This article describes the Life Course Health Development (LCHD) framework, which was created to explain how health trajectories develop over an individual's lifetime and how this knowledge can guide new approaches to policy and research. Using recent research from the fields of public health, medicine, human development, and social sciences, the LCHD framework shows that
Health is a consequence of multiple determinants operating in nested genetic, biological, behavioral, social, and economic contexts that change as a person develops.
Health development is an adaptive process composed of multiple transactions between these contexts and the biobehavioral regulatory systems that define human functions.
Different health trajectories are the product of cumulative risk and protective factors and other influences that are programmed into biobehavioral regulatory systems during critical and sensitive periods.
The timing and sequence of biological, psychological, cultural, and historical events and experiences influence the health and development of both individuals and populations.
Based on the relationship between experience and the biology and psychology of development, the LCHD framework offers a conceptual model for health development and a more powerful approach to understanding diseases. Throughout this article, we illustrate how risk factors, protective factors, and early-life experiences affect people's long-term health and disease outcomes. A better understanding of health development should enable us to manipulate early risk factors and protective factors and help shift our emphasis on treatment in the later stages of disease to the promotion of earlier, more effective preventive strategies and interventions focused on maximizing optimal health development.
Our Changing Understanding of Health
In recent decades our understanding of human health has changed. In the 1970s, older biomedical definitions of health, based on “an absence of disease,” were justly criticized as reductionist and limited in scope (Evans and Stoddart 1990), and clinical researchers began defining health as a dynamic, multifactor, biopsychosocial phenomenon that influences physical, psychological, and social functioning (Engle 1977; Evans 1994; Singer and Ryff 2001). In public health, “field models” were increasingly used to account for the full range of issues that affect human health (Canadian Government 1974) and to guide the development of health research, measurement, and policy (Durch, Bailey, and Stoto 1997; Evans and Stoddart 1990; Halfon et al. 2000; Kindig 1997). Like clinical efforts to reconceptualize health in biopsychosocial terms, field models link determinants from various domains (fields)—including global factors (physical, environmental, socioeconomic), middle-level factors (health care, behavioral interventions), and individual, small-group, and community factors—to explain the origins of disease, health, and well-being (Evans and Stoddart 1990).
Today at the beginning of the 21st century, our understanding of health has broadened. For example, a new definition of health was recently suggested by the European Region of the World Health Organization:
{Health} is the extent to which an individual or group is able, on the one hand, to realize aspirations and satisfy needs, and, on the other hand, to change and cope with the environment. Health is therefore seen as a resource for everyday life, not the objective of living; it is a positive concept emphasizing social and personal resources as well as physical capacities.
(cited in Young 1998, 1)
Our view of disease causation and predisease pathways has also broadened, as it has become clear that health risks are created and maintained by social systems and that the magnitude of those risks is largely a function of socioeconomic disparities and psychosocial gradients (Ben-Shlomo and Kuh 2002; Brunner and Marmot 1999; Hertzman 1994; Hertzman et al. 2001; Singer and Ryff 2001). Accordingly, our efforts to reduce health disparities can no longer be confined only to providing better access and more resources to address the needs of the underserved. We now understand that we must address the underlying social factors that determine these health disparities, including differences in income, employment benefits, and even the very quality of family and social relationships.
Disparities in health outcomes and in the psychosocial factors contributing to them are present early in life and are expressed and compounded during a person's lifetime (Keating and Hertzman 1999; Wadsworth 1999). Risk factors are embedded in a person's biological makeup, manifested in the disparities in a population's health, and maintained by social, cultural, and economic forces (Hertzman 2000). To understand the origins and effects of these health disparities, we increasingly rely on an analysis of biological, psychological, socioeconomic, cultural, and physical environments and their impact on the health of both individuals and populations (Blaine 1999; Boyce et al. 1998; Bronfennbrenner 1979; Hertzman 2000; Lerner 1992). Because research on health disparities has demonstrated the effect of many determinants interacting in various contexts at developmentally sensitive points, we need an integrated conceptual model to translate evidence into policies, practices, and health systems.
The field of developmental science bears an underappreciated relevance for health science and health policy (Cairns, Elder, and Costello 1996). Some early “developmentalists” viewed human development as the unfolding of a genetically predetermined process of maturation and accordingly attributed less influence to environmental factors. More recent developmental theories place greater emphasis on the role of dynamic environment–gene transactions and on the mechanisms through which social contexts induce changes in psychological and biological functions (Gottlieb 1996; Magnusson and Cairns 1996; Sameroff and Fiese 2000). The most recent dynamic contextual developmental models combine gene transactions, changes in social context, and environmental and biological factors to trace the effect of the timing of developmental events on developmental trajectories (Dawson, Ashman, and Carver 2000; Meaney 2001). That is, how genes are expressed is determined by a person's particular physical, psychological, and social environment (Kandel 1998). Consequently, social relationships can actually influence the expression of DNA throughout a person's lifetime (Sameroff and Fiese 2000; Worthman 1999). These understandings have powerful implications for health policy.
Our present scientific understanding of health and of human development has converged around several key points, which are summarized next and then elaborated.
The importance of embedding: Embedding is the process by which experiences are programmed into the structure and functioning of biological and behavioral systems (Hertzman 1999).
The role of risk and protective factors: Researchers in both health and human development agree that both health and development balance gains and growth against deterioration and loss, a balance that involves interactions between protective factors and risk factors (Baltes and Graf 1996; Breslow 1999).
The developmental significance of extended time frames: Current understanding of the development of health and disease highlights how experiences at the beginning of life relate to functional outcomes during the middle and end of life (Ben-Shlomo and Kuh 2002; Hertzman 1994; Meaney 2001; Singer and Ryff 2001; Wadsworth 1999). A life-course developmental perspective addresses the sequencing of events across an entire lifetime and also accounts for intergenerational influences.
The many determinants of health outcomes: Health and developmental science recognize the multidimensionality and complexity of causation, including how environmental, social, psychological, and biological systems interact to influence health and developmental outcomes (Lerner and Benson 2002; Boyce et al. 1998).
Representing health development as functional trajectories: Health and developmental science present changes in functional status over time in terms of trajectories of development.
The Life Course Health Development (LCHD) Framework
Concepts and Principles
Building on this convergence of health and developmental science, we propose a new framework to explain how different environmental, physiological, behavioral, and psychological contexts influence risk profiles and long-term health development trajectories. Accordingly, we define health development as a lifelong adaptive process that builds and maintains optimal functional capacity and disease resistance.
The LCHD framework presented here is based on four related principles that explain how biological factors and environments transform individual biobehavioral functioning across the lifespan or life course.
The multiple contexts of health development.
The design and process of health development.
Mechanisms that account for variation in the trajectories of health development.
The integration of multiple time frames of health development.
Figure 1, adopted from Worthman (1999), suggests how these principles interact during a lifetime. In this figure, the macrocontext, is made up of multiple environments (e.g., physical, social, health care) that interact with the microcontext, which includes the design strategies, processes, and mechanisms of health development, to produce different health outcomes. This figure also illustrates how the design strategies of health development are organized into specific routines, pathways, and functional trajectories.
fig. 1.
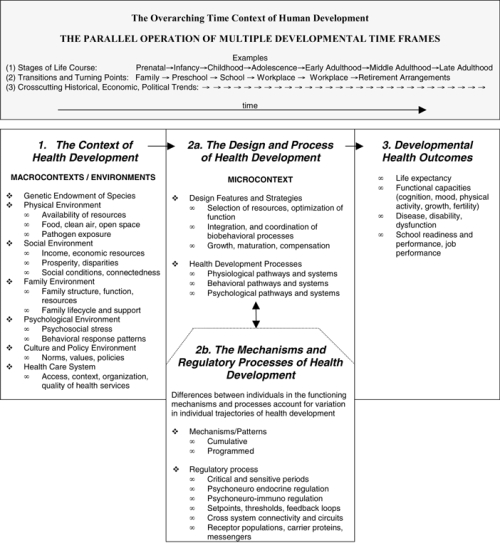
Principle components of the LCHD and their influence on health outcomes. (1) Multiple nested contexts make up the macro-context or environments of health development. These macrocontexts interact with each other and influence and modify (2a) the microcontext. The microcontext includes design features, strategies, and pathways of the health development process. These (2b) regulatory processes that are developmentally programmed mediate and modify the microcontext of health development. Overarching this process are multiple timeframes and specifically timed experiences whose relationships function to integrate and synchronize macro- and microcontexts and to produce variation in (3) developmental health outcomes. Source: Adapted from Worthman 1999, 91.
The Environment of Health Development
Although the basic processes of human development are genetically programmed, gene expression is modified by past and present environments.
In populations, the influence of different environmental contexts can be represented as “macropathways” depicting the interactions between the economic, social, physical, behavioral, cultural, and other environments that mediate, or modify, individual functioning. Macropathways typically involve risk and protective factors that are often correlated (e.g., poverty, geographic proximity, physical environment, limited social capital) and that together define the path of health development (Boyce et al. 1998). Several macropathway models have been proposed to explain how various determinants interact to produce different population health outcomes (Brunner and Marmot 1999; Evans and Stoddart 1990; Hertzman et al. 2001). The existence of the macropathways depicted in these multiple-determinant models is supported by empirical research on how economic change, demographic shifts, and alterations in lifestyles and behaviors affect everyday life and thereby influence growth, reproductive maturation, and fertility, as well as exposure to different risks and protective factors that influence the distribution of disease and the process of aging in populations (Repetti, Taylor, and Seaman 2002; Worthman 1999). In individuals, multiple-determinant models describe the macropathways through which different environmental contexts influence lifestyle, physical activity, and food consumption. These in turn mediate the effects of social, economic, and cultural environments on short- and long-term health and well-being. This mediation is based on the functioning of metabolic and neuroendocrine regulatory “micropathways,” which are described in more detail in the next section.
In any particular environmental context, one or two factors (e.g., access to food, level of psychosocial stress, amount of air pollution) may be especially important to specific health outcomes (e.g., growth, psychological adjustment, exacerbation of asthma), but it is probably rare that a single environmental factor is uncorrelated with other influential factors from the same environment. Moreover, these multiple nested environments are dynamic, and during different stages in life, their relative influence changes (Nordio 1978). For example, family environment has a relatively greater effect on the health development of young children, whereas neighborhood and individual behaviors become more important as they age (Wadsworth 1999). Peer influences predominate in shaping the health development of adolescents, whereas social networks and age-specific service systems are the principal influence in older persons' compensatory adaptations to functional loss (Baltes and Graf 1996).
To understand how multiple nested environments affect individual development, life course sociologists created the concept of “life pathways.” The life pathway concept contrasts with the earlier view of life course as a simple linear trajectory, divided into ages and stages and bounded by the finitude of death (Bury 1998; Featherstone and Hepworth 1998). Research by Elder and his colleagues defined the life path from childhood onward as influenced by the prevailing historical, social, economic, and cultural environments. They described how families used their adaptive roles and capacities to minimize the effect of the 1930s Great Depression on their children's well-being. They also observed how other major historical events occurring during sensitive points in development shaped their life course (Elder 1974; Elder 1998a, 1998b; Elder, Liker, and Cross 1984; Elder, Van Nguyen, and Caspi 1985). An enormous body of life course research describes individual developmental trajectories (life pathways) in accordance with the sequence, impact, and cumulative influence of life events on a range of outcomes from childbearing to transition into and out of the workforce.
