SUMMARY
Caspase-8, an initiator caspase involved in lymphocyte apoptosis, is paradoxically required for lymphocyte proliferation. It is not understood how caspase-8 is controlled during antigenic signaling to allow for activation while averting triggering apoptosis. Here we show that caspase-8 undergoes limited activation upon antigenic stimulation, and this activation is dependent on the paracaspase MALT1. The paracaspase domain of MALT1, in a protease-independent manner, induces caspase-8 activation through direct association. MALT1 diminishes the activation of apoptotic effector caspases, but does not alter the activity of caspase-8 towards c-FLIPL, which is required for antigenic signaling. Mutants of MALT1 that fail to activate caspase-8 and permit c-FLIPL cleavage cannot facilitate NF-κB activation nor IL-2 induction. Our results reveal a mechanism that utilizes a protease potentially deadly to the cell for proliferative signaling and demonstrate a functional connection between the caspase and paracaspase families to enable non-apoptotic processes.
Introduction
Maintenance of immune homeostasis is critical for the elimination of foreign antigens while preventing auto-immunity and hyper-proliferative diseases. This balance is characterized by a rapid clonal expansion of antigen-reactive lymphocytes followed by targeted apoptosis of activated cells. Caspase-8 plays an integral role in lymphocyte apoptosis through engagement by death receptors including CD95 (Fas/Apo-1), tumor necrosis factor receptor 1 (TNFR1), and TRAIL receptors (Krammer et al., 2007). Upon death receptor stimulation, the precursor of caspase-8 (procaspase-8) is recruited to the oligomeric membrane-associated death inducing signaling complex (DISC). There procaspase-8 acquires protease activity upon dimerization (Boatright et al., 2003; Chang et al., 2003) and subsequently undergoes two auto-cleavage events via an interdimer processing mechanism to yield the active mature form (Chang et al., 2003). Activation of caspase-8 in the DISC is regulated by the proteolytically-inactive homolog c-FLIPL, which is also a caspase-8 substrate (Chang et al., 2002; Micheau et al., 2002). Mature caspase-8 is released from the DISC and trans-cleaves effector caspases such as caspase-3 and caspase-7. The effector caspases then undergo a second auto-cleavage event generating mature forms (Liu et al., 2005), which in turn cleave a large number of proteins to dismantle the cell (Figure 1A).
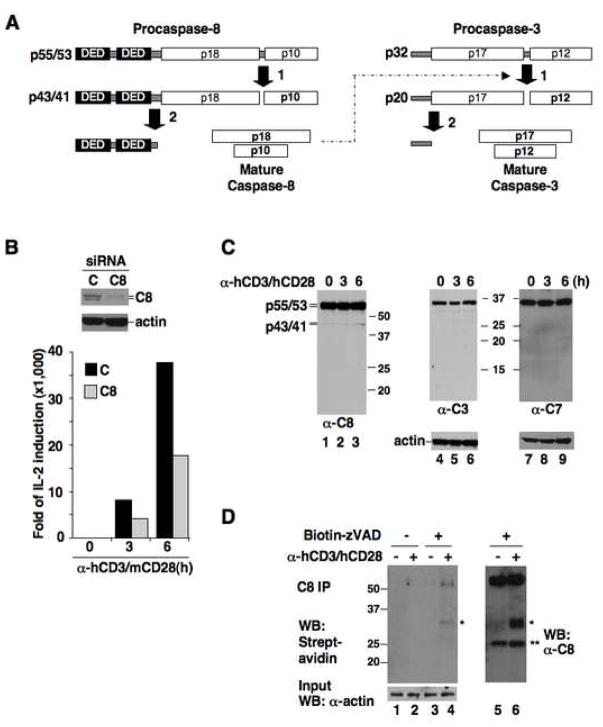
Figure 1. Proliferative function of caspase-8 is related to its activation but not full processing.
(A) Schematic of caspase-8 and caspase-3 processing.
(B) Human primary CD4+ T cells transfected with control (C) or caspase-8 (C8) siRNA plus m/hCD28 were stimulated with α-hCD3/mCD28 antibodies. Caspase-8 expression was analyzed by immunoblotting (top), and IL-2 induction by quantitative RT-PCR (bottom).
(C) CD4+ T cells were stimulated with α-hCD3/hCD28 for the indicated durations, and processing of caspase-8, caspase-3 (C3), and caspase-7 (C7) was analyzed by immunoblotting.
(D) CD4+ cells treated with α-hCD3/hCD28 were incubated with biotin-zVAD-fmk or DMSO. Lysates were immunoprecipitated with α-caspase-8 and analyzed with streptavidin-HRP (left) or α-caspase-8 (right). * uncharacterized bands. ** IgG light chain bands.
