Although demography continues to be the most prominent discipline concerned with population dynamics, involvement of other disciplines is highly desirable. The case for a multidisciplinary approach to population theory has been aptly stated by Kurt Mayer: “Any meaningful interpretation of the cause and effects of population changes must … extend beyond formal statistical measurement of the components of change, i.e. fertility, mortality and migration, and draw on the theoretical framework of several other disciplines for assistance (Mayer 1962).” In noting that the “analysis of the causal determinants and consequences of population change forms the subject matter of population theory,” Mayer inferentially acknowledges the epidemiologic character of population phenomena, for as its etymology indicates, (epi, upon; demos, people; logos, study), epidemiology is the study of what “comes upon” groups of people. More specifically, epidemiology is concerned with the distribution of disease and death, and with their determinants and consequences in population groups. Inasmuch as patterns of health and disease are integral components of population change, epidemiology's reservoir of knowledge about these patterns and their determinants in population groups serves not only as a basis for prediction of population change but also as a source of hypotheses that can be further tested to correct, refine and build population theory. Furthermore, many epidemiologic techniques that have heretofore been limited to the examination of health and disease patterns can be profitably applied as well to the exploration of other mass phenomena, such as fertility control.
A theory of epidemiologic transition, sensitive to the formulations of population theorists who have stressed the demographic, biologic, sociologic, economic and psychologic ramifications of transitional processes, was conceived by this author less than four years ago. Recognition of the limitations of demographic transition theory and of the need for comprehensive approaches to population dynamics stimulated the development of this theory (Van Nort and Karon 1955; Micklin 1968).
Focus of the Theory of Epidemiologic Transition
Conceptually, the theory of epidemiologic transition focuses on the complex change in patterns of health and disease and on the interactions between these patterns and their demographic, economic and sociologic determinants and consequences. An epidemiologic transition has paralleled the demographic and technologic transitions in the now developed countries of the world and is still underway in less-developed societies. Ample evidence may be cited to document this transition in which degenerative and man-made diseases displace pandemics of infection as the primary causes of morbidity and mortality.
The major precepts of the theory of epidemiologic transition are presented below. Smoothed data from the United Nations Model Life Tables (United Nations Department of Social Affairs, 1955), representing a “pooled” cross-cultural view of mortality patterns at various life expectancy levels, provide a useful introduction to the basic propositions.1 The longitudinal view added by historical and contemporary data from several countries provides further documentation; data from individual countries also serve to illustrate some of the peculiar variations of the transition and to support three models that differentiate distinctive patterns of the epidemiologic transition. These models are the classical or western model, as represented here by England and Wales and Sweden; the accelerated transition model, as represented by Japan; and the contemporary or delayed model as represented by Chile and Ceylon.
What begins as an apparently academic exercise, attempting to describe and disentangle the determinants and consequences of changing disease patterns that have accompanied modernization in most western countries, is aimed as well at shedding light on the tenacious population problems of less-developed countries. For example, policy makers tend to see high fertility as the intractable villain, creating acute population pressures and oppressive socioeconomic conditions in developing societies; consequently, programs to treat these onerous problems have been geared almost exclusively to birth control. One of the major practical implications stemming from historical studies of the epidemiologic transition in western countries is that disease control programs may be not only a prerequisite of fertility transition but an effective instrument of socioeconomic development as well.
Mortality and Population Dynamics
Proposition One
The theory of epidemiologic transition begins with the major premise that mortality is a fundamental factor in population dynamics. The clearest indication of mortality's dominant role in population dynamics is implicit in theories of population cycles. The cyclic rises and falls in population size that have been observed in animal and pre-modern human populations reflect sequential phases of population growth and decline; disregarding the possible selective influences of migration, these cyclic movements must ultimately be accounted for in terms of the range of variation in fertility and mortality.
Although the absence of continuous information on the actual levels of fertility and mortality in pre-modern societies precludes deterministic statements about their relative demographic impact, an assessment of the possible range of variations in fertility and mortality does allow probabilistic conclusions. Obviously, the range for fertility is framed by a biologic maximum and a realistic minimum shaped by fecundability, by female survival chances during fertile ages and by marriage and contraceptive practices. Because of the low motivation to limit births and the comparatively ineffective contraceptive methods available in pre-modern societies, the broadest range for fertility was probably about 30 to 50 births per 1,000 population. In contrast, the range for mortality was much greater as there was virtually no fixed upper limit to the death rate. Although 30 deaths per 1,000 population may be a reasonable approximation of mortality's lower asymptote, its upper asymptote in pre-modern societies could have been many times as high in epidemic and famine years. Consequently, even if fertility approached its biologic maximum, depopulation could and did occur as a result of epidemics, wars and famines, which repeatedly pushed mortality levels to high peaks.
The scanty evidence that is available indicates that frequent and violent fluctuations characterized the mortality patterns of pre-modern societies and that the mortality level was extremely high even in the so-called good years. Caught between the towering peaks of mortality from epidemics and other disasters and the high plateaus of mortality dictated by chronic malnutrition and endemic diseases, life expectancy was short and human misery was assured. Several authors have suggested low yet fluctuating life expectancies on the order of 18 for ancient Greece (Angel and Pearson 1953), 22 for Rome, 17 and 35 for medieval Britain (Russell 1958), and 22, 26 and 34 in the sixteenth, seventeenth and eighteenth centuries, respectively, for Geneva (Landis and Hatt 1954). With such short life expectancy at birth, populations were typically young, and population growth was cyclic with only small net increments over long periods of time. Thus, more than any other single factor, fluctuating but always high mortality offers the most likely explanation of the slow rate of world population growth until 1650 A.D.
In the modern period after 1650 the growth curve of world population departed from the cyclic pattern and assumed an exponential form. However, mortality continued to be of overwhelming importance in determining population movements before the Industrial Revolution in the west, as is indicated by a number of studies (Chambers; Eversley 1957; Utterstrom 1965; Vielrose 1965). Vital statistics gleaned from several parish register studies show that both fertility and mortality were extremely variable and moderately high and that the range of variation in mortality was significantly greater than in fertility in the early part of the modern era, as shown in Table 1. No secular downward trend in mortality is apparent in any country before the middle of the eighteenth century, about the same time that population growth began to demonstrate an exponential curve. The initial period of sustained population growth in nearly every country for which reliable data are available corresponds with at least two decisive changes in the death rate. First the fluctuations in mortality became less frequent and less drastic. Second, the initial, slow—sometimes imperceptible—decline in mortality gradually gained momentum and eventually stabilized at relatively low levels in the twentieth century. Thus steady rises in life expectancy, progressively diminishing death rates and more stable and predictable mortality patterns have accompanied the persistent increments in world population.
