Abstract
Temperature-responsive intelligent surfaces, prepared by the modification of an interface with poly(N-isopropylacrylamide) and its derivatives, have been used for biomedical applications. Such surfaces exhibit temperature-responsive hydrophilic/hydrophobic alterations with external temperature changes, which, in turn, result in thermally modulated interactions with biomolecules and cells. In this review, we focus on the application of these intelligent surfaces to chromatographic separation and cell cultures. Chromatographic separations using several types of intelligent surfaces are mentioned briefly, and various effects related to the separation of bioactive compounds are discussed, including wettability, copolymer composition and graft polymer architecture. Similarly, we also summarize temperature-responsive cell culture substrates that allow the recovery of confluent cell monolayers as contiguous living cell sheets for tissue-engineering applications. The key factors in temperature-dependent cell adhesion/detachment control are discussed from the viewpoint of grafting temperature-responsive polymers, and new methodologies for effective cell sheet culturing and the construction of thick tissues are summarized.
Keywords: intelligent materials, temperature-responsive polymer, poly(N-isopropylacrylamide), tissue engineering, living radical polymerization, cell sheet engineering
1. Introduction
Surface modification with thin polymer layers has been used to avoid uncontrolled interactions with cells and proteins in biomedical applications and clinical devices, because protein adsorption and subsequent cell adhesion behaviour are disadvantageous for the desired functions of these materials. Such surface design has been performed by grafting several types of highly hydrophilic polymers, such as poly(ethylene glycol) (Park et al. 1996; Razatos et al. 2000; Winblade et al. 2000), polyacrylamide (PAAm; Ikada et al. 1981; Bamford & Al-Lamee 1996), poly(2-methacryloyloxyethyl phosphorylcholine) (Ishihara et al. 1991; Feng et al. 2005) and poly(2-hydroxyethyl methacrylate) (Koehler et al. 2000; Yoshikawa et al. 2006). These surfaces are resistant to non-specific protein adsorption and cell adhesion, contributing to a material's ‘biocompatibility’, and are able to maintain the functionality of the modified biomaterials for applications such as membranes, bioimplants and sensors (Kato et al. 2003; Kikuchi & Okano 2005).
At the same time, several attractive intelligent surfaces are currently recognized as valuable materials with novel properties of relevance to biomedical research. In particular, temperature-responsive surfaces have been prepared using the thermoresponsive polymer poly(N-isopropylacrylamide) (PIPAAm) and its derivatives (Kikuchi & Okano 2002; Gil & Hudson 2004). PIPAAm exhibits a reversible temperature-dependent phase transition in aqueous solutions at its lower critical solution temperature (LCST) of 32°C (Heskins & Guillet 1968), and this intrinsic thermoresponsive property is widely used in biomedical applications, such as controlled drug and gene delivery systems (Cammas et al. 1997; Chung et al. 1999; Kurisawa et al. 2000; Takeda et al. 2000), enzyme bioconjugates (Chilkoti et al. 1994; Matsukata et al. 1994) and microfluidics (Yu et al. 2003).
Furthermore, PIPAAm-grafted surfaces are being applied to new forms of chromatographic matrices (Kanazawa et al. 1996; Kikuchi & Okano 2004; Ayano & Kanazawa 2006) and cell culture substrates for tissue engineering (Yamada et al. 1990; Shimizu et al. 2002; Yamato & Okano 2004). Grafted chromatographic matrices prepared from PIPAAm and its derivatives allow for the separation of bioactive compounds, including steroid hormones (Kanazawa et al. 1996; Yakushiji et al. 1999), polypeptides and proteins (Kanazawa et al. 1997a,b) and nucleotides in all aqueous mobile phases (Ayano et al. 2006; Kikuchi et al. 2007). This system is useful for controlling both the function and the properties of the stationary phase for high-performance liquid chromatography (HPLC) via the column temperature, which has the advantages of maintaining the biological activity rates of peptides and proteins and reducing contamination from the organic mobile phases commonly used in reverse phase chromatography. Additionally, PIPAAm-grafted cell culture substrates exhibit temperature-responsive cell adhesion/detachment properties. Confluent cells on a PIPAAm-grafted surface can be recovered as contiguous intact cell monolayers, called ‘cell sheets’, by lowering the culture temperature from 37 to 20°C and avoiding the use of digestive enzymes and chelating agents. Consistent cell sheet harvest performances have been demonstrated on PIPAAm-grafted culture surfaces, and this technique has been used to prepare various cell sheet types (Yamato et al. 2007; Yang et al. 2007) for regenerative medicine applications (Nishida et al. 2004; Ohki et al. 2006; Ohashi et al. 2007).
These two applications can only be performed through the accurate design of temperature-responsive intelligent surfaces. For example, the chromatographic separation of bioactive compounds using intelligent surfaces is significantly influenced by the graft configuration of the thermoresponsive polymers, because the polymer graft densities and chain lengths affect the surface wettability, leading to an interaction with the analytes (Kikuchi & Okano 2002; Nagase et al. 2008a,c). Similarly, the thickness of the grafted thermoresponsive polymer layer affects the cell adhesion/detachment properties, because the hydration/dehydration alteration properties of the grafted polymer vary with the distance from the graft substrate (Akiyama et al. 2004; Fukumori et al. 2009).
The present paper focuses on characterizing temperature-responsive intelligent surfaces prepared with stimuli-responsive polymers. We also briefly review the application of these intelligent surfaces to temperature-responsive chromatography and cell culture substrates.
2. Preparation and characterization of temperature-responsive intelligent surfaces
2.1 Radiation-induced graft polymerization
Radiation-induced grafting methods have been widely used for the surface modification of biomaterials (Stannett 1990). Grafting methods can be divided into three categories: (i) the pre-irradiation method, (ii) the peroxide method and (iii) the mutual radiation grafting method. Many types of reactions can be used, such as UV irradiation, electron beam (EB), plasma activation and plasma polymerization.
Using UV irradiation in the presence of benzophenone as a photosensitizer, photopolymerization and photografting of PIPAAm were performed onto polystyrene Petri dishes (Morra & Cassinelli 1997). The IPAAm monomer of 5–40 wt% in 2-propanol with 0.1 per cent benzophenone was poured into Petri dishes and irradiated by UV light (365 nm) for 5–30 min. The existence of grafted PIPAAm was revealed by X-ray photoelectron spectroscopy (XPS) analyses with a take-off angle of 45°. Using these Petri dishes, temperature-induced switching of L-292 mouse fibroblast adhesion was achieved.
Using EB irradiation, uniformly spread IPAAm monomer was polymerized and covalently immobilized onto a tissue culture polystyrene (TCPS) dish surface as shown figure 1a. Various types of cells adhered to and proliferated on hydrophobic PIPAAm-grafted TCPS surfaces at 37°C, but the same cell types did not adhere to hydrated, hydrophilic surfaces at 20°C. Cultured cells on hydrophobic PIPAAm-grafted TCPS at 37°C became detached as cells when the culture's temperature was lowered to 20°C. At 20°C, the surfaces were hydrophilic due to PIPAAm's hydration/dehydration alteration at 32°C. Although EB irradiation requires the use of expensive equipment, this method facilitates the large-scale production of temperature-responsive TCPS: the UpCell surface, which is based on our papers, has been globally launched by Nunc (http://www.nuncbrand.com/en/page.aspx?ID=11850). A temperature-responsive culture dish application will be discussed in §4.
Figure 1.
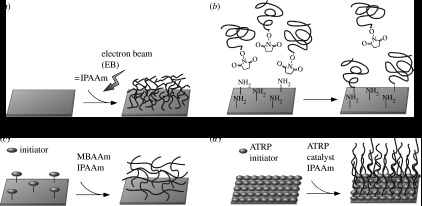
Schematic of surface modification methods using polymers: (a) EB irradiation, (b) ‘grafting to’ and (c) ‘grafting from’ approaches (MBAAm, N,N-methylenebisacrylamide) and (d) surface-initiated living radical polymerization (ATRP, atom transfer radical polymerization).
Recently, Pan et al. (2001) have reported PIPAAm-grafted surfaces prepared by the plasma polymerization method. A plasma glow discharge of IPAAm monomer vapour was used to deposit PIPAAm onto a solid surface, such as silicon, glass (Pan et al. 2001) or a TCPS (Canavan et al. 2005a,b). The spectroscopic data (electron spectroscopy for chemical analysis and Fourier transform infrared spectroscopy (FT-IR)) for this plasma-polymerized IPAAm showed the retention of the monomer structure. The phase transition at 29°C was measured using an atomic force microscope (AFM) method. The phase transition in the plasma-polymerized IPAAm was also responsible for changes in the level of the meniscus when coated capillaries were alternately placed in warm and cold water. The plasma polymerization of IPAAm facilitates a one-step method to fabricate thermally responsive coatings on any substrate; however, this method is not suitable for large-scale production due to difficulties related to continuous treatment and size.
2.2 ‘Grafting to’ approach
A ‘grafting to’ approach has been widely used for the modification of surfaces with stimuli-responsive polymers. This polymer grafting approach consists of two procedures: preparing the polymer with functional groups and reacting the polymer with the surface. This approach has the advantage of controlling the molecular weights of the grafted polymer chains by adjusting the polymerization conditions. However, as in all ‘grafting to’ methods, the polymer graft density is limited because of the sterically restricted reactivity of the surface functional groups, as shown in figure 1b.
Takei et al. (1993, 1994) synthesized two types of PIPAAm: an end-functionalized PIPAAm with a carboxyl end group and a poly(IPAAm-co-acrylic acid (AAc)) copolymer. An end-functional PIPAAm was prepared by the telomerization of IPAAm with 3-mercaptopropionic acid (MPA) using N,N′-azobis(isobutyronitrile) (AIBN) as an initiator in N,N-dimethylformamide (DMF). Poly(IPAAm-co-AAc) was prepared by the radical copolymerization of IPAAm with AAc using AIBN in DMF. Carboxyl groups of these prepared polymers were reacted with amino groups on aminated glass coverslips with poly(styrene-co-aminomethylstyrene) coatings, where they formed a covalent bond. Reactive carboxyl groups along and at the end of the PIPAAm polymer chain produce different graft polymer conformations for multipoint grafted and terminally grafted surfaces. The temperature-responsive change in the wettability of the two types of PIPAAm-grafted surfaces was investigated by measuring the aqueous dynamic contact angles.
Kanazawa et al. (1996) prepared PIPAAm terminally grafted silica beads using a ‘grafting to’ approach for the application to a chromatographic stationary phase. End-carboxyl PIPAAm was prepared, based on the reports by Takei et al. (1993), by using a radical telomerization reaction in the presence of MPA as telogen. Subsequently, the PIPAAm was activated with N-hydroxysuccinimide using dicyclohexylcarbodiimide in dry ethyl acetate. The activated PIPAAm was reacted with (aminopropyl)silica in dioxane. The prepared PIPAAm-modified silica was used as a temperature-responsive chromatographic stationary phase, as discussed later (Kanazawa et al. 1996).
2.3 ‘Grafting from’ approach
The ‘grafting from’ approach has been used to modify surfaces with polymers due to its versatility compared with the ‘grafting to’ approach. In this approach, an initiator is immobilized on a surface, after which active species are generated on the surface to initiate the polymerization of monomers from the surface towards the bulk phase. This method incorporates a relatively large amount of a polymer onto a surface compared with the ‘grafting to’ method.
Yakushiji et al. prepared PIPAAm hydrogel-modified surfaces using the ‘grafting from’ method. Aminated glass surfaces were prepared by silanization with (3-aminopropyl)triethoxysilane for use as glass coverslips (Yakushiji et al. 1998, 1999). The azo initiators were then immobilized onto the surfaces through the formation of amide bonds between the aminated surfaces and the carboxyl-functionalized azo initiators. Subsequent surface-initiated radical polymerization with IPAAm monomer and N,N-methylenebisacrylamide as a cross-linker produced cross-linked PIPAAm hydrogel layers, as shown in figure 1c. The resulting PIPAAm hydrogel-modified surface was also used as a temperature-responsive chromatographic stationary phase. The stationary phase exhibited strong interaction with the analyte due to the penetration of the analyte into the hydrogel layers (Yakushiji et al. 1999).
