Abstract
In most developed countries, as the largest population cohorts approach the age of sixty-five, the impact of population aging on health care expenditures has become a topic of growing interest. This articles examines trends in elderly disability and end-of-life morbidity, estimations of the cost of dying, and models of expenditures as a function of both age and time-to-death and finds broad improvement in mortality and morbidity among the elderly in the developed world. Reduced mortality and low growth in the costs associated with dying could reduce forecasted expenditures, but high growth in expenditures for those not close to death and for nonhospital services could create new economic pressures on health care systems.
Keywords: Health care expenditures, cost-of-dying, time-to-death, aging, demographics, compression of morbidity
In most developed countries, as the largest population cohorts approach the age of sixty-five, the impact of population aging on health care expenditures has become a topic of growing interest in academic and policy circles. The potential economic stakes of the coming demographic transition are substantial. The impact of these demographic trends on expenditures is a function of the number of people in high-use categories, the length of time that they remain in that category, and the cost of the health services they use. Older persons use more health care. In OECD nations, the average per capita expenditures for persons age sixty-five and older are two to eight times more than those for the working-age population and steadily increase with age (CMS 2006; Freund and Smeeding 2002; Mayhew 2000). The proportion of OECD populations in these higher-spending cohorts is projected to grow from 13.0 percent in 2000 to 20.9 percent in 2030 (OECD 2006). Assuming that age-specific spending distributions remain fixed, expenditures as a proportion of GDP are predicted to grow by as much as three percentage points in the first half of the twenty-first century in both the United States (Dang, Antolin, and Oxley 2001) and the European Union (EU) (Bains and Oxley 2004; EPC 2003), which places the sustainability of health care into doubt.
These assumptions may not prove to be accurate, however. Predictions of rapid growth in expenditures based on the growing size of elderly cohorts are not reflected in the data. For example, in the United States, the proportion of the population over age sixty-five grew from 9.8 percent in 1970 to 12.4 percent in 2000 (OECD 2006), yet by one estimate the shift in population age distribution accounted for only 0.2 percentage points of the 4.3 percent inflation-adjusted annual growth in expenditures (Meara, White, and Cutler 2004). Similar results have been observed in other OECD nations. Analyzing individual health services utilization, Barer and colleagues (1989, 2004) estimated the effect of the population's aging on annual service utilization growth in the Canadian province of British Columbia between 1969 and 1996 at less than 0.5 percent, even though the proportion of the population aged sixty-five and older grew from 9.3 to 12.6 percent (BC Government Statistics 2007). In addition, cross-sectional studies of aggregate national spending levels in several countries found the percentage of the population over age sixty-five to be, at best, a weak predictor of expenditures (Gerdtham 1992; Gerdtham et al. 1992; Getzen 1992; Hopkins and MacDonald 2000; Reinhardt, Hussey, and Anderson 2002).
The aging of the population is only one driver of health care expenditures, and the effects of the relatively slow pace of demographic change may be overwhelmed by other factors like the introduction of new technologies and treatments (McClellan 1996, Cutler and McClellan 2001; Meara, White, and Cutler 2004); increased utilization, for example, of drugs and diagnostic tests; and price inflation, particularly given the tight labor markets in health care (Goetghebeur, Forrest, and Hay 2003; Hay 2003; Jacobzone et al. 1998; Koenig et al. 2003). Nevertheless, with the most rapid growth in elderly cohorts still to come, it is important to clarify how their relative spending patterns in old age are likely to compare with those of recent generations, in order to determine whether population aging will remain a toothless tiger or start to bare its teeth.
One key issue is the extent to which higher health care costs at older ages are associated with aging, death, or some combination of the two. Population aging is conventionally used to mean an increase in the percentage of people over the age of sixty-five. This increase reflects changes in the number of people entering a particular age cohort, as well as changes in how long each stays there (i.e., longer life expectancy). To the extent that aging is driven by the higher number of people entering the age cohort, larger cohort sizes at higher-spending ages might be expected to lead to higher expenditures. The effect on expenditures of aging due to lower death rates is less clear. Again, cohort sizes increase, yet the improvement in mortality implies an improvement in health, possibly leading to lower expenditures.
In the last two decades of the twentieth century, age-specific death rates in the United States fell, leading to a net decline in the overall population mortality rate of 2.5 percent (CDC 2006). If mortality rates continue to drop, the effect of the growing cohorts of elderly on future expenditures could be mitigated. Conversely, to the extent that expenditures are associated with age, independent of life expectancy, these lower mortality rates would mean more elderly people and higher expenditures.
One way to analyze the trajectories of health expenditures and morbidity developments at the end of life is to begin with death and work backward. Researchers use mortality-based analyses to answer such questions as, If people are dying at older ages on average, how do health expenditures in the last years of life change with the age at death? How does age affect health expenditures for those people still many years from death? Do changes in mortality parallel similar changes in the health status of people still living? How quickly do people in different age groups deteriorate from good health to death? And most important, are these relationships among age, death, and expenditures changing over time?
To answer these questions, this article reviews the literature on age, mortality, morbidity, and expenditures, particularly the use of time-to-death as a variable for modeling individual health expenditures. Unlike strictly age-based models, time-to-death models count backward from a fixed reference point (a known date of death) and measure expenditures against this backward count. This approach enables us to identify and model more specifically the separate effects of mortality and age. When the effects of mortality are controlled, the estimated relationship between age and expenditures can capture more subtle aspects of the aging process, such as how the utilization of services changes with age, and hence can offer more accurate forecasts of future expenditures.
Our review first presents an analytical framework for understanding the relationship between mortality and morbidity at the end of life and how this relationship might change both over time and with age at death. The framework is based on the “epidemiological transition” debate about whether morbidity is compressing, expanding, or staying the same. We then survey empirical studies of morbidity prevalence to determine the degree of support for these competing theories.
Second, we review the literature that uses individual-level data to measure the “cost of dying.” These studies generally compare the health care costs for a given age group of people who died (decedents) to those still living (survivors). Differences in the costs for decedents versus those of survivors of different ages reflect the “intensity of care,” and changes in age-specific intensity over time in conjunction with demographic forecasts suggest possible future scenarios for aging and expenditures.
The third section of this article introduces the relatively new and growing body of literature that uses individual-level data to develop empirical models of the relationship between expenditures and time-to-death. These studies are a natural extension of the literature on the cost of dying reviewed in the second section but add more quantitative rigor and analyze larger and more complex data sets. They move beyond the binary comparison of decedents and survivors to a more continuous measure of how expenditures change as death approaches. Finally, we examine how such models clarify the relationship between age, mortality, and morbidity among the elderly and enable more accurate expenditure forecasts.
