Abstract
Inflammatory caspases are important effectors of innate immunity. Caspase-12, of the inflammatory caspase subfamily, is expressed in all mammals tested to date, but has acquired deleterious mutation in humans. A single-nucleotide polymorphism introduces a premature stop codon in caspase-12 in the majority of the population. However, in 20% of African descendants, caspase-12 is expressed and sensitizes to infections and sepsis. Here, we examined the modalities by which human caspase-12 confers susceptibility to infection. We have generated a fully humanized mouse that expresses the human caspase-12 rare variant (Csp-12L) in a mouse casp-12−/− background. Characterization of the humanized mouse uncovered sex differences in Csp-12L expression and gender disparity in innate immunity to Listeria monocytogenes infection. The Csp-12L transgene completely reversed the knockout resistance-to-infection phenotype in casp-12−/− males. In contrast, it had a marginal effect on the response of female mice. We found that estrogen levels modulated the expression of caspase-12. Csp-12L was expressed in male mice but its expression was repressed in female mice. Administration of 17-β-estradiol (E2) to humanized male mice had a direct suppressive effect on Csp-12L expression and conferred relative resistance to infection. Chromatin immunoprecipitation experiments revealed that caspase-12 is a direct transcriptional target of the estrogen receptor alpha (ERα) and mapped the estrogen response element (ERE) to intron 7 of the gene. We propose that estrogen-mediated inhibition of Csp-12L expression is a built-in mechanism that has evolved to protect females from infection.
Keywords: SNP, estrogen, inflammatory caspase, innate immunity
Caspases are proteases known for their functions in apoptosis and inflammatory cascades. The central inflammatory caspase, caspase-1, is activated within the inflammasome, a large complex scaffolded by Nod-like receptors (NLR) in response to pathogen invasion or host-derived danger signals (1). Through proteolysis of specific substrates (2), caspase-1 mediates acute inflammation (3), secretion of “alarmins” (4), and an inflammatory form of cell death termed pyroptosis (5, 6), essential processes required for pathogen clearance. The activity of caspase-1 is regulated by related inflammatory caspases, namely caspases-5 and -11, which activate select inflammasomes (7, 8), and caspase-12, which represses caspase-1 catalysis (9).
In humans, caspase-12 has acquired multiple mutations (10). Most notably, a SNP introduces a premature stop codon in exon 4 in the majority of the population. We refer to this common allele as Csp-12S. However, in 20% of African descendants, an arginine replaces the stop, allowing the expression of a full-length protein (Csp-12L) (11, 12). Our previous work has shown that Csp-12L dampens endotoxin responsiveness by blunting the inflammatory response to lipopolysaccharide (LPS). In addition, it has established an association between Csp-12L and susceptibility to severe sepsis and sepsis lethality (11). The function of Csp-12L in blunting immunity is conserved in rodents. Indeed, casp-12−/− mice are resistant to sepsis and clear systemic infections with Listeria monocytogenes more efficiently than wild-type animals (9).
The host response to L. monocytogenes is initiated by the stimulation of pattern recognition receptors (PRRs), which induce the production of antimicrobials and inflammatory cytokines and chemokines. L. monocytogenes is initially recognized by Toll-like receptors (TLRs) at the surface of phagocytes (13). Following phagocytosis and escape into the cytosol, it is sensed by NLRs, namely the Nod1, Ipaf, and Nalp3 inflammasomes (14, 15). The concerted actions of these pathways translate into a robust sterilizing immunity and enhanced pathogen elimination (16).
It is well established that the gender of a host can significantly affect susceptibility to infection (17). Epidemiological clinical data and animal models of various human illnesses including sepsis (18) and listeriosis (19, 20) reveal that males and females handle infections differently.
In this study, we report the characterization of an experimental animal model of the human caspase-12 polymorphism and show that increased resistance to L. monocytogenes correlates with depression of Csp-12L expression by estrogen.
Results and Discussion
Nonsense-Mediated mRNA Decay Targets Csp-12S for Degradation.