Kidoaki et al. (2001) carried out photograft polymerization of IPAAm on dithiocarbamate (iniferter)-derivatized glass substrates prepared by the chloromethylation of glass substrates and subsequent dithiocarbamylation with sodium N,N-diethyldithiocarbamate. UV irradiation of the initiator-immobilized surfaces in the presence of IPAAm monomer at room temperature resulted in polymerization of the grafted chains. To directly characterize the thermoresponsive transition in the PIPAAm graft layer at the microscopic level, the force–distance curve (f–d curve) was measured on the end-grafted PIPAAm surfaces in aqueous solution at 25 and 40°C using an AFM. The approach trace of the f–d curve exhibited a steric repulsion profile at 25°C, whereas the range of the repulsion decreased from 1/10 to 1/20 at 40°C, confirming the ascending heat-induced collapse of the PIPAAm graft layer. The change in the thickness of the graft layer was complementarily measured from the scanning images of the boundary between the grafted and non-grafted regions under well-defined scanning forces.
2.4 Surface-initiated living radical polymerization
In recent years, novel ‘grafting from’ methods have been developed to prepare well-defined polymer brush layers on surfaces. Atom transfer radical polymerization (ATRP), a controlled polymerization technique, is an attractive polymer grafting method because it enables the preparation of surfaces with dense polymer brushes from surface-immobilized ATRP initiators (figure 1d). The dense polymer brush layers exhibit specific properties different from the dilute brush layers prepared by ‘grafting to’ or conventional ‘grafting from’ approaches.
Idota et al. (2006) prepared PIPAAm-grafted glass surfaces using surface-initiated ATRP and characterized these surfaces. The PIPAAm graft layer thickness and its homogeneity on the glass surface were controlled by changing the ATRP reaction time. Temperature-responsive wettability on PIPAAm-grafted surfaces varied with the grafted polymer layer thickness. Additionally, PIPAAm of a controlled molecular weight was densely grafted onto glass capillary luminal surfaces using a similar surface-initiated ATRP, and such a PIPAAm-grafted capillary exhibited substantial effectiveness in thermally regulating aqueous capillary chromatography.
Nagase et al. indicated that PIPAAm brush-grafted silica beads effectively interacted with a hydrophobic analyte. The amount of PIPAAm grafted on the silica bead surfaces exceeded by nearly one order of magnitude that previously reported for polymer hydrogel-modified silica beads prepared by conventional radical polymerization. The surface-initiated ATRP of PIPAAm formed a densely grafted PIPAAm layer on the surface compared with the PIPAAm-grafted surfaces prepared by conventional radical polymerization (Nagase et al. 2007).
Similarly, Mizutani et al. (2008) prepared PIPAAm brushes on poly(4-vinylbenzyl chloride)-coated polystyrene surfaces using surface-initiated ATRP and applied these to temperature-responsive cell culture substrates. The surface characteristics of PIPAAm brushes in relation to endothelial cell (EC) adhesion/detachment were controlled by the PIPAAm layer thickness. By adjusting the polymerization reaction conditions and time, polymer layers supporting confluent cultures of ECs were possible, indicating that PIPAAm brush surfaces prepared by surface-initiated ATRP techniques allow surface selection in the preparation of cell sheets from attachment-dependent cells with a relatively strong adhesive property for tissue engineering applications.
2.5 Surface characterizations of PIPAAm-grafted surfaces
Assessment of PIPAAm-grafted surfaces using instrumental analytical techniques is very important for determining whether the graft has been successful. Both qualitative and quantitative analyses of PIPAAm grafted on inorganic substrates such as silicon, glass and quartz are relatively easily performed by XPS, AFM, ellipsometry, surface plasmon resonance, time-of-flight secondary-ion mass spectroscopy (ToF-SIMS), X-ray reflectometry, neutron reflectometry and so on. By contrast, sensitive and selective detection of grafted PIPAAm on polymeric substrates is required because signals of grafted PIPAAm are generally close to those of the polymeric substrates.
To characterize grafted PIPAAm on a polymeric substrate, surface-oriented spectroscopic methods are frequently used. XPS is a primary choice for the qualitative characterization of grafted PIPAAm on several substrates. On PIPAAm-grafted TCPS surfaces, dense nanoparticle-like domains of PIPAAm were observed by AFM (Akiyama et al. 2007). However, uniform coverage of PIPAAm molecules on the surface was proven by XPS analyses such as the angle-dependent signal of PIPAAm (Uchida et al. 2000) and an absence of π–π* shake-up peaks for polystyrene (Akiyama et al. 2007). The atomic composition of carbon, nitrogen and oxygen on PIPAAm-grafted TCPS was in agreement with the predicted values based on the stoichiometry of the IPAAm monomer (Uchida et al. 2000; Akiyama et al. 2007). Curve fitting of the C1s peak produced the expected composition from the repeating unit of PIPAAm. The sp2-hybridized carbon atom in the carbonyl groups was found at 287.9 eV. The sp3-hybridized carbon peak at 285.0 eV was divided into three components: the two CH3 groups in the isopropyl group and the –CH2– in the PIPAAm backbone; the –CH– unit in the PIPAAm backbone at 285.3 eV; and the –CH– unit adjacent to the –NH– group (–CH–N–) at 286.2 eV. The molar ratio for these three components matched the overall shape of the sp3-hybridized carbon peak, confirming the chemical composition of the grafted PIPAAm (Akiyama et al. 2007). These XPS analyses indicate that the molecular structure of repeating units of IPAAm was retained.
Attenuated total reflection (ATR)/FT-IR is used for quantitative detection. The amount of grafted PIPAAm on PIPAAm-grafted TCPS surfaces was determined by ATR/FT-IR. For TCPS, absorption arising from monosubstituted aromatic rings was observed at 1600 cm−1. Absorption of amide carbonyl derived from PIPAAm grafts appeared in the region of 1650 cm−1. The ratio of peak intensity, I1650/I1600, was used to determine the amount of PIPAAm grafted on the surfaces. Calibration for the amount of grafted PIPAAm was based on a known amount of PIPAAm cast on TCPS surfaces from solution. The amounts of grafted PIPAAm on TCPS have a significant influence on cell adhesion behaviour (Sakai et al. 1996). Bovine ECs adhere onto the PIPAAm-grafted surfaces at coverage rates of less than 2.2 μg cm−2 at 37°C and detach from the surfaces at rates exceeding 0.8 μg cm−2 at 15°C. By contrast, cells cannot adhere and proliferate on PIPAAm-grafted surfaces at 3.0 μg cm−2 at 37°C. The reasons why cells cannot adhere on the surfaces with high amounts of grafted PIPAAm chains will be discussed in §4.1.
Determination of grafted PIPAAm thickness by optical measurements, such as ellipsometry and surface plasmon resonance, is very difficult because the refractive index of PIPAAm is similar to that of the polymeric substrates. We have estimated the thickness of the PIPAAm layer on TCPS surfaces by AFM measurement after UV excimer ablation (Akiyama et al. 2004). Irradiation of an ArF excimer laser (193 nm) by passing a laser pulse through an optical microscope resulted in ablative photodecomposition. To selectively remove the PIPAAm layer on the TCPS substrate, the excimer laser was focused onto the ablated region with dimensions of 30 μm by 25 μm and given a laser fluence of either 10 or 20 mJ cm−2 with a pulse width of 5 ns. The ablation extent of PIPAAm was regulated by the number of excimer laser shots. Staining with hydrophobic fluorescent dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiIC18), was used to confirm whether the hydrophobic TCPS surfaces were exposed by ablation. The fluorescent dye was adsorbed only on hydrophobic surfaces, resulting in selective staining of the exposed hydrophobic TCPS domains. ToF-SIMS imaging data indicated that the laser-ablated TCPS domains were detected only when the fluorescent images of DiIC18 were observed. After selective laser ablation of PIPAAm layers, the thickness was estimated from the gap between the top of the PIPAAm layer and the laser-ablated TCPS surface by AFM measurements. Considering the volume and density of the grafted PIPAAm, the estimated thicknesses were reasonable. Therefore, a combination of the selective laser ablation of PIPAAm and fluorescent imaging facilitates the estimation of grafted PIPAAm thickness on TCPS.
2.6 Temperature-dependent physico-chemical property changes of PIPAAm-grafted surfaces
PIPAAm molecules grafted on a solid interface show temperature-responsive soluble/insoluble changes due to the hydration/dehydration of side groups, resulting in physico-chemical surface property changes, such as a temperature-dependent change in wettability.
Takei et al. (1994) prepared free end linear PIPAAm-grafted surfaces and multipoint attached PIPAAm surfaces by a coupling reaction between the pendant carboxyl groups of poly(IPAAm-co-AAc) and aminated glass coverslips. The temperature-responsive surface wettability change was investigated using the Wilhelmy plate technique. A large contact angle change was obtained for end-grafted PIPAAm surfaces at approximately 24°C, whereas smaller contact angle changes were observed for poly(IPAAm-co-AAc)-grafted surfaces over a wide temperature range. The transition temperatures for both surface types were lower than that of PIPAAm in solution, probably due to the influence of the base coating with the polystyrene derivative, as well as the density of the PIPAAm chains. The small contact angle changes of the PIPAAm multipoint attached surfaces might be due to the restricted chain conformation of the grafted polymers.
Liang et al. (1998) observed the meniscus height in a capillary tube, the inner walls of which were modified by a cross-linked PIPAAm layer formed by UV photopolymerization. A temperature-dependent meniscus change was observed, with a higher meniscus at temperatures below the LCST and a lower meniscus at higher temperatures; a 7 mm difference was seen in the meniscus between 20 and 40°C for a capillary with a diameter of 2 mm. This was due to temperature-responsive wettability changes on the inner wall of the PIPAAm-grafted capillary tube.
We have also reported temperature-dependent changes in the meniscus height within PIPAAm-grafted capillaries resulting from thermoresponsive hydration changes in surface-grafted PIPAAm (Idota et al. 2005). Large changes in meniscus heights were evident because the temperature varied between 30 and 40°C for all PIPAAm-grafted microcapillaries, which increased with a decrease in the diameter of the capillary. The calculated contact angles for PIPAAm-grafted capillaries at specific temperatures remained nearly constant for all of the modified surfaces, regardless of the capillary's inner diameter. Scanning electron microscopy revealed that the changes in the diameters of the capillaries were approximately 1 per cent after PIPAAm modification. Moreover, the changes in the wet thickness of the grafted PIPAAm during phase transitions were nanoscopic, as confirmed by AFM measurements in water (143 nm at 25°C and 68 nm at 35°C). Therefore, the observed large contact-angle changes with temperature were due to hydrophilic/hydrophobic alterations from PIPAAm-grafted surface transitions. Thin immobilized PIPAAm surface layers were seen to control the capillary forces, as evidenced by the surface wettability changes with temperature.
To directly characterize the thermoresponsive structural changes in the PIPAAm graft layer at the microscopic level, the force–distance curve (f–d curve) was measured on an end-grafted PIPAAm surface in aqueous solution at 25 and 40°C, using an AFM (Kidoaki et al. 2001). The approach trace of the f–d curve exhibited a steric repulsion profile at 25°C, whereas the repulsion range decreased from 1/10 to 1/20 at 40°C, confirming the ascending heat-induced collapse of the PIPAAm graft layer. The change in the thickness of the graft layer was complementarily measured from the scanning images of the boundary between the grafted and non-grafted regions under well-defined scanning forces.
