Abstract
Dramatic advances in biological research have revealed the mechanisms underlying many diseases at the molecular level. However, conventional techniques may be inadequate for direct application of this new knowledge to medical treatments. Nanobiotechnology, which integrates biology with the rapidly growing field of nanotechnology, has great potential to overcome many technical problems and lead to the development of effective therapies. The use of nanobiotechnology in drug delivery systems (DDS) is attractive for advanced treatment of conditions such as cancer and genetic diseases. In this review paper for a special issue on biomaterial research in Japan, we discuss the development of DDS based on polymeric micelles mainly in our group for anti-cancer drug and gene delivery, and also address our challenges associated with developing polymeric micelles as super-functionalized nanodevices with intelligent performance.
Keywords: nanomedicine, drug delivery systems, polymeric micelle, non-viral gene delivery, block copolymer, cancer therapy
1. Introduction
Biomaterials have changed medicine in both qualitative and quantitative manners through application to medical technologies such as artificial organs, which partially replace the functions of the original organs. Biomaterials have also received much attention in the field of pharmacy for the formulation of drug delivery systems (DDS). Recently, the trend in materials research in the DDS field has been shifting towards the development of materials with ‘active’ functionality exerting the ability to change their properties responding to specific stimuli. In this regard, materials design based on the nanometre-scaled control of the alignment and assembly of atoms and molecules, termed ‘nanotechnology’, becomes an important concept for constructing effective devices suitable for smart active functions. Application of nanotechnology-based biomaterials to DDS has been strenuously promoted in a global manner through research projects such as the National Nanotechnology Initiative and the Cancer Nanotechnology Plan in the USA, the Nanomedicine Project in Europe and the Nano-DDS project in Japan. It should be noted that a new class of drugs namely ‘biopharmaceutics’ consisting of peptides, proteins and nucleic acids has emerged. To apply such biologically fragile compounds as medicine, development of proper carrier systems is necessary. In this review for the special issue of J. R. Soc. Interface focusing on biomaterials research in Japan, we mainly overview our long-term achievements in the study on polymeric micelles from poly(ethylene glycol) (PEG)–poly(amino acid) block copolymers for drug and gene delivery.
2. Drug delivery systems
Once a drug is administered into the body by injection, it circulates throughout the body; but it is then quickly eliminated by hepatic metabolism and renal excretion. Owing to such an elimination, only a limited portion of the dose reaches the target sites and thus the medicinal effect is reduced. Moreover, the portion of the dose that does not reach the target can be toxic to off-target tissues, leading to undesired side effects. For optimal medicinal effect, the drug concentration must be maintained at an effective dose at the target sites for a certain duration, with minimal dose accumulation at off-target sites; thus, pinpoint therapies allowing control of drug distribution while suppressing side effects are desired in advanced medicine. For these issues, careful design of multifunctional drug carriers with nanoscale dimension has become a popular research subject. Such advanced drug carriers can be intelligently designed based on the inherent properties of the target site such as pH, presence of specific enzymes and tissue-specific markers. In this regard, studies on DDS designed to control drug action in terms of timing, location and dose have been progressively demonstrated (Jones & Leroux 1999; Torchilin 2001; Kabanov et al. 2002; Lavasanifar et al. 2002; Duncan 2003; Haag 2004). For example, in a typical DDS with controlled drug-releasing function, drugs are released slowly and passively from a certain substrate to maintain a desired blood concentration, or are actively released from prodrugs, in which drugs are activated only at target sites by site-specific catabolization. Studies on such ‘nano-DDS’ in Japan have been integrated into the field of materials engineering, which is one of Japan's strongest technologies. Recently, nano-DDS has been assigned as a subject of cooperative research by several Japanese ministries and consists of a number of ongoing projects.
Several obstacles pose a challenge for selective delivery of drugs to desired sites such as solid tumours, as shown in figure 1. The first important issue for the carrier is stability during the course of blood circulation. Drugs must be stably loaded into the carrier without leakage or catabolism by enzymes in the blood. Carriers must avoid glomerular excretion by the kidney and avoid uptake by the reticuloendothelial systems (RES) in the liver, spleen and lung. Since there is a molecular weight threshold for glomerular filtration (42 000–50 000 for water-soluble synthetic polymers), elimination by this mechanism can be avoided by increasing the molecular weights of the carriers. Carriers in the blood circulation may also induce non-specific complement activation and opsonization, resulting in the drug's elimination from the blood compartment due to RES recognition. In this regard, it is crucial to modify the carrier surface with biocompatible materials such as PEG to provide a ‘stealth’ characteristic. The second challenge for DDS is that the carriers must permeate tumour blood vessels to access the target tissue. One of the most important advantages of macromolecular carriers is their preferential accumulation in solid tumours. Such an elevated tumour accumulation is facilitated by microvascular hyperpermeability and impaired lymphatic drainage in tumour tissues, known as the ‘enhanced permeability and retention (EPR) effect’ (Matsumura & Maeda 1986). In this case, the carriers should be small enough (less than 100 nm) to allow effective extravasation from the blood compartment into solid tumours. The EPR effect found by Matsumura and Maeda has become the rule of thumb in ‘passive targeting’. The third challenge is selective uptake into the target cell. In this regard, an attractive strategy is ‘active targeting’, which exploits cell surface receptors by installing moieties onto carriers that specifically recognize target cells. Finally, controlled intracellular trafficking or organelle targeting is important to enhance medicinal effects, such as nuclear targeting in the case of gene delivery. Carriers ranging from 5 to 100 nm in size are internalized into cells by the endocytotic pathway, where endosomes and encapsulated carriers are separated from the cell membrane by a process of inward folding. These endosomes have an acidic pH value (pH∼5.5) and eventually fuse with lysosomes, where drugs can be degraded by a host of lysosomal enzymes. Therefore, careful modulation of both size and surface properties is required for functionality and pinpoint targeting, which allow for the maximum medicinal effect to be realized. As a result, nanobiotechnology-based carriers integrating specific performance enhancing functions have been demonstrated, resulting in creative and innovative supramolecular nanodevices (Nishiyama & Kataoka 2006; Osada & Kataoka 2006).
Figure 1.
Schematic of the biological distribution for carriers administered intravenously. The carriers are required to circulate in the bloodstream by avoiding RES recognition and glomerular excretion by the kidney, and finally must extravasate to the target tissue through the hyperpermeable region of the capillaries (EPR effect). Carrier uptake occurs by non-specific or receptor-mediated endocytosis, followed by transport into the endosome. The delivered drugs and genes should be released into the cytosol before lysosomal degradation.
3. Polymeric micelles for drug delivery systems
In aqueous media, amphiphilic block copolymers, which have a large solubility difference between the hydrophilic and hydrophobic segments, spontaneously form polymeric micelles with a distinct core–shell structure in which a hydrophobic inner core is surrounded by a hydrophilic shell (figure 2; Kataoka et al. 1993). PEG is often used as the hydrophilic segment by virtue of its biocompatibility. A polar PEG shell of the micelle facilitates solubility in water through steric stabilization and also provides biocompatibility and a stealth effect on the polymeric micelles, since PEG prevents the adsorption of proteins and is generally believed to be non-interactive with biological components (Jeon et al. 1991; Otsuka et al. 2001).
Figure 2.
Design of a multifunctional delivery system based on polymeric micelles formed with block copolymers capable of encapsulating drugs and pDNA.
Systematic control of the core-forming block structure provides micelle stability and a wide variety of materials for drug loading, release and activation. Thermodynamic and kinetic stability of the polymeric micelles is an important issue in their application to systemic administration, because their fast dissociation in the blood compartment results in the burst release of loaded drugs, inducing systemic toxicity. In contrast to micelles from small surfactant molecules, polymeric micelles are generally more stable and can retain the loaded drug for a prolonged period of time even in a very diluted condition in the body due to appreciably lowered critical micelle concentration, particularly for polymeric micelles from highly regulated block copolymers with distinct core–shell structure (Kwon et al. 1993). Functional groups, such as amines and carboxylic acids in the core-forming segments, are useful for introducing drugs into the micelle core. In this sense, PEG–polypeptide block copolymers, prepared by the polymerization of the appropriate N-carboxyanhydrides from a terminal amine on PEG, are advantageous because a series of block copolymers with different functional groups in the side chain can be prepared from the same platform. To facilitate micelle formation, a combination of intermolecular forces is available, such as hydrophobic interactions (Bader et al. 1984; Yokoyama et al. 1989; Gref et al. 1994), electrostatic interactions (Harada & Kataoka 1995, 1999; Kabanov et al. 1996; Kataoka et al. 1996; Harada & Kataoka 1999), metal complexation (Yokoyama et al. 1996; Nishiyama et al. 1999) and hydrogen bonding (Kataoka et al. 1998). The variation of the intermolecular force of core-forming segments enables one to regulate micelle stability for prolonged circulation as well as for controlled drug release property.
The sizes of these micelles are determined by thermodynamic parameters and are typically in the size range of several tens of nanometres with a relatively narrow size distribution, similar to the size range of viruses and lipoproteins. Owing to the combination of the high molecular weight and biocompatibility of polymeric micelles, avoidance of renal glomerular filtration and RES uptake is expected, thus providing longevity in blood circulation. This characteristic of polymeric micelles may facilitate tumour accumulation by the EPR effect, namely passive targeting (Matsumura 2008). Furthermore, functionalization of the distal end of PEG chains allows for incorporation of targeting ligands or other biomarkers into the micelle shell, providing control of biodistribution and site-specific cellular uptake, namely active targeting. These polymeric micelle systems may produce highly effective therapeutics, and are also expected to provide a framework for the design of multifunctional and intelligent nanodevices having combined ability for detection, diagnosis, analysis and therapy in a single platform (Pack et al. 2005; Mastrobattista et al. 2006; Sutton et al. 2007; Torchilin 2007; Davis et al. 2008).
