Abstract
Bioactive ceramics have been used clinically to repair bone defects owing to their biological affinity to living bone; i.e. the capability of direct bonding to living bone, their so-called bioactivity. However, currently available bioactive ceramics do not satisfy every clinical application. Therefore, the development of novel design of bioactive materials is necessary. Bioactive ceramics show osteoconduction by formation of biologically active bone-like apatite through chemical reaction of the ceramic surface with surrounding body fluid. Hence, the control of their chemical reactivity in body fluid is essential to developing novel bioactive materials as well as biodegradable materials. This paper reviews novel bioactive materials designed based on chemical reactivity in body fluid.
Keywords: ceramics, bioactivity, hydroxyapatite, calcium phosphate, hybrid, coating
1. Introduction
Bones play an important role in our lives, supporting our bodies and enabling us to perform various motions. The techniques used to repair damaged bones also are important, and when an area of damaged bone is too large for self-repair, the damaged bones must be repaired by using alternative materials, such as autografts, allografts and artificial materials. Autografts, which are transferred from healthy parts of the bones of the same patient, are widely used because they show high performance. However, there are problems related to a limited amount of tissue being available and there is additional damage to the body because the bone tissue is extracted from the patient. Although allografts, which are transferred from other people, are also used, they have problems related to not only limited availability but also with foreign body reactions and infections. Therefore, artificial materials that are safe and free from these limitations are needed to repair bone defects. However, in general, artificial materials implanted in bony defects are encapsulated by fibrous tissue and do not bond to living bone. To solve the problem of the foreign body reaction, bioactive ceramics have received much attention, and some bioactive ceramics are now clinically used as bone substitutes. Bioactive ceramics are generally regarded as ceramics that are designed to induce specific biological activity for repairing damaged organs. For repairing bone tissues, the bioactivity is regarded as the capability to make direct contact with living bone after implantation in bony defects. The phenomenon of new bone formation on the surfaces of bioactive ceramics is called osteoconductivity.
Some bioactive ceramics have already been used to repair bone defects because their bioactivity allows them to achieve tight fixation resulting from direct bonding to living bone. The first bioactive ceramic developed was a glass in the Na2O–CaO–SiO2–P2O5 system, after Hench et al. (1971, 1991). This bioactive glass is known as Bioglass. Since the discovery of Bioglass, many researchers developed various types of bioactive ceramics, such as sintered hydroxyapatite (Ca10(PO4)6(OH)2; Jarcho et al. 1977; LeGeros & LeGeros 1993), glass-ceramic Ceravital (Gross et al. 1993) and glass-ceramic A–W (Kokubo et al. 1982; Kokubo 1993). However, these bioactive ceramics have lower fracture toughness and higher Young's modulus than human cortical bone, so the applications are limited to the replacement of bony parts under low loads and as bone fillers. The development of novel bioactive materials with various mechanical and biological properties, in addition to high affinity for the bone tissue, is therefore needed.
Previous studies (Hench 1991; Kokubo 1991) revealed that bioactive ceramics form a layer of biologically active bone-like apatite on their surfaces after being implanted in bony defects. It is therefore an important condition for ceramic materials to form a bone-like apatite layer on their surfaces after exposure to the body environment to express the property of the direct bonding to living bone. A similar bone-like apatite layer can form on bioactive ceramics if they are immersed in a simulated body fluid (SBF), as proposed by Kokubo and his colleagues. The SBF of Kokubo et al. is an aqueous solution that has almost the same constituents with regard to inorganic species as human extracellular fluid (Kokubo et al. 1990a,b; Cho et al. 1995; Kokubo & Takadama 2006). SBF does not contain any cells or proteins, which means that the apatite layer is formed through the chemical reaction of the bioactive ceramics with the surrounding fluid. It is therefore expected that novel bioactive materials can be designed by controlling the chemical reactivity of the materials in body fluid.
Based on this idea, new types of bioactive materials, such as bioactive glass-ceramics with controlled surface reactions, bioactive organic–inorganic hybrids, bioactive coatings and bioresorbable ceramics, have been developed. The concepts used and the attempts at development of these bioactive materials are described in this paper.
2. Bioactive glass-ceramics
2.1 Glass-ceramics in the MgO–CaO–SiO2 system
Crystallization of a glass is one of the useful techniques to control the chemical reactivity and mechanical properties of the glass. A design of the glass composition for a bioactive glass-ceramic is shown. Bioglass shows high reactivity in the body environment and shows high bone-bonding ability, i.e. bioactivity. This high bioactivity is caused by the high potential for bone-like apatite formation after the reaction of the materials with body fluid. Body fluid is an aqueous solution that is supersaturated with respect to hydroxyapatite, with the following chemical equilibrium. This equation is somewhat simplified and does not account for the incorporation of other ions, such as Mg2+, HPO4− or CO32− ions, during apatite formation from body fluid:
Release of sodium and calcium ions (Na+ and Ca2+) from the glass causes an increase in pH after ion exchange with H3O+ and leads to an increase in the Ca2+ concentration of the surrounding fluid. These chemical reactions result in an increase in the degree of supersaturation with respect to hydroxyapatite. However, the high reactivity produces a thick silica gel layer between the apatite layer and the glass, owing to the reactions
The formation of this thick silica gel layer is not desirable, as the mechanical strength of the silica gel layer is quite low, which reduces bonding between the glass and the bone. Therefore, a design in which the glass does not form a thick silica gel layer is required.
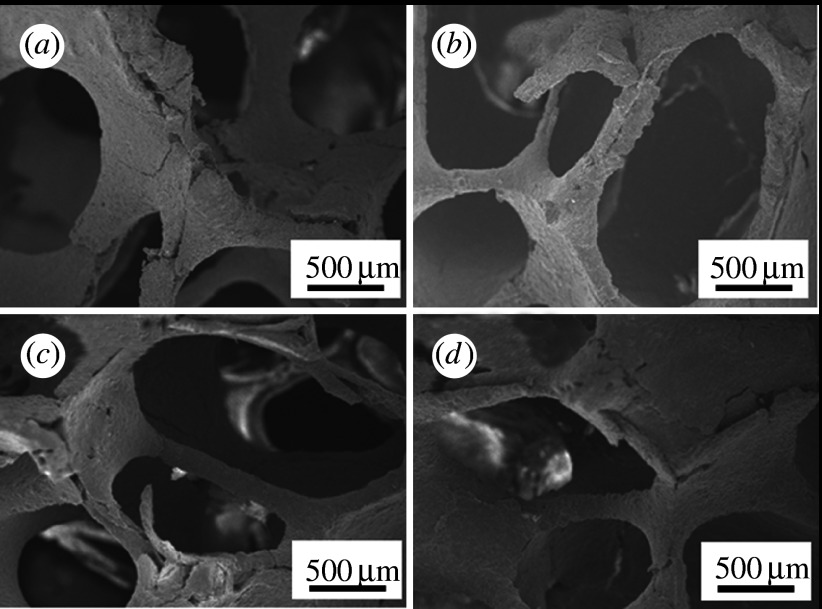
According to a report that evaluates the capability of bone-like apatite formation on glasses in the ternary system CaO–SiO2–P2O5 after exposure to SBF (Ohtsuki et al. 1991a), glasses in the CaO–SiO2 binary system have been identified as basic components for producing bioactive glasses. The typical composition of such a bioactive glass is 50CaO·50SiO2 mol%. However, the reactivity of 50CaO·50SiO2 mol% glass in the body environment is so high that a thick silica gel layer is observed between the apatite layer and the glass after soaking in SBF. MgO was added to CaO–SiO2-based glass-ceramics so as to reduce the silica gel layer, as based on a fundamental study of apatite formation in the ternary MgO–CaO–SiO2 system (Tsuru et al. 1995). Magnesium is one of the major inorganic elements in body fluids and has already been used in bioactive glass-ceramics, such as glass-ceramic A–W, which does not produce such a thick silica gel layer in the body environment. When CaO was partially replaced with MgO, a decrease in the thickness of the silica gel layer between the apatite layer and the glass was observed. A glass with the composition of 10MgO·40CaO·50SiO2 mol% formed apatite in SBF within 3 days, and the apatite layer seemed to be in direct contact with the glass substrate without the formation of a thick silica gel layer between the apatite and the glass. This means that strong bonding of the apatite and the glass substrate is expected. Therefore, it is expected that the 10MgO·40CaO·50SiO2 mol% composition is useful for designing bioactive glass-ceramics. Based on this idea, Ohtsuki et al. (2004) synthesized glass-ceramics from a glass with this composition. At first, a glass with nominal composition of 10MgO·40CaO·50SiO2 mol% was prepared using a conventional melt-quenching technique. A slurry consisting of pulverized glass and ultrapure water was loaded in a polyurethane sponge, which was then heated at various temperatures to give porous glass-ceramics. Porous ceramics with both high bone-bonding ability and bioresorbability are expected to be useful scaffolds for use in bone regeneration. Porous ceramics with continuous pores of approximately 500 μm in diameter were obtained after sintering and crystallization (figure 1). Chang et al. (2000) reported that pores that are 300–500 μm in diameter in hydroxyapatite ceramics were effective for bone ingrowth. Therefore, this porous structure is expected to be useful for the invasion of cells and new bone when these glass-ceramics are implanted into bone defects. Precipitation of parawollastonite and diopside was observed after heat treatment at temperatures above 900°C. The amount of precipitated diopside increased with increasing temperature. The synthesized glass-ceramics formed apatite on their surfaces after soaking in SBF. When the porous glass-ceramic was implanted in a rabbit tibia, it showed bioresorbability (Tanihara et al. 2003). Consequently, glasses in the MgO–CaO–SiO2 system allow for easy production of porous glass-ceramics with bioactivity and bioresorption using a conventional sintering process.