3. Temperature-responsive surfaces for aqueous chromatography
3.1 Aqueous chromatography using temperature-modulated hydrophobic interactions with analytes
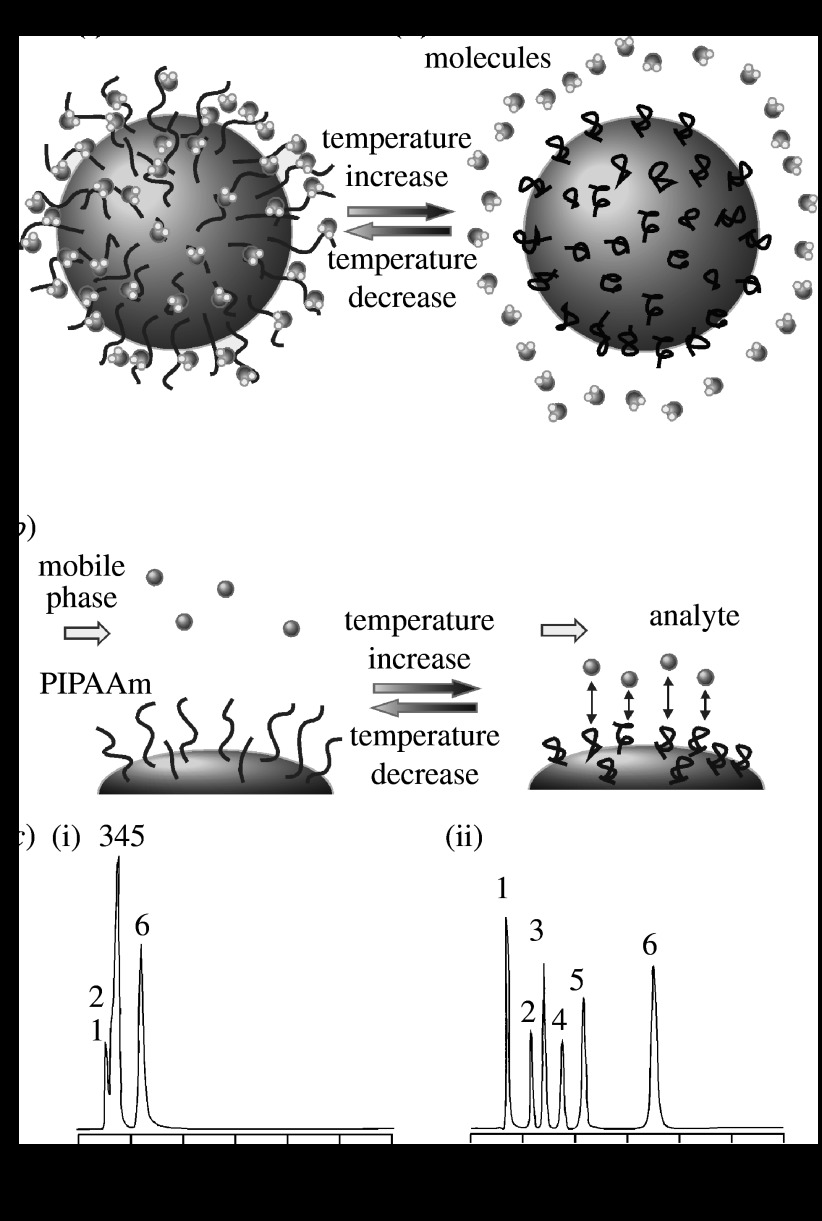
The previous section discussed the temperature-dependent hydrophilic/hydrophobic alteration of PIPAAm-grafted surfaces. This intrinsic surface property is applicable to the chromatographic stationary phase, enabling the control of the hydrophobic interaction (partitioning) with analytes by external temperature changes as shown in figure 2a.
Figure 2.
Concept of temperature-responsive chromatography: (a) hydration and dehydration of grafted polymer under an external temperature change ((i) hydrated, expanded PIPAAm and (ii) dehydrated, shrunken PIPAAm), (b) hydrophobic interaction with analyte and (c) chromatograms ((i) 5°C and (ii) 35°C) of steroid analytes separated on PIPAAm-grafted silica beads. Peaks: 1, benzene; 2, hydrocortisone; 3, prednisolone; 4, dexamethasone; 5, hydrocortisone acetate; 6, testosterone. Adapted from Kanazawa et al. (1996). Copyright 1996, American Chemical Society.
Kanazawa et al. (1996) proposed a new concept for chromatography which uses a temperature-responsive surface with a constant aqueous mobile phase. PIPAAm was grafted onto (aminopropyl)silica using an activated ester–amine coupling method, and the grafted silica beads were then used as a chromatographic stationary phase with Milli-Q water as the mobile phase (figure 2b). The elution profiles of five mixed steroids on the PIPAAm-grafted silica beads depended largely on the temperature of the aqueous mobile phase. The retention times for the hydrophobic steroids increased with an increase in temperature, and the elution order was in agreement with the increasing order of the hydrophobicities of the steroids, as confirmed from the log P values of the steroids, where P is the partition coefficient of the steroids in the 1-octanol/water system (figure 2c). This is because of the temperature-responsive change in the hydrophobic properties of the PIPAAm-grafted silica, which exhibited hydrophilic properties at lower temperatures, while the surfaces became hydrophobic at higher temperatures. Thus, the function of the HPLC stationary phase was controlled by external temperature changes, resulting in new chromatography systems without the addition of organic solvents into the mobile phase.
The graft conformation of the PIPAAm on the surface greatly influenced the temperature-responsive wettability changes. Thus, the graft conformation of PIPAAm on column matrix surfaces should affect the elution behaviour of analytes. Yakushiji et al. (1999) prepared cross-linked PIPAAm hydrogel-modified surfaces to investigate the effects of the three-dimensionally cross-linked structure of PIPAAm layers on both wettability changes and hydrophobic interactions with analytes in response to temperature change. A temperature-responsive surface was prepared by polymerizing IPAAm in the presence of a cross-linker on substrates to which an azo initiator had been covalently bonded. The separation of the relatively hydrophobic steroids was achieved even at lower temperatures. This is owing to the penetration of the analytes into the hydrogel layer. The expanded network of the highly hydrated gel layer allowed for the penetration of steroid molecules into the hydrogel layer, which resulted in changes in the peak width.
The incorporation of hydrophobic sites is an important factor to improve the separation efficiency of temperature-responsive chromatography. Thus, hydrophobic copolymers of IPAAm and n-butylmethacrylate (BMA) with reactive functional groups were introduced onto silica bead surfaces, and the beads were used as column packing materials (Kanazawa et al. 1997a,b, 2000). The elution behaviour of a mixture of five steroids was observed; the capacity factors for the steroids on the copolymer-modified silica beads were much larger than on homopolymer PIPAAm-modified columns. The retention times for the steroids increased remarkably with increased BMA content, indicating that the temperature-responsive elution behaviour of the steroids was significantly affected by the hydrophobicity of the grafted polymer chains on the silica surface (Kanazawa et al. 1997a,b). Protein separation with the copolymer-modified silica beads was explored using insulin chains A and B and β-endorphin fragments 1–27. For a BMA content of 3.2 per cent in the grafted copolymer, these three peptides were successfully separated at 30°C with a 0.5 M NaCl aqueous mobile phase (pH 2.1). The retention times for these peptides were related to the number of hydrophobic amino acid residues in the peptides, indicating that the separation was performed by the hydrophobic interaction between the grafted copolymer and the peptides (Kanazawa et al. 1997a,b). Similarly, phenylthiohydantoin (PTH)–amino acid analyses were performed on poly(IPAAm-co-BMA) copolymer-modified silica beads in the chromatographic stationary phase (Kanazawa et al. 2000). PTH–amino acid interactions with this surface were modulated by changing the column temperature using an isocratic aqueous mobile phase. The retention times observed for the PTH–amino acids depended largely on the column temperature and increased above the transition temperature of the grafted copolymer. This was explained by the increase in the partitioning of the PTH–amino acids onto a hydrophobized surface due to increased dehydration and hydrophobic aggregation of the grafted copolymer chains above the transition temperature.
3.2 Ion-exchange chromatography using temperature-modulated electrostatic interactions with analytes
Ion-exchange chromatography is a versatile tool for separation in pharmaceutical processes, because many biologically active substances have both hydrophobic and ionic regions in their molecular structures. Thus, we hypothesized that the effective separation of biological substances could be performed by the electrostatic interaction of ionic biological substances with thermoresponsive IPAAm copolymers bearing charged side groups as shown in figure 3a.
Figure 3.
Concept of temperature-responsive ion-exchange chromatography: (a) electrostatic interaction with analyte and (b) chromatograms ((i) 10°C and (ii) 50°C) of adenosine nucleotides separated on cationic copolymer-grafted silica beads. Peaks: 1, AMP; 2, ADP; 3, ATP. Adapted from Nagase et al. (2008b). Copyright 2008, American Chemical Society.
Kobayashi et al. (2001) prepared cross-linked poly(IPAAm-co-AAc)-grafted silica bead surfaces and applied them as new column matrix materials that exploit temperature-responsive anionic chromatography to separate basic bioactive compounds, specifically catecholamine derivatives, in aqueous mobile phases. Because this ionic copolymer has a well-known temperature-responsive phase transition and apparent pKa shift (Feil et al. 1992; Kobayashi et al. 2002), the polymer-grafted silica bead surfaces were expected to exhibit simultaneous hydrophilic/hydrophobic and charge density alterations under thermal stimuli. The elution behaviour of catecholamine derivatives from a copolymer-modified bead-packed column was monitored using aqueous mobile phase HPLC under varying temperatures and pH levels. The optimal separation of four catecholamine derivatives was achieved at an elevated temperature of 50°C, at pH 7.0. This is due to the increased hydrophobicity of the stationary phase, as evidenced by the elution of a non-ionic hydrophobic steroid. From these results, the mutual influences of the electrostatic and hydrophobic interactions between the basic catecholamine derivatives and pH/temperature-responsive surfaces were noted. Consequently, the elution of weakly charged bioactive compounds can be easily regulated through the modulation of stationary-phase thermoresponsive hydrophilic/hydrophobic and charge density changes.
Additionally, to enhance the hydrophobic interaction with analytes, N-tert-butylacrylamide (tBAAm) was introduced into the anionic copolymer. Cross-linked poly(IPAAm-co-AAc-co-tBAAm) thin hydrogel layers on silica beads were used as column matrices for the separation of the angiotensin subtypes I–III (Kobayashi et al. 2003). The effective separation of the angiotensin subtypes was performed on a column of these terpolymer thin hydrogel-grafted surfaces as compared with an uncharged control binary copolymer of IPAAm and tBAAm. Although hydrophobic interactions affected the separation of the angiotensin subtypes, the combined electrostatic and hydrophobic interactions resulted in a more pronounced retention. At temperatures below the terpolymer phase transition, hydrophobic interactions predominated, and any changes in the electrostatic interactions were minimal. By contrast, above the phase transition temperature, electrostatic interactions were dramatically reduced as a result of the decreased charge densities of the polymer-grafted surfaces. Thus, peptide retention times were also reduced, exhibiting a maximum near 30–35°C.
On the other hand, to separate acidic biological substances, the cationic copolymer poly(IPAAm-co-N,N-dimethylaminopropylacrylamide-co-BMA)-modified stationary phase was used as a column matrix (Ayano et al. 2006; Kikuchi et al. 2007). The copolymer-grafted surfaces exhibited thermoresponsive changes in charge density as well as hydrophilic/hydrophobic characteristics (Kikuchi & Okano 2004). The copolymer chains dehydrate and molecular aggregation occurs due to weakly deprotonated cationic amino groups in hydrophobized circumstances, resulting in a decrease in the surface charge density. The intrinsic properties of the copolymer-modified surface were applied to regulate adenosine nucleotide retention in HPLC using the aqueous mobile phase. At lower temperatures, adenosine nucleotides exhibited a longer retention time, which was driven by ionic interaction with the positively charged surfaces. With increasing temperatures, their retention time was shortened and a drastic change was observed above the copolymer transition temperature (figure 3b). Furthermore, the copolymer-grafted stationary phase was applied to the separation of oligonucleotides and oligodeoxythymidines using mobile phases with various pH values. Using a pH 4.5 mobile phase, the oligodeoxythymidines were retained and the retention times decreased with an increase in temperature. This was explained by the electrostatic interaction between the grafted copolymer and the dissociated oligodeoxythymidines. At lower temperatures, the grafted copolymer chain hydrated and its cationic amino groups protonated and interacted electrostatically with the anionic dissociated oligodeoxythymidines. By contrast, using pH 3.0 buffer as the mobile phase, the retention times for the oligodeoxythymidines increased with increasing temperature, indicating that the elution behaviours were based on the hydrophobic interaction due to the non-dissociated oligodeoxythymidines at that pH value.
3.3 Affinity chromatography using conformational change of grafted polymer
PIPAAm and its derivatives exhibit conformational changes as well as hydrophilic/hydrophobic alterations. This intrinsic property was used for the stationary phase of a novel affinity chromatography approach.