3.1 Polymeric micelles delivering anti-cancer drugs
The first polymeric micelle developed in our group was one loaded with the anti-cancer drug doxorubicin (Dox). It was found that Dox-conjugated PEG-b-poly(aspartate) (PEG–PAsp) block copolymers spontaneously formed polymeric micelles in aqueous media (figure 3a; Yokoyama et al. 1987, 1989, 1990). Here, Dox was covalently conjugated to side chains of the poly(aspartate) (PAsp) segment by an amide bond between the carboxylic group in PAsp and the primary amino group of the glycosidyl residue in Dox. This conjugation provided sufficient hydrophobicity in the PAsp segment due to the hydrophobicity of Dox, thus forming a stable inner core of polymeric micelles with diameters of several tens of nanometres. The dissociation rate of the micelle in phosphate-buffered saline was estimated to be of the order of days and was quite slow even in the presence of 50 per cent rabbit serum (Yokoyama et al. 1993). A Dox molecule can be physically entrapped in the micellar core by hydrophobic interaction with conjugated Dox in a PAsp segment. It should be noted that physically entrapped Dox exerts the major cytotoxic function, while the conjugated Dox works mainly to increase the micelle stability (Yokoyama et al. 1993). The biodistribution of the PEG–PAsp(Dox) micelle was investigated by radiolabelling the PAsp(Dox) segment of the block copolymer. The micelle showed remarkably prolonged blood circulation, such that a quarter of the injected dose remained in the blood at 24 hours, whereas free Dox disappeared immediately from the blood in a few minutes (Kwon et al. 1994; Yokoyama et al. 1999). The PEG–PAsp(Dox) micelle effectively accumulated into a subcutaneously inoculated tumour (murine colon adenocarcinoma (C-26)), and eventually Dox in the micelle (total of conjugated and physically entrapped Dox) exhibited a 7.4-fold higher tumour accumulation than free Dox at 24 hours due to the EPR effect, achieving significantly higher in vivo anti-tumour activity against C-26 than free Dox (Yokoyama et al. 1991). Furthermore, the efficacy of the PEG–PAsp(Dox) micelle was further improved by controlling block copolymer composition and the dose of Dox, providing a complete cure of the C-26 tumours. The optimized PEG–PAsp(Dox) micelle is currently under phase II clinical trial as NK911 in Japan. Similarly, paclitaxel was also incorporated into the micelle core by hydrophobic interaction. NK105, a paclitaxel-incorporating micelle, proved to have higher efficacy with fewer side effects than free paclitaxel (Hamaguchi et al. 2005). The phase I clinical trial showed that NK105 can be administrated safely as a short infusion without the support of anti-allergic agents (Hamaguchi et al. 2007). A phase II study is now underway in Japan in patients with advanced stomach cancer. 7-Ethyl-10-hydroxy-camptothecin (SN-38), a water-insoluble anti-cancer drug that is a biologically active metabolite of irinotecan hydrochloride (CPT-11), was also loaded into a polymeric micelle of PEG–poly(glutamate) (PEG–PGlu) by hydrophobic interaction (Koizumi et al. 2006). The SN-38-loaded micelle showed markedly enhanced anti-tumour activity compared with free drug, and is thus a promising SN-38-based formulation. Note that the SN-38-loaded polymeric micelle was also effective against metastasis (Sumitomo et al. 2008). This micelle system is currently being tested in a phase I clinical trial as NK012 in Japan and in the USA.
Figure 3.
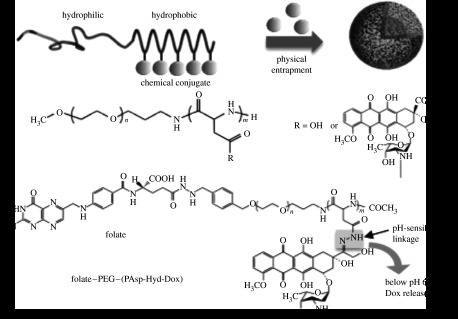
Dox-loaded polymeric micelles prepared by hydrophobic interaction. (a) ‘First-generation’ Dox-loaded micelle comprising PEG–PAsp block copolymer with chemically conjugated Dox. Micelle formation occurs spontaneously and additional Dox is physically entrapped within the hydrophobic core. (b) ‘Second-generation polymeric micelle’ prepared with multifunctionalized block copolymer containing a folate ligand and a pH-sensitive drug linkage for active targeting and selective intracellular Dox release.
Micelle formation is also driven by metal complexation as in the case of cisplatin (CDDP)-containing micelles. CDDP is a well-known platinum metal complex exhibiting a wide range of anti-tumour activities (Rosenber et al. 1969). However, its clinical use is limited due to its significant toxic side effects, such as acute nephrotoxicity and chronic neurotoxicity. CDDP shows a rapid distribution over the whole body and high glomerular clearance within 15 min after intravenous injection. Therefore, many efforts have been devoted to develop a DDS aimed at increasing the blood circulation period and accumulation in solid tumours (Bogdanov et al. 1997; Gianasi et al. 1999). We introduced CDDP into a micelle system by metal complexation between platinums of CDDP and carboxyl groups of PEG–PAsp (figure 4). The complex spontaneously formed a polymeric micelle with a very narrow size distribution (Yokoyama et al. 1996). The PEG–PAsp(CDDP) micelles showed environmentally responsive drug-release behaviour in response to salt concentration; they were stable in distilled water at room temperature, yet the loaded drug was sustainably released for over 50 hours as a result of exchange of chloride ion with CDDP in 150 mM NaCl (Nishiyama et al. 2003). The PEG–PAsp(CDDP) micelle exhibited a sixfold higher accumulation in tumour sites compared with free CDDP at 8 hours. Nevertheless, the in vivo anti-tumour activity was only slightly higher than that of the free CDDP for the same dose (Nishiyama et al. 2001). The micelle composition was then further modified to regulate CDDP release and extend the blood circulation time by using PEG–PGlu instead of PEG–PAsp. This modification was aimed at increasing micelle stability with more hydrophobic PGlu side chains, which contain an additional CH2 (figure 4). The PEG–PGlu(CDDP) micelle showed more sustained release of CDDP (half-value period: more than 90 hours) than the PEG–PAsp(CDDP) micelle (half-value period: approx. 30 hours) with a longer induction period (PEG–PGlu(CDDP): more than 20 hours; and PEG–PAsp(CDDP): approx. 10 hours) under physiological conditions (Nishiyama et al. 2003). Biodistribution of the PEG–PGlu(CDDP) micelle revealed a high plasma platinum level with a longer persistent time (11% of the injected dose at 24 hours) than the PEG–PAsp(CDDP) micelle (1.5% at 24 hours) with decreased accumulation in the liver and spleen. As a consequence of the longer circulation period, the tumour accumulation of the PEG–PGlu(CDDP) micelle was 20-fold higher than that of the free CDDP, indicating tumour-selective targeting due to the EPR effect. Treatment of tumour-bearing mice with the PEG–PGlu(CDDP) micelle by intravenous injection achieved complete tumour regression for five out of six mice, with only minimal body weight loss (within 5% of the initial weight). By contrast, treatment with free CDDP at the same drug dose exhibited tumour regression for only one mouse out of six and significant body weight loss (20% of the initial weight). The PEG–PGlu(CDDP) micelle (Uchino et al. 2005) is currently undergoing a phase I clinical trial as NC-6004 in the UK.
Figure 4.
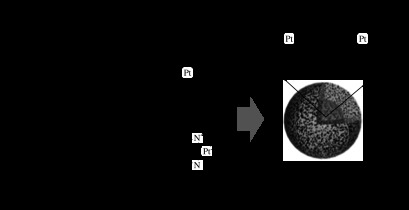
Platinum drug-loaded polymeric micelles formed by metal complexation. Carboxylic groups of PEG–PAsp or PEG–PGlu block copolymers are linked with the platinum of CDDP or DACHPt through coordination bonds.
3.2 Polymeric micelles delivering therapeutic genes
Recent progress in the understanding of the biological mechanisms driving life processes at the molecular level has led to the development of novel nucleic acid-based therapies such as plasmid DNA and siRNA as innovative medicines. Their clinical application, however, is hindered particularly by their instability under physiological conditions as well as low cellular uptake efficiency due to their large molecular weight and anionic nature. When DNA or RNA is directly administered into the blood stream, it is rapidly eliminated, mainly by DNase and RNase attacks. Thus incorporating DNA or RNA into appropriate nanocarriers is necessary for their practical use. For gene therapy, intracellular delivery to the nucleus is required in addition to accumulation within desired tissues. Owing to these crucial requirements, viral vectors such as retroviruses, adenoviruses and adeno-associated vectors have been commonly used as gene carriers in clinical trials for gene therapy. However, problems associated with immune response (Lehrman 1999; Marshall 1999) as well as the possibility of recombination with endogenous genes leading to oncogene effects (Cavazzana-Calvo et al. 2000; Aiuti et al. 2002; Hacein-Bey-Abina et al. 2003) prevent their use in clinical treatments. From the standpoint of preparation, viral vectors are not well suited for efficient mass production and thus are limited in clinical applications.