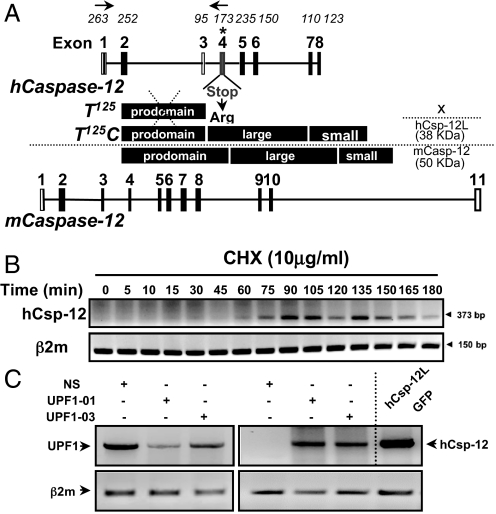
Before reconstructing the human caspase-12 polymorphism in an animal model, we examined whether the SNP in Csp-12S results in the synthesis of a stable and functional CARD-only protein (Fig. 1A). Therefore, we raised antibodies directed against the human caspase-12 prodomain and probed for expression in various tissues and cell lines. We consistently failed to detect a truncated form of caspase-12 (data not shown). We next examined whether the presence of the premature stop codon in Csp-12S targeted its product for degradation by nonsense-mediated mRNA decay (NMD). NMD is a conserved eukaryotic quality-control mechanism that detects and rapidly degrades aberrant mRNAs harboring premature termination codons (ref. 21 and references therein). NMD occurs if translation terminates prematurely 50–55 nucleotides upstream of an exon–exon junction (22). The human Csp-12 premature stop codon is located within the theoretical limits of NMD, 57 nucleotides upstream of the junction between exons 4 and 5. To examine whether NMD degrades Csp-12S mRNA, we treated HeLa cells, which are genotypically Csp-12 S/S, with the protein synthesis inhibitor cycloheximide for different time points. Inhibition of translation blocks NMD and results in accumulation of mRNAs that are otherwise degraded by this surveillance mechanism. Fig. 1B examines caspase-12 mRNA stability by reverse transcriptase (RT)-PCR and shows message accumulation following cycloheximide treatment, suggesting that NMD targets human Csp-12S. To confirm this result, we used RNA interference (RNAi) to deplete the NMD core factor Up-frameshift protein 1 (Upf1). Fig. 1C shows that knockdown of Upf1, using 1 of 2 different specific siRNAs, abolished the degradation of Csp-12S mRNA. Altogether, our results show that Csp-12S does not encode a CARD-only protein as previously predicted and is irrelevant in humans because its mRNA is rapidly turned over by NMD.
Fig. 1.
NMD targets the Csp-12S message for degradation. (A) Exon–intron organization of human and mouse caspase-12 genes. Numbers above the human Csp12 gene structure correspond to exon lengths in base pairs and the asterisk indicates the SNP. Domain structures of the predicted Csp-12S, Csp-12L, and mouse caspase-12 are depicted. (B) Kinetics of Csp-12S mRNA accumulation following treatment with cycloheximide. mRNA levels were analyzed by RT-PCR using the primers depicted in A. β2-microglobulin (β2m) mRNA was used as a standardizing control. (C) RT-PCR analysis of the levels of UPF1 mRNA (Left) and Csp-12 mRNA (Right) in HeLa cells following knockdown of UPF1. mRNA from HEK293T cells expressing a fusion protein between Csp-12L and GFP was used as a positive control.
Generation of a Csp-12L Humanized Mouse.