Paradoxical to its established role in lymphocyte apoptosis, caspase-8 is also essential for lymphocyte activation. Human and mouse lymphocytes defective in caspase-8 show profound defects in proliferation in response to antigen receptor engagement (Chun et al., 2002; Salmena et al., 2003), and this function of caspase-8 requires proteolytic activity (Su et al., 2005). During antigenic signaling, procaspase-8 associates with a complex formed by Bcl10 and MALT1 (Su et al., 2005), which links the receptor proximal signaling events to activation of the transcription factor NF-κB and induction of interleukin-2 (IL-2) (Thome, 2004). Chromosomal translocations resulting in up-regulation and/or gain-of-function mutations of Bcl10 and MALT1 are associated with uncontrolled lymphocyte proliferation and lymphomas (Isaacson and Du, 2004). Bcl10 is an adaptor protein that recruits MALT1 to the receptor-associated lipid rafts, while MALT1 is a member of the paracaspase family, classified by a paracaspase domain that is most similar, yet still distantly related, to the protease domain of caspases (Uren et al., 2000). Recent studies demonstrated the paracaspase domain of MALT1 possesses protease activity cleaving Bcl10 and the NF-κB inhibitor A20 (Coornaert et al., 2008; Rebeaud et al., 2008). However, the protease activity of MALT1 plays a fine-tuning rather than an essential role in antigenic signaling. Paracaspases, like caspases, are found in metazoans ranging from C. elegans to human (Uren et al., 2000), yet the functional relationship between these two related proteases remains unclear.
The dual role of caspase-8 in apoptosis and cell proliferation raises a central question as to how caspase-8 becomes activated in antigenic signaling to enable proliferative signaling while averting triggering apoptosis. In this study, we uncover a mechanism of caspase activation involving hetero-dimerization between caspase-8 and the paracaspase MALT1. The MALT1 paracaspase domain, independently of protease activity, promotes procaspase-8 to undergo limited autoproteolytic processing upon hetero-dimerization. This generates an active form of caspase-8 that exhibits diminished activity towards caspase-3. Yet, MALT1 still enables caspase-8 to cleave c-FLIPL, which akin to caspase-8 is involved in lymphocyte proliferation (Chau et al., 2005; Zhang and He, 2005). Thus, MALT1 controls caspase-8 activation to favor proliferative over apoptotic signaling.
Results
Procaspase-8 is activated but not significantly processed at early stages of T cell activation
To assess the involvement of caspase-8 processing in antigen receptor signaling, we knocked down the expression of caspase-8 in purified human primary CD4+ T cells using siRNA (Figure 1B, top panel). To limit the antigenic response to siRNA-treated cells, we also used a chimeric CD28 -- consisting of the mouse CD28 extracellular domain and the human CD28 cytoplasmic tail (m/hCD28) -- that transmits co-stimulatory signals in transfected human T cells in response to an agonistic anti-mouse CD28 antibody (Parry et al., 2003). Treatment with anti-human CD3 plus anti-mouse CD28 antibodies strongly induced the expression of IL-2 in control siRNA-treated cells as early as 3–6 h. However, IL-2 induction was significantly impaired when caspase-8 was knocked down (Figure 1B, bottom panel), consistent with previous results and indicating a critical function for caspase-8 at the early stages of T cell activation. Yet, during this timeframe only a small amount of the caspase-8 p43/41 processing intermediate was generated, and the fully processed apoptotic mature p18 form was not detectable (Figure 1C).
This limited processing nevertheless suggested that procaspase-8 acquires enzymatic activity upon antigenic stimulation. To confirm, we applied a biotinylated labeling agent that covalently binds the active site of caspases, Bio-zVAD-fmk, to stimulated primary T cells. Procaspase-8 became activated within 1 h upon anti-CD3/CD28 treatment (Figure 1D). Yet despite its activation caspase-8 did not engage the apoptotic pathway, as shown by the lack of appreciable processing and activation of its apoptotic substrates, effector caspases-3 and -7 (Figure 1C). Therefore, during T cell receptor (TCR) signaling, caspase-8 is activated to a limited extent and does not initiate the apoptotic caspase cascade.