Analytical Framework: Relationships between Mortality and End-of-Life Morbidity
As figure 1 shows, U.S. data demonstrate a close relationship between per capita expenditures and death rates, both of which rise steadily with age (CDC 2006; CMS 2006). The literature suggests a direct association between high expenditures and death: in the American Medicare program, the 5 percent of beneficiaries aged sixty-five and over who die each year account for 25 to 30 percent of total expenditures, although these have dropped somewhat over time (Hoover et al. 2002; Lubitz and Riley 1993). Similarly, in the Canadian province of Manitoba, the 1 percent of the adult population who died in 2000/2001 accounted for 21 percent of expenditures (Menec et al. 2004). It is not death itself that drives up expenditures, however, but the morbidity that precedes and may eventually lead to death.
FIGURE 1.
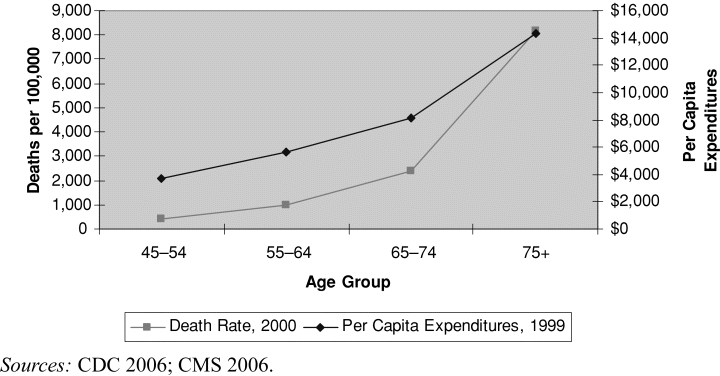
U.S. Per Capita Health Expenditures and Death Rates by Age Group, 1999–2000.
Cutler and Sheiner (1998) provide a useful framework for analyzing aggregate expenditures. They look at expenditures as the sum over all ages of the product of (1) the number of people alive in each age group, (2) the average health status at each age, and (3) the per capita medical spending conditional on health status, which also varies according to age. Note that this framework looks only at averages and ignores the considerable skewing of health status and costs within each age group (Deber, Forget, and Roos 2004). Indeed, sick people of all ages are likely to be expensive, whereas most healthy people in all age cohorts incur relatively few costs.
Most demographically derived predictions of future health care spending focus on numbers of people, assuming that their average health status remains constant and per capita spending grows at rates equal to the rate of inflation, in either the general economy or health care specifically (Dang, Antolin, and Oxley 2001; Miller 2001). As we noted earlier, however, the increasing number of older people is at least partially due to their lower mortality rate. If age-specific mortality rates fall, health status defined in terms of disability or illness—Cutler and Sheiner's second factor—may improve as well. In that case, forecasts of future expenditures may be too high, since they take into account the larger number of people alive at older ages based on improvements in mortality, but not the potential improvement in health status and lower need for health care that might be associated with these gains. In contrast, if mortality gains are associated with greater morbidity in the higher number of survivors, the forecasts may be too low. Empirical data are required.
Epidemiological Transition: The Theory
Considerable theoretical and empirical research has addressed the relationship between morbidity and mortality. The empirical work typically measures the prevalence of morbidity and enters these rates into mortality-based life tables to calculate health-adjusted life expectancies (HALE). Life expectancies are segmented into a healthy period (assumed to be low morbidity) and a period of high morbidity at the end of life. Depending on how morbidity is measured, HALE may also be characterized as disability-free life expectancy (e.g., Crimmins, Saito, and Reynolds 1997; Freedman et al. 2004; Sagardui-Villamor et al. 2005) or disease-free life expectancy (e.g., Mathers, Iburg, and Begg 2006; Mathers et al. 2001, 2004).
The theoretical framework places trends in elderly mortality and morbidity in the context of the epidemiological transition. First introduced by Abdel Omran (1971/2005), the concept of the epidemiological transition characterizes how social, environmental, and health factors combine to change life expectancies, the most common causes of death, and the prevalence of disease among successive population cohorts. For example, medical and social advances that drastically reduced the rates of mortality due to infectious diseases and heart disease have significantly changed the size and composition of the cohorts surviving into old age (Cutler and Richardson 1997; Mathers et al. 2001). Because these are cohorts that before these advances might have died earlier, rates of disease prevalence and morbidity are likely to change as well.
Researchers contemplating the interaction of these cohort effects, medical advances, and socioeconomic factors have reached a wide range of conclusions with respect to the likely implications for morbidity at the end of life. Fries (1980) popularized the concept of “compression of morbidity,” predicting that, for the elderly, the period of morbidity preceding death would shrink over time. He argued that gains in life expectancy would slow to the extent that premature deaths due to disease approached zero. Further reductions in mortality would thus have to occur mainly among the elderly, for whom past gains have been relatively minor. Fries theorized that at some point, medicine would be unable to mitigate the process of natural aging and that human longevity would approach a natural limit. Nonetheless, improvements in lifestyle, socioeconomic conditions, and medicine could reduce chronic conditions within that relatively fixed life span and thus would shorten the period of infirmity preceding death. The next cohorts of elderly would be healthier than they were in the past.
As a counterargument to Fries's compression hypothesis, Olshansky and colleagues (1991) presented a theory of expansion of morbidity. Even though they generally agree with Fries regarding limits to longevity (Olshansky, Carnes, and Cassel 1990), they suggest that even minimal mortality gains at old ages would lead to increasing morbidity associated with people surviving for longer periods with nonfatal chronic conditions.
Figure 2 depicts competing theories of end-of-life morbidity. Each of the three pairs of horizontal bars represents the lifetime experience of population cohorts separated in time and experiencing different rates of both mortality and morbidity. In all cases, life expectancy is assumed to increase. In the first pair (2a), reductions in age-specific mortality rates (leading to longer life expectancy) have exceeded reductions in the age-specific rate of morbidity incidence. Consequently, the period of chronic end-of-life morbidity expands, as represented by the growth in the hatched area of the bar. In the second depiction (2b), the combination of lower age-specific morbidity incidence and an increase in life expectancy leads to a roughly equal postponement of both death and the onset of chronic morbidity. In this scenario, morbidity neither expands nor compresses; instead, the length of the period of chronic end-of-life morbidity remains relatively unchanged. In the final depiction (2c), the relationship between changes in life expectancy and age-specific morbidity is reversed from scenario 2a, with the incidence of morbidity now falling faster than mortality. This is Fries's compression of morbidity scenario.
FIGURE 2.
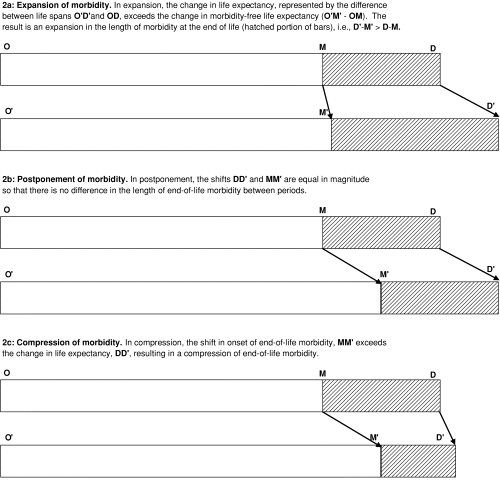
Illustration of Expansion, Postponement, and Compression of Morbidity Theories. Legend: O = beginning of life; M = onset of end-of-life morbidity; D = death. The shift from the upper to the lower bar in each pair (i.e., from OD to O′D′) represents a change in the population's health and life expectancies for a period of calendar time.