On the other hand, non-viral vectors composed of polymers or lipids offer a superior alternative in terms of safety, mass preparation and cost. With these incentives in mind, the research and development of safe and highly functionalized non-viral vectors are being undertaken worldwide (Gebhart & Kabanov 2001; Merdan et al. 2002; Niidome & Huang 2002; Glover et al. 2005; Wagner & Kloeckner 2006) and also in Japan (Kawano et al. 2004; Kogure et al. 2008). In this regard, a polymeric micelle system would be a promising formulation even for delivering nucleic acids due to its advanced and tunable characteristics. A polymeric micelle comprising nucleic acids is formed by polyion complexation between anionically charged DNA or RNA and a block copolymer having a hydrophilic segment and a cationic segment (figure 2; Kataoka et al. 1996; Katayose & Kataoka 1997; Itaka et al. 2004; Matsumoto et al. 2009). Complexation of plasmid DNA (pDNA) with a PEG–polycation such as PEG–poly(lysine) (PEG–PLys) occurs spontaneously, resulting in a polyion complex micelle (PIC micelle or polyplex micelle) with a size of approximately 100 nm (Katayose & Kataoka 1997, 1998; Oupicky et al. 2000). The polyplex micelles exhibit a near neutral zeta potential due to the PEG shell even in the presence of an excess amount of PEG–polycations (Itaka et al. 2003). Therefore, non-specific interaction with serum proteins or cells in the blood compartment is expected to be suppressed. Eventually, long-circulation property and thus tumour accumulation by the EPR effect will be readily expected. Polyplex micelles containing pDNA indeed exhibited efficient gene introduction in cultured cells, and also showed gene expression in the liver following intravenous injection into a mouse tail vein (Harada-Shiba et al. 2002). By packaging DNA into a polymeric micelle, prolonged blood circulation was achieved in which pDNA remained intact after 3 hours, whereas naked pDNA was immediately degraded by nucleases in a few minutes. These results demonstrate that polymeric micelles exhibit great feasibility as gene carriers as well.
4. Evolution of polymeric micelles into supramolecular multifunctional nanodevices
The polymeric micelles described above may be considered ‘first generation’ in the sense that they are monofunctional for the most part. A delivery system with multifunctions is not just a carrier, but rather an innovative supramolecular ‘nanodevice’ in which loaded materials and the carrier are integrated structurally and functionally in a nanometre scale. In this regard, smart polymeric micelles designed with stimuli-responsive properties may improve the therapeutic index towards solid tumours and to reduce side effects. For example, differences in the concentrations of reductive agents such as glutathione between the outside and the inside of a cell and pH reduction in endosomes are feasible targets for chemical differences. Nanocarriers taken into cells via endocytosis are compartmentalized in endosomes (pH∼5.5) where the proton concentration increases to approximately 100-fold relative to the extracellular environment (pH 7.4). Thus, drug carriers with pH-sensitive linkages based on hydrazone, cis-aconityl or acetal groups that degrade at low pH have been prepared (Christie & Grainger 2003). Glutathione is the most abundant reducing agent in the cytoplasm present in mM concentrations inside the cell and only in μM concentrations in the blood compartment (Meister & Anderson 1983). The disulphide bond is known to be stable in the extracellular environment, yet is readily cleaved inside the cell due to the increased concentration of glutathione, thus providing another chemical target allowing for site-specific release.
In contrast to the concept of passive targeting mainly by the EPR effect, the concept of active targeting using specific ligands is an attractive strategy to increase delivery efficiency and to decrease side effects. For such specific ligands, antibodies or their fragments, transferrin, folate, sugars and peptides are often considered (Davis et al. 2008). Cancer-specific antibody is a promising class of tumour-targeting ligands due to its high binding affinity (Torchilin et al. 2003). Transferrin, an iron-binding glycoprotein, is a well-studied ligand for tumour targeting (Schmidt et al. 1986; Sorokin et al. 1989). In rapidly dividing cells, such as malignant cells, transferrin receptor expression on their surfaces is elevated due to an increased cellular demand for iron. For cell-specific delivery towards liver parenchymal cells, a galactose moiety, which is recognized by the asialoglycoprotein (ASGP) receptors, may be introduced onto the surfaces of the carriers (Wu & Wu 1988; Yasugi et al. 1999; Nagasaki et al. 2001; Jeong et al. 2005), since a large number of cell surface receptors that bind and subsequently internalize ASGP are extensively expressed in hepatocytes. Folate is also a promising candidate (Yoo & Park 2004), because the folate-binding receptor is overexpressed in a large fraction of human tumours, but is only minimally distributed in normal tissue (Weitman et al. 1992). Macrophages can be a target for gene therapy in diseases such as Gaucher's disease and human immunodeficiency virus infection. In this case, mannose ligands are used because of the large numbers of mannose receptors expressed on the surfaces of macrophages. Dendritic cells also express a large number of mannose receptors, a feature exploited for the delivery of mannosylated carriers containing antigen-coded pDNA (Wickham 2003). Small peptides have also been used as ligands for cancer targeting. cRGD peptide has a high affinity to the αvβ3 integrin receptor, a cellular transmembrane protein overexpressing in angiogenic vessels, and is often used as such a ligand (Wermuth et al. 1997; Nasongkla et al. 2004). Artery wall-binding peptide is of interest for effective targeting of gene into artery wall cells (Nah et al. 2002). Note that modulation of the surface charge of nanocarriers by peptide conjugation is feasible for regulating their biodistribution after systemic administration (Yamamoto et al. 1999).
4.1 Polymeric micelles delivering anti-cancer drugs
Versatile design and engineering of block copolymers enable preparation of polymeric micelles with targetability to specific tissues. Targeted anti-cancer micelles were prepared by conjugating folate to the distal end of PEG–PAsp block copolymer aimed at increasing tumour accumulation (figure 3b). The folate-conjugated polymeric micelle loaded with Dox indeed showed significantly increased cellular uptake. Cytotoxicity analysis in vitro indicated that the cell growth-inhibitory activity of the folate-conjugated micelle was enhanced, suggesting that this could be an effective approach for ligand-mediated uptake for cancer treatment (Bae et al. 2005a).
The folate-conjugated polymeric micelle was also equipped with additional functionality, allowing for site-specific drug release, exploiting the difference in proton concentration between the extracellular environment and endosomal environments. Dox was conjugated to the core-forming PAsp segment of the PEG–PAsp through a hydrazone bond, which is stable under physiological conditions but cleavable under acidic intracellular environments of endosomes and lysosomes (figure 3b; Bae et al. 2003a,b). Indeed, the intracellular release of conjugated Dox from the micelle was confirmed by confocal laser scanning microscopy using an in vitro tumour model of multicellular tumour spheroids of a C26 cell line (Bae et al. 2005b). In animal tests, pH-sensitive micelles showed effective anti-tumour activity to suppress tumour growth in mice over a broad range of injection doses, whereas toxicity remained extremely low. The micelles were safe when injected at doses up to 40 mg kg−1 with three out of six mice completely cured and no toxic deaths among the treated mice having occurred. This is in sharp contrast to the case of free Dox, in which tumour growth was suppressed by a 10 mg kg−1 dose, but body weight fell substantially due to toxicity. Moreover, all of the mice treated with a 15 mg kg−1 dose of Dox experienced toxic death. The therapeutic efficacy of the micelles designed for site-specific targeting and environment-responsive drug release was significantly improved over that of free Dox.
Notably, the PEG–PAsp(Dox) micelle was even able to accumulate in pancreatic cancer cells (which are well known for their malignancy and difficulty for drug access), by co-administration of TGF-β type I receptor (TβR-I) inhibitor at a low dose (Kano et al. 2007). Administration of the TβR-I inhibitor at a low dose probably plays a role in enhancing the EPR effect in the intractable tumour. The use of the TβR-I inhibitor combined with long-circulation nanocarriers such as polymeric micelles may be of significant clinical and practical importance in treating intractable solid cancers.
Another approach using pH-triggered drug release was reported where an accelerated release of physically incorporated Dox from the micelles was achieved with a decrease in pH (Lee et al. 2003). That study investigated a pH-sensitive polymeric micelle composed of a mixture of PEG–poly(l-histidine) as a pH-sensitive polybase possessing pKa values around the physiological pH and biodegradable PEG–poly(l-lactic acid) block copolymers. The Dox-loaded mixed micelles were stable under physiological pH condition, but were unstable in the pH ranges of the tumour sites. When the mixed micelles were conjugated with folic acid as a targeting moiety, the micelles were more effective in killing tumour cells due to accelerated drug release in the tumour region and folate receptor-mediated tumour uptake. Furthermore, the fusogenic activity of poly(l-histidine) in the endosomes facilitated the cytosolic delivery of Dox to achieve improved cytotoxicity. This approach is also expected to be useful for the treatment of solid tumours in vivo.