MALT1 promotes procaspase-8 activation through the paracaspase domain
In human primary T cells, caspase-8 associates with MALT1 both prior to and after antigenic stimulation (Figure 2A). To examine the functional relationship of caspase-8 to MALT1-induced NF-κB activation, we used caspase-8 wild type or deficient Jurkat cells. In wild type Jurkat cells, co-expression of both MALT1 and its activator Bcl10 strongly activated NF-κB (Figure 2B). However, this activation was impaired in caspase-8 deficient cells, indicating that caspase-8 functions downstream of MALT1. To determine the role of MALT1 in caspase-8 activation, we knocked down MALT1 expression in primary human T cells with siRNA. When the transfected cells were stimulated with anti-CD3/CD28 antibodies, activation of caspase-8 was observed in the control but not in the MALT1 siRNA-transfected cells (Figure 2C). Therefore, caspase-8 activation upon TCR stimulation is dependent on MALT1.
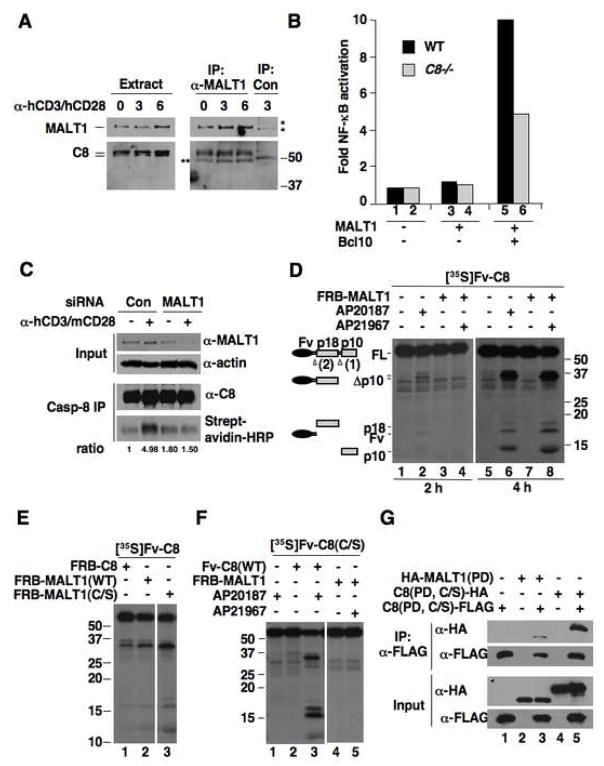
Figure 2. MALT1 associates with caspase-8 and promotes caspase-8 activation through the paracaspase domain.
(A) CD4+ cells were treated with α-hCD3/hCD28 or left untreated. Lysates were immunoprecipitated with α-MALT1 or control antibody, followed by Western blot analysis. *, nonspecific bands. **, IgG heavy chain bands.
(B) Wild type or caspase-8 deficient Jurkat cells were transfected with MALT1 and Bcl10 as indicated, together with an NF-κB-responsive firefly luciferase reporter plasmid. Fold activation of NF-κB are relative to vector-transfected wild type Jurkat cells and are normalized to a co-transfected Renilla luciferase control.
(C) CD4+ cells transfected with control or MALT1 siRNA plus m/hCD28 were stimulated with α-hCD3/mCD28 in the presence of biotin-zVAD-fmk or DMSO. Lysates were immunoprecipitated with α-caspase-8. Lysates and immunoprecipitates were analyzed by Western blot. The relative ratios of biotin-zVAD-labeled caspase-8 to total caspase-8 are given.
(D) In vitro-translated, 35S-labeled Fv-caspase-8 was treated with the homo-dimerizer AP20187 or co-incubated with non-isotope-labeled FRB-MALT1 and treated with the hetero-dimerizer AP21967. 35S-labeled proteins were visualized by autoradiography. See Supp. Fig. 2 for relative expression of unlabeled proteins.
(E) [35S]Fv-caspase-8 was incubated with FRB-C8 or -MALT1 fusion proteins in the presence of AP21967. The two parts are from the same exposure of the same blot.
(F) [35S]Fv-caspase-8(C/S) was incubated with Fv-C8 or FRB-MALT1 and dimerizers. The two parts are from the same exposure of the same blot.
(G) The tagged protease domains (PD) of caspase-8 and MALT1 were expressed in 293T cells as indicated and their interactions were detected by co-immunoprecipitation assay.