TABLE 1.
Birth and Death Rates from the Late Seventeenth through the Eighteenth Century in Sweden and England
| Range of Variation |
|||
|---|---|---|---|
| Area | Period | Birth Rate | Death Rate |
| Narke, central Sweden | 1691–1750 | 28.6–38.1 | 17.1–41.7 |
| Sweden | 1721–1800 | ||
| five-year average | 31.3–37.1 | 21.2–32.9 | |
| annual | 28.7–38.7 | 18.4–52.4 | |
| Worcestershire, England | 1665–1780 | 34.0–47.5 | 26.9–51.6 |
| Nottingham, England | 1700–1795 | 31.6–46.3 | 31.2–48.3 |
Sources: Utterstrom, G. 1965. Two Essays on Population in Eighteenth Century Scandinavia. In Population in History, edited by D.V. Glass and D.E.C. Eversley, pp. 523–48. Chicago: Aldine Publishing Company; Eversley D.E.C. 1957. A Survey of Population in an Area of Worcestershire from 1660 to 1850 on the Basis of Parish Registers. Population Studies 10, 253–79; Chambers, J.D. Three Essays on the Population and Economy of the Midlands, in Glass and Eversley, op. cit., pp. 308–53; Vielrose E., 1965. Elements of the Natural Movement of Populations. Oxford: Pergamon Press, Inc.
The strong association between the level and range of fluctuation in the death rate and the pace of population growth is shown in Figure 1, which is based on annual vital rates for Sweden. As long as perennial epidemics, plagues, famines and wars acted unpredictably and virtually uncontrollably to produce recurring high peaks of mortality, uninterrupted population growth was not likely—even when fertility was persistently high. As fluctuations in mortality became less severe and the peaks less frequent, Swedish population began to grow exponentially; this pattern has been shown by historical studies to have occurred in several other geographically and culturally distinct populations (Chambers; Deprez; Drake 1969; Eversley 1957; Reinhard 1968; Utterstrom 1965; Vielrose 1965).
Figure 1.
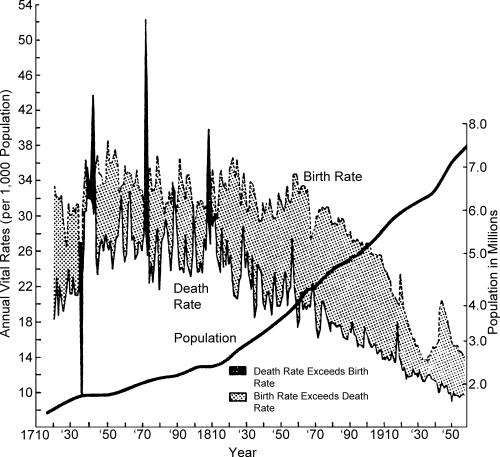
The Transition in Sweden
Source: Vielrose, E. 1965. Elements of the Natural Movement of Populations. Oxford: Pergamon Press, Inc.
Demographic trends for England and Wales, Japan, Ceylon and Chile are compared in Figure 2. In each country an exponential pattern of population growth accompanies the secular downward trend in mortality. In England and Wales, where the transition from high to low vital rates occurred over two centuries, the exponential growth curve was attenuated only after fertility fell and approached the low level of mortality; this pattern is less apparent for Japan, where an accelerated transition occurred over several decades. Although data concerning the relative effects of mortality and fertility on population growth are incomplete for the early transitional period, it seems likely that a significant though temporary increase in fertility may have added momentum to the population explosion set off by steady improvements in survivorship. The influence of fertility is particularly apparent in the rapid population growth of currently developing nations that have not yet completed their transitions; see for example the graphs for Chile and Ceylon in Figure 2. In most of these developing countries, the death rate has declined rapidly in recent years, especially since World War II, and the birth rate has remained high with minor fluctuations. This sudden widening of the demographic gap has produced unprecedentedly high rates of population growth, as can be seen by comparing the growth curves of the four countries in Figure 2.
Figure 2.
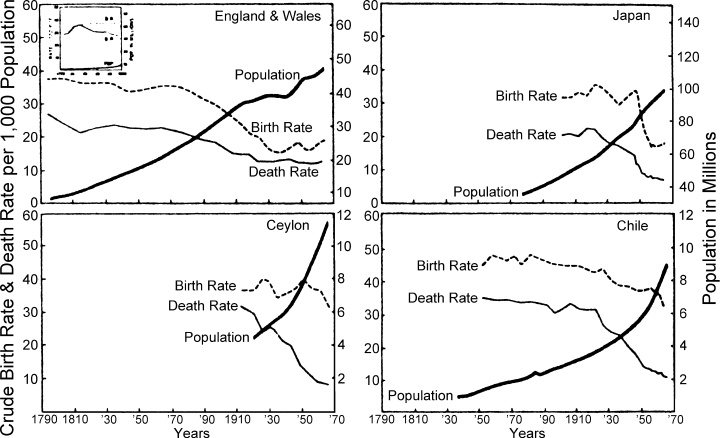
Demographic Trends in Selected Countries
Sources: England and Wales eighteenth century data (inset) and early nineteenth century data (to 1841) are Brownlee's estimates, cited by Glass, D. V., Population and Population Movements in England and Wales, 1700 to 1850, in Glass, D. V. and Eversley, D. E. C. (Editors), Population in History, Chicago, Aldine Publishing Company, 1965, pp. 221–246; data for 1841–50 and 1951–55 are from Glass, D. and Grebenik, E., World Population, 1800–1950, in Habakkuk, H. J. and Postan, M. (Editors), The Cambridge Economic History of Europe, Vol. VI, Cambridge, Cambridge University Press, 1965, pp. 56–138; data since 1955 are from Demographic Yearbook, 1963 and Demographic Yearbook, 1967, New York, United Nations. Data for Japan, 1900–04 to 1958 are from Taeuber, I., Japan's Demographic Transition Re-examined, Population Studies, 14, 28–39, 1960–61; data since 1958 from Demographic Yearbook, op. cit. Data for Chile, 1850–54 to 1960–64, are from Collver, O. A., Birth Rates in Latin America: New Estimates of Historical Trends and Fluctuations, Research Series No. 7, Berkeley, Institute of International Studies, University of California, 1965; data since 1962 from Demographic Yearbook, 1967, op. cit. Data for Ceylon, 1911–13 to 1936 are from International Vital Statistics, Vital Statistics Special Reports, 9, May 2, 1940; 1936–38 to 1946 data from Annual Epidemiologic and Vital Statistics, 1939–1946. Geneva, World Health Organization, 1951; data since 1947 from Demographic Yearbook, 1953, 1963, and 1967, op. cit.