The conformational change in PIPAAm has been used to control the affinity of a target molecule by modulating the surrounding temperature as shown in figure 4a (Yoshizako et al. 2002). In such a system, the adsorbed biomolecules can be completely recovered by lowering the temperature to expand the PIPAAm chains. Cibacron blue (CB), which is known to have a high affinity for serum albumin, was immobilized onto amino-functionalized polymethacrylate bead matrices with an appropriate spacer length. The spacer molecule was used to change the distance between the bead surfaces and the affinity molecule, CB. The matrix surfaces were co-immobilized with end-carboxyl PIPAAm, synthesized through radical telomerization using MPA as the chain transfer agent. The grafted PIPAAm on the surface was fully expanded below the LCST, and its length was comparable with or slightly longer than that of the CB bound with the spacer. At temperatures above the LCST of PIPAAm, the albumin adsorption was large, with the amount decreasing as the temperature was decreased below the LCST. The adsorbed albumin at higher temperatures was easily desorbed with low-temperature treatment, which caused the PIPAAm molecules to hydrate and expand outward. The expansion of the PIPAAm molecules caused the albumin conjugated to the CB to be displaced. Thus, the affinity chromatography required no modification to the mobile-phase pH or ionic strength.
Figure 4.
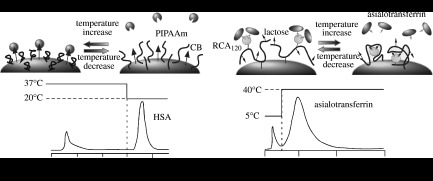
Concept of temperature-triggered affinity control in the context of conformational change in PIPAAm molecules: control of (a) albumin adsorption/desorption and (b) asialotransferrin adsorption/desorption (Ricinus communis agglutinin). Adapted from Yoshizako et al. (2002) and Yamanaka et al. (2003). Copyright 2002 and 2003, American Chemical Society.
Additionally, the temperature-induced conformational change in PIPAAm was used for other types of affinity chromatography, under which a ligand can be moved dynamically in response to external stimuli as shown in figure 4b. Ricinus communis agglutinin (RCA120), galactose-specific lectin and lactose were introduced into the PIPAAm chain, and the copolymer was attached to sepharose beads (Yamanaka et al. 2003). The glycoprotein target, asialotransferrin, was loaded onto a column packed with copolymer-immobilized beads. At 5°C, the column retained the glycoprotein target. With an increase in column temperature, most of the asialotransferrins were eluted at 30°C. This temperature-induced elution was explained by the sugar recognition of RCA120. With an increase in column temperature, the grafted copolymer was dehydrated and collapsed. Co-immobilized RCA120 ligand and lactose hapten were brought into closer proximity to each other, resulting in the immobilized lactose displacing the affinity-bound asialotransferrin from the immobilized RCA120 lectin.
3.4 Novel polymer brush surfaces for temperature-responsive chromatography
As described in §2.4, dense polymer brush surfaces, prepared by surface-initiated ATRP, are attractive for biomedical device applications, due to their intrinsic properties. These surfaces are also useful for the stationary phase of temperature-responsive chromatography.
PIPAAm at a controlled molecular weight was densely grafted onto glass capillary luminal surfaces using a similar surface-initiated ATRP, and the PIPAAm-grafted surfaces were investigated using an observation of their interactions with hydrophobic steroids as analytes (Idota et al. 2006). The steroid elution behaviour with these PIPAAm-grafted capillary surfaces contrasted sharply with that seen in PIPAAm hydrogel-grafted porous monolithic silica capillaries prepared by an EB method, where a peak broadening was observed at high temperatures. This is due to the strong collective aggregation of densely grafted PIPAAm with the capillary lumen, which prevents steroid molecules from diffusing into the polymer brush layers at the interface.
Nagase et al. (2007) indicated that PIPAAm brush-grafted silica beads effectively interacted with hydrophobic analytes. The amount of PIPAAm grafted onto the silica bead surfaces exceeded by nearly one order of magnitude that previously reported with polymer hydrogel-modified silica beads prepared by conventional radical polymerization, because the surface-initiated ATRP of PIPAAm formed a densely grafted PIPAAm layer on the surface. Relatively longer retention times for steroids were observed on the PIPAAm brush-grafted columns compared with those previously reported for other PIPAAm-grafted silica beads, which suggests that densely grafted PIPAAm chains make it possible to control strong hydrophobic interactions with steroids by changing the column temperature. Furthermore, copolymerization of the cationic monomer (dimethylamino)ethylmethacrylate (DMAEMA) was also performed using a similar ATRP procedure, and a poly(IPAAm-co-DMAEMA) brush was grafted onto the silica bead surfaces (Nagase et al. 2008b). The chromatographic retention times for adenosine nucleotides were significantly increased, indicating that strong electrostatic cationic copolymer brush interactions occur between the surfaces and nucleotide analytes.
4. Temperature-responsive surfaces for cell sheet engineering
4.1 First-generation temperature-responsive culture dish
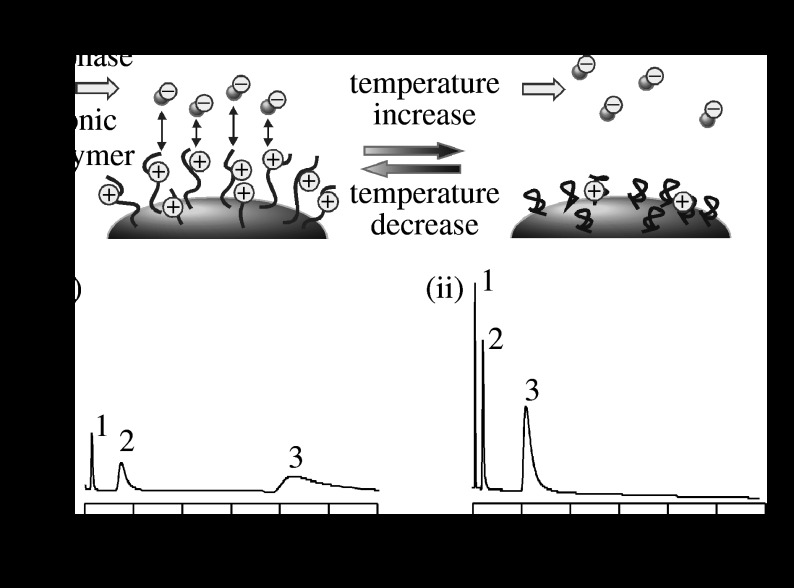
A conventional culture method was used to grow confluent cells on the surface of a TCPS dish. Cells were then harvested through the enzymatic proteolysis of an extracellular matrix (ECM), such as with trypsin, and by chelating Ca2+ ions to disrupt cell–cell junctions, such as with ethylenediamine tetraacetic acid. Since 1990, we have developed a PIPAAm-grafted TCPS dish as a temperature-responsive culture substrate, allowing cultured cells to be recovered simply by lowering the temperature (figure 5; Yamada et al. 1990; Okano et al. 1993).
Figure 5.
First-generation temperature-responsive culture dish. (a) Temperature-dependent wettability changes for PIPAAm-grafted surfaces at 10 and 37°C ((i) hydrophilic surface and (ii) hydrophobic surface). (b) Schematic for the interactions of temperature-responsive surfaces with cells ((i) passive adhesion and (ii) active adhesion).
Temperature-triggered alterations of cell adhesion/detachment were observed on ultrathin PIPAAm-grafted TCPS with a dry thickness of 15 nm; cell adhesion dramatically decreased on a surface grafted with a thicker PIPAAm layer (greater than 30 nm; Akiyama et al. 2004). Within nanometre-scale ultrathin layers, the motion of the grafted PIPAAm chains was strongly restricted by immobilization onto the TCPS surface, resulting in dehydration of the PIPAAm chains. Moreover, hydrophobic environments in the vicinity of the TCPS surface also enhanced the dehydration and aggregation of the PIPAAm chains. We inferred that the hydrophobic environments of TCPS surfaces have little influence on the grafted PIPAAm layer over a thickness of 30 nm, resulting in a pronounced mobility of the grafted PIPAAm chains and hydration. Almost at the same time, another group (Takezawa et al. 1990, 1992) reported cell cultures on TCPS coated with a mixture of PIPAAm and collagen. Neither adhesion nor proliferation of fibroblasts was observed on TCPS coated with only PIPAAm, whereas cells adhered and proliferated on TCPS coated with a mixture of PIPAAm and collagen (Takezawa et al. 1990). These results imply that fibroblasts cannot adhere and proliferate on coatings composed solely of PIPAAm without the affinity interactions of collagen, because free PIPAAm chains are released on account of the restricted immobilization onto hydrophobic polystyrene surfaces. These data prove that the graft architecture and thickness of the PIPAAm immobilized covalently onto TCPS both play a crucial role in the temperature-induced alterations of the hydrophilic/hydrophobic properties and cell adhesion/detachment. During cell detachment induced by lowered temperatures, cell morphological change and detachment were suppressed by an ATP synthesis inhibitor, sodium azide and a tyrosine kinase inhibitor, genistein (Okano et al. 1995; Yamato et al. 1999). An actin filament stabilizer, phalloidin, and its depolymerizer, cytochalasin D, also inhibited cell detachment (Yamato et al. 1999). These results indicate that cell detachment from temperature-responsive TCPS is an active process, which is induced by intracellular events, such as signalling and the reconstruction of cytoskeletons (figure 5b). Moreover, the cells that are recovered from temperature-responsive TCPS dishes retain their cellular structure and functions, whereas trypsinization causes damage to the cell membrane and ECM (Yamada et al. 1990; Okano et al. 1993). Hence, the cell culture method of using a temperature-responsive TCPS dish is considered a powerful tool for investigating the molecular machinery involved in cell-surface detachment.
4.2 Cell sheet engineering
Recently, scientists have predicted that regenerative medicine would permit the resurrection of failed tissues/organs. Tissue engineering using biodegradable scaffolds was proposed by Langer & Vacanti (1993) as a promising methodology to reconstruct in vitro three-dimensional tissues. However, clinical applications using biodegradable scaffold-based tissue engineering have been limited to tissues such as artificial skin (Hansbrough et al. 1997), blood vessels (Shin'oka et al. 2001) and bladder (Atala et al. 2006). This method is not suitable for the regeneration of cell-dense tissues, including cardiac muscle, liver and kidney. In addition, seeded cells survive only on the periphery of the scaffolds due to the limited diffusion of nutrients, such as oxygen and glucose, and waste metabolites.
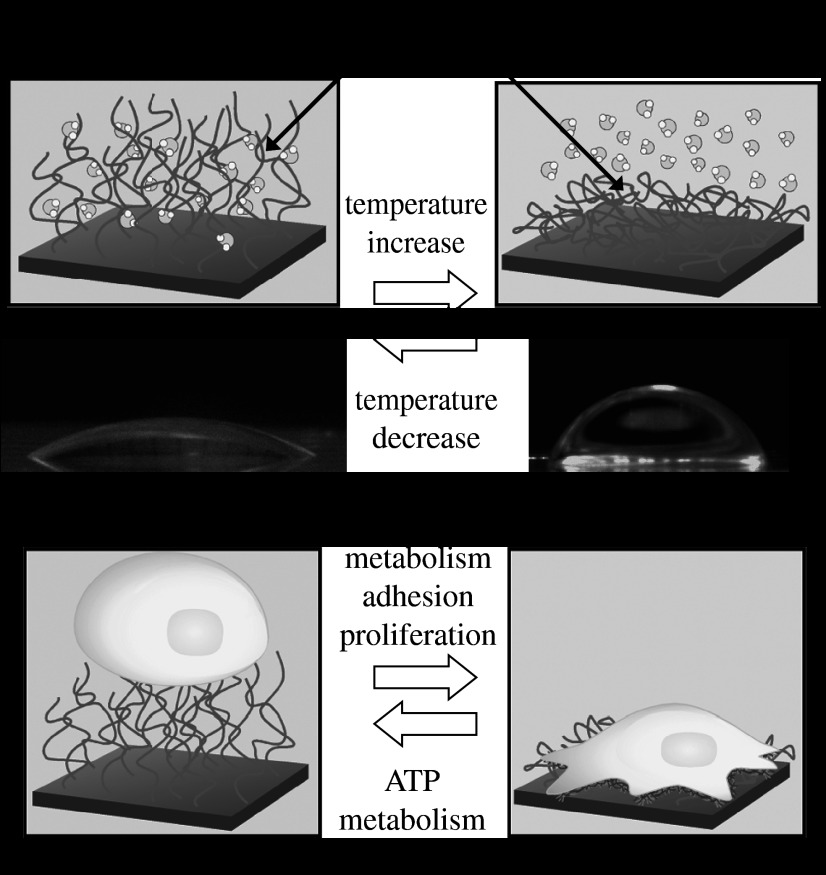
In order to overcome these shortcomings, we have developed a novel concept to construct three-dimensional tissues from cell sheets using temperature-responsive TCPS (Yamato & Okano 2004; Yamato et al. 2007; Yang et al. 2007). A new approach called ‘cell sheet engineering’ was used to construct ideal transplantable tissues composed exclusively of cells. Cell monolayers, confluently cultured on hydrophobized PIPAAm-grafted TCPS surfaces at 37°C, were detached as single cell sheets when the temperature was lowered to 20°C, because the surfaces became hydrophilic below the LCST of PIPAAm (Kushida et al. 1999, 2000, 2005). The membrane proteins and ECM on the cell sheets were retained, because the cell sheets were recovered simply by lowering the temperature, with no enzymatic proteolysis treatment (figure 6a). Because the recovered cell sheets feature the ECM on the underside, it is possible to transfer them onto other materials, such as a culture dish, another cell sheet or biological tissue.