Figure 1.
SEM micrographs of the inside of porous glass-ceramics heat treated at various temperatures. (a) 770°C, (b) 1000°C, (c) 1100°C and (d) 1200°C.
2.2 Glass-ceramics in the ZnO–CaO–SiO2–P2O5–CaF2 system
The MgO–CaO–SiO2 glass-ceramic described in §2.1 is useful in the point of reactivity, but it does not have much stimulation effect for bone formation; therefore, the design of a glass-ceramic that has stimulation effect for bone formation is described in this section. The bioactivity of ceramics is governed by the reaction between the ceramic and the body fluid. Moreover, the bioresorption of ceramics is also governed by a chemical reaction. It is expected that the addition of zinc oxide to a bioactive glass-ceramic may control the reaction between the glass-ceramic and the surrounding body fluid. Zinc oxide was selected because zinc is an essential trace element that shows a stimulatory effect on bone formation (Yamaguchi et al. 1987). Therefore, zinc ions released from a glass-ceramic may enhance bone regeneration in a bony defect. Ito et al. (2000) developed calcium phosphate ceramics designed to release zinc ions. The authors expected that the glass-ceramic system would be useful for releasing zinc because the properties can be easily controlled by changing the composition. Among the bioactive ceramics, glass-ceramic A–W shows high bioactivity and high mechanical strength (Kokubo et al. 1982; Kokubo 1993). The effect of the addition of ZnO to CaO–SiO2–P2O5–CaF2 glass-ceramics, whose compositions were based on a modification of glass-ceramic A–W, on the apatite-forming ability, chemical durability and zinc release, was examined (Kamitakahara et al. 2006a).
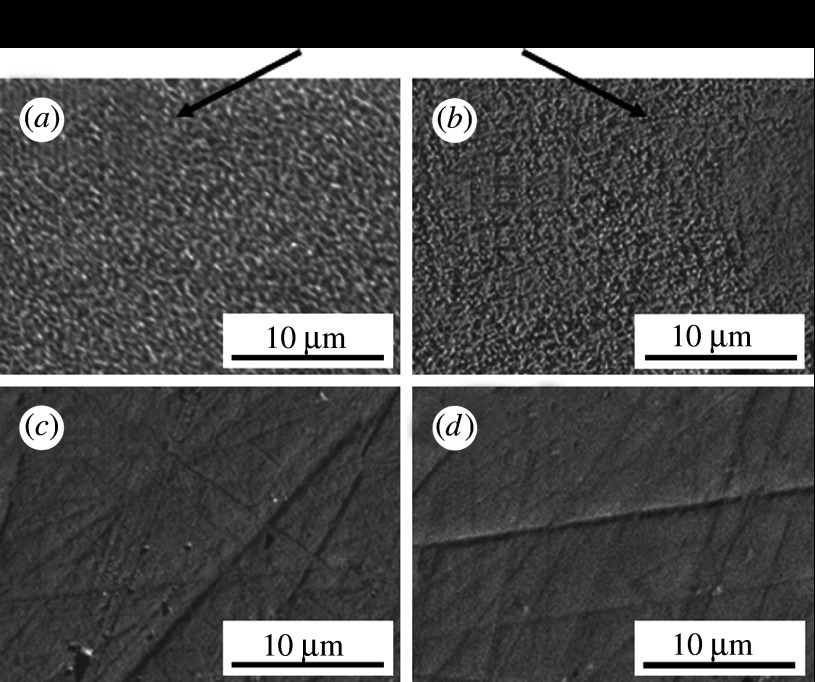
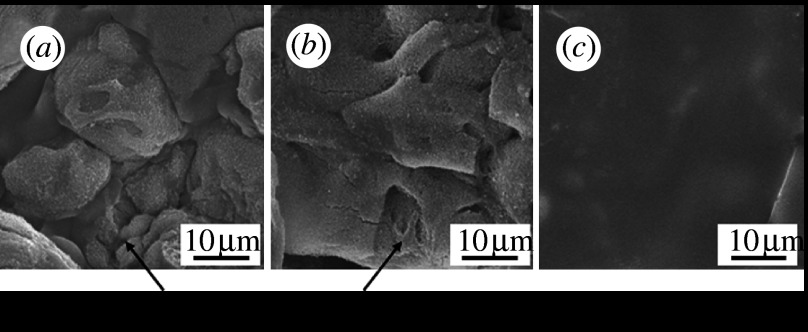
Glasses with the composition xZnO·(57.0−x)CaO·35.4SiO2·7.2P2O5·0.4CaF2 were prepared (where x=0.0, 0.7, 3.6 or 7.1 mol%) using a conventional melt-quenching technique (samples Zn0, Zn0.7, Zn3.6 and Zn7.1, respectively). Compacts of the glass powders were heated to a temperature of 930°C for 4 hours for sintering and crystallization. Glass-ceramics containing apatite and wollastonite were obtained using this procedure. Figure 2 shows SEM micrographs of the surfaces of the glass-ceramics after soaking in SBF for 7 days. Precipitates composed of fine apatite particles were observed on glass-ceramic samples Zn0 and Zn0.7, but they were not observed on samples Zn3.6 and Zn7.1.
Figure 2.
SEM micrographs of the surfaces of glass-ceramics after soaking in SBF for 7 days. (a) Zn0, (b) Zn0.7, (c) Zn3.6 and (d) Zn7.1.
Changes in the element concentrations of SBF after soaking the glass-ceramics are shown in figure 3. The decrease in phosphorus concentration for samples Zn0 and Zn0.7 was because of the consumption of phosphate ions through the formation of apatite on the surfaces of glass-ceramic samples Zn0 and Zn0.7. The increase in silicon concentration indicates the release of silicate ions from the glass-ceramics. The release of silicon from the glass-ceramics decreased significantly with increasing ZnO content. This indicates that the reaction between the glass-ceramics and the SBF was suppressed, and the formation of silanol groups was also suppressed by the addition of ZnO. It is speculated that the chemical durability of the glass-ceramics was improved by the addition of ZnO, as ZnO is an amphoteric oxide, and has a very low solubility in SBF. The suppression of the formation of silanol groups would lead to the suppression of apatite formation on the glass-ceramics, but the glass-ceramics still showed apatite-forming ability when the ZnO content was low. The reactions between glass-ceramics and body fluids govern their bioactivity and bioresorbability. This suggests that the bioresorbability of a glass-ceramic can be controlled to some extent without the loss of bioactivity. The release of zinc was clearly detected in glass-ceramic samples containing 3.6 mol% or more of ZnO, and the amount of zinc increased with increasing ZnO content. From the above results, the addition of an appropriate amount of ZnO may provide a composition that has apatite-forming ability and an appropriate zinc release rate.
Figure 3.
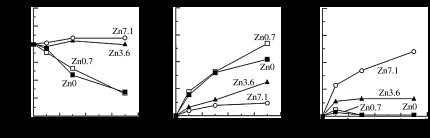
Changes in element concentrations of SBF owing to the soaking of glass-ceramics. (a) P, (b) Si and (c) Zn.