Shifts in Mortality and Disease Patterns
Proposition Two
During the transition, a long-term shift occurs in mortality and disease patterns whereby pandemics of infection are gradually displaced by degenerative and man-made diseases as the chief form of morbidity and primary cause of death. Typically, mortality patterns distinguish three major successive stages of the epidemiologic transition:
The Age of Pestilence and Famine when mortality is high and fluctuating, thus precluding sustained population growth. In this stage the average life expectancy at birth is low and variable, vacillating between 20 and 40 years.
The Age of Receding Pandemics when mortality declines progressively; and the rate of decline accelerates as epidemic peaks become less frequent or disappear. The average life expectancy at birth increases steadily from about 30 to about 50 years. Population growth is sustained and begins to describe an exponential curve.
The Age of Degenerative and Man-Made Diseases when mortality continues to decline and eventually approaches stability at a relatively low level. The average life expectancy at birth rises gradually until it exceeds 50 years. It is during this stage that fertility becomes the crucial factor in population growth.
The Age of Pestilence and Famine represents for all practical purposes an extension of the pre-modern pattern of health and disease. In this stage the major determinants of death are the Malthusian “positive checks,” namely, epidemics, famines and wars. Graunt's study of London's Bills of Mortality (Graunt 1939) in the mid-seventeenth century shows, for example, that nearly three-fourths of all deaths were attributed to infectious diseases, malnutrition and maternity complications; cardiovascular disease and cancer were responsible for less than six per cent.2 (See graph for seventeenth century London in Figure 4.)
Figure 4.
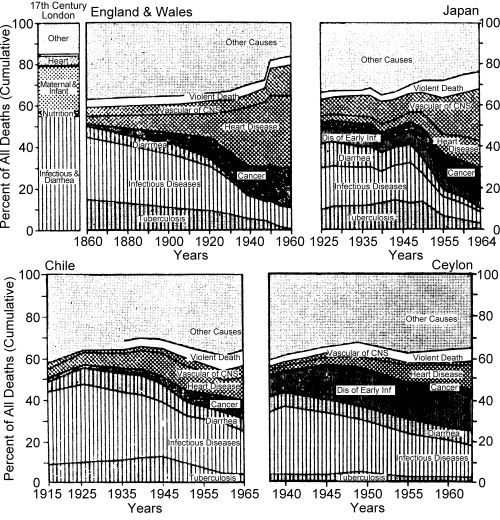
Trends in Cumulative Cause of Death Ratios for Both Sexes in Various Countries
Sources: Data for seventeenth century London in Graunt, J., Natural and Political Observations Made upon the Bills of Mortality, Baltimore, The Johns Hopkins Press, 1939 (first published in London, 1662); data for England and Wales 1848 to 1947 from Logan, W. P. D., Mortality in England and Wales from 1848 to 1947, Population Studies, 4, 132–178, September 1950; data since 1947 from Annual Epidemiologic and Vital Statistics, 1960, Geneva, World Health Organization, 1963. Data for Japan, 1925 are from Report Prepared by the Central Sanitary Bureau of the Home Department, Tokyo, 1925; data for 1935 from International Vital Statistics, Vital Statistics Special Reports, 9, May 2, 1940; data for 1940, 1960 and 1964 are from Annual Epidemiologic and Vital Statistics, 1939–46, 1960 and 1964, op. cit.; data for 1947, 1950, 1955 and 1965 are from Demographic Yearbook, 1953, 1956 and 1967, New York, United Nations. Data for Chile, 1917 and 1926, are from Statistical Yearbook of the Republic of Chile, 1, 1926; data for 1936 are from Vital Statistics Special Reports, 1938; data for 1940–1955 are from Annual Epidemiologic and Vital Statistics, 1939–46, 1950 and 1956, op. cit.; data for 1960–65 are from Demographic Yearbook, 1966 and 1967, op. cit. Data for Ceylon, 1938–1960, are from Annual Epidemiologic and Vital Statistics, 1939–46, 1950, 1956 and 1960, op. cit.; data for 1963–65 are from Demographic Yearbook, 1963 and 1967, op. cit.
United Nations compilations, which were used to calculate the cumulative cause-of-death ratios for successive life expectancy levels, show that disease patterns change markedly as life expectancy rises (Department of Economic and Social Affairs 1962). Two sets of data are given according to the preponderant age structure, whether “young” or “old.” The trends in the cause-of-death ratio for both population structures are given in Figure 3 and indicate the progressive decline in infectious diseases and concomitant increase in degenerative diseases (as indicated by the cardiovascular and cancer categories) as life expectancy improves.
Figure 3.
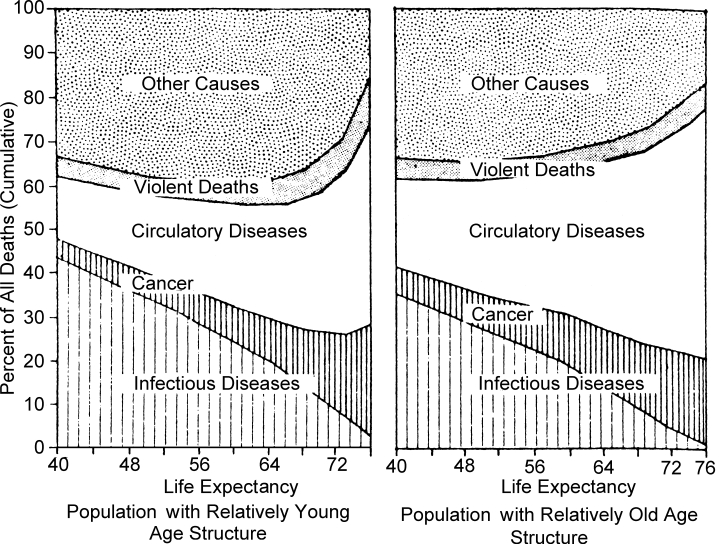
Pattern of Mortality Trends (Standardized Mortality) by Cause-of-Death Groups for Expectation of Life at Birth from 40 to 76 Years
Source: Department of Economic and Social Affairs. 1962. Population Bulletin of the United Nations 6, 110–12.