Even though social environments and experiences influence health development at all stages, those early in life are thought to have a particularly powerful effect on morbidity, largely because of the persistence of biobehavioral attributes that are acquired early in life (Friedman et al. 1993; Haan, Millsap, and Hartka 1986; Schwartz et al. 1995). The 1946 British National Birth Cohort Follow-up Study provided extensive evidence of the effect of early life experiences on cognitive functions (Richards et al. 1999, 2001), physical growth trajectories (Montgomery, Bartley, and Wilkinson 1997), menopause (Hardy and Kuh 1999; Kuh, Wadsworth, and Hardy 1997), blood pressure (Wadsworth et al. 1985), psychotic illness (Jones et al. 1994), respiratory health (Mann, Wadsworth, and Coley 1992), and other serious diseases (DeStavola et al. 2000; Pless et al. 1989). Other studies demonstrated a “dose response” relationship between the exposure to abuse and family dysfunction during childhood and the prevalence, severity, and age of onset of adult disease (Felitti et al. 1998). Several recent reviews connected the relationship between childhood family and social environments and the occurrence of later health and mental health outcomes (Institute of Medicine 2001; Repetti, Taylor, and Seeman 2002; Singer and Ryff 2001). Recent studies of adolescents in Chicago's inner city also identified how particular social networks can determine their risk status and resiliency and influence their life course trajectories (Sampson and Laub 1993, 1996; Sampson, Raudenbush, and Earls 1997).
Early experiences and adaptive responses can significantly influence the trajectory of health development, without having a deterministic effect. Especially in behavioral subsystems, contemporary theories and research stress that people remain relatively malleable throughout life (Lerner 1992). Longitudinal studies of the elderly show that throughout life, environment and context (e.g., culture-specific social relationships, general social support, strategies for maintaining personal mobility, and other factors) are important to modifying functional trajectories such as critical problem-solving and calculation abilities (Baltes, Lindenberger, and Staudinger 1998). The ability to identify and use resources in their environment enables elderly persons to maximize their functional capacities within the parameters defined by early life experiences and genetics. For example, despite measurable declines in the speed and efficiency of information processing, an elderly person is able to maintain higher levels of intellectual and other functioning through the use of various strategies, some that are widespread (e.g., enhancing memory with written notes) and others that may be specific to a particular cultural or economic context (e.g., moving in with children, selecting an “assisted-living” housing arrangement).
The life pathway of health development is defined by the cumulative pattern of experiences of individuals and populations in many contexts, reflecting the importance of developmental transitions, turning points, and trajectories (Moen and Wethington 1999). Environmental insults can cause damage in gradual and independent ways or may cluster together in socially patterned ways (Ben Shlomo and Kuh 2002). For example, a child living in a poor neighborhood may be more likely to witness interpersonal violence and, at the same time, subsist on a poor diet and be exposed to environmental toxins. Over time, the same child is less likely to attend nursery school, to have many educational advantages at home, and to attend an elementary school that facilitates relationships with more advantaged peers. The effects of these experiences and environments are compounded as the child passes through each transition and turning point along his or her life path (Blaine 1999). Small initial effects, such as a reduction in the child's school readiness, may snowball as his school achievement drops relative to that of other children and may be reinforced by less workplace readiness in later years (Phillips, Crouse, and Ralph 1998).
Research on the development of life pathways suggests that nested and correlated social contexts broaden and deepen and become more differentiated over time (Boyce et al. 1998; Blaine 1999). These age-dependent patterns of social influence are most powerful at times of transition between different life phases (Blaine 1999; Graber and Brooks-Gunn 1996; Moen and Wethington 1999). As individuals move along even very constrained life pathways, they may adopt and relinquish roles and identities and transform and modify personal ties and social relationships as they adapt to the demands of their shifting social, psychological, and biological environments. Distinct life paths emerge not only from the correlation of related contexts but also because individual and family responses form coherent biological and cultural strategies of adaptation (Chapman and Scott 2001).
The Design and Process of Health Development
To understand the connection between social context and health, we next examine the development and organization of the micropathways that translate information from social relationships, environmental exposures, and historical events into biological information that alters the functioning of biological processes. In this section we focus on the design and integration of biobehavioral processes into a number of functional systems (e.g. nervous, endocrine, immune, and respiratory) and how regulatory systems program health development trajectories.
The Design and Development of Micropathways
The design of human physiological systems, such as the nervous, endocrine, and immune systems, has evolved in response to the selection pressures of evolution (Worthman 1999). In addition to each system's unique attributes, all systems are functionally enmeshed and physiologically connected to one another. Each one, and the human organism as a whole, is capable of self-organization—of changing and adapting to its particular niche in response to its internal (genetic) programs and external relationships (Baltes and Graf 1996).
Because each system provides an operational context for the others, each one also offers opportunities for and imposes constraints on the others' functioning. Figure 2 shows how pathways can connect the various functional systems, thereby permitting higher levels of integration and functioning. In this simplified schema, the brain translates the cognitive and emotional information it receives into changes in immune and endocrine functions, and the immune system gives sensory information, via the hypothalamus, to the nervous system about the nature and magnitude of an infection.
fig. 2.
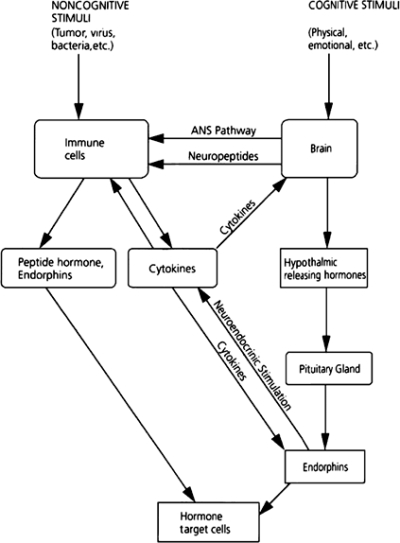
Pathways of communication and information transfer between the brain, hypothalamic-pituitary-adrenal axis, and immune system. This figure depicts how external influence triggers response patterns across systems promoting integrated patterns of response. Source: Brunner and Marmot 1999, 30.
Micropathways develop and adapt as they transfer information from extraorganismic contexts into intraorganismic structures and functional attributes (figures 1 and 2). This process is dependent on the design of the biological, psychological, and behavioral systems and their capacity to change in response to different contexts (Repetti, Taylor, and Seaman 2002). Regulatory systems, such as the nervous, immune, and endocrine systems, appear to be particularly important in transferring information between external and internal environments (Worthman 1999).
For example, by modulating the metabolism, the endocrine system plays a central role in the day-to-day allocation of physiological resources. It mediates the interface between the individual and his or her environment by regulating responses to stresses and challenges through a range of facilitative adjustments and by regulating the rate of growth and the timing of developmental transitions such as puberty and menopause (Worthman 1999). The endocrine system has been compared to a sophisticated computer operating system, with its capacity to switch “application programs” on and off. It can pace growth and even allocate resources to other somatic systems. Furthermore, it can even control growth across time, selectively using current resources to meet immediate adaptive demands in ways that limit or enhance their future availability (Baltes and Graf 1996; Worthman 1999).
The case of fetal malnutrition provides an example of how the endocrine system's functional pathways can be modified in response to environmental stress. Malnourished infants manifest a blunted response of insulin-like growth factor (IGF) to growth hormone and hyperresponsiveness to growth hormone releasing factor (GRHF). This pattern is indicative of receptor-mediated resistance (Barker 1992). As a result of these changes, malnourished infants create a glucose metabolism set point. These physiological changes reveal the developmental pathway that may account for the observed association between early malnutrition and lower adult glucose tolerance and a higher risk of developing diabetes (Barker 1992; Worthman 1999).
Although each person's endocrine system has the same basic design and functional architecture, each functions somewhat differently because of genetic variation and environmental influences, resulting in hormone levels that vary as much as fivefold between individuals (Worthman 1999). Anthropologists like Worthman have begun to explore how different human ecologies may account for the variations in endocrine function by modifying set points; modulating the intensity of the functional interactions among the endocrine, immune, and nervous systems; transforming age-specific developmental changes; and influencing regulatory functions (Worthman 1999). Other biobehavioral systems have evolved in a similar way, with unique design features that influence how they are programmed and how they control changes in functional capacity during the life course.
The micropathways that connect and influence the operation of human biobehavioral systems are not fully functional at birth. Rather, they are adaptively programmed in response to different experiences and to the short- and long-term changes in functional status produced by various feedforward and feedback processes. Despite this complex developmental programming, certain processes of the endocrine, immune, neurological, respiratory, and other systems have similar functional trajectories during a person's lifetime. The developmental trajectory of each of these biobehavioral systems follows the same general pattern: first, steady curvilinear growth in functional capacity; then, maintenance of functional integrity; and finally, compensation in the face of decline, which often proceeds in a less smooth and more steplike pattern (Baltes and Graf 1996). This basic pattern of the “life course functional trajectory” for several systems is depicted in figure 3.
fig. 3.
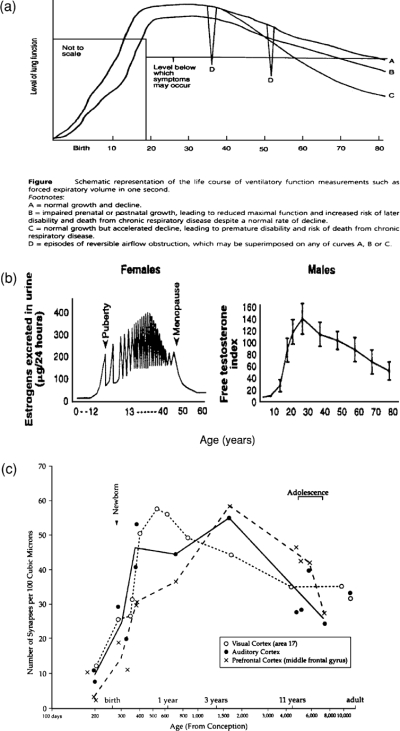
Diagrams demonstrating similarities in the pattern of developmental functional trajectories across physiological systems. Sources: (a) Strachan 1997, 104; (b) Lamberts, van den Beld, and van der Lely 1997, 421; (c) Ramey and Ramey 2000, 132.
Micropathways and Functional Trajectories
The autonomic nervous system (ANS), sympathetic adrenomedullary (SAM) and parasympathetic nervous system (PNS), hypothalamic pituitary adrenal (HPA) axis, and limbic (serotonergic) system have been shown to regulate several biobehavioral pathways that are significant for health (Seeman et al. 1997). Animal and human experiments have documented how these regulatory mechanisms are developmentally programmed in response to different social and behavioral contexts (Liu et al. 1997; Meaney, Aitkin, and Van Berkel 1988; Suomi 1999). Meaney and colleagues demonstrated that rat pups that received more maternal attention and stimulation had more hippocampal glucocorticoid receptors, were less reactive, recovered more rapidly when stressed, and demonstrated less age-related HPA dysregulation (Meaney, Aitken, and Van Berkel 1988). Meaney and colleagues have also shown that different levels of tactile stimulation induce different levels of gene transcription of cortisol releasing factor (CRF), accounting for different behavioral responses to stress (Meaney 2001). Suomi's long-term studies of monkeys showed the relationships among social dominance, patterns of behavioral responses, HPA and autonomic reactivity, and immunocompetency (Suomi 1999). Extrapolating these animal models to humans, Boyce and colleagues described how patterns of autonomic reactivity develop in children in different environmental contexts. They found that different behavioral and autonomic reactivity patterns were associated with the development of acute illness, and they have prospectively mapped the relationship of these reactivity patterns to the development of psychopathology as the children age (Boyce, Alkon, et al. 1995; Boyce, Chesney, et al. 1995; Boyce et al. 2001).