MALT1 possesses a paracaspase domain that shares conspicuous homology to the protease domain of caspases (Uren et al., 2000). This similarity, together with MALT1-dependent caspase-8 activation, led us to investigate whether the MALT1 paracaspase domain can directly activate procaspase-8 through hetero-dimerization. We used a controlled hetero-dimerization system based on Fv (a derivative of FK506-binding protein), FRB (the rapamycin-binding region of the FKBP-rapamycin-binding protein), and the hetero-dimerizer AP21967 that can bind to both Fv and FRB. Strikingly, when Fv-caspase-8 was incubated with FRB-MALT1, caspase-8 was processed in the presence of AP21967 (Figure 2D, lane 8). This activity was specific to MALT1, as incubation with the hetero-dimerizer alone did not result in cleavage of caspase-8 (Supp. Figure 3). MALT1-induced caspase-8 processing is an auto-proteolytic event, as it occurred when a MALT1 active site mutant (Cys464-to-Ser) was used (Figure 2E, lane 3) but not when a caspase-8 active site mutant (Cys360-to-Ser) was used (Figure 2F, lane 5). In addition, MALT1 was not cleaved by caspase-8 during dimerization (Supp. Figure 4). A co-immunoprecipitation assay revealed the MALT1 paracaspase domain binds to the caspase-8 protease domain in vivo (Figure 2G). Together, these data demonstrate a protease activity-independent function for the paracaspase domain of MALT1 that enables caspase-8 activation through hetero-dimerization.
Kinetic analysis showed that MALT1-induced activation of caspase-8 progressed at a slower rate as compared to caspase-8 activation induced a homo-dimerizer, AP20187 (Figure 2D, lanes 4 versus 2), which mimics caspase-8 activation during apoptosis (Chang et al., 2003; Chang and Yang, 2003). This difference suggests that the unique activation mechanism mediated by MALT1 may be a limiting step to prevent uncontrolled caspase-8 activation.
MALT1 alters the substrate specificity of caspase-8
To gain mechanistic insights into the minimal caspase-3 processing during antigenic signaling, we compared the ability of caspase-8 activated by hetero-dimerization with MALT1 to the ability of caspase-8 activated by homo-dimerization to cleave procaspase-3. Fv-caspase-8 showed some activity towards procaspase-3 in the absence of dimerizers (Figure 3A, lane 2), likely due to basal activation under prolonged incubation (data not shown). Notably, addition of FRB-MALT1 decreased Fv-caspase-8-mediated caspase-3 processing. When homo-dimerized, Fv-caspase-8 showed a markedly increased ability to cleave procaspase-3, enabling caspase-3 to proceed with the second self-processing event to generate the mature apoptotic form (lane 5). In contrast, when Fv-caspase-8 was hetero-dimerized with FRB-MALT1, its activity towards caspase-3 was not enhanced above basal levels (lane 6). Additionally, the second caspase-3 self-cleavage event critical for apoptosis induction was not observed. These results suggest that caspase-8 activated by MALT1 has limited capacity in triggering apoptosis.
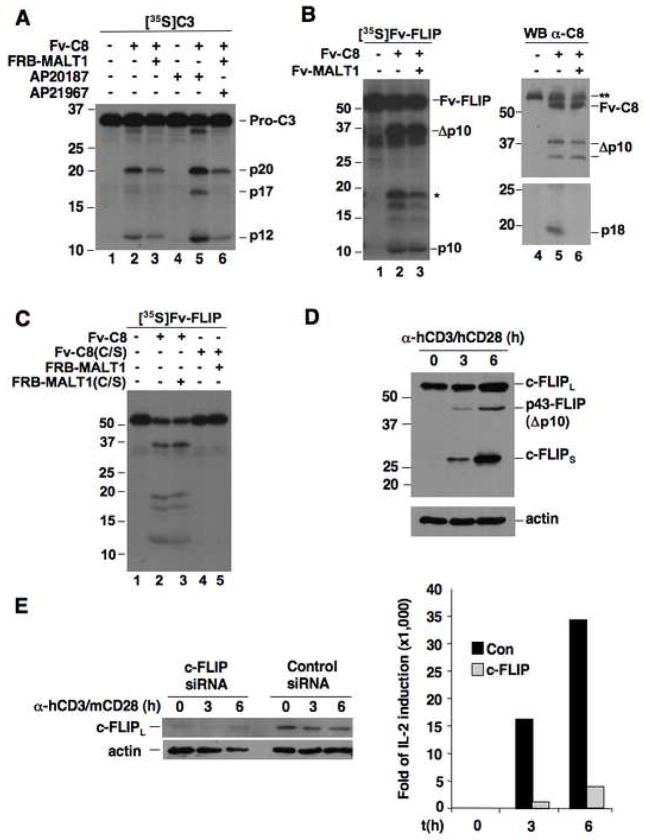
Figure 3. The MALT1-activated caspase-8 cannot generate the apoptotic form of caspase-3 but is capable of cleaving c-FLIPL.
(A) [35S]Caspase-3 was incubated with Fv-Caspase-8 alone or Fv-Caspase-8 plus FRB-MALT1 with or without dimerizer.