Similar trends are described by the cause-of-death statistics for a number of individual countries, as shown in Figure 4. The gradual shift in disease patterns characteristic of the classical transition can be seen in the steady decline of infectious diseases (including tuberculosis and diarrhea) and the moderate increase in cancer and cardiovascular diseases in England and Wales up to 1920. After World War I, the decline of infectious and rise of degenerative diseases is more distinct, and since 1945 the increase in cardiovascular deaths is particularly striking. The shift from infectious to degenerative disease predominance is more readily apparent for Japan, which has experienced an accelerated transition in only a few decades. Among currently developing nations, the transition from infectious to degenerative disease predominance has started but has not yet been completed, as shown by the graphs for Chile and Ceylon in Figure 4. The recession of infectious diseases that began in Chile in the 1920's has been gradual but discernible. In Ceylon this shift was delayed even further until the late 1940's.
The determinants of the transition from infectious to degenerative disease predominance are by no means simple. Their detailed treatment is beyond the scope of this paper; however, it may be useful to mention three major categories of disease determinants.
Ecobiologic determinants of mortality indicate the complex balance between disease agents, the level of hostility in the environment and the resistance of the host. More often than not, however, even these determinants cannot be categorically specified. One outstanding example is the recession of plague in most of Europe toward the end of the seventeenth century. The reasons for this recession are not fully understood, although the mysterious disappearance of the black rat may have been a contributing factor. Nonetheless, it is relatively certain that with the possible exception of smallpox, the recession of plague and many other pandemics in Europe was in no way related to the progress of medical science (McKeown and Brown 1955).
Socioeconomic, political and cultural determinants include standards of living, health habits and hygiene and nutrition. Hygiene and nutrition are included here, rather than under medical determinants because their improvement in western countries was a byproduct of social change rather than a result of medical design.
Medical and public health determinants are specific preventive and curative measures used to combat disease; they include improved public sanitation, immunization and the development of decisive therapies. Medical and public health factors came into play late in the western transition, but have an influence early in the accelerated and contemporary transitions.
The reduction of mortality in Europe and most western countries during the nineteenth century, as described by the classical model of epidemiologic transition, was determined primarily by ecobiologic and socioeconomic factors. The influence of medical factors was largely inadvertent until the twentieth century, by which time pandemics of infection had already receded significantly. The mortality decline in currently developing countries has been more recent and the effect of medical factors has been more direct and more salient, as shown by the contemporary or delayed transition model. In the Afro-Asian countries in particular, the tremendous impact of imported medical technologies on mortality has been magnified by massive public health programs. Although it would be naive to attempt precise identification of the complex determinants in each case, it does seem apparent that the transition in the now developed countries was predominantly socially determined, whereas the transition in the “third world” is being significantly influenced by medical technology.
Relative Risks of Mortality by Age and Sex
Proposition Three
During the epidemiologic transition the most profound changes in health and disease patterns obtain among children and young women. The genuine improvements in survivorship that occur with the recession of pandemics are peculiarly beneficial to children of both sexes and to females in the adolescent and reproductive age periods, probably because the susceptibility of these groups to infectious and deficiency diseases is relatively high.
Childhood survival is significantly and progressively improved as pandemics recede in response to better living standards, advances in nutrition and early sanitation measures and is further enhanced as modern public health measures become available. Data from the U.N. Model Life Tables were used to calculate the trend in the probability of death for various age groups with the transition from life expectancy level 20 to level 74 as shown in Figure 5.
Figure 5.
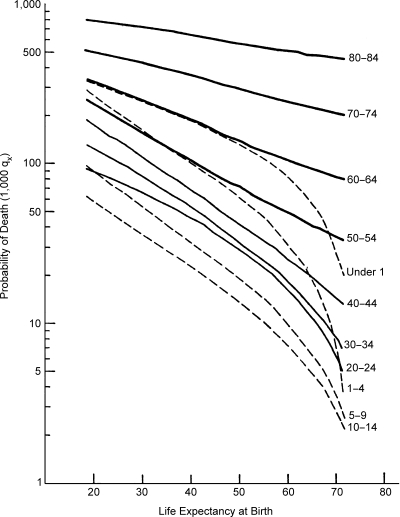
Trends in the Probability of Death (Life Table Mortality) for Age Groups with Increasing Life Expectancy at Birth (Both Sexes)
Source: Department of Social Affairs, Population Branch, Age and Sex Patterns of Mortality: Model Life Tables for Underdeveloped Countries, Population Studies, No. 22, New York, United Nations, 1955.
Although all age groups benefit from the shift in disease patterns and the increase in life expectancy, the decline in childhood mortality is demonstrably the greatest, especially in the one to four year age group. The age-specific death rate trends for England and Wales, Japan and Chile, depicted by the three graphs in Figure 6, reflect this phenomenon. The point in time that marks the beginning of progressive improvement in survival of children (0–15 years), differs from one country to another. In England, childhood mortality has obviously been dropping steadily since the late nineteenth century and in Japan since sometime between the two World Wars. In Chile, although measurable drops in childhood mortality have been registered since 1940, the death rates for infants and children still remain very high; for example, the mortality risks of infants (0–1) and young children (1–4) were, respectively, 5.2 and 3.2 times higher in Chile than in Japan in 1965.
Figure 6.