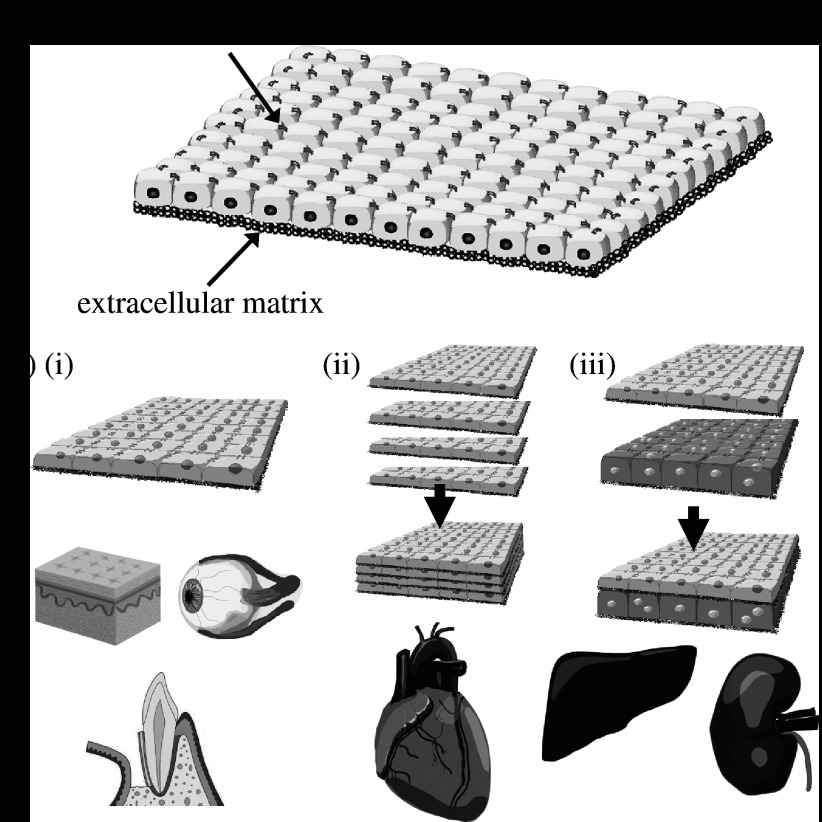
Figure 6.
Concept of cell sheet engineering. (a) Recovered cell sheet from a temperature-responsive TCPS dish retains the extracellular matrix (ECM) beneath the cell sheet and cell–cell junctions. (b(i)) Single cell sheet useful for skin or cornea transplantation, (ii) layered cell sheets used to reconstruct homogeneous three-dimensional tissues, including myocardium, and (iii) heterotypic layered cell sheets used to fabricate laminar structures, including the liver and kidneys.
Fluorescent immunostaining revealed that the fibronectin located beneath cultured cells was removed from the temperature-responsive TCPS dish surface after the temperature had been reduced (Kushida et al. 1999). By contrast, Canavan et al. (2005a,b) reported that in analyses using XPS, ToF-SIMS and fluorescent immunostaining, a component of collagen was observed on the plasma-polymerized PIPAAm-deposited TCPS after detachment of the cell sheets. In order to make a comparison with the plasma polymerization method, the surface characterization of PIPAAm-grafted TCPS prepared by EB irradiation was investigated before and after the recovery of cell sheets by temperature reduction (Akiyama et al. 2007). XPS analyses showed that when the cell sheets were lifted off the PIPAAm-grafted TCPS, some residual proteins and/or peptides were left behind. A comparison of the molar ratio of the amide species estimated from the C1s spectrum before and after cell culture also suggested that PIPAAm grafted by EB irradiation could reduce the protein and/or peptide absorption more efficiently than that grafted by the plasma method. Although the difference between the two methods is unclear, we infer that the EB irradiation method provides a more uniform grafting of PIPAAm onto TCPS surfaces than the plasma polymerization method.
Intrinsically, sheet-like tissues located on the surface of the body, such as epidermal keratinocytes (Yamato et al. 2001) and corneal epithelial cell sheets (Nishida et al. 2004), are easily transplantable (figure 6b(i)). In collaboration with Nishida et al., corneal epithelial cell sheets have been applied to the treatment of patients with unilateral or bilateral total corneal stem cell deficiencies arising from alkali burns or Stevens–Johnson syndrome (Nishida et al. 2004). In patients with a unilateral limbal stem cell deficiency, corneal epithelial cell sheets are cultured from autologous limbal stem cells. Autologous oral mucosal epithelial cell sheets are also applied to bilateral total corneal stem cell deficiencies.
By layering cardiomyocyte sheets harvested from a temperature-responsive TCPS dish (figure 6b(ii)), it was possible to fabricate a cardiac patch and its spontaneous pulsating was easily visible to the naked eye (Shimizu et al. 2002). In collaboration with Sawa et al., layered autologous skeletal myoblast sheets have been used in the treatment of dilated cardiomyopathy (Memon et al. 2005).
Autologous mucosal epithelial cells for the treatment of oesophageal ulcerations were transplanted to prevent stenosis. A clinically relevant large animal model was examined by combining endoscopic submucosal dissection and the endoscopic transplantation of autologous oral mucosal epithelial cell sheets. Four weeks after surgery, complete wound healing with no stenosis was confirmed (Ohki et al. 2006). To regenerate the damaged periodontal support, periodontal treatment using periodontal ligament-derived cell sheets was applied to defects in rats (Hasegawa et al. 2005) and beagle dogs (Akizuki et al. 2005). In both cases, periodontal tissue healing with bone, periodontal ligament and cementum formation was observed. Other applications using cell sheet engineering have been summarized in other reviews (Yamato & Okano 2004; Yamato et al. 2007; Yang et al. 2007). Recent progress towards temperature-responsive culture dishes will be discussed in §5.
5. Second-generation temperature-responsive culture dishes
5.1 Temperature-responsive culture inserts
Oral mucosal epithelial cells are considered to be a promising alternative cell source for regenerative medicine (Pellegrini 2004). Recently, we have also reported successful applications in the treatment of corneal epithelial stem cell deficiency (Nishida et al. 2004) and oesophageal ulceration (Ohki et al. 2006) using autologous oral mucosal epithelial cell sheets. However, a 3T3 murine feeder layer and foetal calf serum (FBS) were needed to fabricate stratified epithelial cell sheets. Because animal-derived materials would induce infection, tissue-engineered cell sheets that use the feeder layer method are classified as xenogenetic products based on the regulations of the US Food and Drug Administration.
In order to eliminate the use of animal-derived materials, a temperature-responsive culture insert with a poly(ethylene terephthalate) membrane (pore size 0.45 μm) was used as a cell culture substrate (figure 7; Murakami et al. 2006a). In the case of a normal cell culture on a PIPAAm-grafted TCPS dish with autologous serum, the harvested cell sheets were fragile, due to limited supplies of nutrients and/or growth factors because of the tight junctions between adjacent epithelial cells, focal contacts and/or hemidesmosome-like adhesion structures between basal cells and the substrate. By contrast, stratified epithelial cell sheets were fabricated using a temperature-responsive culture insert and autologous serum, which allowed nutrients to be supplied from the basal side of the cells through submicrometre-size pores in the inserts. This culture system, which eliminates animal-derived materials such as the 3T3 murine feeder layer and FBS, is a versatile approach for practical applications: the protocol of the clinical trials using autologous oral mucosal epithelial cell sheets was actually based on this system (Murakami et al. 2006b).
Figure 7.
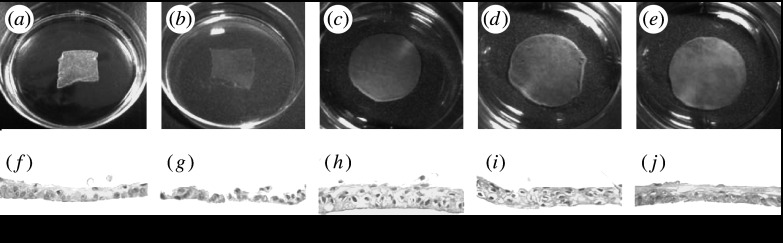
Harvested oral mucosal epithelial cell sheets. Primary canine oral epithelial cells were cultured on (a, b) temperature-responsive culture dishes and (c–e) temperature-responsive culture inserts under the various conditions (a) with or (b–e) without 3T3 feeder layers, in the presence of (a–c) FBS or (d) autologous serum or (e) in the absence of serum. On temperature-responsive culture dishes, square temperature-responsive domains were surrounded by non-cell adhesive area, while all culture insert surfaces were completely grafted with temperature-responsive polymer. (a–e) Macroscopic views and (f–j) haematoxylin and eosin-stained paraffin sections. Adapted from Murakami et al. (2006a).
5.2 Biomolecule-immobilized temperature-responsive culture dishes
To introduce bioactive molecules onto temperature-responsive culture surfaces, we used an isopropylacrylamide analogue, 2-carboxyisopropylacrylamide (CIPAAm), which has a carboxylate side chain (Aoyagi et al. 2000; Ebara et al. 2003). Arg-Gly-Asp-Ser (RGDS) peptides were covalently immobilized via amide bond formation onto a poly(IPAAm-co-CIPAAm)-grafted TCPS dish (figure 8; Ebara et al. 2004a,b). Cells adhered and spread on the RGDS-immobilized temperature-responsive TCPS even in the absence of serum at 37°C. By reducing the temperature to 20°C, the cells were detached from the surfaces. As a brief introduction, we have demonstrated a new method to immobilize biomolecules onto temperature-responsive TCPS via the affinity binding between avidin and biotin (Nishi et al. 2007). Biotinylated biomolecules, inducing RGDS peptides as a model substrate with glycine as a spacer, were easily conjugated onto the streptavidin-immobilized temperature-responsive TCPS without coupling reagents, such as water-soluble carbodiimide and active ester.
Figure 8.
Schematic of affinity control between integrin receptors and biomolecule-immobilized temperature-responsive culture dishes. (a) At 37°C, cells adhered and spread on the RGDS ligand-immobilized temperature-responsive TCPS even in the absence of serum. By reducing the temperature to (b) 20°C, the spread cells were detached from the surfaces.
In addition, the co-immobilization of RGDS and Pro-His-Ser-Arg-Asn (PHSRN) sequences onto the poly(IPAAm-co-CIPAAm)-grafted TCPS enhanced the synergistic role of RGDS binding to α5β1 integrin, resulting in stable adhesion and cell spreading. In particular, PHSRN–G6–RGDS immobilization dramatically delayed the rate of cell detachment because the length of G6 was similar to the spatial distance between the PHSRN and RGDS in the native fibronectin (Ebara et al. 2008).