Improvements of polymeric micelle systems have been demonstrated from the standpoint of both block copolymer structure and drug-loading strategies. Owing to the difficulty of treatment with CDDP due to acute dose-related side effects (such as nephrotoxicity, ototoxicity, neurotoxicity, nausea, vomiting and myelosuppression) and the appearance of intrinsic and acquired resistance, an improved platinum drug, dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt), was developed by Kidani et al. (1977). DACHPt has shown a wide and markedly different spectrum of activities than CDDP, such as lower toxicity than CDDP and no cross-resistance to it in many CDDP-resistant cancers (Rixe et al. 1996; Zhang & Lippard 2003). However, DACHPt is significantly less soluble in water than CDDP (Kidani et al. 1978). To enhance the water solubility of DACHPt as well as pinpoint delivery, DACHPt-loaded micelles were developed by metal complexation between platinum and carboxylic acids contained in a PEG–PGlu block copolymer (figure 4; Cabral et al. 2005). The DACHPt-loaded (PEG–PGlu(DACHPt)) micelles showed prolonged blood circulation and increased tumour accumulation (20-fold greater accumulation of PEG–PGlu(DACHPt) micelles at the tumour site compared with free oxaliplatin, which is a related free drug and a third-generation platinum drug with improved water solubility). Moreover, the optimized micelles exhibited reduced non-specific accumulation in the liver and spleen, resulting in higher specificity to solid tumours. These marked results could be correlated to the extended blood circulation and preferential tumour accumulation of the polymeric micelles against primary and metastatic tumours. The in vivo results suggest that PEG–PGlu(DACHPt) could be an outstanding DDS for platinum-based drugs in the treatment of not only primary tumours but also metastatic tumours (Cabral et al. 2007).
4.2 Polymeric micelles delivering therapeutic genes
For gene delivery directed to clinical application, further developments in carrier design are needed to increase both transgene efficiency and reduce cytotoxicity while maintaining homeostasis. In addition to efficient passive accumulation to target tissues by improving the stealth effect, it will also be necessary to develop new designs for responsive functionality by sensing spatio-temporal signals that promote enhanced cellular uptake and prompt endosome escape and efficient intracellular trafficking to the nucleus (Khalil et al. 2006).
Active targeting is also attractive for site-selective gene delivery. We have developed a lactose-equipped polymeric micelle encapsulating pDNA targeted to hepatocytes, which possess abundant ASGP receptors that recognize lactose moieties (figure 5a; Kataoka et al. 1999). The transfection efficiency of these micelles in HepG2 cells (hepatoma) showed that the lactose micelle was significantly more efficient at transfection than the micelle-lacking lactose (Wakebayashi et al. 2004). A competitive assay using asialofetuin (ASF), a natural ligand against ASGP receptors, which would inhibit uptake of the lactose-installed micelle, suggested that ASGP receptor-mediated endocytosis is a major pathway for the cellular uptake of the lactosylated micelle.
Figure 5.
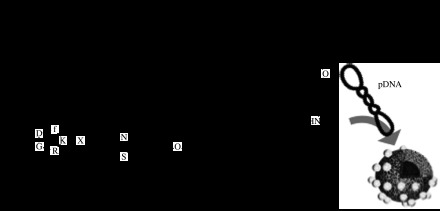
Block copolymers with ligand installed for targeted gene delivery. (a) Lactose for ASGP receptor and (b) cyclic RGD peptides for αvβ3 integrin receptor.
For the treatment of cancer by gene therapy, it is difficult to deliver lethal genes to all malignant cells to extinguish tumour tissues. Therefore, anti-angiogenesis treatment is an attractive strategy (Folkman et al. 1971; Schiffelers et al. 2004; Kim et al. 2005, 2006). This strategy inhibits the formation of new blood vessels and thus cuts off the supply of nutrition to cancer cells. Here, the target is not cancer cells themselves but vascular cells around the tumour. Thereby, a delivery system specifically targeting vascular endothelial cells would be of great benefit. We focused on αvβ3 integrin receptors for carrier design (Hood et al. 2002) because it is generally known that they are expressed on various cell types such as endothelial cells, osteoclasts, macrophages, platelets and melanomas, and that they play a significant role in angiogenesis, vascular intima thickening and the proliferation of malignant tumours (Brooks et al. 1994). Cyclic RGD peptide (c(RGDfK)), which specifically recognizes these receptors, was conjugated at the end of PEG–PLys block copolymer (figure 5b). The c(RGDfK)–PEG–PLys/pDNA polyplex micelle showed a remarkably increased transfection efficiency compared with ligand-free polyplex micelles against HeLa cells expressing αvβ3 integrins (Oba et al. 2007). Flow cytometric analysis revealed a higher uptake of the c(RGDfK)–PEG–PLys/pDNA micelle than the PEG–PLys/pDNA micelle in HeLa cells, consistent with the transfection results. Furthermore, confocal laser scanning microscopic observation revealed that the pDNA in the c(RGDfK)–PEG–PLys micelle preferentially accumulated in the perinuclear region of the HeLa cells within 3 hours of incubation, whereas no such fast and directed accumulation of pDNA to the perinuclear region was observed for the micelles without c(RGDfK) ligands. These results indicate that the increased transfection efficiency induced by the introduction of the c(RGDfK) peptide ligand was attributed to an increase in cellular uptake and also enhanced intracellular trafficking of micelles towards the perinuclear region via αvβ3 integrin receptor-mediated endocytosis. This, in turn, suggested that the cyclic RGD peptide-conjugated polyplex micelle has promising feasibility as a site-specific targetable gene delivery system.
For delivery of nucleic acids such as DNA and siRNA, loaded material should be stably packaged in the carrier but also be released in the targeted cells. In this regard, bioresponsive smart polymeric micelles that dissociate inside of cells in response to chemical stimuli present in the intracellular compartment were designed (Miyata et al. 2004). The inner core of the micelle was cross-linked through disulphide bonds, which are expected to be cleaved inside the cell (figure 6). Owing to disulphide cross-linking of the core, the stability of the micelle was increased. At the same time, the efficient release of packaged pDNA was demonstrated in response to reducing reagent (dithiothreitol), mimicking the intracellular environment (Kakizawa et al. 1999). These distinctive environmental sensitivities were well reflected in the transfection efficiency, as gene transfection was higher, by an order of magnitude, than that of the non-cross-linked system. The cross-linked micelle exhibited appreciable gene expression in parenchymal cells of the mouse liver through intravenous injection (Miyata et al. 2005). From a practical viewpoint, the long-term storage of gene carriers is a critical issue. The disulphide cross-linking micelle maintained the original transfection capacity even after freeze–thaw treatment without the use of any protective reagents, while the non-cross-linked micelles showed significantly lower transfection efficiency after the same treatment.
Figure 6.
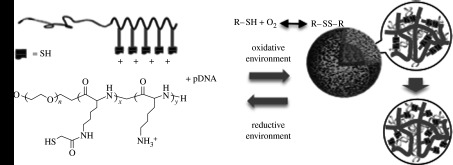
Cross-linked micelles responsive to the intracellular environment. Disulphide cross-linking imparts stability in the extracellular environment, which is readily reversed in the intracellular reductive environment.
Prompt endosome escape of therapeutic genes into the cytosol is a major factor for achieving effective transgene expression. When carriers are internalized by endocytosis, they are transferred to lysosomes via endosomes where nucleases digest foreign nucleic acids at lowered pH. Therefore, quick escape from the endosomes into the cytosol is a critical issue. It is believed that polycations with a low apparent pKa, such as polyethyleneimine (PEI), promote endosome escape by the so-called proton sponge effect (Boussif et al. 1995). However, the binding of polyamines with low pKa to DNA is weaker that that of polyamines with high pKa, thus their complexes are not sufficiently stable in the biological entity. In regard to these issues, we designed a new type of A-B-C triblock copolymer for micelle formation by tandemly aligning two cationic segments in a single polymer strand (Fukushima et al. 2005). In this triblock copolymer, each segment has its own distinctive role: a PEG segment for biocompatibility, a second segment composed of low pKa amines (poly[(3-morpholinopropyl) aspartamide] (PMPA) segments) for buffering capacity and a third segment composed of high pKa amines (PLys segment) for DNA binding (figure 7a). Detailed NMR studies suggested that the micelle formed with the triblock copolymers has a spatially regulated three-layered structure (figure 7b), in which DNA associates only with the PLys segment and the PMPA segments remain free. The transfection activity of the triblock system exhibited one order of magnitude higher transfection compared with the PEG–PLys block copolymer with similar PLys unit length. This transfection efficiency is comparable with that of the PEI/pDNA polyplex, but with a remarkably lower cytotoxicity. These results suggest that well-designed tandem alignment of two cationic segments allows for efficient endosome escape, as initially expected.
Figure 7.
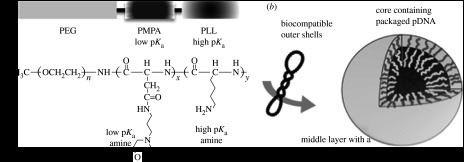
Design of a triblock copolymer for promotion of endosome escape. (a) The triblock copolymer PEG–PMPA–PLys system performs specific functions; the PEG segment improves biocompatibility, the PMPA segment contains inherent buffering capacity to promote early endosome escape and the PLys segment facilitates packaging pDNA in the micelle core. (b) Schematic of the hypothesized three-layered micelle formed from the triblock copolymer and pDNA with a spatially regulated structure.