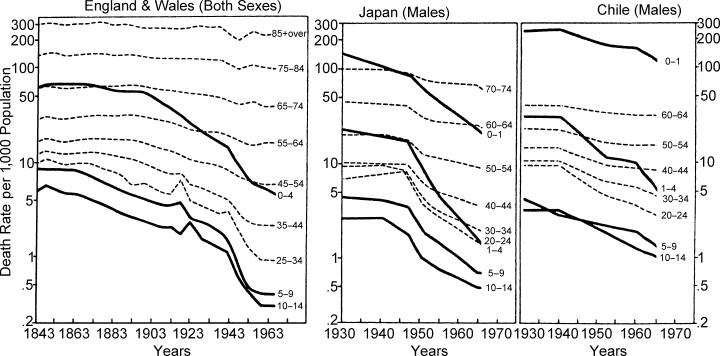
Trends in Age-Specific Death Rates in Various Countries (Semilogarithmic Scale)
Sources: Data for 1841–45 to 1951–55 are from Vielrose, E., Elements of the Natural Movement of Populations, Oxford, Pergamon Press, Inc., 1965 and Demographic Yearbook, 1963 and 1967, New York, United Nations. Data for Japan, 1929–1948, are from Vital Statistics Special Reports, 1938; and Annual Epidemiologic and Vital Statistics, 1939–46, Geneva, World Health Organization; data for 1951–66 are from Demographic Yearbook, 1953 and 1967, op. cit. Data for Chile, 1930–32, are from Vital Statistics Special Reports, 1938; data for 1940–1965 are from Demographic Yearbook, 1953, 1966 and 1967, op. cit.
Data from the U.N. Model Life Tables are plotted in Figure 7 to show the probability of death by age and sex at different life expectancy levels. Females’ risk of dying is less than that for males in the post-reproductive period at all life expectancy levels, but females have a higher probability of death during the adolescent and reproductive age intervals at low life expectancy levels. During the transition from infectious to degenerative disease predominance, women switch from a level of mortality in the reproductive years higher than that of men to a level more advantageous, such that the female's higher relative risk of death disappears at about the level of 50 years life expectancy and becomes lower than that of males thereafter.
Figure 7.
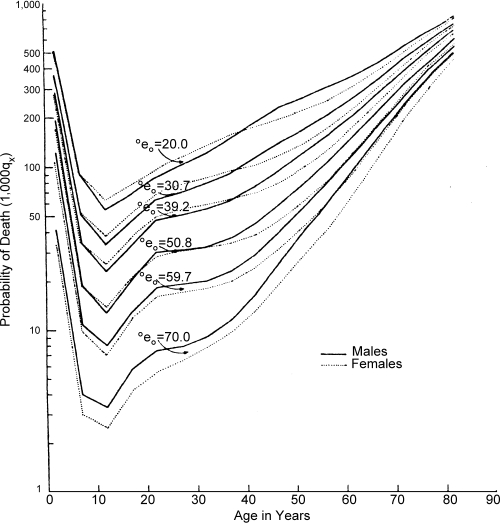
Probability of Death (1,000 qx) by Age and Sex at Various Levels of Life Expectancy at Birth
Source: Department of Social Affairs, Population Branch, Age and Sex Patterns of Mortality: Model Life Tables for Underdeveloped Countries, Population Studies, No. 22, New York, United Nations, 1955.
Age and sex mortality profiles for England and Wales, Japan, Chile and Ceylon are given in Figure 8; in each case the overall mortality risks diminish progressively with time, and the female risk of death, which is greater initially, gradually approaches the male level. The pattern of change in male/female relative risk appears similar for England, Japan and Chile, and although the accelerated timing of the transition in Japan is distinctive, the most recent data for Japan (1960) and Chile (1965) show relative risks that are remarkably similar to that for England in 1947. The phenomenon of superior survival chances for females, which is most strikingly depicted by the English data for 1960 and is typical of western countries, may be replicated in other countries as fertility declines and as modern industrial society develops and places peculiar stresses on male members of society. The pattern of male/female relative risk presented by Ceylon may be typical of many Afro-Asian countries where high fertility and certain cultural factors have been offered in explanation of continued high relative risks for females (El-Badry 1969).
Figure 8.
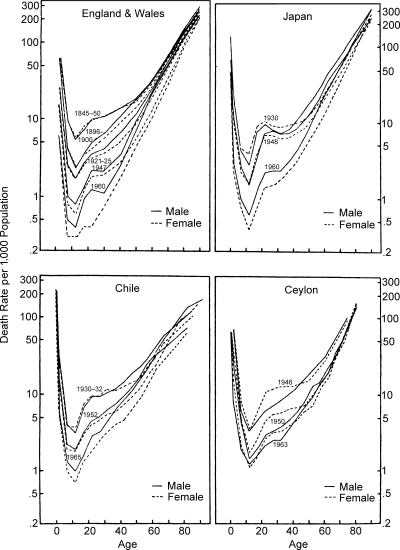
Trends in Age and Sex Profiles of Mortality in Various Countries (Semilogarithmic Scale)
Sources: Data for England and Wales, Japan and Chile from same sources as in Figure 6. Data for Ceylon are from Demographic Yearbook, 1948, 1949–50, 1959, 1960 and 1965, New York, United Nations.
Interacting Transition Variables
Proposition Four
The shifts in health and disease patterns that characterize the epidemiologic transition are closely associated with the demographic and socioeconomic transitions that constitute the modernization complex.
Interactions with Demographic Changes
The decline in mortality that comes with the epidemiologic transition widens the “demographic gap” between birth rates and death rates and hence affects demographic change by bolstering population growth (see Figure 2). In a more subtle manner, the mortality transition affects demographic movements indirectly through its impact on fertility and population composition.
During the course of the epidemiologic transition, sequential changes occur in the age and sex structure and the dependency ratios of populations. During the Age of Pestilence and Famine infectious disease and chronic malnutrition exact a particularly high toll among children and women in the adolescent and reproductive years; only small proportions of the population survive the high mortality of youth. The young dependency ratio is thus quite high and continues to be so in the early stage of the Age of Receding Pandemics; throughout these periods, the population comprises about the same proportions of males and females or a slightly higher proportion of males. As infectious diseases recede and larger segments of the population survive childhood, the dependency ratio becomes more balanced. Finally, during the Age of Degenerative and Man-Made Diseases, the tremendous improvements in survivorship registered among all age groups except the very old are reflected by a more uniform population distribution; at the same time the old dependency ratio increases and the male to female ratio is typically less than unity within the older age groups.
The improvements in female and childhood survival that occur with the shift in health and disease patterns discussed above have distinct and seemingly contradictory effects on fertility. On one hand, the better health and greater longevity increasingly enjoyed by females of reproductive ages tend to enhance fertility performance. On the other hand, the drastic reduction in risks to infants and young children that occurs in the later stages of the transition tends to have an opposite effect on natality; that is, prolonged lactation associated with reduced mortality among infants and toddlers and parental recognition of improved childhood survival tend to lengthen birth intervals and depress overall reproductive performance.