These surfaces facilitated the spreading of cells without serum via affinity interactions between the exposed RGDS ligand-immobilized poly(IPAAm-co-CIPAAm) chains and the integrin receptor at 37°C (above the LCST). Moreover, the cells spread on the surfaces at 37°C were detached spontaneously when the temperature was lowered below the LCST (20°C), as the hydrated poly(IPAAm-co-CIPAAm) chains extended and spatially shielded the cell integrin from accessing the immobilized RGDS ligands. By tailoring the immobilized ligands on temperature-responsive surfaces, temperature-regulated control of affinity interactions between ligands and cell receptors should be achievable. Furthermore, this method allows serum-free cell culturing and trypsin-free cell harvesting, essentially eliminating mammalian-sourced components from the culture process.
5.3 Micropatterned temperature-responsive surfaces
Living tissues and organs consist of multiple types of cells with a spatially ordered architecture, such that heterotypic cellular interactions influence and maintain the development of physiological functions and activities. In order to mimic heterotypic cellular architectures, much attention has been paid to methods for two-dimensional micropatterning of heterotypic cells, such as photolithography (Bhatia et al. 1997), soft lithography (Yousaf et al. 2001; Khetani & Bhatia 2008) or dielectrophoretic forces (Albrecht et al. 2006). In addition, three-dimensional cellular micropatterning has been developed using several cellular printing systems (Tsang & Bhatia 2004; Calvert 2007). However, the regeneration of complicated tissues and organs remains a challenging issue, mainly due to a lack of the following: (i) three-dimensional tissues with microscopically arranged, high-density cells and (ii) vascularized, multifunctional tissues replicating the native structure.
The in vitro formation of capillary networks is required for the creation of thick, multifunctional tissues replicating the native structure. Transplanted tissues must be connected to the blood vascular system in vivo in order to survive and perform their functions. In order to establish a construction method for such three-dimensional tissues with capillary networks, we introduced micropatterned cell sheets by using micropatterned temperature-responsive surfaces prepared by a maskless photolithography method (figure 9). For the rapid prototyping of cellular micropatterning on the substrate, we developed an all-in-one device by using a commercially available liquid crystal display projector to expose patterned images generated on a personal computer (PC) through a reduction lens (Itoga et al. 2004a, 2008). The projected images are easily generated on PC monitors with commercial software; therefore, special control software is not needed for lithographic pattern generation. This device facilitates the preparation of micropatterned hydrogels (Itoga et al. 2004a), polydimethylsiloxane microchannels (Itoga et al. 2004b, 2008; Kobayashi et al. 2004; Nie et al. 2007; Wada et al. 2008) and micropatterned cells (Itoga et al. 2004a,b, 2006, 2008) without the need for photomasks. Temperature-responsive micropatterned surfaces were prepared using a maskless photolithography method (figure 9; Tsuda et al. 2007). On a 20 μm-wide micropatterned PIPAAm domain, which was 60 μm distant from a non-adhesive PAAm domain, ECs selectively adhered to the micropatterned PIPAAm domain at 37°C. Stratified tissue equivalents were constructed by layering fibroblast monolayer sheets with micropatterned EC sheets harvested from temperature-responsive micropatterned surfaces. The fidelity of the micropatterned ECs was maintained within the multilayered tissues after assembly, leading to self-organization into capillary-like networks after a 5-day culture period. This technique holds promise for the study of cell–cell communications and angiogenesis in reconstructed, three-dimensional environments, as well as for the fabrication of tissues with complex, multicellular architecture.
Figure 9.
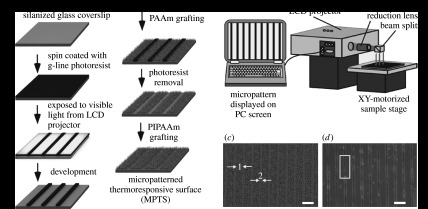
Microfabrication process used to create thermoresponsive micropatterned surfaces. (a) Schematic of the microfabrication process used to create micropatterned surfaces. Photoresist patterns on silanized coverslips are created using a rapid prototyping micropattern exposure system comprising a modified LCD projector and XY-motorized sample stage described in (b). Polyacrylamide (PAAm) is selectively covalently grafted onto the non-photoresist-coated regions of the silanized coverslip. After lift-off and cleaning of the photoresist, PIPAAm is covalently grafted onto the silanized coverslip. (c) Microscopy of visualized pattern formation onto micropatterned surfaces caused by water condensation onto the zones of different surface hydrophilicity: PAAm-grafted 60 μm-wide lane in (1) and PIPAAm-grafted 20 μm-wide lane in (2). (d) Cultured ECs on 20 μm-wide adhesive thermoresponsive domains at 37°C. Adapted from Tsuda et al. (2007).
For the purpose of fabricating complicated three-dimensional tissues, we have focused on the development of novel micropatterned dual temperature-responsive surfaces to enable both the culture and the recovery of patterned heterotypic cell sheets (Tsuda et al. 2005, 2006). Two types of temperature-responsive polymers exhibiting different LCSTs, PIPAAm and BMA-co-grafted PIPAAm, were micropatterned using EB polymerization through stainless masks. Micropatterned surfaces exhibiting hydrophilic/hydrophobic property alterations following a change in temperature allowed the selective adhesion and growth of rat primary hepatocytes (HCs) and bovine carotid ECs. At 27°C, the seeded HCs adhered exclusively to the hydrophobic poly(IPAAm-co-BMA) co-grafted domains and not to the hydrated PIPAAm domains. When the temperature was increased to 37°C, the sequential seeding of ECs allowed exclusive adhesion to the hydrophobized PIPAAm domains, resulting in patterned co-cultures. Co-cultured, patterned cell monolayer cell sheets were recovered as cell sheets by the hydration of both polymer-grafted domains as the temperature was reduced to 20°C. These recovered co-cultured cell sheets could be manipulated; they could be moved onto and sandwiched between other cell sheets, providing a novel technology to prepare tissue-mimicking multilayer materials by overlaying co-cultured cell sheets. In the future, this technology could lead to solutions to the challenges of how to regenerate complicated organs, such as the liver and kidneys.
6. Conclusions
In this review, we have introduced temperature-responsive intelligent surfaces for chromatographic matrices and cell sheet culture substrates. The creation of these intelligent surfaces has been performed using several methodologies, and these intelligent surfaces open up a new frontier in the biomedical field. Temperature-responsive chromatography, involving the modification of thermoresponsive polymers, enables us to separate biological compounds with high biological activity. This separation using intelligent chromatography holds promise for the purification of biologically active compounds, pharmaceutical development and online measurement in clinical practice. Furthermore, temperature-responsive cell culture substrates, prepared by the precise modification of temperature-responsive polymers, allow the recovery of confluent cell monolayers as contiguous living cell sheets. These intelligent cell culture substrates permit novel therapies for difficult-to-treat diseases, with several clinical trials already having been performed. In addition, several other trials related to the preparation of thick and dense tissues and the development of improved cell culture substrates for the effective preparation of cell sheets have also begun. These developments will ultimately lead to the widespread use of cell sheets in clinical therapy. Thus, the development of these novel intelligent surfaces and their use will facilitate progress in the fields of medicine and biology. The results could be of significant importance to various biomedical fields.
Acknowledgments
Part of this work was financially supported by the Formation of Innovation Center for the Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology, 21st Century Center of Excellence (COE) Program, the High-Tech Research Center Program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Core Research for Evolutional Science and Technology (CREST) programme from the Japan Science and Technology Agency, the Development of New Environmental Technology Using Nanotechnology Project of the National Institute of Environmental Science (NIES), commissioned by the Ministry of the Environment, Japan, Grants-in-Aid for Scientific Research (B) no. 19591568 from the Japan Society for the Promotion of Science and Grants-in-Aid for Young Scientists (B) nos 20700398 and 20700399 from MEXT.
Footnotes
One contribution of 10 to a Theme Supplement ‘Japanese biomaterials’.
References
- Akiyama Y., Kikuchi A., Yamato M., Okano T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir. 2004;20:5506–5511. doi: 10.1021/la036139f. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Kushida A., Yamato M., Kikuchi A., Okano T. Surface characterization of poly (N-isopropylacrylamide) grafted tissue culture polystyrene by electron beam irradiation, using atomic force microscopy, and X-ray photoelectron spectroscopy. J. Nanosci. Nanotechnol. 2007;7:796–802. doi: 10.1166/jnn.2007.509. [DOI] [PubMed] [Google Scholar]
- Akizuki T., Oda S., Komaki M., Tsuchioka H., Kawakatsu N., Kikuchi A., Yamato M., Okano T., Ishikawa I. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J. Periodont. Res. 2005;40:245–251. doi: 10.1111/j.1600-0765.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- Albrecht D.R., Underhill G.H., Wassermann T.B., Sah R.L., Bhatia S.N. Probing the role of multicellular organization in three-dimensional microenvironments. Nat. Methods. 2006;3:369–375. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- Aoyagi T., Ebara M., Sakai K., Sakurai Y., Okano T. Novel bifunctional polymer with reactivity and temperature sensitivity. J. Biomater. Sci. Polym. Ed. 2000;11:101–110. doi: 10.1163/156856200743526. [DOI] [PubMed] [Google Scholar]
- Atala A., Bauer S.B., Soker S., Yoo J.J., Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Ayano E., Kanazawa H. Aqueous chromatography system using temperature-responsive polymer-modified stationary phases. J. Sep. Sci. 2006;29:738–749. doi: 10.1002/jssc.200500485. [DOI] [PubMed] [Google Scholar]
- Ayano E., Sakamoto C., Kanazawa H., Kikuchi A., Okano T. Separation of nucleotides with an aqueous mobile phase using pH- and temperature-responsive polymer modified packing materials. Anal. Sci. 2006;12:539–543. doi: 10.2116/analsci.22.539. [DOI] [PubMed] [Google Scholar]
- Bamford C.H., Al-Lamee K.G. Studies in polymer surface modification and grafting for biomedical uses: 2. Application to arterial blood filters and oxygenators. Polymer. 1996;37:4885–4889. doi: 10.1016/0032-3861(96)00538-1. [DOI] [Google Scholar]
- Bhatia S., Yarmush M., Toner M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J. Biomed. Mater. Res. 1997;34:189–199. doi: 10.1002/(SICI)1097-4636(199702)34:2<189::AID-JBM8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Calvert P. Printing cells. Science. 2007;318:208–209. doi: 10.1126/science.1144212. [DOI] [PubMed] [Google Scholar]
- Cammas S., Suzuki K., Sone C., Sakurai Y., Kataoka K., Okano T. Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J. Control. Rel. 1997;48:157–164. doi: 10.1016/S0168-3659(97)00040-0. [DOI] [Google Scholar]