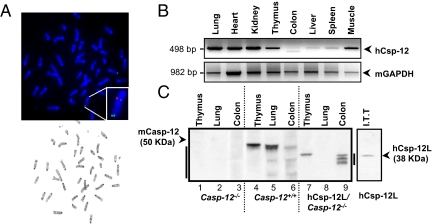
To better understand the role of the human Csp-12L variant in the host response to pathogens, and because of structural differences between human and mouse caspase-12 proteins (Fig. 1A), we generated a mouse model that reproduces the human caspase-12 polymorphism. This “humanized” mouse expresses human Csp-12L, under the control of its endogenous promoter and regulatory elements, in a mouse caspase-12-deficient background. Supporting information (SI) Fig. S1A depicts the bacterial artificial chromosome (BAC) that was used to generate the founder transgenic mouse. To obtain the humanized mouse (casp-12−/−/Csp-12L), Csp-12L BAC transgenic mice were bred onto a casp-12−/− background, following the breeding scheme in Fig. S1B. Integration of the transgene was verified by PCR genotyping and Southern blot analysis (Fig. S1C). To map the exact transgene chromosomal insertion site, we performed sequential G-banding to FISH analysis, which revealed 1 integration site on mouse chromosome 6 (Chr 6qB1/B2) (Fig. 2A). We next examined transgene expression by RT-PCR (Fig. 2B). Interestingly, the expression pattern of Csp-12L mirrored that of mouse caspase-12 in the majority of tissues tested (Fig. 2B and ref. 23), suggesting some conserved regulatory elements between mouse and human promoter/enhancer regions. Multiple splice variants have been identified for human caspase-12 (10). Using antibodies directed against the large subunit of mouse caspase-12, we have previously been able to detect 2 splice variants that migrated as a doublet at ≈38 KDa, in human blood of Csp-12S/L and Csp-12L/L donors (11). Using the same antibodies, we examined Csp-12L protein levels in humanized mouse tissues (10, 33). Mouse caspase-12 was expressed in tissues derived from wild-type but not casp-12−/− or humanized mice (Fig. 2C), and Csp-12L was expressed only in tissues from the humanized mouse (Fig. 2C, lanes 7–9). Consistent with our previous findings in Csp-12S/L and Csp-12L/L donors, we detected multiple Csp-12L splice variants migrating at the 38-kDa level in humanized mouse tissues (e.g., in the colon; Fig. 2C, lane 9).
Fig. 2.
Generation of a humanized mouse expressing the human Csp-12L variant. (A) G-banding to FISH on a primary fibroblast culture derived from a female casp-12−/−/Csp-12L mouse. The human Csp-12L BAC was labeled with Spectrum Orange (red signal) and a control BAC probe for mouse chromosome 6 was labeled with Spectrum Green (green signal). The Csp-12L transgene expression pattern in mouse tissues was examined by RT-PCR (B) and Western blot (C). The right arrowhead in C indicates an in vitro transcribed and translated Csp-12L.
Gender Disparity in Innate Immunity to L. monocytogenes in the Csp-12L Humanized Mouse.
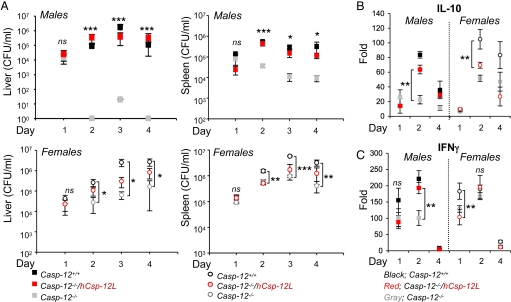
To examine the effect of the transgene on complementing the casp-12−/− mouse phenotype and investigate the function of Csp-12L in inflammation and innate immunity in this model, we subjected wild-type, casapse-12-deficient, and humanized mice to systemic infection with L. monocytogenes. As previously reported (9), casp-12−/− mice were resistant to L. monocytogenes compared to wild-type mice and had lower bacterial content in the spleen and liver at different time points postinfection (pi) (Fig. 3A). Interestingly, the Csp-12L transgene completely reversed the resistance phenotype of male but not female casp-12−/− mice, rendering humanized male mice as susceptible to infection as wild-type male mice (Fig. 3A). On the contrary, on days 2 and 3 pi, humanized female mice remained resistant to L. monocytogenes similar to casp-12−/− female mice, with bacterial content in the spleen and liver significantly lower than that of wild-type female mice. On day 4 pi, the phenotype of humanized female mice became intermediate between that of wild-type and casp-12−/− female mice (Fig. 3A).