2.3 Glass-ceramics in the 3CaO·P2O5–CaO·MgO·2SiO2 system
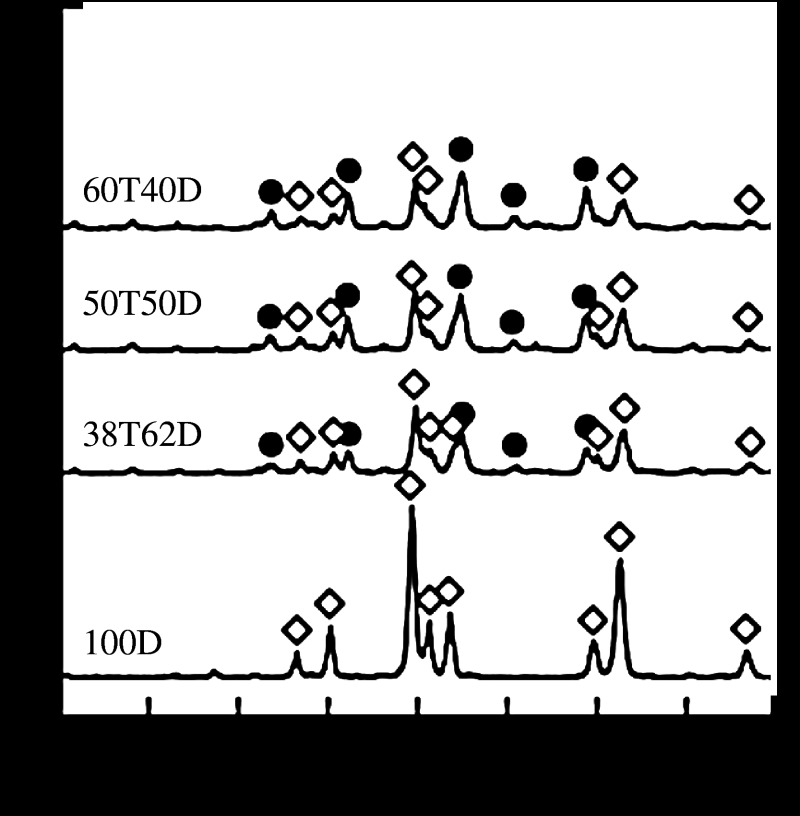
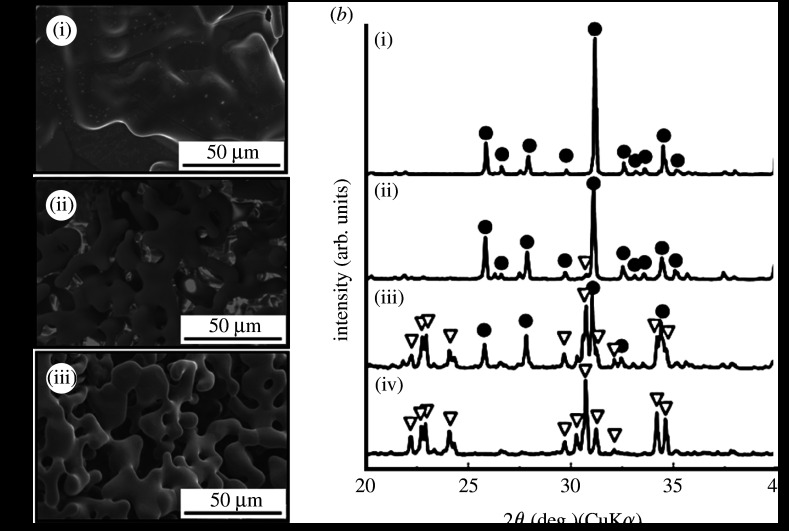
For the design of a scaffold, bioresorbability is important. The design of a glass-ceramic for a scaffold is described. Tricalcium phosphate (3CaO·P2O5, TCP) is known as a bioresorbable ceramic and is already used clinically as an important bone-repair material (Rejda et al. 1977). However, it is not easy to control its bioactivity and bioresorbability, because TCP is a crystalline ceramic. The combination of glass-based ceramics with TCP may lead to novel bioactive and bioresorbable materials for bone regeneration. It has been reported that diopside (CaO·MgO·2SiO2) ceramics show potential for making direct contact with bone, and they show high mechanical strength (Nonami & Tsutsumi 1999). Glass-ceramics containing TCP and diopside would therefore be candidate materials for showing high bioactivity and high mechanical strength in the initial stages after implantation, followed by appropriate degradation during bone regeneration. Kamitakahara et al. (2006b) prepared glass-ceramics containing TCP and diopside from 3CaO·P2O5–CaO·MgO·2SiO2 glasses. Glass-ceramics with the compositions of x(3CaO·P2O5)·(100−x)(CaO·MgO·2SiO2) were prepared, where x=0, 38, 50 or 60 mass% (samples 100D, 38T62D, 50T50D and 60T40D, respectively). To characterize the glass-ceramics, pulverized glasses of each composition were first compacted and heated to obtain the bulk glass-ceramics. Figure 4 shows the powder XRD patterns of the dense glass-ceramics sintered at 1200°C. Both β-TCP and diopside were precipitated in samples 60T40D, 50T50D and 38T62D, while only diopside was precipitated in glass-ceramic sample 100D. β-TCP phase was assumed to be stabilized by some magnesium substitution. The diopside content decreased with increasing TCP content, while the β-TCP content increased with increasing TCP content. After the glass-ceramics were soaked in SBF, fine apatite precipitates were observed on the surfaces of glass-ceramic samples 60T40D and 50T50D within 3 days, and on sample 38T62D within 7 days, as shown in figure 5. The formation of apatite on sample 100D was observed after 14 days of soaking in SBF. The reason why the apatite-forming ability increased with increasing TCP content in the glass composition is attributed to the ease of release of calcium ions from the residual glassy phase in the glass-ceramics into the SBF, as the dense sintered β-TCP shows a low ability for inducing apatite formation in SBF (Ohtsuki et al. 1991b).
Figure 4.
Powder XRD patterns of dense glass-ceramics sintered at 1200°C. Circles, β-TCP; diamonds, diopside.
Figure 5.
SEM micrographs of dense glass-ceramics after soaking in SBF for (a) 3 and (b) 7 days. (i) 60T40D, (ii) 50T50D, (iii) 38T62D and (iv) 100D.
Porous glass-ceramics with continuous pores of approximately 500 μm in size were also successfully prepared with the above compositions when a slurry composed of the glass powders and ultrapure water was loaded in a polyurethane sponge and heated. These porous glass-ceramics containing β-TCP and diopside are expected to be useful as scaffold materials for bone repair.
3. Bioactive hybrids
In the previous section, the design of bioactive glass-ceramics was described. When these glass-ceramics are used as bioresorbable scaffolds, they are finally resorbed, replaced by newly formed bone and mismatch of mechanical properties to bone is not a problem. However, the adjustment of the mechanical properties to bone is important for the design of a material that is implanted for a long period. In spite of the specific biological activity of bioactive ceramics with bone tissue, these bioactive ceramics do not satisfy every clinical application because the mechanical properties of ceramic materials are different from those of natural bone. The development of bioactive materials that show not only bioactivity but also mechanical properties similar to living bone is desired. Living bone is a composite of 70 mass% apatite and 30 mass% collagen (Park & Lakes 1992). Therefore, the combination of an organic substance with bioactive inorganic components would produce a novel bone-repairing material showing not only bioactivity but also mechanical properties similar to living bone.
As discussed above, the CaO–SiO2 binary system can provide a basic composition to form a bone-like apatite layer in the body environment. The detailed investigation of the formation of bone-like apatite layers on a glass with a composition of 50CaO·50SiO2 mol% proposed a mechanism for the induction of heterogeneous nucleation on these materials triggering the formation of bone-like apatite layers in SBF (Ohtsuki et al. 1992). The existence of silanol (Si–OH) groups on the surface of the materials is important to induce heterogeneous nucleation of apatite, as well as the release of Ca2+ from the materials. This finding introduces the idea that bioactive organic–inorganic hybrids can be produced through organic modification of chemical species that allow the formation of Si–OH groups and release of Ca2+ after exposure to body environments. The development of several types of organic–inorganic hybrids was attempted through sol–gel processing, which is a popular process for preparing hybrids of inorganic and organic components because the process can be conducted at low temperature (Mackenzie et al. 1992). Examples of such bioactive hybrids with the ability for bone-like apatite formation are polydimethylsiloxane (PDMS)–CaO–SiO2 (Tsuru et al. 1997; Kamitakahara et al. 2001, 2002), PDMS–CaO–SiO2–TiO2 (Chen et al. 2000), poly(tetramethylene oxide) (PTMO)–CaO–SiO2 (Miyata et al. 2002) and others (Rhee et al. 2002). These were confirmed to show apatite-forming ability in SBF. In these hybrids, the inorganic component, which shows bioactivity, is homogeneously distributed at the molecular level and chemically bonds to the organic component.