In the search for the most suitable chemical structure for a cationic segment, PEG block copolymers with a series of cationic side chains were synthesized through the aminolysis of PEG–poly(β-benzyl l-aspartate) (PBLA), namely an ester–amide exchange reaction. The reaction proceeds via the formation of a succinimide intermediate in the polymer backbone, which is efficiently converted to a polyaspartamide in which quantitative introduction of a desired side chain is demonstrated without undesired side reactions (figure 8a; Nakanishi et al. 2007). The reaction, which enables side chains to be introduced as aspartamides, is crucial in the design of functionalized polymers for biomaterials. A polymer library was prepared using the above-mentioned reaction, from which we have found that PEG–polyaspartamide with an ethylenediamine unit as a side chain (PEG–PAsp(DET); figure 8b) had high transfection efficiency as well as low cytotoxicity (Kanayama et al. 2006). The side chain of the block copolymer exhibits single protonation in physiological pH at 7.4 while exhibiting double protonation at the endosomal pH of 5.5 (figure 8b; Han et al. 2007). Therefore, it is expected that the block copolymer exhibits low cytotoxicity in extracellular conditions due to single protonation. On the other hand, it becomes able to interact with the endosomal membrane due to increased positive charges by double protonation, which eventually results in the promotion of endosome escape. This polymeric micelle provided gene expression in primary cells (Kanayama et al. 2006) as well as in in vivo disease models with low toxicity. The polyplex micelle prepared with PEG–PAsp(DET) and pDNA was also applied to vascular lesions with good results (Akagi et al. 2007). The micelle was instilled intravascularly into rabbit carotid arteries with neointima, and it exhibited appreciable reporter gene transfer into vascular lesions without any vessel occlusion by thrombus. These findings indicate the feasibility of the polyplex system for treating vascular diseases. The PEG–PAsp(DET) polyplex system was also applied for in vivo gene transfer in a bone-defect model of mouse skull bone (Itaka et al. 2007). The polyplex micelle was mixed with a calcium phosphate cement scaffold and installed into the bone defect. Sustained release of PEG–PAsp(DET) from the scaffold and transfection of surrounding cells were observed. By the transfection of constitutively active form of activin receptor-like kinase 6 (caALK6) and runt-related transcription factor 2 (Runx2), osteogenic differentiation was induced in mouse calvarial cells with no sign of inflammation. This result demonstrated the first successful in vivo gene transfer with therapeutic potential using polyplex micelles, and showed that the system holds promise for constructing a practical gene-activated matrix for tissue engineering and also for bone-regenerative medicine. It is worth pointing out the biocompatibility of the PEG–PAsp(DET) polyplex system in addition to efficient in vivo gene transfer. It was demonstrated that the PAsp(DET) polyplex system exhibited no interference of endogenous housekeeping gene expression; thus, cellular homeostasis is maintained (Masago et al. 2007). Biocompatibility with regard to cellular homeostasis is a crucial issue for practical use, especially when gene transfer is applied to primary cells to regulate cell functions such as differentiation.
Figure 8.

(a) Quantitative aminolysis reaction of PBLA via a succinimidyl ring intermediate, by which appropriate compounds can be installed into the polymer backbone by displacement of benzyl groups in PBLA side chains. (b) Chemical structure of PEG–PAsp(DET) where an ethylenediamine unit is installed by aminolysis and its corresponding pH–α (α=[protonated amino groups]/[whole amino groups]) curve of PEG–PAsp(DET) at 37°C in 150 mM NaCl, showing that protonation takes place in two distinct steps.
4.3 Regulation of plasmid DNA packaging in polyplex micelles
Various types of block copolymers have been designed to improve gene expression efficacy by exploring chemical modifications for desirable functions, as described in previous sections. The properties of polyplex micelles must differ from site to site during the delivery process; thus, it is essential to optimize the packaging of DNA into the micelle according to the requirements at each site for improved gene expression efficacy. Packaging of pDNA into a polyplex micelle, pDNA condensation in other words, induced by block catiomer is not so simple, because pDNA has the topological characteristic of supercoiled closed-circular form and its condensation occurs with topological constraint. It was shown that pDNA packaging is affected by both polymer structure and charge ratio between pDNA and PLys. Change in PLys chain length and the charge ratio between pDNA and PLys resulted in the formation of rod-like, toroidal and collapsed sphere structures (Mann et al. 2008). We have found an interesting activity of S1 nuclease, a selective enzyme that is known to cleave single-stranded DNA in condensed pDNA. When pDNA was condensed into rod-like or toroidal structures, it was cleaved into highly ordered distinct fragments, each being 10/12, 9/12, 8/12, 6/12, 4/12, 3/12, 2/12 in length with respect to the original pDNA (Osada et al. 2005). This S1 nuclease activity suggests that double-strand dissociation might be induced in micelle-packaged pDNA. Presumably, the specific cleavage may relate to DNA folding in pDNA condensation, which proceeds under the constraints of helical double-stranded DNA rigidity and the characteristic supercoiled closed-circular topology.
5. Summary
Biomaterials have developed from passive materials that support the functions of target tissues or organs to active and smart materials that themselves act by sensing appropriate signals and exerting their function. Polymeric micelles for DDS have also developed from simple carrier systems that only deliver therapeutic materials to intelligent and multifunctional nanodevices by integrating bioresponsive functions. These advances are mediated by nanobiotechnology, designing the biological activity of engineered materials in terms of physicochemical viewpoints. Polymeric micelles are being developed as total delivery systems integrating multifunctionality to improve their efficacy. Although this review focuses on anti-cancer drugs and pDNA as deliverable materials, we have also developed DDS for other therapeutic materials such as proteins (Jaturanpinyo et al. 2004; Yuan et al. 2005; Kawamura et al. 2007), siRNA (Itaka et al. 2004; Kakizawa et al. 2006; Matsumoto et al. 2009), imaging agents for diagnosis (Imai et al. 2007; Kumagai et al. 2007) and photosensitizers for photodynamic therapy (Ideta et al. 2005; Jang et al. 2005, 2006, 2007) or co-delivery with pDNA for photochemical gene delivery (Nishiyama et al. 2005, 2006, 2007; Arnida et al. 2006). The development of polymeric micelles with smart functions such as environment sensitivity and tissue selectivity may enhance the desired activity of potent bioactive compounds, facilitating their clinical application. Polymeric micelle-based DDS, which can perform versatile functions based on innovative nanobiotechnologies, will lead the way for development of future advanced medicines.
Footnotes
One contribution of 10 to a Theme Supplement ‘Japanese biomaterials’.
References
- Aiuti A., et al. Correction of ADA–SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- Akagi D., Oba M., Koyama H., Nishiyama N., Fukushima S., Miyata T., Nagawa H., Kataoka K. Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther. 2007;14:1029–1038. doi: 10.1038/sj.gt.3302945. [DOI] [PubMed] [Google Scholar]
- Arnida, Nishiyama N., Kanayama N., Jang W.D., Yamasaki Y., Kataoka K. PEGylated gene nanocarriers based on block catiomers bearing ethylenediamine repeating units directed to remarkable enhancement of photochemical transfection. J. Control. Release. 2006;115:208–215. doi: 10.1016/j.jconrel.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Bader H.R., Ringsdorf H., Schmidt B. Watersoluble polymers in medicine. Angew. Makromol. Chem. 1984;123/124:457–485. [Google Scholar]
- Bae Y., Fukushima S., Harada A., Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. 2003a;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- Bae Y., Fukushima S., Harada A., Kataoka K. Preparation and characterization of pH responsive adriamycin-loaded polymeric micelles: drug release profile and in vitro cytotoxicity on human lung cancer SBC-3. J. Control. Release. 2003b;91:237–239. [Google Scholar]
- Bae Y., Jang W.D., Nishiyama N., Fukushima S., Kataoka K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol. Biosyst. 2005a;1:242–250. doi: 10.1039/b500266d. [DOI] [PubMed] [Google Scholar]