Fig. 3.
Csp-12L compensates for loss of mouse caspase-12 in male but not female mice. (A) Casp-12+/+, casp-12−/−, and Csp-12L/casp-12−/− (n = 4/genotype) were challenged intravenously with 5 × 104 CFU of L. monocytogenes, and pathogen clearance was determined in livers and spleens of male and female mice on different days pi. (B and C) RNA extracted from spleens of male and female mice on different days pi with L. monocytogenes was analyzed by qPCR for IL-10 (B) and IFNγ (C) expression. Data represent average expression ± SEM from 4 different mice.
It is well documented that sex-dependent factors affect susceptibility to infection (17–19). This difference in susceptibility is attributed to the effects sex hormones exert on regulating genes involved in inflammation and immunity (24, 25). The increased susceptibility of female mice to L. monocytogenes has been linked to enhanced production of the immunosuppressive cytokine IL-10 and downregulation of the protective cytokine IFNγ by estrogen (ref. 20 and references therein). To examine the effects of Csp-12L on the production of these cytokines, we quantified their levels in the serum of infected mice. Our results show that female mice failed to induce elevated levels of IFNγ despite their higher bacterial burden compared to male mice, but had higher levels of IL-10 (Fig. 3 B and C). Interestingly, similar to what we have observed when assessing bacterial content, Csp-12L had a striking effect on cytokine production in male but not female mice, such that levels of both IL-10 and IFNγ in humanized male mice were comparable to those of wild-type male mice while for females, the humanized mice had similar levels to caspase-12 deficient mice.
The Suppressive Effect of Csp-12L on Bacterial Clearance Is Mediated by Inhibition of Cytokine Production.
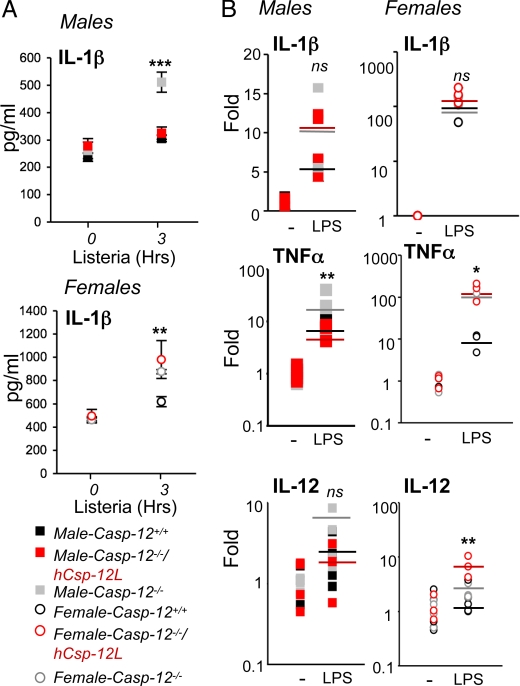
We have previously shown that mouse caspase-12 inhibited caspase-1 catalysis within the inflammasome (9). To examine whether Csp-12L had a similar effect on the inflammasome in response to L. monocytogenes, we infected splenocytes derived from wild-type, casp-12−/−, or humanized mice with L. monocytogenes at a multiplicity of infection (MOI) of 50:1 (bacteria:cell) and measured levels of IL-1β secreted in the media 3 h pi. Fig. 4A shows enhanced IL-1β secretion by casp-12−/− splenocytes compared to wild-type cells from both male and female mice. Interestingly, and consistent with the bacterial clearance results, the presence of the Csp-12L transgene reversed the casp-12−/− IL-1β hyperproduction phenotype in cells derived from humanized male but not female mice (Fig. 4A). These results indicated that, similar to mouse caspase-12, Csp-12L exerts a suppressive effect on the inflammasome. Notably, we examined the levels of the pro-IL1β message among the 3 genotypes and did not find a significant difference among genotypes in response to infection with L. monocytogenes (data not shown). Similarly, the regulation of pro-IL-1β expression was not different in response to treatment of splenocytes from the 3 genotypes and the 2 sexes with LPS (Fig. 4B). TNFα and IL-12, on the other hand, were more robustly induced by casp-12−/− splenocytes compared to wild-type cells, and this induction was repressed by Csp-12L in cells derived from humanized male mice (Fig. 4B).