Epidemiological Transition: The Evidence
The hatched area of the bars in figure 2 can be defined as the difference between total and health-adjusted life expectancy. The compression of morbidity theory predicts that this difference will shrink, either in absolute terms or as a percentage of total life expectancy. The expansion of morbidity theory predicts just the opposite.
One challenge to the empirical literature is reconciling the many different ways in which morbidity can be measured (Murray, Salomon, and Mathers 2000; Robine and Michel 2004). Among the potential measures are disability (i.e., the ability to complete activities of daily living, ADL, and/or instrumental activities of daily living, IADL); self-reported health status; and the prevalence of chronic diseases. These different measures also may move in opposite directions, thus complicating the identification of time trends in population morbidity.
Despite these potential complications, the evidence concerning recent trends in morbidity is quite consistent and generally favors the theory of compression. In many developed countries, most measures of morbidity among the elderly have declined in recent years (Crimmins 2004; Doblhammer and Kytir 2001; Jacobzone, Cambois, and Robine 2000; Jagger, Barberger-Gateau, and Robine 2005; Manton, Stallard, and Corder 1995; Manton, Stallard, and Liu 1993; Robine and Michel 2004; Sagardui-Villamor et al. 2005; Spillman 2004), after a period of relatively stagnant morbidity in the 1970s and early 1980s (CDC 2007; Crimmins 2004; Crimmins, Saito, and Reynolds 1997). For the elderly, the prevalence of disabilities with ADLs and IADLs fell at an annual rate of 1.5 to 2 percent in the 1990s and into the 2000s (CDC 2007; Crimmins, Saito, and Reynolds 1997; Fries 2003; Jacobzone, Cambois, and Robine 2000; Singer and Manton 1998). Improvements of a similar magnitude have also been observed in self-reported ratings of health status (CDC 2007; Crimmins 2004; Doblhammer and Kytir 2001; Jagger, Barberger-Gateau, and Robine 2005). Although the prevalence of many chronic diseases like arthritis, diabetes, and cancer has increased (CDC 2007; Cutler and Richardson 1997; Robine and Michel 2004; Robine, Mormiche, and Sermet 1998), both theory (Manton 1982) and evidence (Crimmins 2004; Mathers, Iburg, and Begg 2006; Mathers et al. 2004; Robine, Mormiche, and Sermet 1998) indicate that the average severity of these diseases is declining, so that the greater prevalence of disease is consistent with falling levels of disability.
To estimate whether end-of-life morbidity has compressed or expanded, morbidity trends must be compared with developments in mortality. The limit to longevity predicted by Fries and Olshansky has not yet been reached (Fries 2003; Jacobzone, Cambois, and Robine 2000; Wilmoth 2000), and age-specific mortality continues to improve, particularly at older ages. Mortality rates for people aged seventy-five and older in the United States fell 1.2 percent annually between 1980 and 2003 (CDC 2006). But since age-specific disability declined even faster (1.5 percent or more), the proportion of life characterized as “disability free” has expanded (Crimmins 2004; Crimmins, Hayward, and Saito 1994; Crimmins, Saito, and Reynolds 1997; Jagger, Barberger-Gateau, and Robine 2005; Manton 1988; Mathers et al. 2004; Singer and Manton 1998). For example, Crimmins, Saito, and Ingegneri (1997) estimated that the portion of life after sixty-five expected to be free of disability rose from 49 percent in 1980 to 51 percent in 1990 (also see Crimmins and Saito 2001). More recently, Sagardui-Villamor and colleagues (2005) estimated substantially larger improvements in Spain between 1986 and 1999, with the disability-free portion growing by 20 percentage points for both sexes.
Combining morbidity prevalence data and mortality incidence data into one calculation presents potential issues concerning timing and cohort effects (Barendregt, Bonneux, and Van der Maas 1994; Mathers and Robine 1997; Murray, Salomon, and Mathers 2000). Simplifying life into a three-stage model—healthy, pre-death morbidity, and death—does not allow for transitions back and forth between the healthy and morbid states or for gradations of morbidity. To address this concern, researchers have introduced multistate models that incorporate rates of incidence and remission from states of morbidity (e.g., Crimmins, Saito, and Reynolds 1997; Manton and Stallard 1996, Mathers and Robine 1997), but empirical applications of these models show relatively little change from the base estimates (Murray, Salomon, and Mathers 2000). As a result, most of the literature continues to use the simpler life-table methods using morbidity prevalence.
It is tempting to conclude that the compression of morbidity evident in the estimations of increasing disability-free life expectancy should translate into less money spent on health care. As a first approximation, we can link the epidemiological transition to expenditure trends, in which the costs in the hatched portions of the bars will be relatively high and the costs in the remainder, relatively low. In that case, a compression-of-cost scenario would predict a relatively short period of high expenditures (often in the period right before death), whereas an expansion scenario would predict a relatively long period of high expenditures associated with longer survival with a chronic disease.
For example, Singer and Manton (1998) argue that if the 1.5 percent annual rate of decline observed in U.S. disability rates were to continue through 2070, it could fully offset the expected economic burden of an aging population and that the support ratio (the number of economically active persons aged twenty to sixty-four relative to the number of chronically disabled persons aged sixty-five and older) could be maintained at 1994 levels. If there were no further improvements in morbidity, however, this ratio would fall by more than 60 percent. Jacobzone, Cambois, and Robine (2000) reached similar conclusions from a broad survey of OECD disability and demographic data, projecting that the growth of long-term care spending as a share of GDP could be completely eliminated by improved disability trends in the United States and could be substantially reduced even in OECD countries with lower projected growth in the working-age population.
Jacobzone and colleagues (1998), however, sounded a note of caution in linking disability trends to developments in health expenditures. Apparent improvements in population morbidity may be due to one or a combination of the following factors: the increased usage of the health care system, more effective health care, and healthier lifestyles. Different combinations of these variables carry different implications for expenditures. In some studies, elderly people's greater independence parallels their greater use of technology and personal aids, suggesting that better—and more expensive—management of symptoms may be more responsible for the observed improvement than better individual health is (Freedman et al. 2004; Manton, Stallard, and Corder 1995; Spillman 2004).
Because health care services may be used to prevent disease and mitigate symptoms, the declines in disability and poor self-rated health cannot, without further research, be directly linked to less consumption of care. The evidence presented in this section regarding morbidity and mortality trends describes developments in age-specific health status, which is the second factor in Cutler and Sheiner's decomposition of health expenditures. To complete the picture, evidence is required for the third factor: medical spending associated with health status. The remaining sections of this article review the literature examining expenditure data in the context of mortality.