We attempted to synthesize organic–inorganic hybrids starting from methacryloxypropyltrimethoxysilane (MPS) and 2-hydroxyethylmethacrylate (HEMA) with the addition of a calcium salt (Ohtsuki et al. 2002; Miyazaki et al. 2003a). MPS has alkoxysilane groups that provide silanol groups after hydrolysis, whereas HEMA provides a hydrophilic polymer matrix in the hybrid. Mixtures of MPS and HEMA with various molar ratios were dissolved in ethanol. The mixtures were polymerized by heat treatment at 75°C with benzoyl peroxide (BPO) as initiator. Then an ethanol solution containing calcium chloride (CaCl2) was added to the polymer solution. The solution obtained was cast in polypropylene containers and dried at room temperature to give bulk gels. A homogeneous transparent gel was obtained at the MPS : HEMA composition 0.1 : 0.9. As higher concentration of MPS led to many cracks in the specimens during the drying process, crack-free specimens were not obtained when the molar ratio of MPS was more than 0.1. Figure 6 shows the appearance of the MPS–HEMA hybrid with MPS : HEMA=0.1 : 0.9. The obtained hybrid showed high flexibility. When the hybrid was soaked in SBF, fine apatite particles covered the surface of the sample within 7 days. This indicates that these hybrids have the potential to show bioactivity. The apatite-forming ability and mechanical properties of the MPS–HEMA hybrids can be controlled by the addition of catalysts during gelation because the siloxane network formed is affected by the catalyst (Uchino et al. in press).
Figure 6.

Appearance of the MPS–HEMA hybrid with MPS : HEMA=0.1 : 0.9 (molar ratio).
This chemical modification using Si–OH and Ca2+ is applicable to the development of bioactive poly(methyl methacrylate) (PMMA) bone cements (Ohtsuki et al. 2001; Miyazaki et al. 2003b; Mori et al. 2003). One of the significant problems of PMMA bone cements is loosening at the interface between the bone and the cement owing to the lack of bioactivity. Although several attempts to obtain bioactive PMMA bone cements by mixing bioactive ceramic powders have been conducted (Shinzato et al. 2000), large amounts of ceramic powder at more than 50 mass% need to be incorporated into the cement to give bioactivity. Moreover, the existence of an interface between the bioactive ceramic powders and the PMMA matrix might be a problem because the bonding strength between them is not so strong. In this system, MPS and MMA can be copolymerized and Si–OH groups are chemically bonded to the PMMA matrix. PMMA powder was mixed with a calcium salt, such as CaCl2, calcium acetate (Ca(CH3COO)2), calcium hydroxide (Ca(OH)2), calcium lactate (Ca(CH3CHOHCOO)2), calcium benzoate (Ca(C6H5COO)2) and calcium methacrylate (Ca(CH2=C(CH3)COO)2), at 20 mass% of the powder. BPO was then added to the powder as a polymerization initiator. MMA liquid was mixed with MPS at 20 mass% of the liquid. N,N-dimethyl-p-toluidine (NDT) was then added to the liquid as a polymerization accelerator. They were mixed with a powder/liquid mass ratio of 2 at 23±2°C. At half of the setting time of the specimens, they were soaked in SBF to examine the apatite-forming ability. After soaking, fine apatite particles were observed on the cements modified with CaCl2, Ca(CH3COO)2, Ca(OH)2 and Ca(CH2=C(CH3)COO)2. Modification of PMMA cement by incorporation of MPS and appropriate calcium salts makes the cement form apatite in the body environment. The solubility of the calcium salts in water decreases in the order CaCl2>Ca(CH3COO)2>Ca(CH3CHOHCOO)2>Ca(C6H5COO)2>Ca(OH)2, and calcium salts highly soluble in water are effective for providing the PMMA cement with apatite-forming ability in SBF. It is noted that the cement modified with Ca(OH)2 formed apatite in SBF, in spite of the solubility of Ca(OH)2 being the lowest among the calcium salts used in this study. The pH of the surrounding solution markedly increased after soaking of the cement modified with Ca(OH)2 in SBF, and this increase in pH would enhance apatite nucleation as OH− is also a component of apatite.
The compressive strength of the modified cements decreased after exposure to SBF, except for the cement modified with Ca(OH)2. This is attributed to the release of Ca2+ ions from the cement into the solution. Among the cements examined in this study, those modified with Ca(CH3COO)2, Ca(OH)2 or Ca(CH3CHOHCOO)2 showed compressive strength near the lower limit (approx. 70 MPa) required by ISO5833. Bioactive PMMA bone cement, able to maintain mechanical strength even in the body environment, can be obtained through the addition of an appropriate selection of calcium salts. In this study, the amount of the inorganic components required for the development of bioactive PMMA cement was significantly reduced in comparison with previous techniques. This is desirable for maintaining the good handling properties of conventional PMMA cements.
4. Bioactive coating
The coating of bone-like apatite on the materials is also a candidate method to obtain mechanical properties similar to bone. As an important factor for artificial materials to show bioactivity is to form bone-like apatite on their surfaces in the body, materials coated with bone-like apatite are also expected to bond to living bone. If composite materials, organic polymers coated with apatite, are synthesized using a process mimicking biomineralization to produce bone as in living tissues, the coated bone-like apatite may accelerate osteoconduction in bony defects. Such a coating design gives the possibility of fabricating novel composite materials that show high bioactivity and mechanical properties similar to those of human bone. Kokubo et al. (1990a) revealed that the apatite formed on bioactive ceramics in SBF is similar to bone apatite. This indicates that a bone-like apatite coating can be achieved using SBF. From the mechanism of formation of apatite on the surface of bioactive glass, it was revealed that silanol (Si–OH) groups are effective for inducing heterogeneous nucleation of hydroxyapatite on the surfaces of substrates. Abe et al. (1990) reported that a bone-like apatite layer could be formed on the surfaces of substrate using a CaO–SiO2-based glass as the source of the apatite-nucleating agent. First, a substrate is apposed to the CaO–SiO2-based glass in SBF. The silicate ions released from the glass attach to the surface of the substrate and induce apatite nucleation (Takadama et al. 2000). The substrates are then soaked in additional SBF or 1.5SBF, which is a solution with ion concentrations 1.5-fold greater than those of SBF to induce apatite crystal growth.
Attempts to introduce silanol groups covalently bonded to organic polymers to induce apatite nucleation have also been reported (Kim et al. 2001; Oyane et al. 2003). Composites consisting of bone-like apatite and biodegradable polymers are attractive materials for bone repair as they are expected to show bioactivity and be resorbed during bone repair. We have reported an apatite–alginate composite using SBF (Hosoya et al. 2004). It is known that alginate can act as a scaffold material for the repair of skin and nerves (Suzuki et al. 1998, 1999). Alginate was reacted with 3-aminopropyltriethoxysilane (APES), which gives silanol groups after hydrolysis. Modification of the alginate with APES gave a gel because the alginate could be cross-linked by dehydration of the silanol groups derived from the APES. The gels obtained were soaked in a CaCl2 solution and subsequently soaked in SBF and apatite was formed on and inside these gels. Modification of alginate with silanol groups induced not only gel formation but also apatite-forming ability in SBF on and inside the alginate gel. An apatite–alginate composite can therefore be produced by modification of alginate with silanol groups and subsequent soaking in CaCl2 solution and SBF. The modification of starch with silanol groups and calcium ions was also attempted (Miyazaki et al. 2007). Starch-based materials are receiving attention as novel bone substitutes (Mendes et al. 2001). Potato starch was dissolved in dimethylsulphoxide and CaCl2, glycidoxypropyltrimethoxysilane (GPS, CH2OCHCH2O(CH2)3Si(OCH3)3) and ultrapure water were then added to the solution. The mass ratios of starch to the total mass of GPS and starch ranged from 0.40 to 0.67, while the molar ratio of CaCl2 to GPS was fixed at 0.05. The specimen with (starch)/(GPS+starch)=x mass% was denoted as ‘Sx’. The prepared sol was dried at 60°C and then soaked in SBF. Figure 7 shows SEM micrographs of the surfaces of the specimens after soaking in SBF for 7 days. Fine apatite particles were observed on the surfaces of S40 and S50, but not S67, which indicates that adequate modification of polymers with silanol groups and calcium ions produces apatite–polymer composites.
Figure 7.
SEM micrographs of the surfaces of starch-based materials after soaking in SBF for 7 days. (a) S40, (b) S50 and (c) S67.
It has been reported that functional groups other than Si–OH also induce heterogeneous nucleation of apatite in SBF. Ti–OH (Li et al. 1994), Ta–OH (Miyazaki et al. 2001a), Zr–OH (Uchida et al. 2001), Nb–OH (Miyazaki et al. 2001b), carboxyl (–COOH; Tanahashi & Matsuda 1997), and sulphonic (–SO3H) groups (Miyazaki et al. in press) induce heterogeneous nucleation of apatite. Therefore, a composite composed of apatite and polymer would be obtained when an organic polymer containing carboxyl groups on the surface and releasing Ca2+ ions into the fluid is soaked in SBF.