Fig. 4.
Csp-12L dampens cytokine production. (A) Splenocytes from male and female mice (n = 4/genotype/sex) were primed overnight with 50 ng/mL of pure LPS and then infected for 3 h with L. monocytogenes. Supernatants were collected and IL-1β levels were determined by ELISA. (B) Splenocytes were treated for 1 h with 1 μg/mL of crude LPS. mRNA was extracted and pro-IL-1β, TNFα, and IL-12 levels were measured by qPCR.
The Expression of the Human caspase-12 Gene Is Modulated by Estrogen.
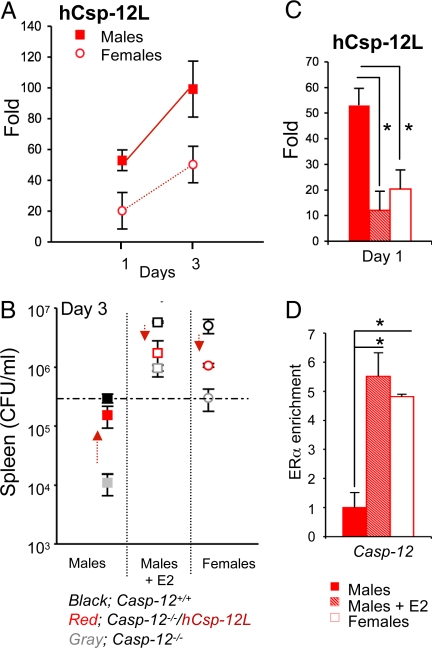
Altogether, our results suggested that while Csp-12L was produced in males, its expression might have been repressed in female mice, supporting the lack of its suppressive effect on cytokine induction and pathogen clearance in this group. We thus hypothesized that estrogen might modulate the expression of Csp-12L and consequently host response to infection. To test this hypothesis, we first examined the levels of Csp-12L in humanized male and female mice throughout the infection. At equivalent bacterial load, on day 1 pi (Fig. 3A), the expression of Csp-12L was clearly repressed by 17-β-estradiol (E2), as it was highest in males compared to females (Fig. 5A). It has been previously reported that LPS and IFNγ regulate caspase-12 expression (9, 23), which is consistent with an induction of Csp-12L during the infection. Nonetheless, Csp-12L levels remained lower in female mice compared to male mice even at the peak of the infection on day 3 pi (Fig. 5A). Interestingly, as Csp-12L levels increased in humanized female mice, they became more susceptible to infection (Fig. 3A, Lower panels, day 4 pi). To examine whether estrogen had a direct suppressive effect on Csp-12L expression, we injected male mice i.p. with E2 and monitored Csp-12L levels and bacterial content at the peak of infection. E2 administration to male mice of all 3 genotypes augmented their susceptibility to L. monocytogenes and increased bacterial load to levels equivalent to those found in female mice (Fig. 5B). However, as described above, within the female group, the humanized mice behaved similar to casp-12−/− mice in that they were both significantly more resistant to infection compared to wild-type mice. Similarly, in the E2-treated group, the humanized mice were as resistant to infection as casp-12−/− mice. Quantification of Csp-12L levels indicated repression of Csp-12L expression by E2 in the treated male humanized mice (Fig. 5C). Therefore, estrogen exerts 2 independent effects in this model, the first enhancing the susceptibility to infection and the second repressing expression of Csp-12L. Because Csp-12L is a negative regulator of bacterial clearance, the net estrogen-dependent increase in susceptibility to L. monocytogenes is less pronounced in female humanized mice (or males injected with E2), in which bacterial clearance is derepressed by inhibition of Csp-12L. It is thus conceivable that repression of Csp-12L expression by estrogen has evolved to protect the susceptible (and childbearing) sex from infection. Interestingly, it appears that this regulatory mechanism is confined to human, but not mouse caspase-12. Quantification of mouse caspase-12 levels in wild-type male and female mice showed equivalent levels of expression between the 2 sexes throughout infection with L. monocytogenes (Fig. S2A). Moreover, injection of wild-type male mice with E2 did not exert any significant repressive effects on mouse caspase-12 expression (Fig. S2A).