The Cost of Dying
Cost-of-dying studies fall into two broad categories. Decedents-only studies count “lifetime costs” (often from eligibility for U.S. Medicare at age sixty-five to death) of known decedents, plot these against time-to-death, and compare total costs and the shares of total costs incurred in the last year(s) of life for decedents of different ages. Decedents-versus-survivors studies compare the health care expenditures of individuals dying in a given period with those in the same age cohort who continue living. The results are often expressed as decedent/survivor cost ratios. The second method is used in a broader range of studies, allowing comparisons across different sectors of the health care system, jurisdictions, and age groups. With health status approximated by proximity to death, cost-of-dying estimates can estimate Cutler and Sheiner's third factor: the intensity of care for a given health status.
Decedent Costs and Age
Although the relationship between health care expenditures and death changes significantly with the age at death, it is important to note which health care sectors are included in the expenditure measurement. For services covered by U.S. Medicare, 30 to 50 percent less is spent in the last year(s) of life on decedents at ages older than eighty-five versus those between sixty-five and seventy-five (Hoover et al. 2002; Levinsky et al. 2001; Lubitz, Beebe, and Baker 1995; Lubitz and Prihoda 1984; Lubitz and Riley 1993; Miller 2001; Yang, Norton, and Stearns 2003), with a greater decline with age in decedents' hospital costs. That is, hospital costs for decedents aged eighty-five and older are estimated to be 50 percent lower than for those between sixty-five and sixty-nine in the United States (Yang, Norton, and Stearns 2003) and as much as 70 percent lower in Denmark (Madsen, Serup-Hansen, and Kristiansen 2002).
These results appear to depend partly on how care is organized and financed. For example, one Canadian study (McGrail et al. 2000) found that hospital costs dropped 30 to 35 percent between the oldest and youngest age groups. This drop is not reflected in the number of days spent in hospital, however; Roos, Montgomery, and Roos (1987) and Menec and colleagues (2004) estimated that the number of inpatient hospital days in Manitoba peaked for decedents aged seventy-five to eighty-four and that there was relatively little difference between the over-eighty-five and the sixty-five-to-seventy-four age groups, implying that although the number of hospital days did not decline linearly with age, the intensity of services received per hospital day did. Lower intensity of hospital care with age was found as well in the U.S. Medicare program (Levinsky et al. 2001; Long and Marshall 2000). In contrast with the Manitoba results, Busse, Krauth, and Schwartz (2002) found age-related declines of nearly 50 percent in the number of days that decedents spent in hospitals in Germany. These findings point to potentially significant differences in the way that hospitals are used in different jurisdictions.
But hospital and Medicare costs are only part of the health care system, and other sectors may not have the same relationship between age and decedent costs. In particular, services such as nursing homes and home care, which are not always covered by public funding, are largely used by the very old (or very ill) and may substitute for hospitals or emergency rooms (Werblow, Felder, and Zweifel 2005). A key policy question is who bears what costs and whether financial barriers to using certain services might affect observed expenditures.
The literature confirms that nonhospital costs-of-dying show trends that are opposite to and often greater than those for hospital and Medicare costs. Decedents' nursing home costs, in particular, rise dramatically with the age at death, typically by a factor of five or more when comparing the youngest and the oldest old (McGrail et al. 2000; Menec et al. 2004; Roos, Montgomery, and Roos 1987; Spillman and Lubitz 2002; Yang, Norton, and Stearns 2003). The costs of sectors outside hospitals and nursing homes are less commonly studied, but the available evidence suggests that home care and outpatient pharmaceutical costs demonstrate a similar, but somewhat weaker, increase as that of nursing home costs (McGrail et al. 2000; Menec et al. 2004; Spillman and Lubitz 2002; Yang, Norton, and Stearns 2003). Expenditures on physicians more closely resemble hospital costs, although for decedents the decline, with age, in spending is somewhat weaker (Madsen, Serup-Hansen, and Kristiansen 2002; Menec et al. 2004). Hoover and colleagues (2002) found that all non-Medicare costs combined doubled for decedents aged eighty-five and older versus sixty-five to seventy-four, while Yang, Norton, and Stearns (2003) showed that Medicaid costs (mainly nursing homes costs at these ages) more than tripled for the same age groups.
When nursing home and non-Medicare costs are combined with hospital and/or Medicare costs to obtain decedents' costs for a more complete spectrum of health care services, a more stable relationship between age and cost emerges. Hoover and colleagues (2002) and Yang, Norton, and Stearns (2003) all found that the total costs for decedents changed minimally with the age at death. In contrast, McGrail and colleagues (2000) estimated a rise of 42 percent in Canadian decedents' costs for ages eighty-five to eighty-seven, as compared with age sixty-six. Such differences, in a jurisdiction with relatively high levels of public funding, may reflect the potential impact of service mix and the extent of public financing. Because the mix of services received changed from primarily hospital-based care for the younger old to primarily nursing home and home care for those eighty-five and older, more generous coverage for nonhospital services (e.g., through Medicaid) could raise the cost differential with age. When putting together all costs, the higher average age at death does not reduce the economic burden to society of caring for the dying, although it does lower the portion for acute care. At best, decedents' costs remain stable with age. But who bears those costs is likely to vary, with lower costs to public payers in systems that leave more of the costs of nursing homes and home care to private insurers and/or individuals and their families.
Although they do not directly estimate expenditures on survivors, the lifetime cost studies do shed light on the important question of whether longer lifetimes entail higher cumulative expenditures. As with other findings in this literature, the difference between the costs of Medicare or hospitals and those for nursing or social care is substantial. Evidence of lifetime Medicare costs indicates that cumulative costs rise at a decelerating rate with age at death to age ninety and then level off (Gornick, McMillan, and Lubitz 1993; Lubitz, Beebe, and Baker 1995). Rather than add more years of expenditure, longer lifetimes are more likely to delay but not increase the years of heavy spending, not unlike scenario 2b from figure 2. When non-Medicare expenditures are considered, however, the conclusions change (Spillman and Lubitz 2000, 2002). Lifetime expenditures for nursing homes and home care grow at an accelerated rate with age at death, pointing more to scenario 2a's expansion of morbidity.
Survivor Costs and Age
Although a higher average age at death may not reduce decedents' average costs, lower mortality rates will nonetheless lower the percentage of decedents in any age group, so that decedents' total costs to society could still fall. This decline would be only temporary, since every cohort will eventually die and incur the costs of dying, but a continued decline in mortality could help spread out what would otherwise be a rapid acceleration in expenditures as the population ages. However, if the expansion of morbidity thesis holds, or if the intensity of care for survivors grows, then the growing survivor cohorts created by lower mortality rates will place heavy demands on the health care system.
Table 1 summarizes the cost-of-dying studies. Here we move from the decedent-only results summarized earlier to studies comparing survivors and decedents within the same age cohort, thus offering insight into the health expenditures of survivors of different ages. Similar to the case of decedent costs, nursing home and non-Medicare survivor costs rise much faster with age than do the costs of hospitals and the Medicare program. Nursing home costs for survivors in the younger ages near sixty-five are very low, and as a consequence of this low base, the growth of costs for older survivors is high and variable. Nursing home costs for ages over eighty-five are estimated to be anywhere from twelve (Roos, Montgomery, and Roos 1987) to more than thirty times (McGrail et al. 2000) those at age sixty-five. Taking into account all non-Medicare costs, when the costs for younger survivors are higher than for nursing homes alone, Hoover and colleagues (2002) calculated a smaller multiple of 3.6 times in comparing those aged eighty-five and older with those aged sixty-five to seventy-four.