Calculations derived from two independent simulation models, illustrate the differential effects of changing mortality on fertility as shown in Table 2. In part A of the table, Ridley, et al., present estimates of the probable reproductive performance of women aged 15 to 50 under varying mortality conditions (Ridley et al. 1967). The REPSIM model from which these estimates were calculated assumed a short postpartum nonsusceptible period after each live birth, thereby “controlling” for the deflationary effect of improved childhood survival on fertility. The REPSIM estimates of the net reproduction rate can be seen to rise steadily with improvements in female survival; in the absence of contraception, the enhanced fertility performance simulated by this model can be largely attributed to the increasing probability of surviving the reproductive period.
TABLE 2.
The Effects of Improved Survivorship on Fertility as Estimated by Two Independent Simulation Models
| Part A: Changes in Fertility Performance with Improvements in Female Survival and in the Absence of Deliberate Fertility Regulation* | ||||
|---|---|---|---|---|
| Life Expectancy Level | Per Cent of Women Surviving from Age 15 to 50 | Estimated Loss in Married Life Due to Mortality | Mean Number of Live Births per Woman | Net Reproduction Rate |
| 31 | 52 | 11.7 Years | 7.1 | 2.0 |
| 41 | 68 | 8.2 | 7.8 | 2.7 |
| 51 | 80 | 5.5 | 8.6 | 3.3 |
| 61 | 89 | 3.4 | 9.0 | 3.9 |
| 71 | 95 | 1.5 | 9.7 | 4.6 |
| Part B: Changes in Fertility Performance with Improvements in Survivorship with Completely Effective Contraception When a Couple is 95% Certain That a Son Will Survive to Father's 65th Birthday** | ||||
| Life Expectancy Level | Probability of Son's Death before Father Reaches Age 65 | Number Male Births for at Least One to Survive Father's 65th Birthday | Percentage Wives Never Bearing Needed Number of Sons | Female Net Reproduction Rate |
| 20 | .695 | 9 | 91.4 | 1.75 |
| 30 | .551 | 6 | 35.3 | 2.39 |
| 40 | .435 | 4 | 6.1 | 2.32 |
| 50 | .323 | 3 | 1.6 | 2.13 |
| 60.4 | .204 | 2 | 0.2 | 1.64 |
| 70.2 | .083 | 2 | 0.2 | 1.81 |
*Source: Ridley, J.C., et al. January, 1967. The Effects of Changing Mortality on Natality: Some Estimates from a Simulation Model. Milbank Memorial Fund Quarterly. 45: 77–97.
**Source: Heer, D.M. April 1966. Births Necessary to Assure Desired Survivorship of Sons under Differing Mortality Conditions, paper presented at annual meeting of the Population Association of America, New York City.
The data given in part B of the same table are based on Heer's simulation model for estimating the effects of son survivorship on reproductive behavior; this model assumes that each couple continues to have children until they are 95 per cent certain that at least one son will survive until the father's 65th birthday and that birth control is practiced and is completely effective whenever couples cease to “want” children (Heer 1966). This model thus shows a decrease in the net reproduction rate as child survival chances improve. Further proof of this is provided by empirical data from Hassan's work (Hassan 1966).
Because the probability of a female surviving the reproductive period usually increases earlier in the transition than improvements in infant and childhood survival, fertility may rise in the early stage of the epidemiologic transition. The tendency of improved infant and childhood survival to depress fertility in the middle and subsequent stages of the transition can be attributed largely to the following factors:
Biophysiologic factors: The increased chance that a live birth will survive infancy and early childhood and result in prolonged lactation tends to lengthen the mother's postpartum period of natural protection against conception. Another run of the REPSIM model, which simulated both long and short postpartum periods as a function of the probable pregnancy outcome, shows a moderately declining net reproduction rate at higher life expectancy levels. These data also indicate that the interval between births increases progressively at all parities as life expectancy rises. Ridley, et al., concluded that the lengthening of birth intervals, particularly among young, highly fecund, low-parity women, has a deflationary effect on ultimate parity and is a major mechanism linking improved survival and lowered fertility.
Socioeconomic factors: The risk of childhood death is lowered by better nutrition and sanitation as socioeconomic conditions improve. As the probability of child survival increases, the desirability of having many children may diminish in response to changes in the social and economic system that cast the child as an economic liability rather than asset. Concomitantly, improvements in birth control technology facilitate the achievement of emerging small family size norms.
Psychologic or emotional factors: Improved infant and childhood survival tends to undermine the complex social, economic and emotional rationale for high parity for individuals and hence high fertility for society as a whole. As couples become aware of the near certainty that their offspring, particularly a son, will survive them, the likelihood of practicing family limitation is enhanced. Not only are compensatory efforts to “make up” for lost children reduced, but the investment of parental energies and emotions may take on a new, qualitative dimension as each child in the small family is provided better protection, care and education.
Interaction with Socioeconomic Changes
The interactions between the epidemiologic and socioeconomic transitions are also complex. Whether the epidemiologic transition is effected chiefly by socioeconomic improvements (as in now developed countries) or by modern public health programs (as has been the case in currently developing countries), the lowering of mortality and of infectious disease tends to increase the effectiveness of labor and hence economic productivity, both through better functioning of adult members of the labor force and through an increase in the proportion of children who survive and mature into productive members of society.
A gross assessment of the economic effects of the mortality transition that occurs with the shift in health and disease patterns can be made by subjecting a given set of consumption-production patterns to the varying mortality conditions outlined by the U.N. Model Life Tables. Based on the axiom that a person consumes a certain amount of wealth (for food, shelter, clothing and so forth) and produces a certain amount, a schedule of consumption-production values was developed by Sauvy to indicate the relative excesses in consumption and output that obtain at various age periods during the life of one man, as shown in Table 3. As indicated by these approximate values, output exceeds consumption in the middle years of life (roughly age 20 to age 65) while the costs of rearing children (providing training and education as well as vital necessities) and the costs of caring for the aged render consumption excessive in the early and later periods of life.
TABLE 3.
Estimated Excesses in Economic Consumption and Output at Various Ages
| Excess in Consumption during Various Age Periods | Excess in Output during Various Age Periods | ||
|---|---|---|---|
| 0–1 | 50 | 20–25 | 260 |
| 1–5 | 225 | 25–30 | 300 |
| 5–10 | 332 | 30–35 | 350 |
| 10–15 | 450 | 35–40 | 350 |
| 15–20 | 350 | 40–45 | 320 |
| 65–70 | 350 | 45–50 | 290 |
| 70–75 | 400 | 50–55 | 260 |
| 75–80 | 500 | 55–60 | 215 |
| 80+ | 650 | 60–65 | 85 |
Source: Sauvy, A. 1969. General Theory of Population. New York: Basic Books, Inc., Publishers, p. 250.