Fig. 5.
Estrogen levels control human caspase-12 expression. (A) Csp-12L expression levels were quantified by qPCR in splenocytes derived from male and female humanized mice on days 1 and 3 pi. (B) E2 was injected i.p. 4 h before infection and was injected daily for 3 days pi. On day 3 pi, bacterial loads were determined in mice treated or not with E2. Data represent average expression ± SEM from 4 mice. (C) The Csp-12L transgene is expressed in the spleen of male mice infected with L. monocytogenes but its expression is repressed in females and males injected with E2. (D) ChIP was performed using anti-mouse ERα or IgG control antibodies in splenocytes derived from humanized mice (males, males treated with E2, or females), and pull-down of human casp-12 was determined by qPCR. Data represent average expression ± SEM from 2 different mice.
To further characterize whether the estrogen effect on human casp-12 was direct, we performed conventional ChIP experiments with anti-estrogen receptor alpha (ERα) antibodies on samples derived from splenocytes of humanized female mice or males treated with E2 and showed that ERα is enriched on the human caspase-12 gene (Fig. 5D). Our results are consistent with a previous report of genomewide analysis of ERα binding sites in MCF7 human breast cancer cells (26) that pulled down the caspase-12 gene as a transcriptional target of ERα (Fig. S3A). On the basis of the ChIP results, a putative estrogen receptor element (ERE) was found embedded in intron 7 of the human caspase-12 gene (Fig. S3B). Consistent with a lack of regulation of mouse caspase-12 by estrogen, alignment of human and mouse caspase-12 genomic DNA sequences indicated that this ERE is not conserved in the mouse caspase-12 gene (Fig. S2B). Taken together, these results suggest direct regulation of human caspase-12 by estrogen and ERα.
In summary, we have generated an animal model that replicates the human caspase-12 polymorphism. Our results indicate that despite having evolved multiple polymorphisms and structural alterations, human Csp-12L is functionally related to its murine ortholog. BAC transgenic expression of Csp-12L compensated for loss of mouse caspase-12 and completely reversed the resistance-to-infection phenotype of casp-12−/− mice. Interestingly, we uncovered an unsuspected hormonal regulatory mechanism that governs human Csp-12L expression during infection. Our results indicate that through estrogen production, females have a built-in mechanism that prevents Csp-12L from being expressed, favoring more robust inflammatory and immune responses to pathogens. Our findings that E2 administration inhibited Csp-12L expression and conferred resistance to infection may have important therapeutic implications for managing infectious diseases in the African population.
Materials and Methods
Assessment of hCsp-12S mRNA Decay.
HeLa cells were treated with 10 μg/ml of cycloheximide for different time points (0–180 min) or were transiently transfected with 100 nM of siRNA (Dharmacon, D-011763–01 and D-011763–03) for 48 h. Nonsilencing siRNA was used as a negative control (QIAGEN, no. 1022076). RNA was extracted from cells and reverse transcribed to cDNA, and semiquantitative RT-PCR was then performed using primers listed in Table S1.