There are several reports of the coating of apatite onto natural polymer substrates, such as carboxymethylated chitin gels (Kawashita et al. 2003), gellan gum gels (Kawashita et al. 2003), carboxymethylated chitin (Kokubo et al. 2004a,b), alginate fibres (Kokubo et al. 2004b) and poly(γ-glutamic acid) gels (Sugino et al. 2008), in SBF if they are treated with calcium salt solutions in advance. We also reported that cloth made of raw silk fibres forms apatite in 1.5SBF (Takeuchi et al. 2003). The surfaces of raw silk consist of sericin, which has approximately 20 mol% acidic amino acids (aspartic and glutamic acids; Komatsu 2000; Shimura & Katagata 2000). This indicates that the surface of raw silk fibres is rich in carboxyl groups and the high content of carboxyl groups in sericin is effective for heterogeneous apatite nucleation. The apatite-forming ability of the sericin fibres can be enhanced by soaking in CaCl2 solutions before soaking in 1.5SBF. The structural effects of sericin on apatite formation were also revealed by examining the ability of apatite formation on sericin films made by different processes (Takeuchi et al. 2005a). Sericin films were prepared from a solution extracted from raw silk fibres under various conditions. Among the films, only the sericin film with the highest β-sheet content showed apatite formation in 1.5SBF. These results suggest that apatite nucleation on sericin depends on the content of β-sheets in addition to the content of carboxyl groups. The effectiveness of β-sheet structure for apatite formation was also examined using a synthesized polypeptide (Takeuchi et al. 2008). These results indicate that not only the number of functional groups but also their arrangement are important factors governing apatite-forming ability.
Synthetic polymer substrates are also useful to prepare apatite–polymer composites. We (Miyazaki et al. 2003c; Kawai et al. 2004) reported that aromatic polyamides containing carboxyl groups (polyamide-COOH) or sulphonic (–SO3H) groups (polyamide-SO3H; Konagaya & Tokai 2000) show apatite-forming ability in 1.5SBF when they contained CaCl2. The amount of apatite formed and the rate of formation increased with increasing amounts of functional groups in the polyamides. These results indicate that functional groups in synthetic polymers can act as effective nucleating sites for apatite. We then compared the induction period for the nucleation of apatite and rate of crystal growth on polyamide-COOH and polyamide-SO3H with incorporated calcium chloride by soaking them in 1.5SBF (Kawai et al. 2005). The induction period for apatite nucleation on polyamide-SO3H was shorter than for polyamide-COOH. Considering that functional groups effective for apatite nucleation are negatively charged in physiological solutions, calcium incorporation in the initial stage is very important for apatite nucleation (Takadama et al. 2001). Therefore, easier association of sulphonic groups with calcium ions may lead to a shorter induction period for apatite nuclei than that with carboxyl groups. However, the rate of crystal growth did not depend on the type of functional group, but depended on the degree of supersaturation of the surrounding solution. Providing this apatite-forming ability to these polymers produces novel materials, which when coated with apatite would be novel bioactive materials for bone regeneration.
5. Bioresorbable ceramics
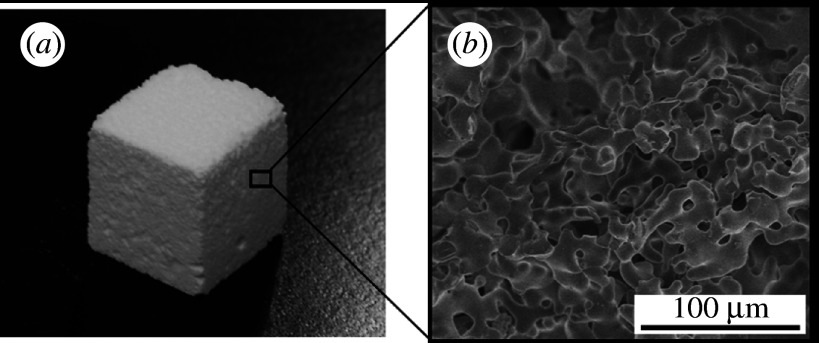
When materials can be resorbed, replaced by newly formed bone, the mismatch of mechanical properties to bone is not a problem. This section describes the design of bioresorbable scaffold ceramics based on calcium phosphate. Bioresorbable ceramics are gradually degraded in bony defects, and are expected to be useful in bone repair. Development of engineering techniques for the regeneration of living tissues leads to the requirement for higher resorbability of scaffold materials, especially in cases of their combination with pharmaceuticals or cells to enhance tissue regeneration. Although β-TCP ceramics have been widely used as conventional resorbable bone substitutes, there is still a requirement for ceramic scaffolds with higher resorbability. α-TCP ceramics are expected to be useful as bone substitutes and as scaffold for tissue engineering when bioresorbability higher than that of β-TCP is required. Because the solubility of α-TCP is higher than that of β-TCP (Chow 1991), a higher degradation rate in the body is expected. As α-TCP is a thermodynamically stable phase at temperatures above approximately 1100°C, α-TCP sintered ceramics can be easily fabricated using conventional sintering processes. We produced α-TCP porous ceramics with 80 per cent continuous pores of approximately 10–50 μm in size by a conventional sintering processing as follows (Kitamura et al. 2004a). β-TCP powder, potato starch and water were mixed to form a slurry. The slurry was dried, heated to 1000°C to burn out the starch, and then the product was sintered at 1400°C, followed by natural cooling in a furnace, to give an α-TCP porous ceramic. Figure 8 shows the appearance and microstructure of the prepared α-TCP porous ceramic. The porosity was easily controlled by the amount of the starch. The porosity governs not only the strength but also the reactivity. The design of surface characteristics can effectively result in higher potential for the induction of apatite formation in the body environment, and it is important to not only control the bioresorbability of TCP ceramics but also to enhance bioactivity (Uchino et al. 2008). The pores in α-TCP porous ceramic are open and continuous to the surface. This structure can be combined with polymers and drugs to produce additional functions. Coating α-TCP with biodegradable polymers can reduce the degradation rate of the α-TCP porous ceramics. Coating with hydroxypropylcellulose was effective in reducing bioresorption and improving workability (Kitamura et al. 2004b). Selection of the type of polymer coated on α-TCP frameworks is an important factor to induce bioactive characteristics. For example, sericin was used as a coating polymer (Takeuchi et al. 2005b) because sericin has the potential to induce bone-like apatite in a physiological environment and may show affinity for bone (Takeuchi et al. 2003). Moreover, when drugs or osteoinductive factors are incorporated in the polymer coating, the α-TCP porous ceramic is expected to show high ability to induce bone formation. To enhance bone regeneration, Saito et al. reported α-TCP porous ceramics combined with a BMP-derived peptide called bone-forming peptide (BFP; Saito et al. 2003, 2006). The combination of α-TCP porous ceramics with BFP was effective in enhancing bone formation, leading to the repair of 20 mm long rabbit radial bone defects. When α-TCP porous ceramics were combined with BFP, the α-TCP porous ceramics with high resorbability acted as useful scaffolds. When the scaffold is implanted with adequate pharmaceuticals or cells into tissue defects, tissue repair is enhanced. In this case, the period when the scaffold is needed to remain becomes short. Therefore, higher resorbability is needed when we combine with pharmaceuticals or cells.
Figure 8.
(a) Appearance and (b) microstructure of α-TCP porous ceramic.
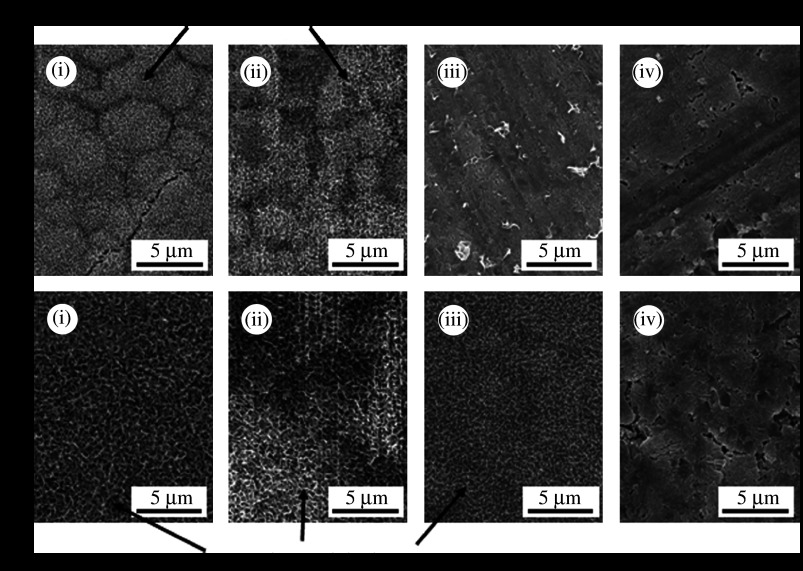
The bioresorption rate was also controlled by the coexistence of α- with β-TCP, based on their different degradation rates. Famery et al. (1994) reported a fabrication process for TCP ceramics with the α- and β-TCP ratio being controlled by the addition of magnesium. We (Oishi et al. 2004; Kamitakahara et al. 2005a) attempted the fabrication of TCP porous ceramics with α- and β-TCP ratios controlled by the addition of Mg, Zn and Fe because additives such as Mg (Ando 1958) and Zn (Kreidler & Hummel 1967) raise the temperature of transformation of TCP from the β- to α-phase. The addition of Mg was most effective in decreasing the porosity and α-TCP content, and a porous body consisting of α- and β-TCP with continuous pores ranging from 10 to 50 μm could be prepared by a conventional sintering method when the Mg content was 0.1 mass% (figure 9). We compared the in vivo degradation rate of the TCP porous ceramic consisting of biphasic α- and β-TCP (α/β-TCP) to that of TCP ceramics consisting of pure α- or β-TCP, and revealed that the biphasic TCP porous ceramic showed lower and higher degradation rates than α- and β-TCP, respectively (Kamitakahara et al. 2005b). The behaviour of the TCP ceramics in the body environment was affected not only by the phase contents but also by the porosity of the ceramics. Materials designed based on their solubility or dissolution rate would also provide novel bioactive materials.
