/emboj/20.18.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- 24.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 25.Stankoff B, et al. Ciliary neurotrophic factor (CNTF) enhances myelin formation: A novel role for CNTF and CNTF-related molecules. J Neurosci. 2002;22:9221–9227. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoo T, et al. p57Kip2 regulates actin dynamics by binding and translocating LIM-kinase 1 to the nucleus. J Biol Chem. 2003;278:52919–52923. doi: 10.1074/jbc.M309334200. [DOI] [PubMed] [Google Scholar]
- 27.Heinen A, Kremer D, Hartung HP, Küry P. p57(kip2)'s role beyond Schwann cell cycle control. Cell Cycle. 2008;7:2781–2786. doi: 10.4161/cc.7.18.6629. [DOI] [PubMed] [Google Scholar]
- 28.Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 29.Kessaris N, et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durand B, Fero ML, Roberts JM, Raff MC. p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr Biol. 1998;8:431–440. doi: 10.1016/s0960-9822(98)70177-0. [DOI] [PubMed] [Google Scholar]
- 31.Zezula J, et al. p21cip1 is required for the differentiation of oligodendrocytes independently of cell cycle withdrawal. EMBO Rep. 2001;2:27–34. doi: 10.1093/embo-reports/kve008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen S, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar P, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 34.Arber S, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- 36.Schroeter M, et al. CD8+ phagocyte recruitment in rat experimental autoimmune encephalomyelitis: Association with inflammatory tissue destruction. Am J Pathol. 2003;163:1517–1524. doi: 10.1016/S0002-9440(10)63508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy KD, de VJ. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]