- Bae Y., Nishiyama N., Fukushima S., Koyama H., Yasuhiro M., Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 2005b;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- Bogdanov A., Wright S.C., Marecos E.M., Bogdanova A., Martin C., Petherick P., Weissleder R. A long-circulating co-polymer in ‘passive targeting’ to solid tumors. J. Drug Target. 1997;4:321–330. doi: 10.3109/10611869708995848. [DOI] [PubMed] [Google Scholar]
- Boussif O., Lezoualch F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo—polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P.C., Clark R.A.F., Cheresh D.A. Requirement of vascular integrin alpha(V)beta(3) for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Cabral H., Nishiyama N., Okazaki S., Koyama H., Kataoka K. Preparation and biological properties of dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt)-loaded polymeric micelles. J. Control. Release. 2005;101:223–232. doi: 10.1016/j.jconrel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Cabral H., Nishiyama N., Kataoka K. Optimization of (1,2-diamino-cyclohexane)platinum(II)-loaded polymeric micelles directed to improved tumor targeting and enhanced antitumor activity. J. Control. Release. 2007;121:146–155. doi: 10.1016/j.jconrel.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M., et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Christie R.J., Grainger D.W. Design strategies to improve soluble macromolecular delivery constructs. Adv. Drug Deliv. Rev. 2003;55:421–437. doi: 10.1016/S0169-409X(02)00229-6. [DOI] [PubMed] [Google Scholar]
- Davis M.E., Chen Z., Shin D.M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- Folkman J., et al. Tumor angiogenesis—therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Fukushima S., Miyata K., Nishiyama N., Kanayama N., Yamasaki Y., Kataoka K. PEGylated polyplex micelles from triblock catiomers with spatially ordered layering of condensed pDNA and buffering units for enhanced intracellular gene delivery. J. Am. Chem. Soc. 2005;127:2810–2811. doi: 10.1021/ja0440506. [DOI] [PubMed] [Google Scholar]
- Gebhart C.L., Kabanov A.V. Evaluation of polyplexes as gene transfer agents. J. Control. Release. 2001;73:401–416. doi: 10.1016/S0168-3659(01)00357-1. [DOI] [PubMed] [Google Scholar]
- Gianasi E., Wasil M., Evagorou E.G., Keddle A., Wilson G., Duncan R. HPMA copolymer platinates as novel antitumour agents: in vitro properties, pharmacokinetics and antitumour activity in vivo. Eur. J. Cancer. 1999;35:994–1002. doi: 10.1016/S0959-8049(99)00030-1. [DOI] [PubMed] [Google Scholar]
- Glover D.J., Lipps H.J., Jans D.A. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- Gref R., Minamitake Y., Peracchia M.T., Trubetskoy V., Torchilin V., Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- Haag R. Supramolecular drug-delivery systems based on polymeric core-shell architectures. Angew. Chem. Int. Ed. 2004;43:278–282. doi: 10.1002/anie.200301694. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T., et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer. 2005;92:1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi T., et al. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br. J. Cancer. 2007;97:170–176. doi: 10.1038/sj.bjc.6603855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Bae Y., Nishiyama N., Miyata K., Oba M., Kataoka K. Transfection study using multicellular tumor spheroids for screening non-viral polymeric gene vectors with low cytotoxicity and high transfection efficiencies. J. Control. Release. 2007;121:38–48. doi: 10.1016/j.jconrel.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Harada A., Kataoka K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block-copolymers with poly(ethylene glycol) segments. Macromolecules. 1995;28:5294–5299. doi: 10.1021/ma00119a019. [DOI] [Google Scholar]
- Harada A., Kataoka K. Chain length recognition: core-shell supramolecular assembly from oppositely charged block copolymers. Science. 1999;283:65–67. doi: 10.1126/science.283.5398.65. [DOI] [PubMed] [Google Scholar]
- Harada-Shiba M., Yamauchi K., Harada A., Takamisawa I., Shimokado K., Kataoka K. Polyion complex micelles as vectors in gene therapy—pharmacokinetics and in vivo gene transfer. Gene Ther. 2002;9:407–414. doi: 10.1038/sj.gt.3301665. [DOI] [PubMed] [Google Scholar]
- Hood J.D., Bednarski M., Frausto R., Guccione S., Reisfeld R.A., Xiang R., Cheresh D.A. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- Ideta R., et al. Nanotechnology-based photodynamic therapy for neovascular disease using a supramolecular nanocarrier loaded with a dendritic photosensitizer. Nano Lett. 2005;5:2426–2431. doi: 10.1021/nl051679d. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kaneko E., Asano T., Kumagai M., Ai M., Kawakami A., Kataoka K., Shimokado K. A novel contrast medium detects increased permeability of rat injured carotid arteries in magnetic resonance T2 mapping imaging. J. Atheroscler. Thromb. 2007;14:65–71. doi: 10.5551/jat.14.65. [DOI] [PubMed] [Google Scholar]
- Itaka K., Yamauchi K., Harada A., Nakamura K., Kawaguchi H., Kataoka K. Polyion complex micelles from plasmid DNA and poly(ethylene glycol)–poly(l-lysine) block copolymer as serum-tolerable polyplex system: physicochemical properties of micelles relevant to gene transfection efficiency. Biomaterials. 2003;24:4495–4506. doi: 10.1016/S0142-9612(03)00347-8. [DOI] [PubMed] [Google Scholar]
- Itaka K., Kanayama N., Nishiyama N., Jang W.D., Yamasaki Y., Nakamura K., Kawaguchi H., Kataoka K. Supramolecular nanocarrier of siRNA from PEG-based block catiomer carrying diamine side chain with distinctive pKa directed to enhance intracellular gene silencing. J. Am. Chem. Soc. 2004;126:13 612–13 613. doi: 10.1021/ja047174r. [DOI] [PubMed] [Google Scholar]
- Itaka K., Ohba S., Miyata K., Kawaguchi H., Nakamura K., Takato T., Chung U.I., Kataoka K. Bone regeneration by regulated in vivo gene transfer using biocompatible polyplex nanomicelles. Mol. Ther. 2007;15:1655–1662. doi: 10.1038/sj.mt.6300218. [DOI] [PubMed] [Google Scholar]
- Jang W.D., et al. Supramolecular nanocarrier of anionic dendrimer porphyrins with cationic block copolymers modified with polyethylene glycol to enhance intracellular photodynamic efficacy. Angew. Chem. Int. Ed. 2005;44:419–423. doi: 10.1002/anie.200461603. [DOI] [PubMed] [Google Scholar]
- Jang W.D., Nakagishi Y., Nishiyama N., Kawauchi S., Morimoto Y., Kikuchi M., Kataoka K. Polyion complex micelles for photodynamic therapy: incorporation of dendritic photosensitizer excitable at long wavelength relevant to improved tissue-penetrating property. J. Control. Release. 2006;113:73–79. doi: 10.1016/j.jconrel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Jang W.D., Nishiyama N., Kataoka K. Supramolecular assembly of photofunctional dendrimers for biomedical nano-devices. Supramol. Chem. 2007;19:309–314. doi: 10.1080/10610270701324089. [DOI] [Google Scholar]
- Jaturanpinyo M., Harada A., Yuan X.F., Kataoka K. Preparation of bionanoreactor based on core-shell structured polyion complex micelles entrapping trypsin in the core cross-linked with glutaraldehyde. Bioconjug. Chem. 2004;15:344–348. doi: 10.1021/bc034149m. [DOI] [PubMed] [Google Scholar]
- Jeon S.I., Lee J.H., Andrade J.D., Degennes P.G. Protein surface interactions in the presence of polyethylene oxide; 1. Simplified theory. J. Colloid Interface Sci. 1991;142:149–158. doi: 10.1016/0021-9797(91)90043-8. [DOI] [Google Scholar]
- Jeong Y.I., Seo S.J., Park I.K., Lee H.C., Kang I.C., Akaike T., Cho C.S. Cellular recognition of paclitaxel-loaded polymeric nanoparticles composed of poly(γ-benzyl l-glutamate) and poly(ethylene glycol) diblock copolymer endcapped with galactose moiety. Int. J. Pharm. 2005;296:151–161. doi: 10.1016/j.ijpharm.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Jones M.C., Leroux J.C. Polymeric micelles—a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999;48:101–111. doi: 10.1016/S0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Kabanov A.V., Bronich T.K., Kabanov V.A., Yu K., Eisenberg A. Soluble stoichiometric complexes from poly(N-ethyl-4-vinylpyridinium) cations and poly(ethylene oxide)-block-polymethacrylate anions. Macromolecules. 1996;29:6797–6802. doi: 10.1021/ma960120k. [DOI] [Google Scholar]
- Kabanov A.V., Lemieux P., Vinogradov S., Alakhov V. Pluronic((R)) block copolymers: novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002;54:223–233. doi: 10.1016/S0169-409X(02)00018-2. [DOI] [PubMed] [Google Scholar]
- Kakizawa Y., Harada A., Kataoka K. Environment-sensitive stabilization of core–shell structured polyion complex micelle by reversible cross-linking of the core through disulfide bond. J. Am. Chem. Soc. 1999;121:11 247–11 248. doi: 10.1021/ja993057y. [DOI] [Google Scholar]
- Kakizawa Y., Furukawa S., Ishii A., Kataoka K. Organic–inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer. J. Control. Release. 2006;111:368–370. doi: 10.1016/j.jconrel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kanayama N., Fukushima S., Nishiyama N., Itaka K., Jang W.D., Miyata K., Yamasaki Y., Chung U.I., Kataoka K. A PEG-based biocompatible block catiomer with high buffering capacity for the construction of polyplex micelles showing efficient gene transfer toward primary cells. ChemMedChem. 2006;1:439–444. doi: 10.1002/cmdc.200600008. [DOI] [PubMed] [Google Scholar]