Figure 9.
(a) SEM micrographs ((i) Mg1.0, (ii) Mg0.1 and (iii) Mg0) and (b) XRD patterns of TCP ceramics (down triangles, α-TCP; circles, β-TCP) with different Mg contents ((i) Mg1.0 (ii) Mg0.5, (iii) Mg0.1 and (iv) Mg0). Sample Mgx contains x mass% of Mg.
6. Conclusion and perspective
This paper reviewed novel bioactive ceramic-based materials designed based on chemical reactivity in body fluid. We can develop various bioactive ceramic-based materials, such as glass-ceramics, calcium phosphate ceramics, hybrids and composites. As each material has different advantages, the development of various bioactive materials would be useful and contribute to the medical field. Material design considering the interaction with proteins or cells will be also important. Not only the chemical properties but also the surface charge and morphology are important for these interactions. Material designs based on various aspects must be developed in the future.
Footnotes
One contribution of 10 to a Theme Supplement ‘Japanese biomaterials’.
References
- Abe Y., Kokubo T., Yamamuro T. Apatite coating on ceramics, metals and polymers utilizing a biological process. J. Mater. Sci. Mater. Med. 1990;1:233–238. doi: 10.1007/BF00701082. [DOI] [Google Scholar]
- Ando J. Phase diagrams of Ca3(PO4)2–Mg3(PO4)2 and Ca3(PO4)2–CaNaPO4 systems. Bull. Chem. Soc. Jpn. 1958;31:201–205. doi: 10.1246/bcsj.31.201. [DOI] [Google Scholar]
- Chang B.-S., Lee C.-K., Hong K.-S., Youn H.-J., Ryu H.-S., Chung S.-S., Park K.-W. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials. 2000;21:1291–1298. doi: 10.1016/S0142-9612(00)00030-2. [DOI] [PubMed] [Google Scholar]
- Chen Q., Miyata N., Kokubo T., Nakamura T. Bioactivity and mechanical properties of PDMS-modified CaO–SiO2–TiO2 hybrids prepared by sol–gel process. J. Biomed. Mater. Res. 2000;51:605–611. doi: 10.1002/1097-4636(20000915)51:4<605::AID-JBM8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Cho S.B., Nakanishi K., Kokubo T., Soga N., Ohtsuki C., Nakamura T., Kitsugi T., Yamamuro T. Dependence of apatite formation on silica gel on its structure: effect of heat treatment. J. Am. Ceram. Soc. 1995;78:1769–1774. doi: 10.1111/j.1151-2916.1995.tb08887.x. [DOI] [Google Scholar]
- Chow L.C. Development of self-setting calcium phosphate cements. J. Ceram. Soc. Jpn. 1991;99:954–964. [Google Scholar]
- Famery R., Richard N., Boch P. Preparation of α- and β-tricalcium phosphate ceramics, with and without magnesium addition. Ceram. Int. 1994;20:327–336. doi: 10.1016/0272-8842(94)90050-7. [DOI] [Google Scholar]
- Gross U.M., Müller-Mai C., Voigt C. Ceravital® bioactive ceramics. In: Hench L.L., Wilson J., editors. An introduction to bioceramics. World Scientific; Singapore: 1993. pp. 105–124. [Google Scholar]
- Hench L.L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991;74:1487–1510. doi: 10.1111/j.1151-2916.1991.tb07132.x. [DOI] [Google Scholar]
- Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K., Jr Bonding mechanism at interface of ceramic prosthetic materials. J. Biomed. Mater. Res. Symp. 1971;2:117–141. doi: 10.1002/jbm.820050611. [DOI] [Google Scholar]
- Hosoya K., Ohtsuki C., Kawai T., Kamitakahara M., Ogata S., Miyazaki T., Tanihara M. A novel covalently crosslinked gel of alginate and silane with the ability to form bone-like apatite. J. Biomed. Mater. Res. 2004;71A:596–601. doi: 10.1002/jbm.a.30189. [DOI] [PubMed] [Google Scholar]
- Ito A., Ojima K., Naito H., Ichinose N., Tateishi T. Preparation, solubility and cytocompatibility of zinc-releasing calcium phosphate ceramics. J. Biomed. Mater. Res. 2000;50:178–183. doi: 10.1002/(SICI)1097-4636(200005)50:2<178::AID-JBM12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Jarcho M., Kay J.L., Gumaer R.H., Drobeck H.P. Tissue, cellular, and subcellular events at a bone–ceramic apatite interface. J. Bioeng. 1977;1:79–92. [PubMed] [Google Scholar]
- Kamitakahara M., Kawashita M., Miyata N., Kokubo T., Nakamura T. Bioactivity and mechanical properties of polydimethylsiloxane (PDMS)–CaO–SiO2 hybrids with different PDMS contents. J. Sol–Gel Sci. Technol. 2001;21:75–81. doi: 10.1023/A:1011261617377. [DOI] [PubMed] [Google Scholar]
- Kamitakahara M., Kawashita M., Miyata N., Kokubo T., Nakamura T. Bioactivity and mechanical properties of polydimethylsiloxane (PDMS)–CaO–SiO2 hybrids with different calcium contents. J. Mater. Sci. Mater. Med. 2002;13:1015–1020. doi: 10.1023/A:1020324101682. [DOI] [PubMed] [Google Scholar]
- Kamitakahara M., Ohtsuki C., Oishi M., Ogata S., Tanihara M., Miyazaki T. Control of the microstructure of porous tricalcium phosphate: effects of addition of Mg, Zn and Fe. J. Jpn. Soc. Powder Powder Metallurgy. 2005a;52:356–359. doi: 10.2497/jjspm.52.356. [DOI] [Google Scholar]
- Kamitakahara M., Ohtsuki C., Oishi M., Ogata S., Miyazaki T., Tanihara M. Preparation of porous biphasic tricalcium phosphate and its in vivo behavior. Key Eng. Mater. 2005b;284–286:281–284. [Google Scholar]
- Kamitakahara M., Ohtsuki C., Inada H., Tanihara M., Miyazaki T. Effect of ZnO addition on bioactive CaO–SiO2–P2O5–CaF2 glass-ceramics containing apatite and wollastonite. Acta Biomater. 2006a;2:467–471. doi: 10.1016/j.actbio.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Kamitakahara M., Ohtsuki C., Kozaka Y., Ogata S., Tanihara M., Miyazaki T. Preparation of porous glass-ceramics containing whitlockite and diopside for bone repair. J. Ceram. Soc. Jpn. 2006b;114:82–86. doi: 10.2109/jcersj.114.82. [DOI] [Google Scholar]
- Kawai T., Ohtsuki C., Kamitakahara M., Miyazaki T., Tanihara M., Sakaguchi Y., Konagaya S. Coating of an apatite layer on polyamide films containing sulfonic groups by a biomimetic process. Biomaterials. 2004;25:4529–4534. doi: 10.1016/j.biomaterials.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Kawai T., Ohtsuki C., Kamitakahara M., Tanihara M., Miyazaki T., Sakaguchi Y., Konagaya S. A comparative study of apatite deposition on polyamide films containing different functional groups under a biomimetic condition. J. Ceram. Soc. Jpn. 2005;113:588–592. doi: 10.2109/jcersj.113.588. [DOI] [Google Scholar]
- Kawashita M., Nakao M., Minoda M., Kim H.-M., Beppu T., Miyamoto T., Kokubo T., Nakamura T. Apatite-forming ability of carboxyl group-containing polymer gels in a simulated body fluid. Biomaterials. 2003;24:2477–2484. doi: 10.1016/S0142-9612(03)00050-4. [DOI] [PubMed] [Google Scholar]
- Kim H.-M., Uenoyama M., Kokubo T., Minoda M., Miyamoto T., Nakamura T. Biomimetic apatite formation on polyethylene photografted with vinyltrimethoxysilane and hydrolyzed. Biomaterials. 2001;22:2489–2494. doi: 10.1016/S0142-9612(00)00437-3. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Ohtsuki C., Ogata S., Kamitakahara M., Tanihara M. Microstructure and bioresorbable properties of α-TCP ceramic porous body fabricated by direct casting method. Mater. Trans. 2004a;45:983–988. doi: 10.2320/matertrans.45.983. [DOI] [Google Scholar]