Specific alterations in interactions between genes and environment and disturbances in homeostatic equilibrium and dysregulation due to stress are now being linked to the development of health disorders like cardiovascular disease, hypertension, cancer, and cognitive decline (Brunner and Marmot 1999; McEwen 1998). The concept of allostasis—the ability to achieve stability through change—and the allostatic load hypothesis connect an individual's psychosocial environment to diseases and functional declines by way of dysregulation in various neuroendocrine systems (McEwen and Stellar 1993). Examples of the adaptive price of stress-induced wear and tear (“weathering”) on the organism include pushing the endocrine system toward diabetes or the cardiovascular system toward coronary artery disease and hypertension. For example, in a prospective study of older Americans that measured allostatic load by monitoring different hormones, those individuals with less allostatic load showed a lower trajectory of decline and were less likely to develop cardiovascular disease (Seeman et al. 1997). Likewise, the cascading influence of family stress and conflict on the development of physical and mental disorders in children due to dysregulation of the HPA, SAM, and PNS is increasingly well documented (Gunnar 1998; Hart, Gunnar, and Cicchetti 1996; Repetti, Taylor, and Seeman 2002). The mechanisms and biological markers of reactivity and regulatory patterns may prove valuable as indicators of stress, allostatic load, and predictors of developmental health problems (Boyce et al. 2001; Gunnar 1998; Seeman et al. 1997). For example, the future measurement of biomarkers, such as cortisol or corticotrophin releasing factor (CRF) levels in young children, or a set of emerging biomarkers, may have uses in identifying children at developmental risk for disease or disabilities that will be diagnosed decades later.
The allostatic load hypothesis is one example of how selective optimization and compensation serve as basic design strategies of life course health development. As biobehavioral plasticity and/or resource capacity decreases over time, the remaining resources are used selectively to optimize function. When demands exceed the organism's capacity to respond, these compensatory mechanisms reach their limits, and the system's functional level begin to decline (Baltes and Graf 1996). A more complete and operational understanding of the origins and expression of predisease pathways and life course health development pathways must account for basic strategies of health development like these (Singer and Ryff 2001).
Two examples help illustrate how micropathways develop and function in response to the contextual influences that shape human development (Holt and Sly 2000).
Research on the onset, evolution, and impact of childhood asthma has provided evidence of how the immune, respiratory, and nervous systems interact to determine pathways of asthma onset and morbidity (Holt, Sly, and Bjorksten 1997; Klinnert et al. 2001; Leung 1997; Martinez and Holt 1999). Early life events influence the development of the lungs and the responsiveness of the immune system, as well as a person's susceptibility to infection and allergenic and toxic challenges to the airways (Holt and Sly 1997). The critical periods and pathways in and by which these early influences on asthma operate now appear to include key prenatal and postnatal exposure and response patterns (Holt and Sly 1997; Leung 1997). In addition, early infectious illnesses and exposure to allergens can turn on and off and amplify genetically programmed immune responses (Holt and Sly 1997; Martinez and Holt 1999). The early programming of these response patterns appears to have a significant influence on the trajectory of adult lung function (Strachan 1997). For example, lifelong measurements of forced expiratory volume demonstrate that lower trajectories of function over time are associated not only with premature disability and disease but also with an accelerated decline in functional health status and an earlier expression of disease in adults (Kerstjens et al. 1997; Weiss 1995).
Another example from developmental psychopathology demonstrates the short- and long-term effects of impaired early relationships on children's long-term development (Cicchetti and Aber 1998). A good illustration of this phenomenon is the effect of maternal depression on infant development. Relative to control mothers, depressed mothers express less positive and more negative affect, are less attentive and engaged with their infants, and, when engaged, are more intrusive and controlling and fail to respond adaptively to their infants' emotional signals (Dawson, Hessel, and Frey 1994). Their infants have shorter attention spans, less motivation to master tasks, elevated heart rates, elevated cortisol levels, and reduced EEG activity in the right frontal cortex, all of which correlate with the experience of negative affect in adults (Dawson, Hessel, and Frey 1994). Longitudinal data on infants of depressed mothers indicate that elevated heart rates and cortisol can persist and may represent a functional programming of the child's autonomic set point. If true, this may explain in part Coghill and colleagues' (1986) finding that after controlling for the maternal depression status when the child was four years old, maternal depression during an infant's first year of life was predictive of the child's cognitive ability at that age.
Mechanisms That Account for Variation in the Trajectories of Health Development
Health development can be understood as the interaction between cumulative and programming mechanisms, which are controlled by genes, experiences, and past adaptive responses (Powers and Hertzman 1997). Similar influences, or mechanisms, are known by different names in other fields; for example, in the neurobiology of synaptic development, cumulative mechanisms are described as “experience dependent,” and programming mechanisms are “experience expectant”(Greenough, Black, and Wallace 1987).
Cumulative mechanisms refer to effects that are largely independent of a particular developmental stage and/or exposure timing and instead are dose or exposure dependent. The traditional public health understanding of chronic disease implicitly relied on cumulative pathways of disease. Cumulative models of disease are based on the relationship between the number of social risk factors that a child experiences and his or her intellectual attainment (Sameroff et al. 1987; Sameroff et al. 1993), or on the cumulative effects on various outcomes from a lifelong exposure to a specific risk factor such as cigarette smoke (Elford, Whincup, and Shaper 1991). In addition, numerous studies have examined the cumulative impact of risk factors, for example, for coronary heart disease (CHD), including obesity, hypertension, cholesterol, and cigarette smoking (Epstein 1996; Kuh and Davey Smith 1997; Kromhout, Menotti, and Blackburn 1994). Ben-Shlomo and Kuh have further refined the classification of cumulative mechanisms to define patterns of risk clustering and “chains of risk” where one adverse or beneficial exposure tends to lead to another (Ben-Shlomo and Kuh 2002).
Programming mechanisms refer to the strong, independent effect of risks, exposures, and adaptive responses during sensitive or critical developmental periods, many of which occur early in life. The existence of programming effects and the evidence for critical or sensitive periods in developing biological systems have been known for decades. Recently, however, the range and extent of programming effects of biological and psychophysiological processes have become more widely appreciated and are now used to explain long-term health development (Ben-Shlomo and Kuh 2002; Leon 1998; Lucas 1991, 1998). The programming model concentrates on the effect of earlier experiences on later outcomes.
Critical or “sensitive” periods are those stages of functional development when a regulatory pathway is being constructed or modified and the developing organism is particularly responsive and sensitive to favorable or unfavorable environmental factors. When an early environmental stimulus or insult occurs during a critical or sensitive period, it programs a long-term or permanent change in an organism's functional system (Ben-Shlomo and Kuh 2002; Lucas 1991; Wadsworth 1999). Hormones, antigens, and drugs all can serve as programming agents that deactivate, activate, or alter functional pathways. Programmed long-term adaptations are the result of interactions between genes and the environment in which environmental factors influence and help set the operating parameters of specific genes during critical and sensitive developmental periods (Gottlieb 1996; Sylva 1997; Wadsworth 1999). It is useful to distinguish between a critical and sensitive period, and the qualitative and quantitative effects of exposures during these distinct periods (Ben-Shlomo and Kuh 2002). As opposed to a critical period when a developmental path is determined, a sensitive period of development is a time when a favorable or unfavorable exposure has a stronger effect than it would have at other times.
The work of David Barker (and his colleagues) at the Medical Research Council (MRC), Environmental Epidemiology Unit, in Southampton, England, in the 1980s and 1990s pointed to the importance of early life factors in the programming of risk for chronic disease in adults during critical periods (Barker 1998). Using historical cohort designs, Barker's group analyzed birth weight data and measures of development in the first years of life and found extensive evidence that adult somatic response patterns were programmed in early life. That is, birth weight, placenta size, and weight gain and growth in the first year of life were found to be associated with cardiovascular disease and other chronic illness (e.g., diabetes, hypertension) in the fifth and sixth decades (Barker 1998; Martyn, Barker, and Osmond 1996; Rich-Edwards et al. 1997). While initially viewed with some skepticism (Kuh and Ben-Shlomo 1997; Lucas, Fewtrell, and Cole 1999), Barker's contention regarding the programming effects of fetal and early childhood malnutrition has been supported by recent reviews of physiological pathways that may provide mechanisms for fetal programming (Barker and Osmond 1986; Leon 1998; Lucas 1998; Seckl 1998). These reviews describe how adverse maternal environments (malnutrition; stress; exposure to tobacco, drugs, and alcohol) can modulate placental hormonal function, alter fetal hormonal response patterns, and influence fetal tissue programming of vascular responses, hypothalamic pituitary axis (HPA) activity, insulin-glucose homeostasis, and renal structures (Seckl 1998).
These programming and cumulative models reveal the nature of health development, which we will explore more fully when we consider the LCHD's implications for health policy. Programming models show how and why early experiences matter for later outcomes. Sensitive periods, early learning, induced development, and the programming of somatic systems for decades of function—all have significance for aging and decline. In addition, these potential long-term effects raise important policy questions about the potential value of concentrating resources on improving and optimizing the initial developmental states versus concentrating on reducing risks and promoting positive outcomes for persons already exposed to less optimal initial conditions (Smith 1999; Wadsworth 1999).
The health development trajectory shows how early experiences and exposure to risk factors may affect later health status, including the trajectory of decline (figure 4). The heuristic value of trajectories is supported by a significant body of evidence showing that number, type, timing, and context of risk and protective factors differentially affect outcomes (Boyce et al 1998; Sameroff et al. 1993; Steinberg and Avenevoli 2000) through cumulative and programming mechanisms (Ben-Shlomo and Kuh 2002; Powers and Hertzman 1997). Risk factors lower health development trajectories, and promoting factors help raise trajectories. As figure 4 suggests, health development outcomes can be understood as the product of competing positive and negative influences over time. For example, in a child with asthma, exposure to allergens or infection during periods when the immune system is being programmed, as well as the modifying influence of adaptive or maladaptive parenting, may influence the nature, frequency, and persistence of asthma attacks and long-term lung functioning. Other risk factors, such as additional exposure to allergens and poor pest control or poor access to health care, can diminish the child's functional respiratory trajectory. In contrast, support for breast feeding, management of antigen exposure, behavioral and educational interventions, and appropriate access to good-quality health care all can serve as protective factors that minimize asthma exacerbations and maximize functional outcomes. For both individuals and populations, the number and type of risk factors and protective factors can result in different health trajectories and disparities in health outcomes.
fig. 4.
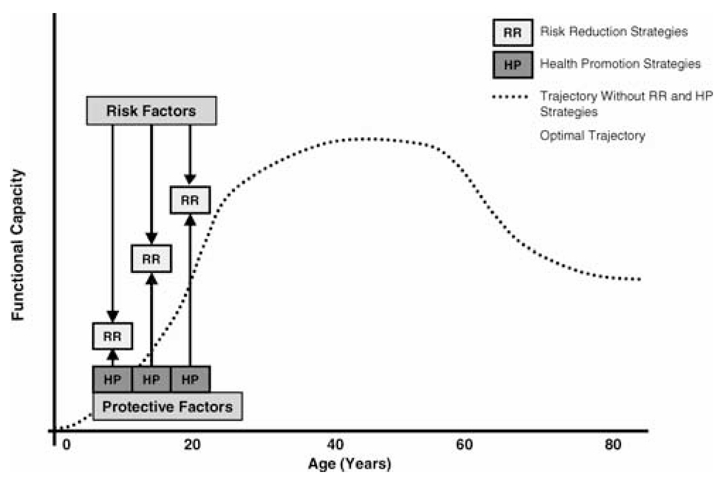
How risk reduction and health promotion strategies influence health development. This figure illustrates how risk reduction strategies can mitigate the influence of risk factors on the developmental trajectory, and how health promotion strategies can simultaneously support and optimize the developmental trajectory. In the absence of effective risk reduction and health promotion, the developmental trajectory will be suboptimal (dotted curve). Source: Halfon, Inkelas, and Hochstein 2000, 455.