TABLE 1.
Summary of Cost-of-Dying Studies
| Study | Year(s) of Data | Age Group(s) Studied | Sector(s) of Health Care System Included | Lifetime Comparison ? | Intra-cohort Comparison ? | Decedent/Survivor Cut-Off | Decedent- Age Trend | Survivor- Age Trend | Decedent/Survivor Ratio | Other Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Lubitz and Prihoda 1984 | 1977, 1978 | 67–69, 70–74, 75–79, 80–84, 85+ | Medicare expenditures | n | y | 1 year compared to 2 or more years | Costs fall by 43% from ages 67–69 to ages 85+ | Costs rise by 50% from ages 67–69 to ages 85+ | For all ages hospital cost ratio was 7.3, physician cost ratio 3.9; decedent/survivor ratio in 2nd-to-last year of life was 2.3 | Average hospital length of stay is stable with age and hospitalization rate falls for decedents, while both rise for survivors |
| Lubitz and Riley 1993 | 1976, 1979, 1983, 1988 | 65 and higher | Medicare expenditures | n | y | Last year vs. more than 1 year | In 1976 costs fell 40% from ages 65–69 to ages 85+, in 1988 fell 34% | In 1976 costs rose 57% from ages 65–69 to ages 85+, in 1988 rose 69% | 10.7 for 65–69 to 4.1 for 85+ for both sample years | Conclude that policy changes (Prospective Payment System and hospice addition to Medicare) have had little effect |
| Lubitz, Beebe, and Baker 1995 | 1974–1988 | 65–101 (at death) | Medicare expenditures | y | n | Last 2 years/age 65 to last 2 years | Costs fall by 32% from ages 70 to 90 | No trend | Average costs in last 2 years of life relative to years 3–10 before death range between 1.6 for age 100 and 2.1 for age 80 | |
| Gornick, McMillan, and Lubitz 1993 | 1974–1988 | 80, 90, 100 (at death) | Medicare expenditures | y | n | 16-years-to-death costs fall by 32% for death at age 100 vs. 80 | Costs in past 16 years rise by 19% from ages 80 to 90 and fall 10% from ages 90 to 100 | From 1.4 (age 100) to 2.1 (age 80) | Graphs indicate decedent and survivor experience begin to separate as much as 8 years before death | |
| Long and Marshall 2000 | Unspecified | 75–79, 80–84, 85–89, 90+ | Hospitals only, managed care admissions | n | y | Last year of life vs. more than 1 year | Costs rise by 5% from ages 75–79 to 80–84 and fall 40% from ages 80–84 to 90+ | Costs rise by 5% from ages 75–79 to 80–84 and fall 32% from ages 80–84 to 90+ | From 2.2 to 3.0, no apparent age trend | Decline in decedent costs more linear and higher for last month vs. last year of life, intensity of treatment for decedents declines in oldest vs. youngest age group |
| Levinsky et al. 2001 | 1996 | 65–74, 75–84, 85+ | Hospitals only, managed care admissions | n | n | Last year of life, no survivors included | Costs in last year of life decline 33% between youngest and oldest ages; hospital and ICU admissions decrease 30% and 50%, respectively; use of ventilators and respirators down 67% | No survivors in data | Decedents using hospice no more or less expensive than those not using hospice; by place of death highest for hospital, then nursing home, then residence | |
| Miller 2001 | 1974–1990 | 65+, 75, 85, 95 | Medicare expenditures | y | n | No explicit cut-off: each year can be compared | Costs fall by 48% from ages 75 to 95 | Costs in the 9th year before death rise by 11% from ages 75 to 95 | Last year divided by 9th-to-last year ranges from 7.1 for age 75 to 3.3 for age 95 | Effect on spending of death appears to begin four years in advance, less for older |
| Van Weel and Michels 1997 | 1991–1995 | 65+ | Dutch acute care and primary care | y | n | Last 18 months/age 65 to last 18 months | No trend, one cohort, 8,000 Dutch florins per year | No trend, one cohort, 4,200 Dutch florins per year | 1.9 for all ages > 65 | Estimate from insurance data that costs in last 18 months of life for ages 65+ were 3 times those in earlier years |
| Madsen, Serup-Hansen, and Kristiansen 2002 | 1995 | All ages | Danish acute care and primary care | n | y | Last year vs. more than 1 year | Hospital costs fall by 70% from ages 65 to 95+; primary care costs fall by 33% from ages 65 to 85+ | Hospital costs rise by 66% from ages 65 to 95+; primary care costs rise by 12% from ages 65 to 85 then fall by 29% from 85 to 95+ | Hospital from 10 (65) to 1.8 (95); primary from 1.2 (65) to 0.6 (95) | |
| Spillman and Lubitz 2000 | 1974–1996 | 65 and higher | Medicare expenditures, nursing home, and home care costs | y | n | Last 2 years/age 65 to last 2 years | Total costs rise by 22% from ages 70 to age 90; Medicare costs fall 37%; nursing home costs rise by factor of 5x | No trend | Not comparing individuals in 1 time period, rather comparing last 2 years to earlier history among same cohort, mix changes to nursing homes | |
| Hoover et al. 2002 | 1992–1996 | 65–74, 75–84, 85+ | Medicare expenditures, nursing home, and home care costs | y | y | Last year vs. more than 1 year | Total costs stable from ages 65–74 to 85+; Medicare costs fall 35%; non-Medicare costs rise by factor of 2x | Total costs rise 144% from ages 65–74 to 85+; Medicare costs rise 56%; non-Medicare costs rise by factor of 3.6x | Total cost from 2.7 (ages 85+) to 6.5 (ages 65–74) | Medicare ratio from 3.6 (ages 85+) to 8.7 (ages 65–74) and non-Medicare from 2.1 to 3.7 for same ages |
| Roos, Montgomery, and Roos 1987 | 1973–1982 | 45–64, 65–74, 75–84, 85+ | Canadian acute care, nursing home, and primary care | n | y | Each of last 4 years vs. 8 years or more | Last 4 years hospital days rise 36% from ages 65–74 to 75–84, then fall 14% to 85+; nursing home days rise by factor of 6.4x from 65–74 to 85+ | Hospital days rise 133% from ages 65–74 to 85+; nursing home days rise by factor of 11.8x over same ages | Based on 4-year average, hospital from 6.5 to 3.5; nursing home from 4 to 2.5 | Hospital utilization is affected by pending death as much as 8 years in advance; this effect declines with age; primary care and nursing home utilization are not significantly affected by age at death or time to death |
| McGrail et al. 2000 | 1986, 1993 | 65, 75–76, 85–87, 90–93 | Canadian acute care, nursing home, home care, and primary care | n | y | Last year vs. more than 1 year | Ages 65 to 90–93, medical falls 32% in 1986 and 36% in 1993; social/nursing rises by a factor of 6.9x in 1986 and 5.2x in 1993 | Ages 65 to 90–93, medical rises 150% in 1986 and 144% in 1993; social/nursing rises by a factor of 30x in 1986 and 42.5x in 1993 | 1993 medical from 18.1 (65) to 4.8 (90–93); social/nursing from 13.5 (65) to 2.0 (90–93) | Total costs for decedents rise 40%–50% in between 65 and 90–93 while for survivors rise 8x–9x |