- Kitamura M., Ohtsuki C., Iwasaki H., Ogata S., Tanihara M., Miyazaki T. The controlled resorption of porous α-tricalcium phosphate using a hydroxypropylcellulose coating. J. Mater. Sci. Mater. Med. 2004b;15:1153–1158. doi: 10.1023/B:JMSM.0000046399.40310.47. [DOI] [PubMed] [Google Scholar]
- Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991;12:155–163. doi: 10.1016/0142-9612(91)90194-F. [DOI] [PubMed] [Google Scholar]
- Kokubo T. A/W glass-ceramics: processing and properties. In: Hench L.L., Wilson J., editors. An introduction to bioceramics. World Scientific; Singapore: 1993. pp. 75–88. [Google Scholar]
- Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Kokubo T., Shigematsu M., Nagashima Y., Tashiro M., Nakamura T., Yamamuro T., Higashi S. Apatite- and wollastonite-containing glass-ceramics for prosthetic application. Bull. Inst. Chem. Res. Kyoto Univ. 1982;60:260–268. [Google Scholar]
- Kokubo T., Ito S., Huang Z.T., Hayashi T., Sakka S., Kitsugi T., Yamamuro T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A–W. J. Biomed. Mater. Res. 1990a;24:331–343. doi: 10.1002/jbm.820240306. [DOI] [PubMed] [Google Scholar]
- Kokubo T., Kushitani H., Sakka S., Kitsugi T., Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A–W. J. Biomed. Mater. Res. 1990b;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- Kokubo T., Hanakawa M., Kawashita M., Minoda M., Beppu T., Miyamoto T., Nakamura T. Apatite formation on non-woven fabric of carboxymethylated chitin in SBF. Biomaterials. 2004a;25:4485–4488. doi: 10.1016/j.biomaterials.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kokubo T., Hanakawa M., Kawashita M., Minoda M., Beppu T., Miyamoto T., Nakamura T. Apatite-forming ability of alginate fibers treated with calcium hydroxide solution. J. Mater. Sci. Mater. Med. 2004b;15:1007–1012. doi: 10.1023/B:JMSM.0000042686.44977.81. [DOI] [PubMed] [Google Scholar]
- Komatsu K. Chemical and structural characteristics of wild cocoon and silk. In: Hojo N., editor. Structure of silk yarn, part B: chemical structure and processing of silk yarn. Science Publishers, Inc; Enfield, NH: 2000. pp. 21–46. [Google Scholar]
- Konagaya S., Tokai M. Synthesis of ternary copolyamides from aromatic diamine (m-phenylenediamine, diaminodiphenylsulfone), aromatic diamine with carboxyl or sulfonic group (3,5-diaminobenzoic acid, 2,4-diaminobenzenesulfonic acid), and iso- or terephthaloyl chloride. J. Appl. Polym. Sci. 2000;76:913–920. doi: 10.1002/(SICI)1097-4628(20000509)76:6<913::AID-APP18>3.0.CO;2-1. [DOI] [Google Scholar]
- Kreidler E.R., Hummel F.A. Phase equilibria in the system Ca3(PO4)2–Zn3(PO4)2. Inorg. Chem. 1967;6:524–528. doi: 10.1021/ic50049a021. [DOI] [Google Scholar]
- LeGeros R.Z., LeGeros J.P. Dense hydroxyapatite. In: Hench L.L., Wilson J., editors. An introduction to bioceramics. World Scientific; Singapore: 1993. pp. 139–180. [Google Scholar]
- Li P., Ohtsuki C., Kokubo T., Nakanishi K., Soga N., de Groot K. The role of hydrated silica, titania and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994;28:7–15. doi: 10.1002/jbm.820280103. [DOI] [PubMed] [Google Scholar]
- Mackenzie J.D., Chung Y.J., Hu Y. Rubbery ormosils and their applications. J. Non-Cryst. Solids. 1992;147 & 148:271–279. doi: 10.1016/S0022-3093(05)80629-5. [DOI] [Google Scholar]
- Mendes S.C., Reis R.L., Bovell Y.P., Cunha A.M., van Blitterswijk C.A., de Bruijn J.D. Biocompatibility testing of novel starch-based materials with potential application in orthopaedic surgery: a preliminary study. Biomaterials. 2001;22:2057–2064. doi: 10.1016/S0142-9612(00)00395-1. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Kim H.-M., Kokubo T., Kato H., Nakamura T. Induction and acceleration of bonelike apatite formation on tantalum oxide gel in simulated body fluid. J. Sol–Gel Sci. Technol. 2001a;21:83–88. doi: 10.1023/A:1011265701447. [DOI] [Google Scholar]
- Miyazaki T., Kim H.-M., Kokubo T., Ohtsuki C., Nakamura T. Apatite-forming ability of niobium oxide gels in a simulated body fluid. J. Ceram. Soc. Jpn. 2001b;109:929–933. [Google Scholar]
- Miyata N., Fuke K., Chen Q., Kawashita M., Kokubo T., Nakamura T. Apatite-forming ability and mechanical properties of PTMO-modified CaO–SiO2 hybrids prepared by sol–gel processing: effect of CaO and PTMO contents. Biomaterials. 2002;23:3033–3040. doi: 10.1016/S0142-9612(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Ohtsuki C., Tanihara M. Synthesis of bioactive organic–inorganic nano-hybrid for bone repair through sol–gel processing. J. Nanosci. Nanotechnol. 2003a;3:511–515. doi: 10.1166/jnn.2003.221. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Ohtsuki C., Kyomoto M., Tanihara M., Mori A., Kuramoto K. Bioactive PMMA bone cement prepared by modification with methacryloxypropyltrimethoxysilane and calcium chloride. J. Biomed. Mater. Res. 2003b;67A:1417–1423. doi: 10.1002/jbm.a.20042. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Ohtsuki C., Akioka Y., Tanihara M., Nakao J., Sakaguchi Y., Konagaya S. Apatite deposition on polyamide films containing carboxyl group in a biomimetic solution. J. Mater. Sci. Mater. Med. 2003c;14:569–574. doi: 10.1023/A:1024000821368. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Yasunaga S., Ishida E., Ashizuka M., Ohtsuki C. Effects of cross-linking agent on apatite-forming ability and mechanical property of organic–inorganic hybrids based on starch. Mater. Trans. 2007;48:317–321. doi: 10.2320/matertrans.48.317. [DOI] [Google Scholar]
- Miyazaki, T., Imamura, M., Ishida, E., Ashizuka, M. & Ohtsuki, C. In press. Apatite formation abilities and mechanical properties of hydroxyethylmethacrylate-based organic–inorganic hybrids incorporated with sulfonic groups and calcium ions. J. Mater. Sci. Mater. Med. ( 10.1007/s10856-008-3556-5). [DOI] [PubMed]
- Mori A., Ohtsuki C., Sugino A., Kuramoto K., Miyazaki T., Tanihara M., Osaka A. Bioactive PMMA-based bone cement modified with methacryloxypropyltrimethoxysilane and calcium salts—effects of calcium salts on apatite-forming ability. J. Ceram. Soc. Jpn. 2003;111:738–742. doi: 10.2109/jcersj.111.738. [DOI] [Google Scholar]
- Nonami T., Tsutsumi S. Study of diopside ceramics for biomaterials. J. Mater. Sci. Mater. Med. 1999;10:475–479. doi: 10.1023/A:1008996908797. [DOI] [PubMed] [Google Scholar]
- Ohtsuki C., Kokubo T., Takatsuka K., Yamamuro T. Compositional dependence of bioactivity of glasses in the system CaO–SiO2–P2O5—its in vitro evaluation. J. Ceram. Soc. Jpn. 1991a;99:1–6. [Google Scholar]
- Ohtsuki C., Kokubo T., Neo M., Kotani S., Yamamuro T., Nakamura T., Bando Y. Bone-bonding mechanism of sintered β-3CaO·P2O5. Phosphorus Res. Bull. 1991b;1:191–196. [Google Scholar]
- Ohtsuki C., Kokubo T., Yamamuro T. Mechanism of apatite formation on CaO–SiO2–P2O5 glasses in a simulated body fluid. J. Non-Cryst. Solids. 1992;143:84–92. doi: 10.1016/S0022-3093(05)80556-3. [DOI] [Google Scholar]
- Ohtsuki C., Miyazaki T., Kyomoto M., Tanihara M., Osaka A. Development of bioactive PMMA-based cement by modification with alkoxysilane and calcium salt. J. Mater. Sci. Mater. Med. 2001;12:895–899. doi: 10.1023/A:1012876108210. [DOI] [PubMed] [Google Scholar]