The Integration of Multiple Time Frames of Health Development
The contexts, processes, and mechanisms of health development are organized according to biological, behavioral, cultural, and historical time frames, each influencing individuals and populations in
The pattern of critical and/or sensitive periods during the life span;
The transitions and turning points in health development;
The interactions among developmental time scales.
Critical/Sensitive Periods during the Life Span
Even early in the 20th century, children's experiences were believed to determine and predict their adult health and mortality (Bloom 1964; Draper 1924). It was not until the 1980s and early 1990s that research was able to demonstrate how experiences in early childhood led to chronic disease in individuals and populations through specific time-dependent biological and social processes (Davey Smith and Kuh 2001; Gottlieb 1996; Kuh and Ben-Shlomo 1997; Sameroff et al. 1987; Sroufe 1997). During these past two decades, epidemiological studies have found an independent predictive effect for adult disease and disability of prenatal and early childhood factors such as the baby's birth weight, weight gain during infancy, and growth rate and height; the maternal socioeconomic circumstances and attachment to the child; the child's educational attainment; and divorce by the parents and their smoking behavior (Barker 1998; Hertzman 1994; Powers and Hertzman 1997; Rodgers 1994; Wadsworth 1991). Some of these exposures exert their influence over several years, whereas others exert influences only during narrow developmental time frames.
The development of the nervous systems demonstrates the importance of critical periods during which a person must undergo evolutionarily normative experiences and their associated neural activities in order to create an optimal adult pattern of neural connections (Carlson and Earls 1997; Penn and Shatz 1999). For example, studies of the structure and function of the visual area of the cerebral cortex in primates show that the establishment of neural connections and their subsequent pruning depend on the type of visual stimulation provided by the environment (Hubel and Wiesel 1965; Hubel, Wiesel, and LeVay 1977). Likewise, the complexity and rapidity of neural development creates a critical period of neuronal vulnerability that extends from the end of the second trimester of gestation well into the first decade of childhood (Huttenlocher 1990; Huttenlocher and Dabholkar 1997; Penn and Shatz 1999). The difference between identical environmental deficits occurring during critical versus noncritical periods is exemplified by visual impairment and the development of amblyopia in humans. Cataracts in a child who is younger than 18 months that are not promptly treated can lead to the loss of much of his or her vision, whereas cataracts in a person in his or her 60s have no known adverse consequence on cortical organization because of the relative stability of mature neuronal connections.
Brain functioning and basic neuronal connectivity are shaped by experience (Eisenberg 1995, 1999; Penn and Shatz 1999). The trajectory and timing of brain development and the growth of neuronal connectivity are dependent on the interplay between the genetic program and a variety of external events and experiences. Genes determine the basic trajectory of neural connections, and experience-dependent and experience-expectant neuronal activities share the details (Greenough, Black, and Wallace 1987).
Neuronal responses during critical periods are experience expectant. That is, programmed nerve cells take advantage of experience and stimuli that are normally available to a species to guide the development of specific attributes of individuals of that species. In other words, nerve cells are genetically structured to use expected inputs from sensory stimuli to program their developing functions. The visual and auditory systems rely upon the expected presence of the visual and auditory environments in which humans evolved. Experience-dependent plasticity is the brain's ability to learn from its own unique and cumulative experiences and to function and respond to specific environmental cues. For example, the human capacity to learn a language is experience expectant, programmed by exposure to any language, whereas learning English rather than Japanese is experience dependent. These constructs are the synaptic equivalents, respectively, of the programming and the cumulative mechanisms just described.
Age-specific experiences shape the structural and functional attributes of a developing nervous system. Suomi's (1999) studies of cross-fostered monkeys and Meaney's (2001) studies of cross-fostered rats dramatically showed how changing early rearing environments can transform the expression of genetically programmed neurotransmitter production and behavioral outcomes. Although no analogous neuromaturational cellular-level studies have been done in humans, epidemiological studies suggest that brain function in later life can also be influenced by experiences in early life, with differences in educational attainment accounting for a fourfold difference in the risk of the Alzheimer's type of dementia (D'Arcy 1994). The notion of critical and sensitive periods has also increasingly been used to consider the optimal timing of altering a disease trajectory, such as the hypothesized critical period when nonsteroidal anti-inflammatory drugs may influence the risk of Alzheimer's disease (Breitner and Zandi 2001).
The Importance of Transitions and Turning Points in Health Development
A second significant timing-related issue for health development is the series of biological, psychological, and social transitions and turning points that individuals experience during their lives. As noted earlier, developmental plasticity and vulnerability are complementary expressions of the same idea, with episodes of rapid change creating periods of enhanced vulnerability. This relationship implies that developmental trajectories can be altered more readily during sensitive periods of rapid developmental change than during other periods. Each transition represents an important point in development during which adverse and beneficial inputs can have a relatively greater effect on future health. Life transitions, such as starting nursery school, entering middle school, or entering or leaving the workforce, impose stress on adaptive and regulatory systems, requiring the developing individual to adapt to new routines and to adopt new response patterns. In young children, neuroendocrine changes are associated with the development of social competence in a new peer group. Other simultaneous physiological and social developmental transitions may take place during puberty or menopause. In future research, these transition and turning points are likely to provide important clues to the nature of biological and behavioral programming (Boyce et al. 2001). How life's transitions and turning points are managed can lead to different stress response patterns, different levels of allostatic load, and different functional trajectories.
Multiple Time Scales
A third critical feature of human development is the role of multiple time scales and chronological contexts whose shifting relationships are responsible for important variations in health development trajectories. Biological, psychological, cognitive, and social developments occur on different time scales, each with its own developmentally significant transitions and turning points. For example, biological processes are regulated by the organism's “biological clock,” which is genetically programmed and influenced by various physiological feedback mechanisms. The biological clock determines the onset of puberty and the emergence of reproductive capacity for males and females, but it also is influenced by social and cultural changes, as evidenced by the shifting age of puberty onset and the changing age of menarche (Worthman 1999).
Psychological time frames are reflected in the particular stages of psychological development and are influenced by transactions between the neurodevelopment process and social experiences. Freud, Erickson, Piaget, Levenson, and many other developmental psychologists illustrated how these psychological processes may be constructed and how they function. At a cultural level, socially defined stages such as “middle childhood” and “adolescence” and societal expectations based on chronological age and outward appearance represent additional time frames.
A key feature of these different biological, psychological, and cultural time scales is the fact that critical developmental events may occur in varying relationships to one another in the same person. For example, adolescents may enter high school at the same chronological age but function at different levels of emotional maturity and physiological development. The synchronization of these different time scales, which have been shaped by the forces of evolution, permits social, cultural, psychological, and biological processes to interact harmoniously. During periods of rapid historical, cultural, or social changes, these entrained time scales can push and pull on one another, leading to disjointed interactions. For example, adolescents now become reproductively mature at a younger age while at the same time, adolescence is being extended into the early to middle 20s, without the need to marry or enter the workforce at the age of 16 to 20, which was the norm a century ago. For many adolescents, the social pressure for psychosexual autonomy directly clashes with this prolonged dependence on family. Similarly, now that families have historically unprecedented residential mobility, many frail elderly persons have become isolated, uncared for, and subject to depression. These subtle changes in the relationships among different specific developmental time scales may have profound consequences for the adaptive response of those regulatory systems that determine health development (Worthman 1999).
These examples show the importance of timing in health development. The timing of experiences during physiologically sensitive periods, the relationship of health development to externally defined social transitions, and the synchronization of developmental events and transitions are likely to play an important role in health development. They also highlight a critical feature of development: experiences missed at one stage of life may not be as effective if they are provided later, even if they are more intensive. Unlike financial resources, which can be equalized over time through interest payments, developmental inputs are not necessarily fungible. Lost years and missed developmental opportunities often cannot be recaptured or can be recaptured only at an extremely high cost. It appears that many developmental options, choices, and resources are available only during specific developmental windows, with a disproportionate number in the early years of life.
In summary, these four principles—context, process, mechanism, and timing—constitute the central components of the LCHD framework. Health development is shaped by the dynamic and continuous interaction between biology and experiences and is framed by the constantly changing developmental contexts over the lifetime. These nested contexts include child rearing, access to resources, employment and health care, and the psychological environment that mediates behavior and stress responses to the trials and tribulations of daily life. The dynamic interaction between biology and experience also is shaped by biobehavioral pathways that are genetically programmed and adaptively influenced by individuals, families, and social experiences and environments. Differences in the health development trajectories of individuals and populations reflect the cumulative and programmed effects of risk and protective factors on health development.
From Developmental Health to a Policy Framework That Develops Health
The LCHD framework offers a developmentally unified way of interpreting new research findings and identifies research issues that deserve immediate attention. This framework challenges policymakers to rethink the following to improve the nation's health:
How can the health of individuals and populations be better measured?
How should health and its related services be organized and designed?
How should we pay for health care and invest in health?
What should be the underlying principles of a national research agenda for medicine and public health?
Health Measurement Applications
Reconceptualizing Health Measurement
The principles of the LCHD framework offer a radically different conceptualization of individual and population health in the United States. Currently, the health of individuals and populations is measured according to health outcomes—disease, disability, dysfunction, and mortality (Haggerty 1983; Young 1998). The most widely used measures of health are based on deficits, using levels of decline to define health status (Patrick and Erickson 1993). Even relatively integrative measures like the health-related quality of life (HRQL) focus on the extent of declines from a hypothetical state of “full health” (Patrick and Erickson 1993). In contrast, developmental health looks at the potential and unexpressed health of individuals and populations (Hertzman 1999), thereby coming much closer to the definition presented earlier.
U.S. policymakers, health providers, and researchers are currently less able to define and measure subtle variations in how healthy people are and can be (Breslow 1999). A developmental framework for health measurement, however, begins with the premise that even putatively “healthy” (free of disease or measurable decline) persons harbor important hidden differences in health status, as reflected in their health development potential. These differences result in varying levels of resilience that have profound implications for future health status and development in the face of risks and adversity.
According to the LCHD framework, differences in developmental health trajectories are likely to explain much of the variance in the nature and rate of later declines in health. An LCHD-informed approach not only measures an individual's deficits but also calculates his or her health assets, much as investors assess both their assets and liabilities to determine their actual financial position. Measuring positive developmental health assets supports health policies based on building individual and community health assets, a concept already used in the field of community development (McKnight 1999), and encourages the use of health measurement constructs that identify positive health and well-being and not merely disease and deficits (Andrews and Ben-Arieh 1999; Breslow 1999; Lerner and Benson 2002).
Population Health Monitoring
The LCHD framework's new approach to measuring a population's health and presenting its health indicators could be used as part of a national, more developmentally oriented method to reach Healthy People goals and to select, frame, and report on Healthy People indicators (Chrvala and Bulger 1999; Halfon et al. 2000). The LCHD framework could also indicate potential relationships among factors like early literacy at age three (Whitehurst and Lonigan 1998), reading readiness at age five, reading ability at age eight, 11th-grade achievement tests (Phillips, Crouse, and Ralph 1998), and the decline of cognitive abilities in later life (Katzman 1993). Using this approach, a community could
Chart and link community members' cognitive outcomes and trajectories.
Predict the need for respiratory care, by measuring the onset and severity of asthma in children, their access to and quality of care, the community's success in eliminating pests and allergens, and long-term respiratory function in its elders.
Track children and adults longitudinally and use time series data for individuals and populations to guide policies and interventions.