| Yang, Norton, and Stearns 2003 | 1992–1998 | 65 and higher | Medicare, Medicaid, hospital, nursing homes, and out-of-pocket costs | n | y | 1 year or less to death versus 1 year or more to death | Total costs in last year of life stable; hospital costs fall 50% from 65–69 to 90–94; nursing home costs rise by factor of 5x | Total costs rise by a factor of 4x from ages 65–69 to 90–94; hospital costs rise slightly; nursing home costs rise more than 20x | From 6 at age 65 to 1.5 at age 95 | Medicare and hospital costs fall sharply with age for decedents and rise gradually for survivors; Medicaid and nursing home costs rise sharply with age for decedents and survivors |
| Busse, Krauth, and Schwartz 2002 | 1989–1995 | 20–85+ in 5-year bands | German hospital days and costs | n | y | Each of last 3 years vs. more than 3 years | Average hospital days fall for 85+ vs. 65–74 by 36% in last year; 50%–60% in 2nd- and 3rd- to-last years | Average hospital days rise by 80% for ages 85+ vs. 65–74 not in last 3 years of life | The fall in average hospital days for decedents is nearly equally attributed to lower probability of entering hospital and lower average lengths of stay |
Unlike the case of decedents' costs, survivors' hospital and Medicare costs continue to rise with age and are 50 to 70 percent higher for the older old versus the younger old (Hoover et al. 2002; Lubitz, Beebe, and Baker 1995; Lubitz and Riley 1993; Madsen, Serup-Hansen, and Kristiansen 2002). Again, studies that measure inpatient hospital days instead of costs estimate a higher growth rate with increasing age (Busse, Krauth, and Schwartz 2002; Menec et al. 2004; Roos, Montgomery, and Roos 1987), suggesting that the intensity of hospital care may diminish for the oldest categories. The total costs from all components of the health care system for survivors more than double from age sixty-five to seventy-four to age eighty-five and older (Hoover et al. 2002) and continue to grow for even older ages. McGrail and colleagues (2000) compared sixty-five-year-olds with those aged ninety to ninety-three and found that the total costs for survivors in the older cohort were eight to nine times as high.
The combination of slow or no growth in decedents' total costs and rapid growth in survivors' costs with age leads to an increasingly small difference between decedents and survivors at older ages. The ratio of decedents' to survivors' costs for all services falls from six times or higher at age sixty-five to less than three times higher at ages older than eighty-five (Hoover et al. 2002; McGrail et al. 2000; Yang, Norton, and Stearns 2003). A smaller difference between decedents' and survivors' costs at older ages is perhaps not surprising. Since the remaining life expectancy of survivors is much shorter at older ages, the distinction between decedents and survivors is less clear.
Time Trends in Decedents' and Survivors' Costs
The evidence thus far compiled from the cost-of-dying literature identifies a number of conflicting forces that could affect health care systems as the population ages. On one hand, decedents in all age groups cost more than survivors do, suggesting that lower mortality rates would reduce expenditures. Countering this, as more people survive to older ages, the difference between decedents' and survivors' costs falls, so that further mortality gains at these ages offer a much smaller cost benefit. As the average age at death rises with declining mortality, a greater percentage of the population may experience a longer period of morbidity, so that at the population level, the expansion of morbidity may appear to hold.
The debate between the compression versus the expansion of morbidity introduced in the first section of this article is not so much about how end-of-life morbidity changes with age as about how end-of-life morbidity at any age changes over time. One key issue, as the proponents of the compression of morbidity theory might argue, is whether the high cost of elderly survivors is likely to change over time. The typical ninety-year-old of the future may be different in regard to health status, lifestyle, and preferences from the ninety-year-old of today and consequently may use the health care system in different ways. Potential future changes can be detected only by using current changes as a basis for projecting future developments.
Few cost-of-dying studies directly estimate time trends in either the ratios or the survivors' and decedents' costs separately. General trends can be inferred by comparing studies from different time periods, but care must be taken to ensure that the data and methods supporting the studies are consistent and comparable.
Spillman and Lubitz (2000) and Lubitz, Beebe, and Baker (1995) used the same methodology and the same (Medicare) data set, which enables us to calculate changes in Medicare costs from age sixty-five until two years before death and Medicare costs over the last two years of life for different ages. Table 2 shows the results of this calculation for ages seventy and ninety. Both end-of-life care and regular care grew faster for younger than for older ages, and survivors' costs for all ages grew much faster than decedents' costs did.
TABLE 2.
Changes in Medicare Expenditures, 1989–1996
| Medicare Expenditures Last 2 Years of Lifea | Medicare Expenditures prior to Last 2 Years of Lifea, b | Lifetime Medicare Expenditures | ||||
|---|---|---|---|---|---|---|
| Age at Death | 1989 | 1996c | 1989 | 1996 | 1989 | 1996 |
| 70 | $22,590 | $39,000 | $12,921 | $33,302 | $35,511 | $72,302 |
| 90 | $15,237 | $25,000 | $47,778 | $105,042 | $63,015 | $130,042 |
| Inflation-adjusteda | ||||||
| 70 | $34,337 | $39,000 | $19,640 | $33,302 | $53,977 | $72,302 |
| 90 | $23,160 | $25,000 | $72,623 | $105,042 | $95,783 | $130,042 |
| Growth, 1989–1996a | ||||||
| 70 | 14% | 70% | 34% | |||
| 90 | 8% | 45% | 36% | |||
1989 expenditures are in 1990 dollars, and 1996 expenditures are in 1996 dollars. CPI inflation during this period grew 26%, and medical care CPI grew 52%. Inflation-adjusted results are in 1996 dollars, adjusted for medical CPI. Growth rates reflect inflation adjustment.
Expenditures before the last 2 years of life begin at age 65 and therefore represent a different number of years for different age groups.
Figures for 1996 Medicare expenditures in the last 2 years of life are inexact, since they were obtained from a chart.
In contrast to the 1989–1996 comparison, a direct time trend analysis of Medicare expenditures provided by Lubitz and Riley (1993) for the period 1976–1988 found that inflation-adjusted decedent and survivor costs grew at close to the same rate for all ages, with survivor costs growing slightly faster than decedent costs for ages sixty-five to seventy-four and decedent costs growing faster at ages beyond seventy-five. Interestingly growth rates for both decedent and survivor costs increased with age: 1988 costs for ages sixty-five to sixty-nine were approximately 32 percent higher than 1976, while costs for ages eighty-five and older grew 44 percent. Overall, for all ages over sixty-five, growth in survivor costs was 43 percent versus 40 percent growth in decedent costs.