- Ohtsuki C., Miyazaki T., Tanihara M. Development of bioactive organic–inorganic hybrid for bone substitutes. Mater. Sci. Eng. C. 2002;22:27–34. doi: 10.1016/S0928-4931(02)00109-1. [DOI] [Google Scholar]
- Ohtsuki C., Miyazaki T., Kishiro K., Kamitakahara M., Tanihara M. Bioactivity of MgO-CaO-SiO2 porous glass-ceramics. J. Ceram. Soc. Jpn. Suppl. 2004;112:S809–S812. [Google Scholar]
- Oishi M., Ohtsuki C., Kitamura M., Kamitakahara M., Ogata S., Miyazaki T., Tanihara M. Fabrication and chemical durability of porous bodies consisting of biphasic tricalcium phosphates. Phosphorus Res. Bull. 2004;17:95–100. [Google Scholar]
- Oyane A., Kawashita M., Nakanishi K., Kokubo T., Minoda M., Miyamoto T., Nakamura T. Bonelike apatite formation on ethylene-vinyl alcohol copolymer modified with silane coupling agent and calcium silicate solutions. Biomaterials. 2003;24:1729–1735. doi: 10.1016/S0142-9612(02)00581-1. [DOI] [PubMed] [Google Scholar]
- Park J.B., Lakes R.S. Biomaterials. Plenum Press; New York, NY: 1992. pp. 185–222. [Google Scholar]
- Rejda B.V., Peelen J.G., de Groot K. Tricalcium phosphate as a bone substitute. J. Bioeng. 1977;1:93–97. [PubMed] [Google Scholar]
- Rhee S.H., Choi J.Y., Kim H.-M. Preparation of a bioactive and degradable poly(ε-caprolactone)/silica hybrid through a sol–gel method. Biomaterials. 2002;23:4915–4921. doi: 10.1016/S0142-9612(02)00251-X. [DOI] [PubMed] [Google Scholar]
- Saito A., Suzuki Y., Ogata S., Ohtsuki C., Tanihara M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochimi. Biophys. Acta. 2003;1651:60–67. doi: 10.1016/s1570-9639(03)00235-8. [DOI] [PubMed] [Google Scholar]
- Saito A., Suzuki Y., Kitamura M., Ogata S., Yoshihara Y., Masuda S., Ohtsuki C., Tanihara M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an α-tricalcium phosphate scaffold. J. Biomed. Mater. Res. 2006;77A:700–706. doi: 10.1002/jbm.a.30662. [DOI] [PubMed] [Google Scholar]
- Shimura K., Katagata Y. Chemical structure of silk fibroin. In: Hojo N., editor. Structure of silk yarn, part B: chemical structure and processing of silk yarn. Science Publishers, Inc; Enfield, NH: 2000. pp. 3–20. [Google Scholar]
- Shinzato S., Kobayashi M., Mousa W.F., Kamimura M., Neo M., Kitamura Y., Kokubo T., Nakamura T. Bioactive polymethyl methacrylate-based bone cement: comparison of glass beads, apatite- and wollastonite-containing glass-ceramic, and hydroxyapatite fillers on mechanical and biological properties. J. Biomed. Mater. Res. 2000;51:258–272. doi: 10.1002/(SICI)1097-4636(200008)51:2<258::AID-JBM15>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Sugino A., Miyazaki T., Ohtsuki C. Apatite-forming ability of polyglutamic acid hydrogels in body environment. J. Mater. Sci. Mater. Med. 2008;19:2269–2274. doi: 10.1007/s10856-007-3327-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nishimura Y., Tanihara M., Suzuki K., Nakamura T., Shimizu Y., Yamawaki Y., Kakimaru Y. Evaluation of a novel alginate gel dressing: cytotoxicity to fibroblasts in vitro and foreign-body reaction in pig skin in vivo. J. Biomed. Mater. Res. 1998;39:317–322. doi: 10.1002/(SICI)1097-4636(199802)39:2<317::AID-JBM20>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Tanihara M., Ohnishi K., Suzuki K., Endo K., Nishimura Y. Cat peripheral nerve regeneration across 50 mm gap repaired with a novel nerve guide composed of freeze-dried alginate gel. Neurosci. Lett. 1999;259:75–78. doi: 10.1016/S0304-3940(98)00924-0. [DOI] [PubMed] [Google Scholar]
- Takadama H., Kim H.-M., Miyaji F., Kokubo T., Nakamura T. Mechanism of apatite formation induced by silanol groups—TEM observation. J. Ceram. Soc. Jpn. 2000;108:118–121. [Google Scholar]
- Takadama H., Kim H.-M., Kokubo T., Nakamura T. Mechanism of biomineralization of apatite on a sodium silicate glass: TEM-EDX study in vitro. Chem. Mater. 2001;13:1108–1113. doi: 10.1021/cm0008718. [DOI] [Google Scholar]
- Takeuchi A., Ohtsuki C., Miyazaki T., Tanaka H., Yamazaki M., Tanihara M. Deposition of bone-like apatite on silk fiber in a solution that mimics extracellular fluid. J. Biomed. Mater. Res. 2003;65A:283–289. doi: 10.1002/jbm.a.10456. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Ohtsuki C., Miyazaki T., Kamitakahara M., Ogata S., Yamazaki M., Furutani Y., Kinoshita H., Tanihara M. Heterogeneous nucleation of apatite on protein: structural effect of silk sericin. J. R. Soc. Interface. 2005a;2:373–378. doi: 10.1098/rsif.2005.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Ohtsuki C., Kamitakahara M., Ogata S., Tanihara M., Miyazaki T., Yamazaki M., Furutani Y., Kinoshita H. Biodegradation of porous alpha-tricalcium phosphate coated with silk sericin. Key Eng. Mater. 2005b;284–286:329–332. [Google Scholar]
- Takeuchi A., Ohtsuki C., Kamitakahara M., Ogata S., Miyazaki T., Tanihara M. Biomimetic deposition of hydroxyapatite on a synthetic polypeptide with β sheet structure in a solution mimicking body fluid. J. Mater. Sci. Mater. Med. 2008;19:387–393. doi: 10.1007/s10856-007-3179-2. [DOI] [PubMed] [Google Scholar]
- Tanahashi M., Matsuda T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 1997;34:305–315. doi: 10.1002/(SICI)1097-4636(19970305)34:3<305::AID-JBM5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tanihara, M., Miyazaki, T., Ogata, S. & Ohtsuki, C. 2003 Design and development of functional biocompatible hybrid materials for medical applications. In Handbook of organic–inorganic hybrid materials and nanocomposites, vol. 2 (ed. H. Nalwa), pp. 265–293. Los Angeles, CA: American Scientific Publishers.
- Tsuru, K., Ohtsuki, C. & Osaka, A. 1995 Effect of MgO and BaO on bioactivity of CaO-SiO2 glasses. In Proc. XVII Int. Cong. on Glass, vol. 5 (ed. G. Fangtian), pp. 85–90. Beijing, China: Chinese Ceramic Society.
- Tsuru K., Ohtsuki C., Osaka A., Iwamoto T., Mackenzie J.D. Bioactivity of sol–gel derived organically modified silicates, part I: in vitro examination. J. Mater. Sci. Mater. Med. 1997;8:157–161. doi: 10.1023/A:1018523203667. [DOI] [PubMed] [Google Scholar]
- Uchida M., Kim H.-M., Miyaji F., Kokubo T., Nakamura T. Bonelike apatite formation induced on zirconia gel in a simulated body fluid and its modified solutions. J. Am. Ceram. Soc. 2001;84:2041–2044. [Google Scholar]
- Uchino T., Yamaguchi K., Kawachi G., Kikuta K., Kamitakahara M., Ohtsuki C. Formation of hydroxyapatite on ceramics consisting of tricalcium phosphate in a simulated body fluid. J. Ceram. Soc. Jpn. 2008;116:96–99. doi: 10.2109/jcersj2.116.96. [DOI] [Google Scholar]
- Uchino, T. Ohtsuki, C., Kamitakahara, M., Miyazaki, T., Hayakawa, S. & Osaka, A. In press. Synthesis of bioactive HEMA-MPS-CaCl2 hybrid gels: effects of catalysts in the sol–gel processing on mechanical properties and in vitro hydroxyapatite formation in a simulated body fluid. J. Biomater. Appl. [DOI] [PubMed]
- Yamaguchi M., Oishi H., Suketa Y. Stimulatory effect of zinc on bone formation in tissue culture. Biochem. Pharmacol. 1987;36:4007–4012. doi: 10.1016/0006-2952(87)90471-0. [DOI] [PubMed] [Google Scholar]