Use various markers in several domains to track and understand related and temporally linked health trajectories.
These steps could better monitor the health of both individuals and populations. Using a more integrated approach, a community could determine its health assets and deficits, create a basis for long-range forecasting, and promote positive health development outcomes for individuals and the community as a whole (Chrvala and Bulger 1999; Halfon et al. 2000).
Individual Health Monitoring
The LCHD framework can also be used to identify biological markers of developmental stress in early childhood that not only identify current exposures but also may be programming the child for enhanced risk in future years. Using the LCHD approach, measurement strategies could become more linked across physiological systems and developmental time frames. For example, to understand the relationship of a person's particular temperament, social competence, and adrenal cortical activity, new measurement strategies could collect data from all these domains and across time (Gunnar et al. 1997). A better understanding of the mediating factors and better measurement strategies could lead to a better definition of the developmental pathways that influence trajectories and the potential of biomarkers to gauge the function and mutability of any given trajectory (Boyce et al. 2001; Dawson, Ashman, and Carver 2000).
The Organization of Health Systems and Interventions for Change
Research evidence indicates that developmental trajectories can be redirected by population-based strategies that transform children's early life experiences. This concept was used in such well-known population-based interventions as the Elmira New York Nurse Home Visitation Study, the Abecedarian Project, and the Perry Pre-School Project (Campbell and Ramey 1995; Olds et al. 1997; Ramey et al. 1992; Weikart, Kamii, and Radin 1994). These studies showed that when children or families at risk receive comprehensive interventions that transform basic contexts and relationships by means of, for example, parenting education and enriched preschool environments, their developmental trajectory can be significantly altered, compared with the trajectories of children who did not receive such interventions (Karoly et al. 1997). Even when changes in IQ were not significant or sustained, these early intervention programs substantially raised noncognitive skills and social attainment (Heckman 1999). Studies have demonstrated that success is possible with the participation of a wide range of community institutions and organizations, including schools and social service agencies (Keating and Hertzman 1999). The United States, Canada, England, and Australia are currently implementing comprehensive strategies to improve child development outcomes based on the results of these and similar intervention studies (Halfon and McLearn 2002).
Designing Integrated Health Interventions
In addition to supporting population-based strategies for organizing health care, the LCHD framework implies a health care system designed to achieve the goal of optimizing developmental health. Whereas the conventional clinical practice is to focus on a relatively few specific causes of poor health outcomes, the LCHD framework suggests that health-promoting interventions may be more effective if organized into integrated health management pathways (Peterson and Kane 1997). For example, the onset and severity of chronic obstructive pulmonary disease (COPD) in adults may be changed by intervening in the childhood or adolescence of individuals with reactive airways (Holt and Sly 2000) and by addressing the biological, emotional, cognitive, and environmental determinants of poor respiratory outcomes. These interventions typically include environmental manipulations to reduce exposure to antigens, more aggressive and responsive clinical interventions to minimize the secondary complications of chronic inflammation, and psychological interventions designed to optimize the adaptive response to a chronic disease (Halfon and Newacheck 2000). Modifying several behavioral risk factors simultaneously (diet, exercise, and stress management) is the strategy of choice for multibehavioral change programs for primary and secondary prevention (Singer and Ryff 2001). Likewise, in the case of insulin-dependent diabetes, multilevel programs for weight control, glucose management, diet control, exercise, and stress management have a synergistic influence when linked together (Wing et al. 1986). Similar multilevel behavioral change strategies have been shown to work for coronary heart disease (Ornish et al. 1998) and may also improve other diseases.
Creating Life Course Health Development Management Systems
The value of an integrated approach to promoting health development can be seen in the growing use of comprehensive approaches to disease management and the initiation of “health management” programs. Health management programs, whose goals are to prevent disease and promote health, could be part of a set of lifelong health management strategies like those now being developed for heart disease, diabetes, and other common chronic and degenerative adult medical conditions (Peterson and Kane 1997; Wagner, Austin, and Von Korff 1996). Recent advertisements by Blue Shield of California illustrate the potential popular appeal of a life course approach (figure 5).
fig. 5.

Blue Shield of California advertising campaign marketing their health insurance products using a life course approach. Printed with permission.
Organization of Developmental Health Services
The predominant organizational form of health services in the United States is managed care. It is epitomized by the health maintenance organization (HMO), which focuses on the vertical integration of services to provide primary, secondary, and tertiary medical care. The goal of a vertical integration of care services is to ensure access to a continuum of medical services that can efficiently maintain health in a reasonably healthy adult in the maintenance phase of life, or the flat part of the developmental trajectory curve.
A truly developmentally focused health service organization would focus instead on improving the health of the entire population and over their entire life course. Because the HMOs' vertical integration strategies would be insufficient, they could be supplemented by longitudinal service integration, reflecting changing life course health development concerns (Halfon, Inkelas, and Hochstein 2000). A longitudinally integrated service delivery system would try to minimize developmental risks and optimize health development trajectories by not only linking health service provisions but also integrating the relationships among all the organizations serving individuals at different stages in their lives. These would replicate at the institutional level the continuity once provided by single providers in small stable communities, providers that might have been familiar with individuals over several decades and across several generations. Such a health development system would be responsible for optimizing its population's health development for an extended time and would reduce the incentives for shifting costs to later stages of life when health care becomes a government responsibility alone.
What we are proposing is more than a reframing of what Alpert, Charney, and Starfield meant by good-quality primary care (Starfield 1998). Although there are important similarities in form and function, we believe that the level of integration proposed within and across a health development system is qualitatively and quantitatively greater than “good primary care.” The type of integration proposed would require changes in the systems that support and maintain the delivery of health services, as well as in the other systems linked to health care, such as early and primary education, care of people with developmental disabilities, and child and family welfare. It will also require policy and programmatic changes at the practice, health care system, community, and policy levels.
For organizations and health systems to become more longitudinally integrated, we will need to develop health information systems, redefine health management pathways, and provide fiscal incentives to organizations to promote long-term health. We have suggested elsewhere that combining vertical, horizontal, and longitudinal integration strategies would permit the construction of a so-called health development organization (HDO), a comprehensive system that would differ from an HMO by its focus on health development and its horizontal linkage of health, education, and social services at the community level (Halfon, Inkelas, and Hochstein 2000).
There are several possible ways of achieving the type of vertical, horizontal, and longitudinal integration contemplated in the HDO.
Medicare and Medicaid could begin to incorporate LCHD principles into their payment methods and regulatory framework. For example, they could pay for the coordination and integration of care over time as part of a health or disease management pathway or pay for specific services that address adaptive mechanisms during critical or sensitive periods. If this approach succeeds in improving outcomes, other purchasers of health care may then have good reason to demand greater accountability for these results through HEDIS (Health Plan Employer Data and Information Set) or other accountability systems.
To reframe and market LCHD principles, the federal government could create a clearinghouse for information about LCHD issues (modeled on the Cochrane Collaboration). This clearinghouse would be responsible for compiling the best available clinical information about LCHD, which might include research on the development and effectiveness of biomarkers for allostatic load or the effectiveness of early family interventions to improve children's behavioral development.
Finally, the federal government could offer incentives, particularly in pediatric care, that favored those services that had demonstrated success in preventive and developmental health, as well as credit insurers and providers that did more to augment the health assets (as measured by the various strategies discussed earlier) of individuals and communities.
Health Care Financing
The growing percentage of the gross domestic product devoted to health care underscores the importance to the national economy of exactly how the United States “produces health” (Evans 1994). The U.S. health system does not spend money in a manner that is likely to optimize the health of the population, both because its current financing policy is largely designed to respond to illness after the fact and because care is paid for as an insured loss. Although the full range of health care financing issues is beyond the scope of this article, as a nation we must decide how developmental health care services are to be financed. It has long been theorized that the optimization of population health requires a greater emphasis on investment (Mushkin 1962). Potentially disproportionate economic and social returns are available from investments during infancy, childhood, and adolescence (Heckman 1999) because early investments increase productivity for decades into the future (Grossman 1972) and because, as the LCHD evidence suggests, such investments can delay and even eliminate future health care costs. Nonetheless, we will not be able neither to solve the fiscal crisis of modern health care nor to provide the appropriate incentives to transform the organization and delivery of health services by simply spending more on preventive services and calling it an investment, especially when policymakers and payers are looking to reduce expenditures. Because of our long-standing and pervasive emphasis on cost-cutting policies, the consideration of new and creative ways to invest in health, not only through prevention, but also through proven long-term health development strategies will be difficult to sell to policymakers.
The U.S. health system has trouble conceiving of health expenditures as an investment but continues to spend most of its money for medical care at the end of life, when the health development trajectory is in relative decline (Cutler and Meara 1999). Although this spending meets essential humanitarian goals, it also ensures that expenditures will remain high and focused on consumption rather than investment, and it guarantees that future health and functional status gains will be minimal and expenditures will continue to be concentrated in the last year of life. Because of how health care has been framed in the United States, routine expenditures are typically based on short-term financing strategies and utilize health insurance products designed to cover episodic care for discrete illnesses or injuries (until recently, Medicaid insurance was on a month-to-month eligibility schedule). Economists classify all medical expenditures as consumption, and payment mechanisms are structured to compensate for “losses” to providers. There is currently no mechanism to record health care expenditures as “investments” whose future value will be measured and whose relative “performance” can be compared with that of alternative investments that can be potentially recouped by the individual or society. Without a fundamental conceptualization of health spending as an investment or the ability to measure investments that are “embodied” in people and to distinguish them from consumption, the currently available finance tools (health insurance) will probably continue to fail to support the optimization of health development.
The LCHD framework suggests the potential benefits of long-term investments in health that begin early in life and continue across the life span. Given the federal government's significant financial interest in delaying and avoiding health care costs and increasing national productivity, government, not the private sector, appears to be the most likely candidate to initiate new LCHD-type financing strategies that increase investment in health capital. Although a prevention- and investment-oriented approach to health expenditure, it would require different kinds, and possibly greater expenditures in the short term. Without a powerful vision for how this might be done, in a way that can fiscally outperform our current health insurance, it will be difficult to convince policymakers and payers that new “investments” are anything more than relabeled “expenditures.”
The only policy wave that is beginning to crest and form the impetus for a new and more proactive approach to health care financing is the emerging genetic revolution in medical care. The large increase in health care costs that are likely to accompany breakthroughs in genomics and its promise for predictive medical care might serve as a new incentive to help us think more boldly and broadly about investment. With functional genomics, and a focus on genetic risk that manifests across decades, greater long-term health care investments will be necessary to modify developmental risks.
Because the value of shifting the emphasis on health spending to childhood seems well established, we should not need to wait 50 years for these early investments to bear fruit in later life (Smith 1999). For example, shifting our spending on coronary heart disease “back” by ten to 20 years by promoting healthier lifestyles or a more aggressive treatment of cardiac risk factors in middle adulthood could demonstrate much of the potential investment value of prevention soon enough to make these expenditures far easier to justify politically and economically than trying to move immediately to prevent CHD by modifying adolescent or young adult behavior or diet—despite the obvious value of exercise and healthy diets throughout life. Similarly, incorporating long-term health investment strategies into medium-term activities designed to forestall adolescent health risks is another strategy that policymakers can use to justify near-term expenditures, based on shorter investment horizons (childhood to adolescence) but at the same time supporting longer-term investment horizons (childhood to retirement age).