- Kano M.R., et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc. Natl Acad. Sci. USA. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Kwon G.S., Yokoyama M., Okano T., Sakurai Y. Block-copolymer micelles as vehicles for drug delivery. J. Control. Release. 1993;24:119–132. doi: 10.1016/0168-3659(93)90172-2. [DOI] [Google Scholar]
- Kataoka K., Togawa H., Harada A., Yasugi K., Matsumoto T., Katayose S. Spontaneous formation of polyion complex micelles with narrow distribution from antisense oligonucleotide and cationic block copolymer in physiological saline. Macromolecules. 1996;29:8556–8557. doi: 10.1021/ma961217+. [DOI] [Google Scholar]
- Kataoka K., Ishihara A., Harada A., Miyazaki H. Effect of the secondary structure of poly(l-lysine) segments on the micellization in aqueous milieu of poly(ethylene glycol)–poly(l-lysine) block copolymer partially substituted with a hydrocinnamoyl group at the N-epsilon-position. Macromolecules. 1998;31:6071–6076. doi: 10.1021/ma971838i. [DOI] [Google Scholar]
- Kataoka K., Harada A., Wakebayashi D., Nagasaki Y. Polyion complex micelles with reactive aldehyde groups on their surface from plasmid DNA and end-functionalized charged block copolymers. Macromolecules. 1999;32:6892–6894. doi: 10.1021/ma990973n. [DOI] [Google Scholar]
- Katayose S., Kataoka K. Water-soluble polyion complex associates of DNA and poly(ethylene glycol)–poly(l-lysine) block copolymer. Bioconjug. Chem. 1997;8:702–707. doi: 10.1021/bc9701306. [DOI] [PubMed] [Google Scholar]
- Katayose S., Kataoka K. Remarkable increase in nuclease resistance of plasmid DNA through supramolecular assembly with poly(ethylene glycol)–poly(l-lysine) block copolymer. J. Pharm. Sci. 1998;87:160–163. doi: 10.1021/js970304s. [DOI] [PubMed] [Google Scholar]
- Kawamura A., Harada A., Kono K., Kataoka K. Self-assembled nano-bioreactor from block ionomers with elevated and stabilized enzymatic function. Bioconjug. Chem. 2007;18:1555–1559. doi: 10.1021/bc070029t. [DOI] [PubMed] [Google Scholar]
- Kawano T., Okuda T., Niidome T. Biodistribution of DNA-complex of dendritic poly(l-lysine) after intravenous injection. Mol. Ther. 2004;9:S315. doi: 10.1016/j.ymthe.2004.06.732. [DOI] [Google Scholar]
- Khalil I.A., Kogure K., Akita H., Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- Kidani Y., Inagaki K., Saito R., Tsukagoshi S. Synthesis and anti-tumor activities of platinum(II) complexes of 1,2-diaminocyclohexane isomers and their related derivatives. J. Clin. Hematol. Oncol. 1977;7:197–202. [Google Scholar]
- Kidani Y., Inagaki K., Iigo M., Hoshi A., Kuretani K. Anti-tumor activity of 1,2-diaminocyclohexane-platinum complexes against sarcoma-180 ascites form. J. Med. Chem. 1978;21:1315–1318. doi: 10.1021/jm00210a029. [DOI] [PubMed] [Google Scholar]
- Kim W.J., Yockman J.W., Lee M., Jeong J.H., Kim Y.H., Kim S.W. Soluble Flt-1 gene delivery using PEI-g-PEG-RGD conjugate for anti-angiogenesis. J. Control. Release. 2005;106:224–234. doi: 10.1016/j.jconrel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Kim W.J., Yockman J.W., Jeong J.H., Christensen L.V., Lee M., Kim Y.H., Kim S.W. Anti-angiogenic inhibition of tumor growth by systemic delivery of PEI-g-PEG-RGD/pCMV-sFlt-1 complexes in tumor-bearing mice. J. Control. Release. 2006;114:381–388. doi: 10.1016/j.jconrel.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Kogure K., Akita H., Yamada Y., Harashima H. Multifunctional envelope-type nano device (MEND) as a non-viral gene delivery system. Adv. Drug Deliv. Rev. 2008;60:559–571. doi: 10.1016/j.addr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Koizumi F., Kitagawa M., Negishi T., Onda T., Matsumoto S., Hamaguchi T., Matsumura Y. Novel SN-38-incorporating polymeric micelles, NK012, eradicate vascular endothelial growth factor-secreting bulky tumors. Cancer Res. 2006;66:10 048–10 056. doi: 10.1158/0008-5472.CAN-06-1605. [DOI] [PubMed] [Google Scholar]
- Kumagai M., et al. Iron hydroxide nanoparticles coated with poly(ethylene glycol)-poly(aspartic acid) block copolymer as novel magnetic resonance contrast agents for in vivo cancer imaging. Colloids Surf. B Biointerfaces. 2007;56:174–181. doi: 10.1016/j.colsurfb.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Kwon G., Naito M., Yokoyama M., Okano T., Sakurai Y., Kataoka K. Micelles based on AB block copolymers of poly(ethylene oxide) and poly(β-benzyl l-aspartate) Langmuir. 1993;9:945–949. doi: 10.1021/la00028a012. [DOI] [Google Scholar]
- Kwon G.S., Suwa S., Yokoyama M., Okano T., Sakurai Y., Kataoka K. Enhanced tumor accumulation and prolonged circulation times of micelle-forming poly(ethylene oxide-aspartate) block copolymer–adriamycin conjugates. J. Control. Release. 1994;29:17–23. doi: 10.1016/0168-3659(94)90118-X. [DOI] [Google Scholar]
- Lavasanifar A., Samuel J., Kwon G.S. Poly(ethylene oxide)-block-poly(l-amino acid) micelles for drug delivery. Adv. Drug Deliv. Rev. 2002;54:169–190. doi: 10.1016/S0169-409X(02)00015-7. [DOI] [PubMed] [Google Scholar]
- Lee E.S., Na K., Bae Y.H. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release. 2003;91:103–113. doi: 10.1016/S0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–518. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- Mann A., Richa R., Ganguli M. DNA condensation by poly-l-lysine at the single molecule level: role of DNA concentration and polymer length. J. Control. Release. 2008;125:252–262. doi: 10.1016/j.jconrel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Marshall E. Clinical trials—gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- Masago K., Itaka K., Nishiyama N., Chung U.I., Kataoka K. Gene delivery with biocompatible cationic polymer: pharmacogenomic analysis on cell bioactivity. Biomaterials. 2007;28:5169–5175. doi: 10.1016/j.biomaterials.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Mastrobattista E., van der Aa M.A.E.M., Hennink W.E., Crommelin D.J.A. Artificial viruses: a nanotechnological approach to gene delivery. Nat. Rev. Drug Discov. 2006;5:115–121. doi: 10.1038/nrd1960. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Christie R.J., Nishiyama N., Miyata K., Ishii A., Oba M., Koyama H., Yamasaki Y., Kataoka K. Environment-responsive block copolymer micelles with a disulfide cross-linked core for enhanced siRNA delivery. Biomacromolecules. 2009;10:119–127. doi: 10.1021/bm800985e. [DOI] [PubMed] [Google Scholar]
- Matsumura Y. Poly (amino acid) micelle nanocarriers in preclinical and clinical studies. Adv. Drug Deliv. Rev. 2008;60:899–914. doi: 10.1016/j.addr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer-chemotherapy—mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- Meister A., Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Merdan T., Kopecek J., Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv. Drug Deliv. Rev. 2002;54:715–758. doi: 10.1016/S0169-409X(02)00046-7. [DOI] [PubMed] [Google Scholar]
- Miyata K., Kakizawa Y., Nishiyama N., Harada A., Yamasaki Y., Koyama H., Kataoka K. Block catiomer polyplexes with regulated densities of charge and disulfide cross-linking directed to enhance gene expression. J. Am. Chem. Soc. 2004;126:2355–2361. doi: 10.1021/ja0379666. [DOI] [PubMed] [Google Scholar]
- Miyata K., Kakizawa Y., Nishiyama N., Yamasaki Y., Watanabe T., Kohara M., Kataoka K. Freeze-dried formulations for in vivo gene delivery of PEGylated polyplex micelles with disulfide crosslinked cores to the liver. J. Control. Release. 2005;109:15–23. doi: 10.1016/j.jconrel.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Nagasaki Y., Yasugi K., Yamamoto Y., Harada A., Kataoka K. Sugar-installed block copolymer micelles: their preparation and specific interaction with lectin molecules. Biomacromolecules. 2001;2:1067–1070. doi: 10.1021/bm015574q. [DOI] [PubMed] [Google Scholar]
- Nah J.W., Yu L., Han S.O., Ahn C.H., Kim S.W. Artery wall binding peptide-poly (ethylene glycol)-grafted-poly(l-lysine)-based gene delivery to artery wall cells. J. Control. Release. 2002;78:273–284. doi: 10.1016/S0168-3659(01)00499-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Park J.S., Jang W.D., Oba M., Kataoka K. Study of the quantitative aminolysis reaction of poly(β-benzyl l-aspartate) (PBLA) as a platform polymer for functionality materials. React. Funct. Polym. 2007;67:1361–1372. doi: 10.1016/j.reactfunctpolym.2007.08.009. [DOI] [Google Scholar]
- Nasongkla N., Shuai X., Ai H., Weinberg B.D., Pink J., Boothman D.A., Gao J.M. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew. Chem. Int. Ed. 2004;43:6323–6327. doi: 10.1002/anie.200460800. [DOI] [PubMed] [Google Scholar]
- Niidome T., Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- Nishiyama N., Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006;112:630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Nishiyama N., Yokoyama M., Aoyagi T., Okano T., Sakurai Y., Kataoka K. Preparation and characterization of self-assembled polymer–metal complex micelle from cis-dichlorodiammineplatinum(II) and poly(ethylene glycol)–poly(α,β-aspartic acid) block copolymer in an aqueous medium. Langmuir. 1999;15:377–383. doi: 10.1021/la980572l. [DOI] [Google Scholar]