- Canavan H.E., Cheng X., Graham D.J., Ratner B.D., Castner D.G. Cell sheet detachment affects the extracellular matrix: a surface science study comparing thermal liftoff, enzymatic, and mechanical methods. J. Biomed. Mater. Res. 2005a;75A:1–13. doi: 10.1002/jbm.a.30297. [DOI] [PubMed] [Google Scholar]
- Canavan H.E., Cheng X., Graham D.J., Ratner B.D., Castner D.G. Surface characterization of the extracellular matrix remaining after cell detachment from a thermoresponsive polymer. Langmuir. 2005b;21:1949–1955. doi: 10.1021/la048546c. [DOI] [PubMed] [Google Scholar]
- Chung J.E., Yokoyama M., Yamato M., Aoyagi T., Sakurai Y., Okano T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate) J. Control. Rel. 1999;62:115–127. doi: 10.1016/S0168-3659(99)00029-2. [DOI] [PubMed] [Google Scholar]
- Chilkoti A., Chen G., Stayton P.S., Hoffman A.S. Site-specific conjugation of a temperature-sensitive polymer to a genetically engineered protein. Bioconjug. Chem. 1994;5:504–507. doi: 10.1021/bc00030a004. [DOI] [PubMed] [Google Scholar]
- Ebara M., Yamato M., Hirose M., Aoyagi T., Kikuchi A., Sakai K., Okano T. Copolymerization of 2-carboxyisopropylacrylamide with N-isopropylacrylamide accelerates cell detachment from grafted surfaces by reducing temperature. Biomacromolecules. 2003;4:344–349. doi: 10.1021/bm025692t. [DOI] [PubMed] [Google Scholar]
- Ebara M., Yamato M., Aoyagi T., Kikuchi A., Sakai K., Okano T. Temperature-responsive cell culture surfaces enable ‘on–off’ affinity control between cell integrins and RGDS ligands. Biomacromolecules. 2004a;5:505–510. doi: 10.1021/bm0343601. [DOI] [PubMed] [Google Scholar]
- Ebara M., Yamato M., Aoyagi T., Kikuchi A., Sakai K., Okano T. Immobilization of cell-adhesive peptides to temperature-responsive surfaces facilitates both serum-free cell adhesion and noninvasive cell harvest. Tissue Eng. 2004b;10:1125–1135. doi: 10.1089/ten.2004.10.1125. [DOI] [PubMed] [Google Scholar]
- Ebara M., Yamato M., Aoyagi T., Kikuchi A., Sakai K., Okano T. A novel approach to observing synergy effects of PHSRN on integrin–RGD binding using intelligent surfaces. Adv. Mater. 2008;10:3034–3038. doi: 10.1002/adma.200702308. [DOI] [Google Scholar]
- Feil H., Bae Y.H., Feijen J., Kim S.W. Mutual influence of pH and temperature on the swelling of ionizable and thermosensitive hydrogels. Macromolecules. 1992;25:5528–5530. doi: 10.1021/ma00046a063. [DOI] [Google Scholar]
- Feng W., Zhu S., Ishihara K., Brash J.L. Adsorption of fibrinogen and lysozyme on silicon grafted with poly(2-methacryloyloxyethyl phosphorylcholine) via surface-initiated atom transfer radical polymerization. Langmuir. 2005;21:5980–5987. doi: 10.1021/la050277i. [DOI] [PubMed] [Google Scholar]
- Fukumori K., Akiyama Y., Yamato M., Kobayashi J., Sakai K., Okano T. Temperature-responsive glass coverslips with an ultrathin poly(N-isopropylacrylamide) layer. Acta Biomater. 2009;5:470–476. doi: 10.1016/j.actbio.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Gil E.S., Hudson S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004;29:1173–1222. doi: 10.1016/j.progpolymsci.2004.08.003. [DOI] [Google Scholar]
- Hansbrough J.F., Mozingo D.W., Kealey G.P., Davis M., Gidner A., Gentzkow G.D. Clinical trials of a biosynthetic temporary skin replacement, dermagraft-transitional covering, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J. Burn Care Rehabil. 1997;18:43–51. doi: 10.1097/00004630-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Yamato M., Kikuchi A., Okano T., Ishikawa I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005;11:469–478. doi: 10.1089/ten.2005.11.469. [DOI] [PubMed] [Google Scholar]
- Heskins M., Guillet J.E. Solution properties of poly(N-isopropylacrylamide) J. Macromol. Sci. Chem. A. 1968;2:1441–1455. doi: 10.1080/10601326808051910. [DOI] [Google Scholar]
- Idota N., Kikuchi A., Kobayashi J., Sakai K., Okano T. Microfluidic valves comprising nanolayered thermoresponsive polymer-grafted capillaries. Adv. Mater. 2005;17:2723–2727. doi: 10.1002/adma.200402068. [DOI] [Google Scholar]
- Idota N., Kikuchi A., Kobayashi J., Akiyama K., Sakai K., Okano T. Thermal modulated interaction of aqueous steroids using polymer-grafted capillaries. Langmuir. 2006;22:425–430. doi: 10.1021/la051968h. [DOI] [PubMed] [Google Scholar]
- Ikada Y., et al. Blood compatibility of hydrophilic polymers. J. Biomed. Mater. Res. 1981;15:697–718. doi: 10.1002/jbm.820150507. [DOI] [PubMed] [Google Scholar]
- Itoga K., Yamato M., Kobayashi J., Kikuchi A., Okano T. Cell micropatterning using photopolymerization with a liquid crystal device commercial projector. Biomaterials. 2004a;25:2047–2053. doi: 10.1016/j.biomaterials.2003.08.052. [DOI] [PubMed] [Google Scholar]
- Itoga K., Yamato M., Kobayashi J., Kikuchi A., Okano T. Micropatterned surfaces prepared using a liquid crystalprojector-modified photopolymerization device and microfluidics. J. Biomed. Mater. Res. 2004b;69A:391–397. doi: 10.1002/jbm.a.30010. [DOI] [PubMed] [Google Scholar]
- Itoga K., Kobayashi J., Yamato M., Kikuchi A., Okano T. Maskless liquid-crystal-display projection photolithography for improved design flexibility of cellular micropatterns. Biomaterials. 2006;27:3005–3009. doi: 10.1016/j.biomaterials.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Itoga K., Kobayashi J., Tsuda Y., Yamato M., Okano T. Second-generation maskless photolithography device for surface micropatterning and microfluidic channel fabrication. Anal. Chem. 2008;80:1323–1327. doi: 10.1021/ac702208d. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Ziats N.P., Tierney B.P., Nakabayashi N., Anderson J.M. Protein adsorption from human plasma is reduced on phospholipid polymers. J. Biomed. Mater. Res. 1991;25:1397–1407. doi: 10.1002/jbm.820251107. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Yamamoto K., Matsushima Y., Takai N., Kikuchi A., Sakurai Y., Okano T. Temperature-responsive chromatography using poly(N-isopropylacrylamide)-modified silica. Anal. Chem. 1996;68:100–105. doi: 10.1021/ac950359j. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Kashiwase Y., Yamamoto K., Matsushima Y., Kikuchi A., Sakurai Y., Okano T. Temperature-responsive liquid chromatography. 2. Effects of hydrophobic groups in N-isopropylacrylamide copolymer-modified silica. Anal. Chem. 1997a;69:823–830. doi: 10.1021/ac961024k. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Yamamoto K., Kashiwase Y., Matsushima Y., Takai N., Kikuchi A., Sakurai Y., Okano T. Analysis of peptides and proteins by temperature-responsive chromatographic system using N-isopropylacrylamide polymer-modified columns. J. Pharm. Biomed. Anal. 1997b;15:1545–1550. doi: 10.1016/S0731-7085(96)02004-3. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Sunamoto T., Matsushima Y., Kikuchi A., Okano T. Temperature-responsive chromatographic separation of amino acid phenylthiohydantoins using aqueous media as the mobile phase. Anal. Chem. 2000;72:5961–5966. doi: 10.1021/ac0004658. [DOI] [PubMed] [Google Scholar]
- Kato K., Uchida E., Kang E.T., Uyama Y., Ikada Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003;28:209–259. doi: 10.1016/S0079-6700(02)00032-1. [DOI] [Google Scholar]
- Khetani S.R., Bhatia S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- Kidoaki S., Ohya S., Nakayama Y., Matsuda T. Thermoresponsive structural change of a poly(N-isopropylacrylamide) graft layer measured with an atomic force microscope. Langmuir. 2001;17:2404–2407. doi: 10.1021/la001522v. [DOI] [Google Scholar]
- Kikuchi A., Okano T. Intelligent thermoresponsive polymeric stationary phases for aqueous chromatography of biological compounds. Prog. Polym. Sci. 2002;27:1165–1193. doi: 10.1016/S0079-6700(02)00013-8. [DOI] [Google Scholar]
- Kikuchi A., Okano T. Temperature responsive, polymer-modified surfaces for green chromatography. Macromol. Symp. 2004;207:217–227. doi: 10.1002/masy.200450319. [DOI] [Google Scholar]
- Kikuchi A., Okano T. Nanostructured designs of biomedical materials: applications of cell sheet engineering to functional regenerative tissues and organs. J. Control. Rel. 2005;101:69–84. doi: 10.1016/j.jconrel.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Kobayashi J., Okano T., Iwasa T., Sakai K. Temperature-modulated interaction changes with adenosine nucleotides on intelligent cationic, thermoresponsive surfaces. J. Bioact. Compat. Polym. 2007;22:575–588. doi: 10.1177/0883911507084294. [DOI] [Google Scholar]
- Kobayashi J., Kikuchi A., Sakai K., Okano T. Aqueous chromatography utilizing pH-/temperature-responsive polymer stationary phases to separate ionic bioactive compounds. Anal. Chem. 2001;73:2027–2033. doi: 10.1021/ac0013507. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Kikuchi A., Sakai K., Okano T. Aqueous chromatography utilizing hydrophobicity-modified anionic temperature-responsive hydrogel for stationary phases. J. Chromatogr. A. 2002;958:109–119. doi: 10.1016/S0021-9673(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Kikuchi A., Sakai K., Okano T. Cross-linked thermoresponsive anionic polymer-grafted surfaces to separate bioactive basic peptides. Anal. Chem. 2003;75:3244–3249. doi: 10.1021/ac026364m. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Yamato M., Itoga K., Kikuchi A., Okano T. Preparation of microfluidic devices using micropatterning of a photosensitive material by a maskless, liquid-crystal-display projection method. Adv. Mater. 2004;16:1997–2001. doi: 10.1002/adma.200400312. [DOI] [Google Scholar]
- Koehler J.A., Ulbricht M., Belfort G. Intermolecular forces between a protein and a hydrophilic modified polysulfone film with relevance to filtration. Langmuir. 2000;16:10 419–10 427. doi: 10.1021/la000593r. [DOI] [Google Scholar]
- Kurisawa M., Yokoyama M., Okano T. Gene expression control by temperature with thermo-responsive polymeric gene carriers. J. Control. Rel. 2000;69:127–137. doi: 10.1016/S0168-3659(00)00297-2. [DOI] [PubMed] [Google Scholar]
- Kushida A., Yamato M., Konno C., Kikuchi A., Sakurai Y., Okano T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999;45:355–362. doi: 10.1002/(SICI)1097-4636(19990615)45:4<355::AID-JBM10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kushida A., Yamato M., Konno C., Kikuchi A., Sakurai Y., Okano T. Temperature-responsive culture dishes allow nonenzymatic harvest of differentiated Madin–Darby canine kidney (MDCK) cell sheets. J. Biomed. Mater. Res. 2000;51:216–223. doi: 10.1002/(SICI)1097-4636(200008)51:2<216::AID-JBM10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kushida A., Yamato M., Isoi Y., Kikuchi A., Okano T. A noninvasive transfer system for polarized renal tubule epithelial cell sheets using temperature-responsive culture dishes. Eur. Cell. Mater. 2005;10:23–30. doi: 10.22203/ecm.v010a03. [DOI] [PubMed] [Google Scholar]
- Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Liang L., Feng X., Liu J., Rieke P.C., Fryxell G.E. Reversible surface properties of glass plate and capillary tube grafted by photopolymerization of N-isopropylacrylamide. Macromolecules. 1998;31:7845–7850. doi: 10.1021/ma9802881. [DOI] [Google Scholar]
- Matsukata M., Takei Y., Aoki T., Sanui K., Ogata N., Sakurai Y., Okano T. Effect of molecular architecture of poly(N-isopropylacrylamide)–trypsin conjugates on their solution and enzymatic properties. Bioconjug. Chem. 1994;116:682–686. doi: 10.1021/bc950082u. [DOI] [PubMed] [Google Scholar]