(B) [35S]Fv-FLIP was incubated with Fv-C8 and Fv-MALT1 as indicated. The reaction mixes were analyzed by autoradiography (left) and anti-caspase-8 immunoblotting (right). *, the large subunit of FLIP that is generated in vitro. **, endogenous caspase-8 in the reticulocyte lysates.
(C) [35S]Fv-FLIP was co-incubated with the indicated Fv-C8 and FRB-MALT1 proteins. The reaction mixes were analyzed as in (A).
(D) CD4+ T cells were stimulated with α-hCD3/hCD28. c-FLIP proteins and actin were detected by Western blot.
(E) Human CD4+ T cells were transfected with control or c-FLIP siRNA plus m/hCD28. Cells were stimulated with α-hCD3/mCD28 beads. IL-2 induction was measured by quantitative RT-PCR.
To determine whether caspase-8 activated by MALT1 is enzymatically active towards other substrates, we turned to the caspase-8-like proteolytically inactive molecule c-FLIPL. In the DISC, c-FLIPL regulates caspase-8 activation and is cleaved to p43-FLIP by caspase-8 (Chang et al., 2002; Micheau et al., 2002). When an Fv fusion of FLIP was incubated with Fv-caspase-8 and Fv-MALT1, it was strongly cleaved (Figure 3B, lane 3). This cleavage was due to caspase-8 enzymatic activity because the protease inactive mutant of MALT1, but not the protease inactive mutant of caspase-8 (C/S), could still induce c-FLIPL cleavage (Figure 3C). In addition, MALT1 alone had no activity towards c-FLIPL (Supp. Figure 5). Intriguingly, caspase-8 retained its ability to cleave c-FLIPL even when its own processing to the p18 apoptotic form was diminished by MALT1 (Figure 3B, lane 6). This was consistent with the slower kinetics of MALT1-induced caspase-8 activation compared to caspase-8 activation by homo-dimerization (Figure 2D). More importantly, this result indicates that caspase-8 does not need to undergo full processing to cleave c-FLIPL.
We confirmed caspase-8-mediated c-FLIPL cleavage event to be physiologically relevant by assessing the processing and role of c-FLIPL in primary human lymphocytes upon anti-CD3/CD28 stimulation. Previous reports have shown that the cleaved form of c-FLIPL strongly activate NF-κB (Kataoka and Tschopp, 2004). Notably, c-FLIPL was rapidly cleaved after stimulation, generating the p43-FLIP product (Figure 3D). In addition, the levels of both c-FLIPL and c-FLIPS were enhanced after anti-CD3/CD28 stimulation. Mouse lymphocytes deficient in c-FLIPL fail to proliferate in response to antigenic stimulation (Chau et al., 2005; Zhang and He, 2005). We knocked down the expression of c-FLIPL in primary human T cells using siRNA. Depletion of c-FLIPL in these cells resulted in a profound defect in CD3/CD28-mediated IL-2 production (Figure 3E), indicating an essential role of c-FLIPL in human lymphocyte signaling.
The ability of MALT1 to activate caspase-8 and enable c-FLIPL cleavage is required for antigenic signaling
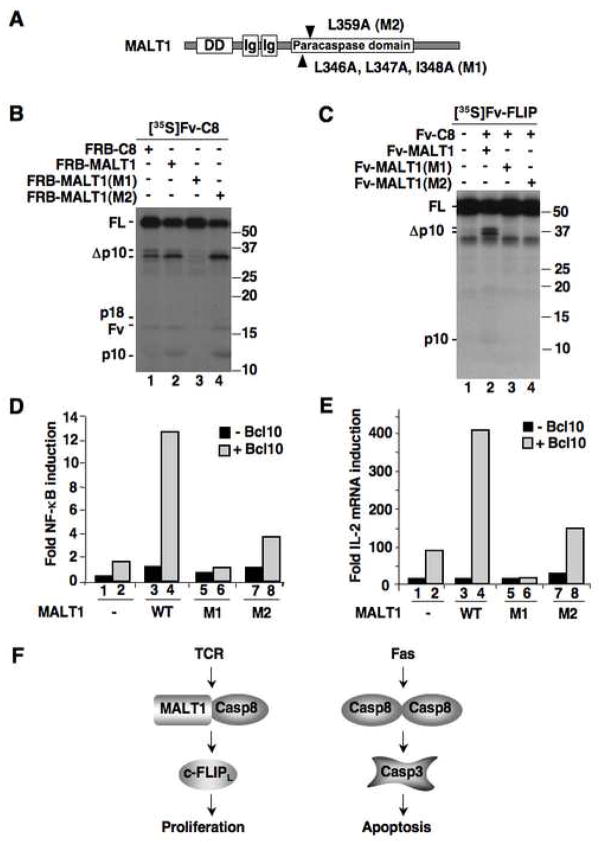
To assess the functional role of MALT1-induced caspase-8 activation and subsequent c-FLIPL cleavage, we mutated amino acids within highly conserved regions of the paracaspase domain of MALT1 that did not overlap reported binding sites to other proteins (Figure 4A). Full length MALT1 containing either mutation 1 or mutation 2 retained the ability to bind to Bcl10 and TRAF6, and both mutants were still able to be polyubiquitinated when co-expressed with TRAF6 (Supp. Figures 6 and 7). However, mutant 1 was unable to activate caspase-8 and prevented caspase-8 mediated cleavage of c-FLIPL (Figure 4B, C). The effect of mutant 2 is less robust; it could promote caspase-8 activation but not c-FLIPL cleavage (Figure 4C). A co-immunoprecipitation assay revealed that MALT1 binding to caspase-8 is diminished by mutation 2 but not by mutation 1 (Supp. Figure 8), suggesting that binding is necessary but not sufficient for MALT1 to activate caspase-8. The dissimilar effects of mutant 2 on caspase-8 activation and c-FLIPL