- Nishiyama N., Kato Y., Sugiyama Y., Kataoka K. Cisplatin-loaded polymer–metal complex micelle with time-modulated decaying property as a novel drug delivery system. Pharm. Res. 2001;18:1035–1041. doi: 10.1023/A:1010908916184. [DOI] [PubMed] [Google Scholar]
- Nishiyama N., Okazaki S., Cabral H., Miyamoto M., Kato Y., Sugiyama Y., Nishio K., Matsumura Y., Kataoka K. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003;63:8977–8983. [PubMed] [Google Scholar]
- Nishiyama N., et al. Light-induced gene transfer from packaged DNA enveloped in a dendrimeric photosensitizer. Nat. Mater. 2005;4:934–941. doi: 10.1038/nmat1524. [DOI] [PubMed] [Google Scholar]
- Nishiyama N., Arnida, Jang W.D., Date K., Miyata K., Kataoka K. Photochemical enhancement of transgene expression by polymeric micelles incorporating plasmid DNA and dendrimer-based photosensitizer. J. Drug Target. 2006;14:413–424. doi: 10.1080/10611860600834508. [DOI] [PubMed] [Google Scholar]
- Nishiyama N., Jang W.D., Kataoka K. Supramolecular nanocarriers integrated with dendrimers encapsulating photosensitizers for effective photodynamic therapy and photochemical gene delivery. N. J. Chem. 2007;31:1074–1082. doi: 10.1039/b616050f. [DOI] [Google Scholar]
- Oba M., Fukushima S., Kanayama N., Aoyagi K., Nishiyama N., Koyama H., Kataoka K. Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing αvβ3 and αvβ5 integrins. Bioconjug. Chem. 2007;18:1415–1423. doi: 10.1021/bc0700133. [DOI] [PubMed] [Google Scholar]
- Osada K., Kataoka K. Drug and gene delivery based on supramolecular assembly of PEG–polypeptide hybrid block copolymers. Pept. Hybrid Polym. 2006;202:113–153. doi: 10.1007/12_084. [DOI] [Google Scholar]
- Osada K., Yamasaki Y., Katayose S., Kataoka K. A synthetic block copolymer regulates S1 nuclease fragmentation of supercoiled plasmid DNA. Angew. Chem. Int. Ed. 2005;44:3544–3548. doi: 10.1002/anie.200500201. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Nagasaki Y., Kataoka K. Self-assembly of poly(ethylene glycol)-based block copolymers for biomedical applications. Curr. Opin. Colloid Interface Sci. 2001;6:3–10. doi: 10.1016/S1359-0294(00)00082-0. [DOI] [Google Scholar]
- Oupicky D., Konak C., Ulbrich K., Wolfert M.A., Seymour L.W. DNA delivery systems based on complexes of DNA with synthetic polycations and their copolymers. J. Control. Release. 2000;65:149–171. doi: 10.1016/S0168-3659(99)00249-7. [DOI] [PubMed] [Google Scholar]
- Pack D.W., Hoffman A.S., Pun S., Stayton P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- Rixe O., Ortuzar W., Alvarez M., Parker R., Reed E., Paull K., Fojo T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem. Pharmacol. 1996;52:1855–1865. doi: 10.1016/S0006-2952(97)81490-6. [DOI] [PubMed] [Google Scholar]
- Rosenber B., Vancamp L., Trosko J.E., Mansour V.H. Platinum compounds—a new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Schiffelers R.M., et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J.A., Marshall J., Hayman M.J., Ponka P., Beug H. Control of erythroid-differentiation—possible role of the transferrin cycle. Cell. 1986;46:41–51. doi: 10.1016/0092-8674(86)90858-5. [DOI] [PubMed] [Google Scholar]
- Sorokin L.M., Morgan E.H., Yeoh G.C.T. Transformation-induced changes in transferrin and iron-metabolism in myogenic cells. Cancer Res. 1989;49:1941–1947. [PubMed] [Google Scholar]
- Sumitomo M., Koizumi F., Asano T., Horiguchi A., Ito K., Asano T., Kakizoe T., Hayakawa M., Matsumura Y. Novel SN-38-incorporated polymeric micelle, NK012, strongly suppresses renal cancer progression. Cancer Res. 2008;68:1631–1635. doi: 10.1158/0008-5472.CAN-07-6532. [DOI] [PubMed] [Google Scholar]
- Sutton D., Nasongkla N., Blanco E., Gao J.M. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007;24:1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- Torchilin V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release. 2001;73:137–172. doi: 10.1016/S0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- Torchilin V.P. Micellar nanocarriers: pharmaceutical perspectives. Pharm. Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- Torchilin V.P., Lukyanov A.N., Gao Z.G., Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc. Natl Acad. Sci. USA. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino H., et al. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br. J. Cancer. 2005;93:678–687. doi: 10.1038/sj.bjc.6602772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Kloeckner J. Gene delivery using polymer therapeutics. Polym. Ther. I Polym. Drugs Conjug. Gene Deliv. Syst. 2006;192:135–173. doi: 10.1007/12_023. [DOI] [Google Scholar]
- Wakebayashi D., Nishiyama N., Yamasaki Y., Itaka K., Kanayama N., Harada A., Nagasaki Y., Kataoka K. Lactose-conjugated polyion complex micelles incorporating plasmid DNA as a targetable gene vector system: their preparation and gene transfecting efficiency against cultured HepG2 cells. J. Control. Release. 2004;95:653–664. doi: 10.1016/j.jconrel.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Weitman S.D., Lark R.H., Coney L.R., Fort D.W., Frasca V., Zurawski V.R., Kamen B.A. Distribution of the folate receptor Gp38 in normal and malignant-cell lines and tissues. Cancer Res. 1992;52:3396–3401. [PubMed] [Google Scholar]
- Wermuth J., Goodman S.L., Jonczyk A., Kessler H. Stereoisomerism and biological activity of the selective and superactive αvβ3 integrin inhibitor cyclo(-RGDfV-) and its retro-inverso peptide. J. Am. Chem. Soc. 1997;119:1328–1335. doi: 10.1021/ja961908l. [DOI] [Google Scholar]
- Wickham T.J. Ligand-directed targeting of genes to the site of disease. Nat. Med. 2003;9:135–139. doi: 10.1038/nm0103-135. [DOI] [PubMed] [Google Scholar]
- Wu G.Y., Wu C.H. Receptor-mediated gene delivery and expression in vivo. J. Biol. Chem. 1988;263:14 621–14 624. [PubMed] [Google Scholar]
- Yamamoto Y., Nagasaki Y., Kato M., Kataoka K. Surface charge modulation of poly(ethylene glycol)–poly(d,l-lactide) block copolymer micelles: conjugation of charged peptides. Colloids Surf. B Biointerfaces. 1999;16:135–146. doi: 10.1016/S0927-7765(99)00065-X. [DOI] [Google Scholar]
- Yasugi K., Nakamura T., Nagasaki Y., Kato M., Kataoka K. Sugar-installed polymer micelles: synthesis and micellization of poly(ethylene glycol)–poly(d,l-lactide) block copolymers having sugar groups at the PEG chain end. Macromolecules. 1999;32:8024–8032. doi: 10.1021/ma991066l. [DOI] [Google Scholar]
- Yokoyama M., Inoue S., Kataoka K., Yui N., Sakurai Y. Preparation of adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer—a new type of polymeric anticanceragent. Makromol. Chem. Rapid Commun. 1987;8:431–435. doi: 10.1002/marc.1987.030080903. [DOI] [Google Scholar]
- Yokoyama M., Inoue S., Kataoka K., Yui N., Okano T., Sakurai Y. Molecular design for missile drug—synthesis of adriamycin conjugated with immunoglobulin-G using poly(ethylene glycol)-block-poly(aspartic acid) as intermediate carrier. Makromol. Chem. Macromol. Chem. Phys. 1989;190:2041–2054. [Google Scholar]
- Yokoyama M., Miyauchi M., Yamada N., Okano T., Sakurai Y., Kataoka K., Inoue S. Polymer micelles as novel drug carrier—adriamycin-conjugated poly(ethylene glycol)–poly(aspartic acid) block copolymer. J. Control. Release. 1990;11:269–278. doi: 10.1016/0168-3659(90)90139-K. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Okano T., Sakurai Y., Ekimoto H., Shibazaki C., Kataoka K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991;51:3229–3236. [PubMed] [Google Scholar]
- Yokoyama M., Sugiyama T., Okano T., Sakurai Y., Naito M., Kataoka K. Analysis of micelle formation of an adriamycin-conjugated poly(ethylene glycol)–poly(aspartic acid) block copolymer by gel permeation chromatography. Pharm. Res. 1993;10:895–899. doi: 10.1023/A:1018921513605. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Okano T., Sakurai Y., Suwa S., Kataoka K. Introduction of cisplatin into polymeric micelle. J. Control. Release. 1996;39:351–356. doi: 10.1016/0168-3659(95)00165-4. [DOI] [Google Scholar]
- Yokoyama M., Okano T., Sakurai Y., Fukushima S., Okamoto K., Kataoka K. Selective delivery of adiramycin to a solid tumor using a polymeric micelle carrier system. J. Drug Target. 1999;7:171–186. doi: 10.3109/10611869909085500. [DOI] [PubMed] [Google Scholar]
- Yoo H.S., Park T.G. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J. Control. Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Yuan X.F., Yamasaki Y., Harada A., Kataoka K. Characterization of stable lysozyme-entrapped polyion complex (PIC) micelles with crosslinked core by glutaraldehyde. Polymer. 2005;46:7749–7758. doi: 10.1016/j.polymer.2005.02.121. [DOI] [Google Scholar]
- Zhang C.X., Lippard S.J. New metal complexes as potential therapeutics. Curr. Opin. Chem. Biol. 2003;7:481–489. doi: 10.1016/S1367-5931(03)00081-4. [DOI] [PubMed] [Google Scholar]