L. monocytogenes Challenge and Estradiol Treatment of Mice.
For in vivo infections, L. monocytogenes strain 10403s was grown to midlogarithmic phase (OD600 of 0.1 = 4 × 108 CFU/mL). Approximately 5 × 104 CFU in 200 μL PBS were injected into the lateral tail vein. For in vivo experiments with E2, mice received a daily i.p. injection of E2 (50 μg/kg) diluted in 200 μL corn oil. On days 1, 2, 3, and 4 pi, spleens and livers were collected and were homogenized in a 10-mL volume of a 0.2% Nonidet P-40 solution. Serial dilutions (1:10, 1:100, 1:1,000) were plated on tryptic soy agar and bacterial colonies were enumerated.
Splenocyte Culture and Infection.
Splenocytes were treated for 1 h with 1 μg/mL Escherichia coli LPS (serotype 055:B5). IL-1β, TNFα, and IL-12 levels were quantified by qPCR. For mouse IL-1β production, cells were primed overnight with 50 ng/mL LPS and then infected for 3 h with L. monocytogenes at a MOI of 50 (bacteria:cell). Ninety minutes pi, 20 μg/mL of gentamicin were added on infected wells to kill extracellular bacteria.
Statistical Analysis.
Statistical analysis was performed using ANOVA and an unpaired Student's t test.
SI.
More detailed methods are found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Dr. Vincent Giguere (McGill University) for advice with the ChIP experiments. This work was supported by grants from the Canadian Institutes for Health Research and the Canadian Foundation for Innovation (to M.S.). M.S. is a Canadian Institutes for Health Research New Investigator.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.R.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813362106/DCSupplemental.
References
- 1.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: A danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Shao W, et al. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 3.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: Two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 4.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 5.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 6.Labbé K, Saleh M. Cell death in the host response to pathogens. Cell Death Differ. 2008;43:380–390. doi: 10.1038/cdd.2008.91. [DOI] [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 9.Saleh M, et al. Enhanced bacterial clearance and sepsis resistance in caspase-12 deficient mice. Nature. 2006;440:1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 10.Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun. 2002;293:722–726. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- 11.Saleh M, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–79. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- 12.Xue Y, et al. Spread of an inactive form of caspase-12 in humans is due to recent positive selection. Am J Hum Genet. 2006;78:659–670. doi: 10.1086/503116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 14.Opitz B, et al. Listeria monocytogenes activated p38 MAPK and induced IL-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J Immunol. 2006;176:484–490. doi: 10.4049/jimmunol.176.1.484. [DOI] [PubMed] [Google Scholar]
- 15.Warren SE, et al. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leber JH, et al. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4:e6. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terres G, Morrison SL, Habicht GS. A quantitative difference in the immune response between male and female mice. Proc Soc Exp Biol Med. 1968;127:664–667. doi: 10.3181/00379727-127-32768. [DOI] [PubMed] [Google Scholar]
- 18.Beery TA. Sex differences in infection and sepsis. Crit Care Nurs Clin North Am. 2003;15:55–62. doi: 10.1016/s0899-5885(02)00028-x. [DOI] [PubMed] [Google Scholar]
- 19.Gellin BG, Broome CV. Listeriosis. JAMA. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 20.Pasche B, et al. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect Immun. 2005;73:5952–5960. doi: 10.1128/IAI.73.9.5952-5960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shyu AB, Wilkinson MF, van Hoof A. Messenger RNA regulation: To translate or to degrade. EMBO J. 2008;27:471–481. doi: 10.1038/sj.emboj.7601977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem. 2007;76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- 23.Kalai M, et al. Regulation of the expression and processing of caspase-12. J Cell Biol. 2003;162:457–467. doi: 10.1083/jcb.200303157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morell V. Zeroing in on how hormones affect the immune system. Science. 1995;269:773–775. doi: 10.1126/science.7638587. [DOI] [PubMed] [Google Scholar]
- 25.Salem ML. Estrogen, a double-edged sword: Modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004;3:97–104. doi: 10.2174/1568010043483944. [DOI] [PubMed] [Google Scholar]
- 26.Carroll JS, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.