Using the Model Life Table data for males, the appropriate value from Sauvy's consumption-output schedule was multiplied by the average number of survivors within each age period to yield indicators for total excess consumption (C)—the excess consumption of the young (Cy) plus the excess consumption of the old (C0)—and the total excess output (O) at each of several life expectancy levels. For any population to “break even” economically, the total excess consumption for young and old persons must be at least equalled by the total excess output in the middle years; that is Cy+ C0= O or C/O = 1 defines an economic equilibrium.
The ratios of consumption to output (C/O) at various life expectancy levels, which are depicted in Figure 9, illustrate the relative excess in consumption when life expectancy is 20 or 30 years. Conversely, at life expectancy levels of 40 years and above, output exceeds consumption, with the optimum excess in output occurring when life expectancy reaches 50 years. Thereafter, largely as a result of the consumption demands of an aging population, the consumption/output ratio rises, although it remains less than one. The steady rise in the proportion of excess output subsequently consumed by the aged (C0/O) is indicated by the shaded portion of the graph.
Figure 9.
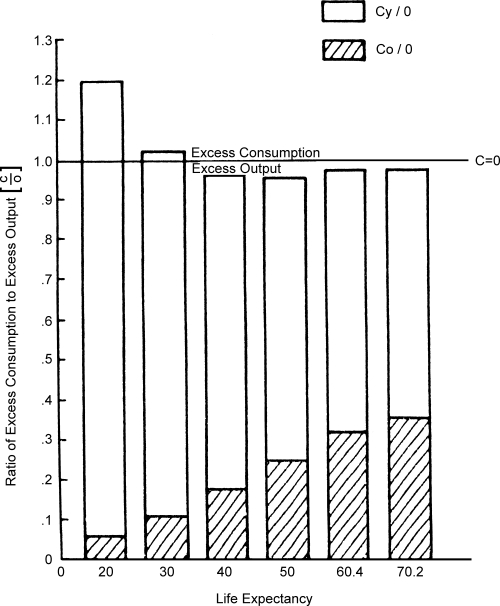
Relative Excesses in Consumption and Output for Stable Male Population at Various Life Expectancy Levels
Sources: Calculated, as described in the text, using Department of Social Affairs, Population Branch, Age and Sex Patterns of Mortality: Model Life Tables for Underdeveloped Countries, Population Studies, No. 22, New York, United Nations, 1955: and Sauvy, A., General Theory of Population, New York, Basic Books, Inc., Publishers, 1969.
From this rather crude assessment, it may be inferred that the relatively large excess output at life expectancy levels of 40 and 50 represents a surplus over subsistence needs that may be applied to capital and technological development. And, in fact, the economic “take-off” and “drive to maturation” periods in several countries, including England and Wales, Sweden and Japan, correspond to such life expectancy levels (Rostow 1960).
Basic Models of the Epidemiologic Transition
Proposition Five
Peculiar variations in the pattern, the pace, the determinants and the consequences of population change differentiate three basic models of the epidemiologic transition: the classical or western model, the accelerated model and the contemporary or delayed model. Through the description, analysis and comparison of mortality patterns in many societies and at different points in time, distinctive core patterns of the epidemiologic transition emerge. The fundamental purpose of delineating these models is to visualize the different matrices of determinants and consequences associated with mortality (and fertility) patterns and to elucidate some of the fundamental issues confronting population policy-makers. As illustration, three models of the epidemiologic transition are sketched below.
The Classical (Western) Model of Epidemiologic Transition
The classical model describes the gradual, progressive transition from high mortality (above 30 per 1,000 population) and high fertility (above 40 per 1,000) to low mortality (less than 10 per 1,000) and low fertility (less than 20 per 1,000) that accompanied the process of modernization in most western European societies. Following a stage of pestilence and famine that prevailed during the pre-modern and early modern periods, a slow unsteady rate of mortality decline gradually gave way to more precipitous declines around the turn of the twentieth century, by which time fertility had already turned downward.
It is noteworthy that socioeconomic factors were the primary determinants of the classical transition. These were augmented by the sanitary revolution in the late nineteenth century and by medical and public health progress in the twentieth century. Exponential population growth and sustained economic development were major correlates of the secular downward trend of mortality. In the late phase of the classical transition (i.e., in the second and third decades of the twentieth century) degenerative and man-made diseases displaced infections as the leading causes of mortality and morbidity. A distinguishing feature of this model is that the disequilibrating effects of explosive population growth were minimized, inasmuch as pandemics and famines receded slowly enough for economic growth to become sustained before low fertility determinants acted to narrow the demographic gap and temper spiralling population growth.
The Accelerated Epidemiologic Transition Model
The accelerated epidemiologic transition model describes the accelerated mortality transition that occurred most notably in Japan. Both the fluctuating mortality in the Age of Pestilence and Famine and the gradual (early) phase of the Age of Receding Pandemics followed a pattern similar to, though later than, the classical model. A major distinction of the accelerated model is that the period taken for mortality to reach the 10 per 1,000 level was much shorter than that for the classical model, as can be seen by comparing the graphs for England and Wales and Japan in Figure 2. The shift to the Age of Degenerative and Man-Made Diseases was also much faster (Figure 4). Accompanying this shift was the selective improvement in survival of children under 15 (Figure 6) and of women (Figure 8), typical of the classical model. These changes, however, occurred over relatively short periods of time.
Most of the countries fitting this model had begun a slow process of modernization prior to the drop in mortality in the twentieth century, which was determined by sanitary and medical advances as well as by general social improvements. In these countries, national and individual aspirations favored a controlled rate of population increase and provided the intense motivation needed to lower fertility in a relatively short period of time. Abortion, especially in Japan, has played a major role in the rapid fertility transition depicted by this model.