The greatest opportunities for investment occur during the first 20 years of life. Several recent analyses of U.S. and Canadian policies show how public expenditures in health and human services, particularly for children, could be better used to maximize the return or investment (Karoly et al. 1997; Keating and Hertzman 1999; McCain and Mustard 1999). Unfortunately, the prevailing pattern of family and social spending on health development does not take full advantage of these investment opportunities. Typically when individuals begin a family, they are just beginning their lifetime earning cycle, and have fewer resources to invest in health (Becker 1993; Brown 1989). A greater public investment in health development is justifiable. However, investing in health as a public expense is further complicated by the fact that managed care organizations (MCOs) and health insurers are more interested in “curing” diseases in adults than in promoting long-term health in children. The result is that even though families, school districts, communities, health insurers, and MCOs all claim to be acting on behalf of the young, the existing pattern of expenditures and investments does not match the front-weighted pattern of human health development.
Implications for Future Research
Much about the mechanisms, determinants, and development of health and illness is not yet understood. Future research should include more integrative longitudinal studies of children that examine life course effects. These studies should begin at or before conception in order to assess the maternal neuroendocrine environment before pregnancy (Wadhwa, Sandman, and Garite 2001). They should collect data during the predictable biological, psychological, and social transitions when humans must adapt to new environments and when interactions between genes and the environment can be measured most easily. What happens during critical and sensitive periods and their biological and psychological effects should be explored further. We need to understand how early programming initiates predisease pathways and how these pathways relate to specific disease states. We also need to understand how developing functional systems interact, react with, and program one another. Such efforts are particularly relevant for unraveling the recently observed increase in childhood asthma, adult onset diabetes, and mental health problems.
As we begin to have a better understanding of the relationship between biological and developmental issues, we should turn to social and contextual issues, such as how family, culture, and other social contexts affect health and how developmental and community assets can promote health and well-being. Because genetic factors account for only part of the variance in developmental outcomes, the value of genomics will depend on our ability to understand and map what Arnold Sameroff termed the “human environtype” (Sameroff and Fiese 2000).
The National Children's Study, planned by a consortium of government agencies and led by the National Institute for Child Health and Human Development, is an important next step. It proposes to track a large cohort of children from as early in life as possible and will measure biological, psychological, social, and cultural influences on health development for the first two decades of their lives. Similar millennium cohort studies are being launched in Australia, the United Kingdom, and Canada and will address many of the new research topics emerging from a LCHD approach.
Only a more comprehensive and integrated research agenda can determine how communities can address medical and social concerns that result in significant health disparities. The challenge for health policy is to identify and act on the relationships between the individual and the environment at those points at which the cost of intervention is lowest and the effectiveness of intervention is highest. All signs point to the need to intervene in the many factors that now maintain the disparities between socioeconomic status and health outcome, including the specific biobehavioral pathways that link social context with health outcomes (Keating and Hertzman 1999). No approach is likely to be as effective as addressing the socioeconomic disparities themselves, however.
Conclusions
The Life Course Health Development (LCHD) framework offers a new approach to health measurement, health system design, and long-term investment in health development and also suggests new directions for research. Perhaps the most important implication of the LCHD framework is the need to treat health development as a long-term investment. By underscoring the investment opportunity of early childhood, the LCHD framework shows how the health of the elderly is connected to the health of the young. Accordingly, rather than simply treating the consequences of decline in old age, promoting health from the beginning of life should improve health and well-being both at midlife and in later years while at the same time reducing the cost of treating degenerative diseases. As the genomics revolution begins, the LCHD model provides a developmentally oriented guide to health investment, health policy, and health research.
Acknowledgments
The authors wish to thank Robert Brook, Arleen Leibowitz, Bernard Guyer, Philip Lee, Leon Eisenberg, Edward Schor, Peter V. Long, Frank Oberklaid, and Richard M. Lerner for their helpful comments on earlier versions of this article and to Phinney Ahn for her invaluable research assistance.
Support for this work was provided by the federal Maternal and Child Health Bureau for the National Center for Infant and Early Childhood Health Policy, grant #5U93MC00099.
References
- Andrews AB, Ben-Arieh A. Measuring and Monitoring Children's Well-Being Across the World. Social Work. 1999;44(2):105–15. doi: 10.1093/sw/44.2.105. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Graf P. Psychological Aspects of Aging: Facts and Frontiers. In: Magnusson D, editor. The Lifespan Development of Individuals: Behavioral, Neurobiological, and Psychosocial Perspectives. Cambridge: Cambridge University Press; 1996. pp. 427–60. [Google Scholar]
- Baltes PB, Lindenberger U, Staudinger UM. Life Span Theory in Development Psychology. In: Lerner RM, Damon W, editors. Handbook of Child Psychology. 5th ed. Vol. 1. New York: Wiley; 1998. pp. 1029–144. Theoretical Models of Human Development. [Google Scholar]
- Barker DJP. Fetal and Infant Origins of Adult Disease. London: Latimer Trend; 1992. [Google Scholar]
- Barker DJP. Mothers, Babies, and Health in Later Life. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- Barker DJP, Osmond C. Infant Mortality, Childhood Nutrition, and Ischaemic Heart Disease in England and Wales. Lancet. 1986;1:1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Becker GS. Human Capital: A Theoretical and Empirical Analysis, with Special Reference to Education. 3d ed. Chicago: University of Chicago Press; 1993. [Google Scholar]
- Ben-Shlomo Y, Kuh D. A Life Course Approach to Chronic Disease Epidemiology: Conceptual Models, Empirical Challenges and Interdisciplinary Perspectives. International Journal of Epidemiology. 2002;31:285–93. [PubMed] [Google Scholar]
- Blaine D. The Life Course, the Social Gradient, and Health. In: Marmot M, Wilkinson RG, editors. Social Determinants of Health. New York: Oxford University Press; 1999. pp. 64–80. [Google Scholar]
- Bloom BS. Stability and Change in Human Characteristics. New York: John Wiley; 1964. [Google Scholar]
- Boyce WT, Alkon A, Tschann JM, Chesney MA, Alpert BS. Dimensions of Psychobiologic Reactivity: Cardiovascular Responses to Laboratory Stressors in Preschool Children. Annals of Behavioral Medicine. 1995;17(4):315–23. doi: 10.1007/BF02888596. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chesney M, Alkon-Leonard A, Tschann JM, Adams S, Chesterman B, Cohen F, Kaiser P. Psychobiologic Reactivity to Stress and Childhood Respiratory Illnesses: Results of Two Prospective Studies. Psychosomatic Medicine. 1995;57:411–22. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Frank E, Jensen PS, Kessler RC, Nelson CA, Steinberg L. Social Context in Developmental Psychopathology: Recommendations for Future Research from the MacArthur Network on Psychopathology and Development. Development and Psychopathology. 1998;10:143–64. doi: 10.1017/s0954579498001552. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ MacArthur Assessment Battery Working Group, and MacArthur Foundation Research Network on Psychopathology and Development. Autonomic Reactivity and Psychopathology in Middle Childhood. British Journal of Psychiatry. 2001;179:144–50. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Breitner JCS, Zandi PP. Do Nonsteroidal Antiinflammatory Drugs Reduce the Risk of Alzheimer's Disease? New England Journal of Medicine. 2001;345:1567–8. doi: 10.1056/NEJM200111223452110. [DOI] [PubMed] [Google Scholar]
- Breslow L. From Disease Prevention to Health Promotion. Journal of the American Medical Association. 1999;281:1030–3. doi: 10.1001/jama.281.11.1030. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, Mass.: Harvard University Press; 1979. [Google Scholar]
- Brown J. Why Do Wages Increase with Tenure? On the Job Training and Life-Cycle Wage Growth Observed within Firms. American Economics Review. 1989;79:971–91. [Google Scholar]
- Brunner E, Marmot M. Social Organization, Stress, and Health. In: Marmot M, Wilkinson RG, editors. Social Determinants of Health. New York: Oxford University Press; 1999. pp. 17–43. [Google Scholar]
- Bury M. Postmodernity and Health. In: Scambler G, Higgs P, editors. Modernity, Medicine and Health: Medical Sociology Towards 2000. London: Routledge; 1998. pp. 1–28. [Google Scholar]
- Cairns RB, Elder GH, Costello EJ. Developmental Science. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Campbell FA, Ramey CT. Cognitive and School Outcomes for High-Risk African American Students at Middle Adolescence: Positive Effects of Early Intervention. American Education Research Journal. 1995;32(4):743–72. [Google Scholar]
- Canadian Government. A New Perspective of Health of Canadians. Ottawa: Department of National Health and Welfare; 1974. Lalonde Report. [Google Scholar]
- Carlson M, Earls F. Psychological and Neuroendocrinological Sequelae of Early Social Deprivation in Institutionalized Children in Romania. Annals of the New York Academy of Science. 1997;807:410–28. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Chapman DA, Scott KG. The Impact of Maternal Intergenerational Risk Factors on Adverse Developmental Outcomes. Developmental Review. 2001;21:305–25. 10.1006/drev.2000.0523. [Google Scholar]
- Chrvala CA, Bulger RJ. Leading Health Indicators for Healthy People 2010: Final Report. Washington, D.C.: Institute of Medicine, National Academy of Sciences; 1999. [PubMed] [Google Scholar]
- Cicchetti D, Aber JL. Contextualism and Developmental Psychopathology. Developmental Psychopathology. 1998;10(2):137–41. doi: 10.1017/s0954579498001540. [DOI] [PubMed] [Google Scholar]
- Coghill SR, Caplan HL, Alexandra H, Robson KM, Kumar R. Impact of Maternal Postnatal Depression on Cognitive Development of Young Children. British Medical Journal. 1986;292:1165–7. doi: 10.1136/bmj.292.6529.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DM, Meara E. The Concentration of Medical Spending: An Update. 1999. National Bureau of Economic Research Working Paper Series, no. 7279.