These differences in trends in Medicare costs indicate how health care provision and consumption may have changed in past decades. After a period where the highest growth in Medicare costs occurred at the oldest ages, the more recent data suggest higher growth in expenditures for younger age cohorts and for surviving populations. The timing of the change coincided fairly closely with the period in which morbidity prevalence rates among the elderly began to decline and health-adjusted life expectancy began to grow relative to total life expectancy (see first section of this article). The relationship between these coincidental trends is complex. More aggressive treatment for survivor conditions may be part of a broader focus on health among the new elderly cohorts that has contributed to their improving morbidity. Additionally, higher growth in costs for survivors could be caused by a higher success rate of treatment so that mortality rates fall and costs that would previously have been attributed to decedents now accrue to survivors. Relatively less growth in decedent expenditures and among the oldest cohorts could be due as much to changes in the health system as to individual health status. Despite mixed evidence on the cost-reducing potential of changes to end-of-life care such as hospice care, do-not-resuscitate orders, and other advanced directives (Scitovsky 1994), these developments may have had some effect.
McGrail and colleagues (2000) provide some additional context to the Medicare trends by examining hospital and social/nursing costs among decedents and survivors in British Columbia, Canada, for the years 1986 and 1993. In contrast to the Medicare data during the same time period, total inflation-adjusted per capita costs actually declined between the beginning and the end of the period for all ages. During the period, provincial budgets in Canada were under some pressure, leading to constrained health care spending. Nevertheless, the relative patterns among decedents and survivors and among different ages confirm those from the Medicare studies considered earlier. Decedent medical costs—mainly hospital and physician visits—declined more than survivor costs and experienced their largest decline for the oldest age group, ninety to ninety-three. Nursing costs behaved differently from medical costs in that decedent costs rose over the period while survivor costs fell. Both the largest rises in decedent nursing costs and the largest drops in survivor nursing costs were experienced by the youngest age groups, so that the difference between the two widened significantly for the youngest old and was nearly unchanged for the oldest old.
The contrast between developments in medical and social/nursing costs in British Columbia in combination with the Medicare trends from the United States tells a story that fits relatively well with an improvement in elderly morbidity and changes in intensity and mix of health care service provision. Medical costs are evidently growing more quickly among survivors than among decedents; and among decedents they are growing more quickly in younger than older populations. At the same time, social/nursing costs are growing faster among decedents, particularly the younger decedents. The combination of reduced social care costs for survivors and increases for decedents among the younger age groups supports the assertion that in recent years, cohorts of seniors (survivors) are increasingly independent. With younger survivors accounting for the smallest decrease in medical costs at the same time as younger decedents are receiving the largest increase in social/nursing services, it appears—albeit from a relatively limited sample—that health care systems are improving their ability to identify and care for patients with the best prospects of recovery while providing social care for those who cannot be cured. For older cohorts, aggressive medical care is being scaled back while the cost of social care remains elevated for decedents and survivors alike.
Time-to-Death Models of Health Care Expenditures
One step beyond comparing decedent and survivor populations within age cohorts is calculating expenditures for known decedents in regular time intervals counting backward from the date of death (e.g., Miller 2001; Roos, Montgomery, and Roos 1987). Using these data, costs can then be modeled as a function of time-to-death, providing a comprehensive estimate of the effect of impending death on expenditure trajectories. Compared with relative cost-of-dying studies, such models avoid issues that might be raised by dividing populations into decedents and survivors based on what is essentially an arbitrary, albeit convenient, threshold of time left alive. There is no theoretical reason why the point at which individuals enter their last twelve months of life should mark a natural health transition, such as that represented by the transition to the hatched portion of the bars in figure 2.
Indeed, the exploratory work of Roos, Montgomery, and Roos (1987) indicates that the transition may occur much earlier than one year before death. These writers modeled hospital and nursing home days as a function of age, sex, and time-to-death, with time-to-death represented by a series of dummy variables (taking the value 1 or 0) for each of the last eight years of life. This method compares utilization in these last eight years with that by all persons known to be at least nine years from death.
The results are stratified by age. People younger than seventy-five used both hospitals and nursing homes in each of the last eight years of life much differently from the individuals with more than nine years to live, implying that the group who eventually died had a higher morbidity for an extended period of time before death. For ages seventy-five to eighty-four, the difference remained significant in all years for nursing homes, but for hospitals the difference was significant only through the sixth year before death. At ages eighty-five and older, the effect of time-to-death on hospital utilization was visible only in the last year of life, whereas for nursing home days, it remained significant in the last four years.
As in the cost-of-dying analyses, the time-to-death model used by Roos, Montgomery, and Roos found a diminishing difference between decedents and survivors from younger to older ages. But where cost-of-dying expresses this difference as a declining ratio between the costs for those in the last year of life versus all others, the time-to-death model expresses it by a decreasing length of time at which the difference is significant when the analysis is repeated further and further away from the event of death. By focusing the analysis on the length of time before death during which decedents and survivors of the same age can be distinguished from one another, the time-to-death empirical approach relates more directly to the question of end-of-life morbidity and health-adjusted life expectancy described in the first section of this article and depicted in figure 2. To be sure, health care expenditures may be only an indirect indication of health status. But the time before death at which decedents' and survivors' use of the health care system begins to separate provides a useful empirical estimate for the point at which, on average, healthy life ends and end-of-life morbidity begins. Roos, Montgomery, and Roos's paper provides the foundation for an emerging literature of time-to-death expenditure modeling, which we review in the remainder of this section.
Estimating the Duration of End-of-Life Morbidity
As was the case in the cost-of-dying literature, in time-to-death models much depends on the way that time-to-death is incorporated into the model and how the surviving population is characterized. As can be seen from the survey in table 3, the majority of the time-to-death models include only known decedents in their sample (Seshamani and Gray 2002, 2004a, 2004b; Zweifel, Felder, and Meiers 1999). When only decedents are included, the comparison is typically between a baseline of observations furthest from death and every subsequent observation as death approaches. The baseline for comparison in the literature reviewed here ranges from the sixteenth year (Seshamani and Gray 2004b) to the eighth quarter before death (Seshamani and Gray 2004a; Stearns and Norton 2004; Zweifel, Felder, and Meiers 1999). The results show that expenditures begin to rise over a very long time period as death approaches, even from a baseline as far back as sixteen years for hospital expenditures, and that the difference becomes significant at the thirteenth year before death (Seshamani and Gray 2004b).
TABLE 3.