The Contemporary (or Delayed) Epidemiologic Transition Model
The contemporary model describes the relatively recent and yet-to-be completed transition of most developing countries. Although slow, unsteady decline in mortality began in some of these countries shortly after the turn of the century, rapid and truly substantial declines in mortality have been registered only since World War II. Public health measures have been a major component of the imported, internationally sponsored medical package that has played a decisive role in setting the stage for astronomic population growth in these economically handicapped countries. In other words, these programs have successfully manipulated mortality downward while leaving fertility at substantially high levels. Both national and international programs of “population control” designed to hasten fertility decline artificially are prominent features of this model for countries where death control has far outstripped birth control. Despite unmistakable gains in the survival of women and children, infant and childhood mortality remains excessively high in most of these countries and in some, females of reproductive age continue to have higher mortality risks than males in the same age group. Although most countries in Latin America, Africa and Asia fit this model, important differences between these areas suggest the utility of developing submodels, particularly with regard to the varying responses of fertility and socioeconomic conditions to national development programs.
Summary
Despite the inherent difficulties in attempting to structure a matrix that includes all the complex vital factors of population dynamics, the need to do so is urgent. A vast array of social, economic and demographic as well as epidemiologic factors shape the course of population change, and although it is doubtful that one comprehensive, all inclusive population theory will ever be formulated, scholars in various disciplines will continue to develop and refine segments of the theory.
The theory of epidemiologic transition, which has been sketched in this brief essay, represents the continuing efforts of this author to crystallize the mechanisms of interaction that characterize the patterns, determinants and consequences of health and disease changes in a variety of social contexts. The basic strategy is not only to describe and compare the mortality transitions of various societies, but more importantly, to lend theoretical perspective to the process of population change by relating mortality patterns to demographic and socioeconomic trends—both longitudinally and cross-sectionally—through the development of models. With elaboration and refinement of such models, comparative analyses of the epidemiologic transition in various population groups can provide information needed to treat at least some of the many problems associated with disequilibrating population movements.
Acknowledgments
I want to express special thanks to Dr. John Cassel, Chairman of the Department of Epidemiology, School of Public Health, and to Dr. Moye Freyman, Director, Carolina Population Center, University of North Carolina, for their continuing support, valuable suggestions and constructive criticism throughout this project. Thanks also to Sandra Burden, social research assistant for her valuable contributions to this project; to Jeannette Cannon, Ann Herrin, Anita Leung, Çiçek Yener, statistical research assistants; to Cynthia Snow, editorial assistant; and to Libby Cline, secretary.
Endnotes
Data for the U.N. Model Life Tables are gathered from a number of countries around the world, with some unavoidable over-representation of countries that are now developed because their vital statistics are more complete and more accurate than those for less developed countries.
These figures are quoted only to indicate the relative magnitude of the problem as the deficiencies in reporting and diagnosis are well recognized.
References
- Angel, Pearson . In: World Population and Production: Trends and Outlook. Woytinsky WS, Woytinsky ES, editors. New York: Twentieth Century Fund; 1953. Cited in. [Google Scholar]
- Chambers JD. Glass and Eversley. Three Essays on the Population and Economy of the Midlands; pp. 308–53. op. cit. [Google Scholar]
- Department of Economic and Social Affairs. Population Bulletin of the United Nations. No. 6. New York: United Nations; 1962. pp. 110–12. [Google Scholar]
- Deprez P. Glass and Eversley. The Demographic Development of Flanders in the Eighteenth Century; pp. 608–30. op. cit. [Google Scholar]
- Drake M. Population and Society in Norway, 1735–1865. Cambridge, England: Cambridge University Press; 1969. [Google Scholar]
- El-Badry MA. Higher Female than Male Mortality in Some Countries of South Asia: A Digest. Journal of the American Statistical Association. 1969;64:1234–44. doi: 10.1080/01621459.1969.10501052. December. [DOI] [PubMed] [Google Scholar]
- Eversley DEC. A Survey of Population in an Area of Worcestershire from 1660 to 1850 on the Basis of Parish Registers. Population Studies. 1957;10:253–79. [Google Scholar]
- Graunt J. Natural and Political Observations Made upon the Bills of Mortality. Baltimore: The Johns Hopkins Press; 1939. this book was originally published in London in 1662. [Google Scholar]
- Hassan S. Influence of Child Mortality on Population Growth. Ann Arbor, Michigan: University Microfilms; 1966. [Google Scholar]
- Heer DM. Births Necessary to Assure Desired Survivorship of Sons under Differing Mortality Conditions. Paper presented at the Annual Meeting of the Population Association of America; New York City. [Google Scholar]
- Landis PH, Hatt PK. Population Problems: A Cultural Interpretation. New York: American Book Company; 1954. [Google Scholar]
- Mayer K. Developments in the Study of Population. Social Research. 1962;29:292–320. Autumn. [Google Scholar]
- McKeown T, Brown RG. Medical Evidence Related to English Population Change in the Eighteenth Century. Population Studies. 1955;9:119–41. [Google Scholar]
- Micklin M. Urban Life and Differential Fertility: A Specification of the Theory of the Demographic Transition. Presented at the annual meetings of the Population Association of America; Boston. [Google Scholar]
- Reinhard M, Armengaud A, Dupaquier J. Histoire Generale De La Population Mondiale. Paris: 1968. Editions Montchrestien. [Google Scholar]
- Ridley JC, et al. The Effects of Changing Mortality on Natality: Some Estimates from a Simulation Model. Milbank Memorial Fund Quarterly. 1967;45:77–97. January. [Google Scholar]
- Rostow WW. Stages of Economic Growth: An Anti-Communist Manifesto. New York: Cambridge University Press; 1960. [Google Scholar]
- Russell JC. Late Ancient and Medieval Population. Transactions of the American Philosophical Society. 1958;48 part 3. June. [Google Scholar]
- Sauvy A. General Theory of Population. New York: Basic Books, Inc., Publishers; 1969. [Google Scholar]
- United Nations Department of Social Affairs. Population Studies. No. 22. New York: United Nations; 1955. Population Branch, Age and Sex Patterns of Mortality: Model Life Tables for Under-Developed Countries. [Google Scholar]
- Utterstrom G. Two Essays on Population in Eighteenth Century Scandinavia. In: Glass DV, Eversley DEC, editors. Population in History. Chicago: Aldine Publishing Company; 1965. pp. 523–48. [Google Scholar]
- Van Nort L, Karon BP. Demographic Transition Re-examined. American Sociological Review. 1955;20:523–27. October. [Google Scholar]
- Vielrose E. Elements of the Natural Movement of Populations. Oxford: Pergamon Press, Inc.; 1965. [Google Scholar]