- D'Arcy C. Education and Socioeconomic Status as Risk Factors for Dementia: Data from the Canadian Study of Health and Aging. Neurobiology of Aging. 1994;14:S40. [Google Scholar]
- Davey Smith G, Kuh D. William Ogilvy Kermack and the Childhood Origins of Adult Health and Disease. International Journal of Epidemiology. 2001;30:696–703. doi: 10.1093/ije/30.4.696. [DOI] [PubMed] [Google Scholar]
- Dawson G, Ashman SB, Carver LJ. The Role of Early Experience in Shaping Behavioral and Brain Development and Its Implications for Social Policy. Development and Psychopathology. 2000;12(4):695–712. doi: 10.1017/s0954579400004089. [DOI] [PubMed] [Google Scholar]
- Dawson G, Hessel D, Frey K. Social Influences on Early Developing Biological and Behavioral Systems Related to Risk for Affective Disorder. Development and Psychopathology. 1994;6:759–79. [Google Scholar]
- DeStavola BL, Hardy R, Kuh D, Dos Santos Silva I, Wadsworth MEJ, Swerdlow AJ. Birth Weight, Childhood Growth and Risk of Breast Cancer in a British Cohort. British Journal of Cancer. 2000;83:964–8. doi: 10.1054/bjoc.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper G. Human Constitution: A Consideration of Its Relationship to Disease. Philadelphia: Saunders; 1924. [Google Scholar]
- Durch JS, Bailey LA, Stoto MA. Committee on Using Performance Monitoring to Improve Community Health. Improving Health in the Community: A Role for Performance Monitoring. Washington, D.C.: National Academy Press; 1997. [Google Scholar]
- Eisenberg L. The Social Construction of the Human Brain. American Journal of Psychiatry. 1995;152(11):1563–75. doi: 10.1176/ajp.152.11.1563b. [DOI] [PubMed] [Google Scholar]
- Eisenberg L. Experience, Brain, and Behavior: The Importance of a Head Start. Pediatrics. 1999;103:1031–5. doi: 10.1542/peds.103.5.1031. [DOI] [PubMed] [Google Scholar]
- Elder GH. Children of the Great Depression: Social Change in Life Experience. Chicago: University of Chicago Press; 1974. [Google Scholar]
- Elder GH., Jr. The Life Course and Human Development. In: Lerner RM, Damon W, editors. Handbook of Child Psychology. 5th ed. Vol. 1. New York: Wiley; 1998a. pp. 939–92. Theoretical Models of Human Development. [Google Scholar]
- Elder GH., Jr. The Life Course as Developmental Theory. Child Development. 1998b;69(1):1–12. [PubMed] [Google Scholar]
- Elder GH, Jr., Liker JK, Cross CE. Parent-Child Behavior in the Great Depression: Life Course and Intergenerational Influences. In: Baltes PB, Brin OG Jr., editors. Life-Span Development and Behavior. Vol. 6. New York: Academic Press; 1984. pp. 109–58. [Google Scholar]
- Elder GH, Jr., Van Nguyen T, Caspi A. Linking Family Hardship to Children's Lives. Child Development. 1985;56:361–75. [PubMed] [Google Scholar]
- Elford J, Whincup p, Shaper AG. Early Life Experience and Adult Cardiovascular Disease: Longitudinal and Case-Control Studies. International Journal of Epidemiology. 1991;129:919–33. doi: 10.1093/ije/20.4.833. [DOI] [PubMed] [Google Scholar]
- Engle G. The Need for a New Medical Model: A Challenge for Biomedicine. Science. 1977;197(4286):129–96. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- Epstein FH. Cardiovascular Disease Epidemiology. A Journey from the Past into the Future. Circulation. 1996;93:1755–64. doi: 10.1161/01.cir.93.9.1755. [DOI] [PubMed] [Google Scholar]
- Evans RG. Health Care as a Threat to Health: Defense, Opulence, and the Social Environment. Daedalus. 1994;123(4):21–42. [Google Scholar]
- Evans RG, Stoddart GL. Producing Health, Consuming Health Care. Social Science and Medicine. 1990;31(12):1347–63. doi: 10.1016/0277-9536(90)90074-3. [DOI] [PubMed] [Google Scholar]
- Featherstone M, Hepworth M. Ageing, the Lifecourse, and the Sociology of Embodiment. In: Scambler G, Higgs P, editors. Modernity, Medicine and Health: Medical Sociology Towards 2000. London: Routledge; 1998. pp. 147–75. [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Apitz AM, Edwards V, Koss MP, Marks JS. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. American Journal of Preventive Medicine. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Tucker JS, Tomlinson-Keasey C, Schwartz JE, Wingard DC, Criqui MH. Does Childhood Personality Predict Longevity? Journal of Personality and Social Psychology. 1993;65(1):176–85. doi: 10.1037//0022-3514.65.1.176. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Developmental Psychobiological Theory. In: Cairns RB, Elder GH, Costello EJ, editors. Developmental Science. Cambridge: Cambridge University Press; 1996. pp. 63–77. [Google Scholar]
- Graber JA, Brooks-Gunn J. Transitions and Turning Points: Navigating the Passage from Childhood through Adolescence. Developmental Psychology. 1996;32(4):768–76. [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and Brain Development. Child Development. 1987;50(3):539–59. [PubMed] [Google Scholar]
- Grossman M. On the Concept of Health Capital and the Demand for Health. Journal of Political Economy. 1972;80(2):223–55. 10.1086/259880. [Google Scholar]
- Gunnar MR. Quality of Early Care and Buffering of Neuroendocrine Stress Reactions: Potential Effects on the Developing Human Brain. Preventive Medicine. 1998;27:208–11. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, Social Competence, and Adrenocortical Activity in Preschoolers. Development and Psychobiology. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Haan N, Millsap R, Hartka E. As Time Goes By: Change and Stability in Personality over Fifty Years. Psychology and Aging. 1986;1:220–32. doi: 10.1037//0882-7974.1.3.220. [DOI] [PubMed] [Google Scholar]
- Haggerty RJ. Epidemiology of Childhood Disease. In: Mechanic D, editor. Handbook of Health, Health Care, and the Health Professions. New York: Free Press; 1983. pp. 101–19. [Google Scholar]
- Halfon N, Ebener P, Sastry N, Wyn R, Ahn PL, Hernandez J, Wong D. California Health Report. Santa Monica, Calif.: RAND Corporation; 2000. DRU-1592-TCWF. [Google Scholar]
- Halfon N, Inkelas M, Hochstein M. The Health Development Organization: An Organizational Approach to Achieving Child Health Development. Milbank Quarterly. 2000;78(3):447–97. doi: 10.1111/1468-0009.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon N, McLearn KT. Families with Children under 3: What We Know and Implications for Results and Policy. In: Halfon N, McLearn KT, Schuster MA, editors. Child Rearing in America: Conditions of Families with Young Children. Cambridge: Cambridge University Press; 2002. pp. 376–412. [Google Scholar]
- Halfon N, Newacheck PW. Characterizing the Social Impact of Asthma in Childhood. In: Weiss K, Buist AS, Sullivan SD, editors. Socioeconomic Impact of Asthma. New York: Marcel Dekker; 2000. pp. 23–54. [Google Scholar]
- Hardy R, Kuh D. Reproductive Characteristics and the Age at Inception of the Perimenopause in a British National Cohort. American Journal of Epidemiology. 1999;149(7):612–20. doi: 10.1093/oxfordjournals.aje.a009861. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Altered Neuroendocrine Activity in Maltreated Children Related to Symptoms of Depression. Development and Psychopathology. 1996;8:201–14. [Google Scholar]
- Heckman JJ. Policies to Foster Human Capital. National Bureau of Economic Research. Working Paper no. 7288. 1999. [accessed May 17, 2002]. Available at http://www.nber.org/papers/w7288.
- Hertzman C. The Lifelong Impact of Childhood Experiences: A Population Health Perspective. Daedalus. 1994;123(4):167–80. [Google Scholar]
- Hertzman C. Population Health and Human Experiences. In: Keating DP, Hertzman C, editors. Developmental Health and the Wealth of Nations: Social, Biological and Educational Dynamics. New York: Guilford Press; 1999. pp. 21–40. [Google Scholar]
- Hertzman C. The Case for an Early Childhood Developmental Strategy. Isuma. 2000;1(2):11–18. [Google Scholar]
- Hertzman C, Power C, Matthews S, Manor O. Using an Interactive Framework of Society and Lifecourse to Explain Self-Rated Health in Early Adulthood. Social Science and Medicine. 2001;53:1575, 85. doi: 10.1016/s0277-9536(00)00437-8. 10.1016/S0277-9536(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Holt PG, Sly PD. Allergic Respiratory Disease: Strategic Targets for Primary Prevention during Childhood. Thorax. 1997;52:1–4. doi: 10.1136/thx.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Sly PD. Prevention of Adult Asthma by Early Intervention during Childhood: Potential Value of New Generation Immunomodulatory Drugs. Thorax. 2000;55:700–3. doi: 10.1136/thorax.55.8.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Sly PD, Bjorksten B. Atopic versus Infectious Diseases in Childhood: A Question of Balance? Pediatric Allergy Immunology. 1997;8:53–8. doi: 10.1111/j.1399-3038.1997.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Binocular Interaction in Striate Cortex of Kittens Reared with Artificial Squint. Journal of Neurophysiology. 1965;28:1041–59. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of Ocular Dominance Columns in Monkey Striate Cortex. Philosophical Transactions of the Royal Society London–Biological Science. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric Study of Human Cerebral Cortex Development. Neuropsychologia. 1990;28:517–27. doi: 10.1016/0028-3932(90)90031-i. 10.1016/0028-3932(90)90031-I. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional Differences in Synaptogenesis in Human Cerebral Cortex. Journal of Comparative Neurology. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Health and Behavior: The Interplay of Biological, Behavioral, and Societal Influences. Washington, D.C.: National Academy Press; 2001. [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child Development Risk Factors for Adult Schizophrenia in the British 1946 Birth Cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kandel ER. A New Intellectual Framework for Psychiatry. American Journal of Psychiatry. 1998;155(4):457–69. doi: 10.1176/ajp.155.4.457. [DOI] [PubMed] [Google Scholar]
- Karoly L, Greenwood PW, Everingham SS, Hoube J, Kilburn MR, Rydell CP, Sanders M, Chiesa J. Investing in Our Children: What We Know and Don't Know about the Costs and Benefits of Early Childhood Interventions. Santa Monica, Calif.: RAND Corporation; 1997. [Google Scholar]
- Katzman R. Education and the Prevalence of Dementia and Alzheimer's Disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- Keating DP, Hertzman C. Developmental Health and the Wealth of Nations: Social, Biological and Educational Dynamics. New York: Guilford Press; 1999. [Google Scholar]
- Kerstjens H, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by Age and Smoking Status: Facts, Figures, and Fallacies. Thorax. 1997;52:820–7. doi: 10.1136/thx.52.9.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig DA. Purchasing Population Health: Paying for Results. Ann Arbor: University of Michigan Press; 1997. [Google Scholar]
- Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DYM, Mrazek DA. Onset and Persistence of Childhood Asthma: Predictors from Infancy. Pediatrics. 2001;108 doi: 10.1542/peds.108.4.e69. e69. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Menotti A, Blackburn H. The Seven Countries Study: A Scientific Adventure in Cardiovascular Epidemiology. Utrecht: Brouwer Offset; 1994. [Google Scholar]
- Kuh DL, Ben-Shlomo Y. A Life Course Approach to Chronic Disease Epidemiology. New York: Oxford University Press; 1997. [Google Scholar]
- Kuh DL, Davey Smith G. A Life Course and Adult Chronic Disease: An Historical Perspective with Particular Reference to Coronary Heart Disease. In: Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease Epidemiology. New York: Oxford University Press; 1997. pp. 15–44. [Google Scholar]
- Kuh DL, Wadsworth MEJ, Hardy R. Women's Health in Midlife: The Influence of the Menopause, Social Factors and Health in Earlier Life. British Journal of Obstetrics and Gynecology. 1997;107:923–33. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- Lamberts SWJ, Beld AW, Lely A. The Endocrinology of Aging. Science. 1997;278:419–24. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- Leon DA. Fetal Growth and Adult Disease. European Journal of Clinical Medicine. 1998;52(S1):S72-–S82. [PubMed] [Google Scholar]
- Lerner RM. Dialectics, Developmental Contextualism, and the Further Enhancement of Theory about Puberty and Psychosocial Development. Journal of Early Adolescence. 1992;12:366–88. [Google Scholar]