processing also suggest that MALT1 may play an additional role in directing caspase-8 activity after proteolytic processing. To assess the ability of these mutants to activate NF-κB and induce IL-2 production, we introduced them into Jurkat cells. Both MALT1 mutants showed defects in NF-κB activation upon overexpression with Bcl10 (Figure 4D), and both were also defective in the production of IL-2 mRNA as well as IL-2 receptor mRNA (IL-2R) (Figure 4E and Supp. Figure 9). In these assays, mutant 1 exhibited more pronounced defects compared to mutant 2, correlating with their effects on caspase-8 activation and c-FLIPL cleavage. These results indicate that the ability of MALT1 to activate caspase-8 and enable cleavage of c-FLIPL is crucial for TCR signaling.
Figure 4. MALT1-induced caspase-8 activation is required for TCR signaling.
(A) Schematic representation of MALT1 mutations.
(B) [35S]Fv-C8 was incubated with FRB fusion of caspase-8, MALT1 and MALT1 mutants in the presence of AP21967. See Supp. Figure 2 for relative expression of unlabeled proteins.
(C) [35S]Fv-FLIP was incubated with equal amounts of Fv-C8 and Fv-MALT1 proteins as indicated.
(D) Jurkat cells were transfected with MALT1 proteins with or without Bcl10, plus an NF-κB responsive luciferase reporter plasmids. Data is representative of three independent experiments.
(E) Jurkat cells were transfected with MALT1 plasmids with or without Bcl10. IL-2 mRNA expression was measured by quantitative RT-PCR. Data is representative of three independent experiments.
(F) Differential activation of caspase-8 in proliferative and apoptotic signaling. Whereas caspase-8 is activated by homo-dimerization during apoptotic signaling, caspase-8 hetero-dimerizes with MALT1 in response to TCR signaling. This hetero-dimer lacks the capacity to activate apoptotic substrates, but retains activity towards c-FLIPL to enable proliferation.
Discussion
Maintenance of homeostasis demands cell proliferation to be balanced by apoptosis. This requires apoptotic machinery be kept intact in proliferating cells to ensure eventual cell death. Embedment of pro-apoptotic proteins such as caspase-8 in proliferative signaling effectively contributes to this protection. However, this engenders a perplexing issue of how caspase-8 activation is differentially regulated to effect opposing cellular outcomes. In the current study, we reveal that MALT1, through its paracaspase domain, promotes caspase-8 activation during TCR signaling. MALT1 diminishes the ability of caspase-8 to activate the apoptotic substrate caspase-3, but does not alter c-FLIPL cleavage. Therefore, MALT1 likely activates caspase-8 for proliferative signaling while avoiding apoptosis (Figure 4F).
Among all cysteine proteases, paracaspases are the closest to caspases, yet the similarity between them is modest and they lack a region corresponding to the small subunit of caspases (Uren et al., 2000). The difference between them may be key to the limited caspase-8 activation and altered substrate specificity. Conceivably, caspase-8 activation may also be limited by other mechanisms such as the small amount of caspase-8 present in the MALT1 complex and the dramatic up-regulation of the anti-apoptotic c-FLIPS (Figure 3D). In addition, MALT1-activated caspase-8 may not be released into thecytosol to cleave caspase-3. Nevertheless, it is remarkable that caspase activation can be uniquely controlled by a distantly related protease. Future biochemical and structural analysis of the caspase-8:MALT1 complex will likely enrich our understanding of the issues governing caspase activation. Because both caspases and paracaspases are well conserved in metazoans ranging from C. elegans to humans, it is tempting to speculate that paracaspase-mediated caspase activation, and the phenomenon of limited activation, may be general mechanisms for non-apoptotic functions of caspases.