- Memon I.A., et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J. Thorac. Cardiovasc. Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Mizutani A., Kikuchi A., Yamato M., Kanazawa H., Okano T. Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials. 2008;29:2073–2081. doi: 10.1016/j.biomaterials.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Morra M., Cassinelli C. Thermal recovery of cells cultured on poly(N-isopropylacrylamide) surface-grafted polystyrene dishes. In: Ratner B.D., Castner D.G., editors. Surface modification of polymeric biomaterials. Plenum Press; New York, NY: 1997. pp. 175–181. [Google Scholar]
- Murakami D., Yamato M., Nishida K., Ohki T., Takagi R., Yang J., Namiki H., Okano T. The effect of micropores in the surface of temperature-responsive culture inserts on the fabrication of transplantable canine oral mucosal epithelial cell sheets. Biomaterials. 2006a;27:5518–5523. doi: 10.1016/j.biomaterials.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Murakami D., Yamato M., Nishida K., Ohki T., Takagi R., Yang J., Namiki H., Okano T. Fabrication of transplantable human oral mucosal epithelial cell sheets using temperature-responsive culture inserts without feeder layer cells. J. Artif. Organs. 2006b;9:185–191. doi: 10.1007/s10047-006-0342-3. [DOI] [PubMed] [Google Scholar]
- Nagase K., Kobayashi J., Kikuchi A., Akiyama Y., Kanazawa H., Okano T. Interfacial property modulation of thermoresponsive polymer brush surfaces and their interaction with biomolecules. Langmuir. 2007;23:9409–9415. doi: 10.1021/la700956b. [DOI] [PubMed] [Google Scholar]
- Nagase K., Kobayashi J., Kikuchi A., Akiyama Y., Kanazawa H., Okano T. Effects of graft densities and chain lengths on separation of bioactive compounds by nanolayered thermoresponsive polymer brush surfaces. Langmuir. 2008a;24:511–517. doi: 10.1021/la701839s. [DOI] [PubMed] [Google Scholar]
- Nagase K., Kobayashi J., Kikuchi A., Akiyama Y., Kanazawa H., Okano T. Preparation of thermoresponsive cationic copolymer brush surfaces and application of the surface to separation of biomolecules. Biomacromolecules. 2008b;9:1340–1347. doi: 10.1021/bm701427m. [DOI] [PubMed] [Google Scholar]
- Nagase K., Kobayashi J., Kikuchi A., Akiyama Y., Annaka M., Kanazawa H., Okano T. Influence of graft interface polarity on hydration/dehydration of grafted thermoresponsive polymer brushes and steroid separation using all-aqueous chromatography. Langmuir. 2008c;24:10 981–10 987. doi: 10.1021/la801949w. [DOI] [PubMed] [Google Scholar]
- Nie F.-Q., Yamada M., Kobayashi J., Yamato M., Kikuchi A., Okano T. On-chip cell migration assay using microfluidic channels. Biomaterials. 2007;28:4017–4022. doi: 10.1016/j.biomaterials.2007.05.037. [DOI] [PubMed] [Google Scholar]
- Nishi M., et al. The use of biotin–avidin binding to facilitate biomodification of thermoresponsive culture surfaces. Biomaterials. 2007;28:5471–5476. doi: 10.1016/j.biomaterials.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Nishida K., et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004;351:1187–1194. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- Ohashi K., et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007;13:880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- Ohki T., Yamato M., Murakami D., Takagi R., Yang J., Namiki H., Okano T., Takasaki K. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704–1710. doi: 10.1136/gut.2005.088518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J. Biomed. Mater. Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- Okano T., Yamada N., Okuhara M., Sakai H., Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic–hydrophobic polymer surfaces. Biomaterials. 1995;16:297–303. doi: 10.1016/0142-9612(95)93257-E. [DOI] [PubMed] [Google Scholar]
- Pan Y.V., Wesley R.A., Luginbuhl R., Denton D.D., Ratner B.D. Plasma polymerized N-isopropylacrylamide: synthesis and characterization of a smart thermally responsive coating. Biomacromolecules. 2001;2:32–36. doi: 10.1021/bm0000642. [DOI] [PubMed] [Google Scholar]
- Park K.D., Suzuki K., Lee W.K., Lee J.E., Kim Y.H., Sakurai Y., Okano T. Platelet adhesion and activation on polyethylene glycol modified polyurethane surfaces. Measurement of cytoplasmic calcium. ASAIO J. 1996;42:M876–M881. doi: 10.1097/00002480-199609000-00117. [DOI] [PubMed] [Google Scholar]
- Pellegrini G. Changing the cell source in cell therapy? N. Engl. J. Med. 2004;351:1170–1172. doi: 10.1056/NEJMp048199. [DOI] [PubMed] [Google Scholar]
- Razatos A., Ong Y.L., Boulay F., Elbert D.L., Hubbell J.A., Sharma M.M., Georgiou G. Force measurements between bacteria and poly(ethylene glycol)-coated surfaces. Langmuir. 2000;16:9155–9158. doi: 10.1021/la000818y. [DOI] [Google Scholar]
- Sakai H., Doi Y., Okano T., Yamada N., Sakurai Y. Thermo-responsive polymer surfaces for cell culture: analysis of the surfaces and control of the cell attachment/detachment. In: Ogata N., Kim S.W., Feijen J., Okano T., editors. Advanced biomaterials in biomedical engineering and drug delivery systems. Springer; Tokyo, Japan: 1996. pp. 229–230. [Google Scholar]
- Shimizu T., Yamato M., Isoi Y., Akutsu T., Setomaru T., Abe K., Kikuchi A., Umezu M., Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ. Res. 2002;90:e40–e48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- Shin'oka T., Imai Y., Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N. Engl. J. Med. 2001;344:532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- Stannett V.T. Radiation grafting—state-of-the-art. Int. J. Radiat. Appl. Instrum. Part C. Radiat. Phys. Chem. 1990;35:82–87. doi: 10.1016/1359-0197(90)90062-M. [DOI] [Google Scholar]
- Takeda N., Nakamura E., Yokoyama M., Okano T. Temperature-responsive polymeric carriers incorporating hydrophobic monomers for effective transfection in small doses. J. Control. Rel. 2000;95:343–355. doi: 10.1016/j.jconrel.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Takei Y.G., Aoki T., Sanui K., Ogata N., Okano T., Sakurai Y. Temperature-responsive bioconjugates. 1. Synthesis of temperature-responsive oligomers with reactive end groups and their coupling to biomolecules. Bioconjug. Chem. 1993;4:42–46. doi: 10.1021/bc00019a006. [DOI] [PubMed] [Google Scholar]
- Takei Y.G., Aoki T., Sanui K., Ogata N., Okano T., Sakurai Y. Dynamic contact angle measurement of temperature-responsive surface properties for poly(N-isopropylacrylamide) grafted surfaces. Macromolecules. 1994;27:6163–6166. doi: 10.1021/ma00099a035. [DOI] [Google Scholar]
- Takezawa T., Mori Y., Yoshizato K. Cell culture on a thermo-responsive polymer surface. Biotechnology. 1990;8:854–856. doi: 10.1038/nbt0990-854. [DOI] [PubMed] [Google Scholar]
- Takezawa T., Yamazaki M., Mori Y., Yonaha T., Yoshizato K. Morphological and immuno-cytochemical characterization of a heterospheroid composed of fibroblasts and hepatocytes. J. Cell Sci. 1992;101:495–501. doi: 10.1242/jcs.101.3.495. [DOI] [PubMed] [Google Scholar]
- Tsang V.L., Bhatia S.N. Three-dimensional tissue fabrication. Adv. Drug Deliv. Rev. 2004;56:1635–1647. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tsuda Y., Kikuchi A., Yamato M., Nakao A., Sakurai Y., Umezu M., Okano M. The use of patterned dual thermoresponsive surfaces for the collective recovery as co-cultured cell sheets. Biomaterials. 2005;26:1885–1893. doi: 10.1016/j.biomaterials.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Tsuda Y., Kikuchi A., Yamato M., Chen G., Okano T. Heterotypic cell interactions on a dually patterned surface. Biochem. Biophys. Res. Commun. 2006;348:937–944. doi: 10.1016/j.bbrc.2006.07.138. [DOI] [PubMed] [Google Scholar]
- Tsuda Y., Shimizu T., Yamato M., Kikuchi A., Sasagawa T., Sekiya S., Kobayashi J., Chen G., Okano T. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials. 2007;28:4939–4946. doi: 10.1016/j.biomaterials.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Uchida K., Sakai K., Ito E., Kwon O.H., Kikuchi A., Yamato M., Okano T. Temperature-dependent modulation of blood platelet movement and morphology on poly(N-isopropylacrylamide)-grafted surfaces. Biomaterials. 2000;21:923–929. doi: 10.1016/S0142-9612(99)00260-4. [DOI] [PubMed] [Google Scholar]
- Wada K., Taniguchi A., Kobayashi J., Yamato M., Okano T. Live cells-based cytotoxic sensorchip fabricated in a microfluidic system. Biotechnol. Bioeng. 2008;99:1513–1517. doi: 10.1002/bit.21718. [DOI] [PubMed] [Google Scholar]
- Winblade N.D., Nikolic I.D., Hoffman A.S., Hubbell J.A. Blocking adhesion to cell and tissue surfaces by the chemisorption of a poly-l-lysine-graft-(poly(ethylene glycol); phenylboronic acid) copolymer. Biomacromolecules. 2000;1:523–533. doi: 10.1021/bm000040v. [DOI] [PubMed] [Google Scholar]
- Yakushiji T., Sakai K., Kikuchi A., Aoyagi T., Sakurai Y., Okano T. Graft architectural effects on thermoresponsive wettability changes of poly(N-isopropylacrylamide)-modified surfaces. Langmuir. 1998;14:4657–4662. doi: 10.1021/la980090+. [DOI] [Google Scholar]
- Yakushiji T., Sakai K., Kikuchi A., Aoyagi T., Sakurai Y., Okano T. Effects of cross-linked structure on temperature-responsive hydrophobic interaction of poly(N-isopropylacrylamide) hydrogel-modified surfaces with steroids. Anal. Chem. 1999;71:1125–1130. doi: 10.1021/ac980677t. [DOI] [Google Scholar]
- Yamada N., Okano T., Sakai H., Karikusa F., Sawasaki Y., Sakurai Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem., Rapid Commun. 1990;11:571–576. doi: 10.1002/marc.1990.030111109. [DOI] [Google Scholar]
- Yamanaka H., Yoshizako K., Akiyama Y., Sota H., Hasegawa Y., Shinohara Y., Kikuchi A., Okano T. Affinity chromatography with collapsibly tethered ligands. Anal. Chem. 2003;75:1658–1663. doi: 10.1021/ac0263768. [DOI] [PubMed] [Google Scholar]
- Yamato M., Okano T. Cell sheet engineering. Mater. Today. 2004;7:42–47. doi: 10.1016/S1369-7021(04)00234-2. [DOI] [Google Scholar]
- Yamato M., Okuhara M., Karikusa F., Kikuchi A., Sakurai Y., Okano T. Signal transduction and cytoskeletal reorganization are required for cell detachment from cell culture surfaces grafted with a temperature-responsive polymer. J. Biomed. Mater. Res. 1999;44:44–52. doi: 10.1002/(SICI)1097-4636(199901)44:1<44::AID-JBM5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Yamato M., Utumi M., Kushida A., Konno C., Kikuchi A., Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473–480. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- Yamato M., Akiyama Y., Kobayashi J., Joseph Y., Kikuchi A., Okano T. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog. Polym. Sci. 2007;32:1123–1133. doi: 10.1016/j.progpolymsci.2007.06.002. [DOI] [Google Scholar]
- Yang J., Yamato M., Shimizu T., Sekine H., Ohashi K., Kanzaki M., Ohki T., Nishida K., Okano T. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007;28:5033–5043. doi: 10.1016/j.biomaterials.2007.07.052. [DOI] [PubMed] [Google Scholar]
- Yoshikawa C., Goto A., Tsujii Y., Fukuda T., Kimura T., Yamamoto K., Kishida A. Protein repellency of well-defined, concentrated poly(2-hydroxyethyl methacrylate) brushes by the size-exclusion effect. Macromolecules. 2006;39:2284–2290. doi: 10.1021/ma0520242. [DOI] [Google Scholar]
- Yoshizako K., Akiyama Y., Yamanaka H., Shinohara Y., Hasegawa Y., Carredano E., Kikuchi A., Okano T. Regulation of protein binding toward a ligand on chromatographic matrixes by masking and forced-releasing effects using thermoresponsive polymer. Anal. Chem. 2002;74:4160–4166. doi: 10.1021/ac025523z. [DOI] [PubMed] [Google Scholar]
- Yousaf M.N., Houseman B.T., Mrksich M. Using electroactive substrates to pattern the attachment of two different cell populations. Proc. Natl Acad. Sci. USA. 2001;98:5992–5996. doi: 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Mutlu S., Selvaganapathy P., Mastrangelo C.H., Svec F., Fréchet J.M.J. Flow control valves for analytical microfluidic chips without mechanical parts based on thermally responsive monolithic polymers. Anal. Chem. 2003;75:1958–1961. doi: 10.1021/ac026455j. [DOI] [PubMed] [Google Scholar]