- Lerner RM, Benson PL. Developmental Assets and Asset-Building Communities: Implications for Research, Policy, and Practice. Boston: Kluwer Academic Publishers; 2002. In press. [Google Scholar]
- Leung D. Immunologic Basis of Chronic Allergic Diseases: Clinical Messages from the Laboratory Bench. Pediatric Research. 1997;45(5):559–68. doi: 10.1203/00006450-199711000-00001. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lucas A. Programming by Early Nutrition in Man. In: Bock BR, Whelan J, editors. Ciba Foundation Symposium: The Childhood Environment and Adult Disease. New York: Wiley; 1991. pp. 56–76. [Google Scholar]
- Lucas A. Programming by Early Nutrition: An Experimental Approach. Journal of Nutrition. 1998;128:401S–6S. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- Lucas A, Fewtrell MS, Cole TJ. Fetal Origins of Adult Disease: The Hypothesis Revisited. British Medical Journal. 1999;316:245–9. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson D, Cairns RB. Developmental Science: Toward a Unified Framework. In: Cairns RB, Elder GH, Costello EJ, editors. Developmental Science. Cambridge: Cambridge University Press; 1996. pp. 7–30. [Google Scholar]
- Mann SL, Wadsworth MEJ, Colley JRT. Accumulation of Factors Influencing Respiratory Illness in Members of a National Birth Cohort and Their Offspring. Journal of Epidemiology and Community Health. 1992;46:286–92. doi: 10.1136/jech.46.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FD, Holt PG. Role of Microbial Burden on Aetiology of Allergy and Asthma. Lancet. 1999;354(supp. 2):12–15. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- Martyn CN, Barker DJP, Osmond C. Mothers' Pelvic Size, Fetal Growth, and Coronary Heart Disease in Men in the UK. Lancet. 1996;348:1264–8. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- McCain MN, Mustard JF. Early Years Study. Reversing the Real Brain Drain: Final Report. Toronto: Ontario Children's Secretariat; [Google Scholar]
- McEwen BS. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the Individual: Mechanisms Leading to Disease. Archives of Internal Medicine. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- McKnight JL. Two Tools for Well-Being: Health Systems and Communities. Journal of Perinatology. 1999;19:S12–S15. doi: 10.1038/sj.jp.7200235. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal Care, Gene Expression, and the Transmission of Individual Differences in Stress Reactivity across Generations. Annual Reviews in Neuroscience. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Van Berkel C. Effect of Neonatal Handling on Age-Related Impairments Associated with the Hippocampus. Science. 1988;239:766–8. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Moen P, Wethington E. Midlife Development in a Life Course Context. In: Willis SL, Reid JD, editors. Life in the Middle: Psychological and Social Development in Middle Age. New York: Academic Press; 1999. pp. 3–24. [Google Scholar]
- Montgomery SM, Bartley MJ, Wilkinson RG. Family Conflict and Slow Growth. Archives of Diseases in Childhood. 1997;77:326–30. doi: 10.1136/adc.77.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushkin SJ. Health as an Investment. Journal of Political Economy. 1962;70(2):129–57. 10.1086/258730. [Google Scholar]
- Nordio S. Needs in Child and Maternal Care: Rational Utilization and Social-Medical Resources. Rivista Italiana di Pediatria. 1978;4:3–20. [Google Scholar]
- Olds DL, Eckenrode J, Henderson CR, Klitzman H, Powers J, Cole R, Sidora K, Morris P, Pettitt LM, Luckey D. Long-Term Effects of Home Visitation on Maternal Life Course, Child Abuse and Neglect, and Children's Arrests: Fifteen Year Follow-up of a Randomized Trial. Journal of the American Medical Association. 1997;278(8):637–43. [PubMed] [Google Scholar]
- Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C, Brand RJ. Intensive Lifestyle Changes for Reversal of Coronary Heart Disease. Journal of the American Medical Association. 1998;280(23):2001–7. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Erickson P. Health Status and Health Policy: Quality of Life in Health Care Evaluation and Resource Allocation. New York: Oxford University Press; [Google Scholar]
- Penn AA, Shatz CJ. Brain Waves and Brain Wiring: The Role of Endogenous and Sensory-Driven Neural Activity in Development. Pediatric Research. 1999;45(4):447–58. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- Peterson KW, Kane DP. Beyond Disease Management: Population-Based Health Management. In: Todd WE, Nash D, editors. Disease Management: A Systems Approach to Improving Patient Outcomes. Chicago: American Hospital Publishing; 1997. pp. 305–45. [Google Scholar]
- Phillips M, Crouse J, Ralph J. Does the Black-White Test Score Gap Widen after Children Enter School? In: Jencks C, Philips M, editors. The Black-White Test Score Gap. Washington, D.C.: Brookings Institution Press; 1998. pp. 229–72. [Google Scholar]
- Pless IBP, Cripps HA, Davies JMC, Wadsworth MEJ. Chronic Physical Illness in Childhood and Psychological and Social Circumstances in Adolescence and Early Adult Life. Developmental Medicine and Child Neurology. 1989;31:746–55. doi: 10.1111/j.1469-8749.1989.tb04070.x. [DOI] [PubMed] [Google Scholar]
- Powers C, Hertzman C. Social and Biological Pathways Linking Early Life and Adult Disease. British Medical Bulletin. 1997;53(1):210–21. doi: 10.1093/oxfordjournals.bmb.a011601. [DOI] [PubMed] [Google Scholar]
- Ramey CT, Bryant DM, Wasik BH, Sparling JJ, Fendt KH, LaVange LM. Infant Health and Developmental Program for Low Birth Weight, Premature Infants: Program Elements, Family Participation and Child Intelligence. Pediatrics. 1992;89:454–65. [PubMed] [Google Scholar]
- Ramey SL, Ramey CT. Early Childhood Experiences and Developmental Competence. In: Danziger S, Waldfogel J, editors. Securing the Future: Investing in Children from Birth to College. New York: Russell Sage Foundation; 2000. pp. 122–50. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky Families: Family Social Environments and the Mental and Physical Health of Offspring. Psychological Bulletin. 2002;128:330–66. [PubMed] [Google Scholar]
- Richards M, Kuh D, Hardy R, Wadsworth MEJ. Lifetime Cognitive Function and Timing of the Natural Menopause. Neurology. 1999;53:308–14. doi: 10.1212/wnl.53.2.308. [DOI] [PubMed] [Google Scholar]
- Richards M, Hardy R, Kuh D, Wadsworth MEJ. Birth Weight and Cognitive Function in the British 1946 Birth Cohort: Longitudinal Population-Based Study. British Medical Journal. 2001;322:199–203. doi: 10.1136/bmj.322.7280.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JS, Stamfer MJ, Manson JE, Rosner B, Hankinson SE, Golditz GA, Willett WC, Hennekens CH. Birth Weight and Risk of Cardiovascular Disease in a Cohort of Women Followed-up since 1976. British Medical Journal. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers B. Pathways between Parental Divorce and Adult Depression. Journal of Child Psychology and Psychiatry. 1994;35:1289–1308. doi: 10.1111/j.1469-7610.1994.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Fiese BH. Transactional Regulation: The Developmental Ecology of Early Intervention. In: Shonkoff JP, Meisels SJ, editors. Handbook of Early Childhood Intervention. 2d ed. Cambridge: Cambridge University Press; 2000. pp. 135–59. [Google Scholar]
- Sameroff AJ, Seifer R, Baldwin A, Baldwin C. Stability of Intelligence from Preschool to Adolescence: The Influence of Social and Family Risk Factors. Child Development. 1993;64:80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Barocas R, Zax M, Greenspan S. Intelligence Quotient Scores of 4-Year-Old Children: Social Environment Risk Factors. Pediatrics. 1987;79:343–50. [PubMed] [Google Scholar]
- Sampson RJ, Laub JH. Crime in the Making: Pathways and Turning Points through Life. Cambridge, Mass.: Harvard University Press; 1993. [Google Scholar]
- Sampson RJ, Laub JH. Socioeconomic Achievement in the Life Course of Disadvantaged Men: Military Service as a Turning Point, circa 1940–1965. American Sociological Review. 1996;61:347–67. [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and Violent Crime: A Multilevel Study of Collective Efficacy. Science. 1997;277:918–24. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Friedman HS, Tucker JS, Tomlinson-Keasey C, Wingard DL, Criqui MH. Sociodemographic and Psychosocial Factors in Childhood as Predictors of Adult Mortality. American Journal of Public Health. 1995;85:1237–45. doi: 10.2105/ajph.85.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR. Physiologic Programming of the Fetus. Emerging Concepts in Perinatal Endocrinology. 1998;25(4):939–62. [PubMed] [Google Scholar]
- Seeman TE, Singer B, Rowe JW, Horowitz R, McEwen BS. The Price of Adaptation—Allostatic Load and Its Health Consequences: MacArthur Studies of Successful Aging. Archives of Internal Medicine. 1997;157:2259–68. [PubMed] [Google Scholar]
- Singer BH, Ryff CD. New Horizons in Health: An Integrative Approach. Washington, D.C.: National Academy Press; 2001. [PubMed] [Google Scholar]
- Smith J. Healthy Bodies and Thick Wallets. The Dual Relation between Health and Economic Status. Journal of Economic Perspectives. 1999;13(2):145–66. [PMC free article] [PubMed] [Google Scholar]
- Sroufe L A. Psychopathology as an Outcome of Development. Development and Psychopathology. 1997;9(2):251–68. doi: 10.1017/s0954579497002046. [DOI] [PubMed] [Google Scholar]
- Starfield B. Primary Care: Balancing Health Needs, Services, and Technology. New York: Oxford University Press; 1998. [Google Scholar]
- Steinberg L, Avenevoli S. The Role of Context in the Development of Psychopathology: A Conceptual Framework and Some Speculative Propositions. Child Development. 2000;71(1):66–74. doi: 10.1111/1467-8624.00119. [DOI] [PubMed] [Google Scholar]
- Strachan DP. Respiratory and Allergic Diseases. In: Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease Epidemiology. New York: Oxford University Press; 1997. pp. 101–20. [Google Scholar]
- Suomi SJ. Developmental Trajectories, Early Experiences, and Community Consequences: Lessons from Studies with Rhesus Monkeys. In: Keating DP, Hertzman C, editors. Developmental Health and the Wealth of Nations. New York: Guilford Press; 1999. pp. 185–200. [Google Scholar]
- Sylva K. Critical Periods in Childhood Learning. British Medical Bulletin. 1997;53(1):185–97. doi: 10.1093/oxfordjournals.bmb.a011599. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Garite TJ. The Neurobiology of Stress in Human Pregnancy: The Implications for Prematurity and Development of the Fetal Central Nervous System. Progress in Brain Research. 2001;133:131–42. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- Wadsworth MEJ. The Imprint of Time: Childhood, History and Adult Life. New York: Oxford University Press; 1991. [Google Scholar]
- Wadsworth MEJ. Early Life. In: Marmot M, Wilkinson RG, editors. Social Determinants of Health. New York: Oxford University Press; 1999. pp. 44–63. [Google Scholar]
- Wadsworth MEJ, Cripps HA, Midwinter RA, Colley JRT. Blood Pressure at Age 36 Years and Social and Familial Factors, Cigarette Smoking and Body Mass in a National Birth Cohort. British Medical Journal. 1985;291:1534–8. doi: 10.1136/bmj.291.6508.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EH, Austin BT, Von Korff M. Organizing Care for Patients with Chronic Illness. Milbank Quarterly. 1996;74(4):511–44. [PubMed] [Google Scholar]
- Weikart DP, Kamii CK, Radin N. Perry Preschool Progress Report. Ypsilanti, Mich.: Ypsilanti Public Schools; 1994. [Google Scholar]
- Weiss ST. Early Life Predictors of Adult Chronic Obstructive Lung Disease. European Respiratory Review. 1995;31(5):303–9. [Google Scholar]
- Whitehurst GJ, Lonigan CJ. Child Development and Emergent Literacy. Child Development. 1998;69(3):848–72. [PubMed] [Google Scholar]
- Wing RR, Epstein LH, Norwalk MP, Lamparski DM. Behavioral Self Regulation in the Treatment of Patients with Diabetes Mellitus. Psychological Bulletin. 1986;99:78–89. [PubMed] [Google Scholar]
- Worthman CM. Epidemiology of Human Development. In: Panter-Brick C, Worthman CM, editors. Hormones, Health, and Behavior: A Socio-Ecological and Lifespan Perspective. Cambridge: Cambridge University Press; 1999. pp. 47–104. [Google Scholar]
- Young TK. Population Health: Concepts and Methods. New York: Oxford University Press; 1998. [Google Scholar]