Importantly, MALT1 does not prevent the cleavage of c-FLIPL by caspase-8. It may be that MALT1-activated caspase-8 is still capable of cleaving c-FLIPL. Alternatively, because c-FLIPL can be cleaved upon hetero-dimerization with procaspase-8 (Chang et al., 2002; Micheau et al., 2002), MALT1 may not affect c-FLIPL cleavage by procaspase-8. Further studies are needed to distinguish these possibilities. c-FLIPL and its cleavage have been implicated in lymphocyte signaling in mice. Our studies confirm a role of c-FLIPL in IL-2 induction and c-FLIPL processing during TCR signaling in primary human T cells. The generation of p43-FLIP may link MALT1-induced caspase-8 activation to downstream signaling events, because p43-FLIP is reported to activate NF-κB via RIP1, TRAF1, and TRAF2 (Kataoka and Tschopp, 2004).
Besides caspase-8 and c-FLIPL, the other DISC component, FADD, has also been associated with lymphocyte proliferative signaling (Zhang et al., 1998), and may play a role in recruiting caspase-8 to MALT1. The global mechanisms defining the action of these dual-function proteins are not well understood. However, the current work reveals a dramatic ability of the paracaspase MALT1 to activate caspase-8 and, at the same time, to restrain its potential lethal function. MALT1 is likely an important component of the regulatory mechanisms governing these dual function proteins that couple apoptosis with proliferation.
Experimental Procedures
Reagents, plasmids, and siRNAs
Biotinylated-zVAD (MP Biomedicals), Dithiobis[succinimidylpropionate] (DSP) (Pierce Biotechnology), and human IL-7 (PHC-0074) (Invitrogen) were purchased from the indicated companies. AP20187 and AP21967 were kindly provided by the ARIAD Pharmaceuticals, Inc. Monoclonal antibodies against caspase-8 (C15) (Dr. M. Peter), MALT1 (Dr. M.-Q. Du), FLIP (NF6) (Alexis Biochemicals), and caspase-3 (Santa Cruz Biotechnology), and polyclonal antibodies against Bcl10 (Santa Cruz Biotechnology), caspase-7 (Cell Signaling Technology), and actin (Sigma) were obtained from the indicated source. Magnetic beads coated with α-human CD3 plus either α-human CD28 or α-mouse CD28 were prepared as described (Parry et al., 2003).
The following plasmids were previously described: Fv-Caspase-8(206), Fv-Caspase-8(206, C/S), Fv-FLIP, Caspase-8 (PD, C/S)-HA, and Caspase-8 (PD, C/S)-FLAG (Chang et al., 2003; Chang et al., 2002; Chang and Yang, 2003); HA-MALT1 and FLAG-Bcl10 (Hu et al., 2006a; Hu et al., 2006b); and the chimeric mouse/human CD28 (m/hCD28) (Parry et al., 2003). Fv-MALT1, FRB-MALT1, HA-MALT1 (PD) and FLAG-MALT1(PD) contained the paracaspase domain (aa 326-567) of MALT1. FRB-MALT1 also contained aa 2021-2113 of FRAP followed by a linker (Gly-Gly-Gly-Gly-Ser). Mutations were generated using the Quik-Change Site-Directed Mutagenesis Kit (Stratagene). All constructs were confirmed by DNA sequencing.
Cells and siRNA transfection
Primary human CD4+ T cells were obtained from the Human Immunology Core, University of Pennsylvania. Wild type (A3) and caspase-8 deficient (I9) Jurkat cells were purchased from ATCC. siRNA obtained from QIAGEN (Caspase-8: Hs_CASP8_11 SI02661946, MALT1: combination of SI00091910 and custom sequence AACCCAGAGTCAAAGGCAGTC). c-FLIP siRNA was obtained from Dharmacon (siGENOME SMARTpool M-003772-06). Primary cells (5×106/sample) were transfected using the Amaxa electroporator, and cultured for 72 h in the presence of 10ng/mL hIL-7 before being stimulated with anti-CD3/CD28 antibodies.
Immunoprecipitation and labeling of active caspases
HA- and FLAG-tagged proteins were expressed in 293T cells and co-immunoprecipitation (IP) assays were performed as described (Hu et al., 2006b). For assaying MALT1:caspase-8 interaction in primary human T cells, cells were lysed in 50 mM HEPES pH 7.5, 150 mM NaCl, 0.5% NP-40, 1.5 mM NaVO4, protease inhibitors, and 3 mM DSP for 30 min on ice. Cross-linking was stopped with 50 mM Tris pH 7.5. Lysates were incubated with α-MALT1 or an isotype-matching control antibody plus Protein A/G beads. For labeling active caspases, 1×107 cells were incubated with labeling buffer (30 mM NaCl, 25 mM Tris-HCl pH 8.0, 1 mM DTT, and 100 μM biotinylated-zVAD) for 30 min at 37°C. Cells were lysed with 3 rounds of freeze-thaw. Lysates were incubated with α-caspase-8 and Protein A/G beads. Biotinylated proteins were detected with ImmunOperoxidase-streptavidin (MP Biomedicals). Protein bands were quantified by NIH Imaging software.
In vitro dimerization and processing
The experiments were performed essentially as described (Chang et al., 2003; Chang and Yang, 2003).
Real-time PCR and reporter assay
RNA was extracted using the TaqMan Gene Expression Cells-to-Ct Kit (Ambion #AM1728). Real-Time PCR was performed using TaqMan Gene Amplification primers and probes (Applied Biosystems IL-2: Hs00174114_m1, IL-2Rα: Hs00166229_m1, 18S: Hs99999901_s1) and TaqMan Universal PCR Master Mix (Applied Biosystems #4304437). For NF-κB reporter assay, Jurkat cells were transfected with the indicated plasmids plus a κB-firefly luciferase reporter plasmid (gift of Dr. Virginia Shapiro) and a Renilla luciferase control reporter. After 16h, luciferase activity was analyzed using the Dual-Luciferase Reporter Assay System (Promega #E1910).
Supplementary Material
Acknowledgments
We thank Emily Fischer and Elise Giantomaso for technical assistance, Drs. Virginia Shapiro, Michael May, Ming-Qing Du, and Marcus Peter for reagents, and Dr. M. Celeste Simon for use of the Amaxa Electroporator. We also thank the Human Immunology Core of the University of Pennsylvania Center for AIDS Research for providing purified human primary T cells and ARIAD Pharmaceuticals, Inc. for the dimerization systems. HK is a predoctoral appointee of the NIH grant T32CA09140. Supported by NIH grants AI057838 (to JLR), CA108872 and AI071274, Leukemia & Lymphoma Society Scholar Award, and American Gastroenterology Association Funderburg Research Scholar Award (to XY).
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Capacio VL, Peter ME, Yang X. Interdimer processing mechanism of procaspase-8 activation. Embo J. 2003;22:4132–4142. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. Embo J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DW, Yang X. Activation of procaspases by FK506 binding protein-mediated oligomerization. Sci STKE. 2003;2003:PL1. doi: 10.1126/stke.2003.167.pl1. [DOI] [PubMed] [Google Scholar]
- Chau H, Wong V, Chen NJ, Huang HL, Lin WJ, Mirtsos C, Elford AR, Bonnard M, Wakeham A, You-Ten AI, et al. Cellular FLICE-inhibitory protein is required for T cell survival and cycling. J Exp Med. 2005;202:405–413. doi: 10.1084/jem.20050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, Staal J, Sun L, Chen ZJ, Marynen P, Beyaert R. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol. 2008;9:263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- Hu S, Alcivar A, Qu L, Tang J, Yang X. cIAP2 inhibits antigen receptor signaling by targeting Bcl10 for degradation. Cell Cycle. 2006a;5:1438–1442. doi: 10.4161/cc.5.13.2866. [DOI] [PubMed] [Google Scholar]
- Hu S, Du MQ, Park SM, Alcivar A, Qu L, Gupta S, Tang J, Baens M, Ye H, Lee TH, et al. cIAP2 is a ubiquitin protein ligase for BCL10 and is dysregulated in mucosa-associated lymphoid tissue lymphomas. J Clin Invest. 2006b;116:174–181. doi: 10.1172/JCI25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644–653. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol. 2004;24:2627–2636. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- Liu H, Chang DW, Yang X. Interdimer processing and linearity of procaspase-3 activation. A unifying mechanism for the activation of initiator and effector caspases. J Biol Chem. 2005;280:11578–11582. doi: 10.1074/jbc.M414385200. [DOI] [PubMed] [Google Scholar]
- Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grutter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- Rebeaud F, Hailfinger S, Posevitz-Fejfar A, Tapernoux M, Moser R, Rueda D, Gaide O, Guzzardi M, Iancu EM, Rufer N, et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat Immunol. 2008;9:272–281. doi: 10.1038/ni1568. [DOI] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell. 2000;6:961–967. doi: 10.1016/s1097-2765(00)00094-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J Exp Med. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.