Summary of Time-to-Death Studies
| Study | Year(s) of Data | Age Group(s) Studied | Sector(s) of Health Care System Included | Baseline for Comparison | Effect of Time-to-Death | Effect of Age, Controlled for Time-to-Death | Age and Time-to-Death Combined Effect | Time Trend in Age and Time-to-Death Effects | Other Findings |
|---|---|---|---|---|---|---|---|---|---|
| Roos, Montgomery, and Roos 1987 | 1973–1982 | 45–64, 65–74, 75–84, 85+ | Canadian acute care, nursing home, and primary care | 9 or more years before death versus each of the last 8 years of life | More significant for nursing home days than for hospital days, especially at older ages | Age controlled for time-to-death still has a positive and significant effect with the exception of hospital days for ages 85+; the effect of age diminishes for both hospitals and nursing homes with older strata | The effect of time-to-death diminishes for both nursing home and hospital days; at ages 65–74, all eight years before death significantly different from year 9 or more; for 85+ only last year different for hospital and last four years for nursing home | Not available | |
| Zweifel, Felder, and Meiers 1999 | 1981–1992, 1991–1994 | All ages, 65+, deceased only | Swiss sick fund, broad multi-sector | 8th quarter before death | Quarters 1–6 significantly different from 8 in first time period; in second time period only quarters 1–3 (all ages) and quarter 1 (65+) significantly different | Negative effect, statistically insignificant, for ages 65+; significant effect for all age sample (positive 1981–1992, negative 1991–1994); coefficient for Age2 is reverse sign in all cases | Quarters 1–6 significantly different from 8 in first time period; in second time period only quarters 1–3 (all ages) and quarter 1 (65+) significantly different; in second time period, effect of time-to-death appears reduced at older versus younger ages | Between first and second time period, effect of time-to-death appears reduced for all ages and older ages | In first sample, using 20th quarter before death as comparison, significant difference remains only to quarter 7; coefficients can be negative closer to quarter 20 (e.g., quarter 19 expected expenditures less than quarter 20) |
| Seshamani and Gray 2004b | 1963–1999 | Dying at ages 65+ in 1970 and after | UK data from a single hospital | 16th year before death | Significant for years 1 to 13 | Age is positive and significant; Age2 negative but not significant | Significant for years 1 to 13; time-to-death/expenditure curve gets flatter with older ages (i.e., time-to-death is less significant with age) | Time-to-death/expenditure curve gets flatter with more recent cohorts (i.e., time-to-death is recently less significant) | |
| Seshamani and Gray 2004a | 1963–1999 | Dying at ages 65+ in 1970 and after | UK data from a single hospital | 20th quarter before death | Quarters 1–3, 5, and 8 significantly different from 20; quarter 1 has negative coefficient (due to curtailed length-of-stay) | On expenditures, statistically insignificant both for Age and Age2; on probability of utilization, age is significant and positive | Quarters 1–3, 5, and 8 significantly different from 20; quarter 1 has negative coefficient (due to curtailed length-of-stay); in last quarter of life, costs peak at ages 80–85, decline thereafter | Not available | Different effects of age than Zweifel, Felder, and Meiers; age effect is parabolic, rising to age 85 and falling thereafter |
| Stearns and Norton 2004 | 1992–1998 | 66–99, survivors and decedents | Medicare | Not specified, assumed to be all persons 9 or more quarters from death | Significant in each quarter of the last two years of life | Remains almost as strong as uncontrolled effect of age; in both cases the coefficient for ages 66–70 through 90–95 are positive and significant relative to higher ages; age 70–75 is the highest expenditure age range | Significant in each quarter of the last two years of life; age has a negative influence on the effect of time-to-death for all quarters before death; this effect is not so much on likelihood of utilization as on intensity of utilization | Not available | The effect of age is on likelihood of use; expenditures given use are not sensitive to age; model results used in projections predict Medicare expenses in 2020 9% lower than models not using time-to-death |
| Seshamani and Gray 2002 | 1963–1999 | Dying at ages 65+ in 1970 and after | UK data from a single hospital | Data from (2004b) are used to project UK hospital expenditures 2002–2026; projections using time-to-death are 12% lower than baseline; expenditures due to last year of life as share of total projected to fall for every age group | |||||
| Breyer and Felder 2006 | 1999 | All ages | Swiss sick fund, broad multi-sector | Entire population surviving 43 months or more beyond 1999 | Stable (visible on graph) effect for time-to-death in each of the four years before death | Effect of Age is negative; effect of Age2 positive; significance not given | Time-to-death has a fairly consistent (rising) effect in the last four years of life on expenditures, but survivor expenses rise steadily with age, so relative comparison falls | Not available | Projections for 2050 assuming rise in life expectancy are compared using (1) time-to-death estimates, (2) age only survivor status-naïve, (3) time-to-death with medical technology growth of 1%; the technology effect in (3) dramatically outweighs the difference between (1) and (2) |
| Werblow, Felder, and Zweifel 2005 | 1999–2004 | All ages | Swiss sick fund, broad multi-sector | Compared to all individuals 5 or more years from death | Time-to-death is a linear variable in this model; found to be significant for total costs, and to have greatest effect on hospital costs | Effect of Age and Age2 are of opposite sign, but signs change depending on the service measured; broad result is that age has minor effect on expenditures, peaking at around 80 for the elderly, but age is more significant and positive for social and physician care | The coefficient of the interaction of the binary dummy variable for death and age is consistently negative and significant, so that older age reduces the effect of death (except for nursing homes); interaction with time-to-death is not available | Not available | Time-to-death outweighs the effects of age for hospital costs, but age is the more important effect for long-term care and home care costs |
Studies whose baseline group for comparison includes persons with no known date of death are relatively few and use varying methodologies, so it is difficult to identify any consistent conclusions from their results. In their study of Medicare expenditures, Stearns and Norton (2004) used as their baseline cohort those persons known to be nine or more quarters from death and found that the expenditures in each of the last eight quarters of life differed significantly from the average of this baseline. The effect of time-to-death appears even stronger than when only decedents are included, an unsurprising result, since low-utilization survivors are now captured in the comparison.
Werblow, Felder, and Zweifel (2005) and Breyer and Felder (2006) also found a strong effect for time-to-death in their Swiss sickness fund data, using a slightly different technique for incorporating survivors and for time-to-death modeling in general. Instead of representing each year or quarter with a dummy variable, time-to-death is represented as a linear variable taking the value 1 in the last year or quarter of life, 2 in the second-to-last year or quarter, and so on. For the surviving cohort, the time-to-death variable is maximized at the minimum known time of life. For example, any person known to live more than five years is assigned a time-to-death of sixty months, even if their actual time-to-death is much higher (Werblow, Felder, and Zweifel 2005). Unfortunately, from the perspective of this review, representing time-to-death as a linear variable makes it impossible to identify a notional transition point—that is, the beginning of the hatched bars in figure 2—that is made possible with dummy variables for each individual time period.
Time-to-Death and Age
Findings from time-to-death models generally confirm the cost-of-dying result that impending death increases expenditures but that the magnitude of the difference tends to diminish at older ages. In Oxford, England, Seshamani and Gray (2002, 2004a, 2004b) found that hospital costs in the last years peaked at ages eighty to eighty-five and fell thereafter. For survivors, Breyer and Felder (2006) calculated, using Swiss sickness fund data, their costs as rising steadily with age. The result of comparing age-expenditure curves for decedents and survivors is a time-to-death effect that declines with age, especially in the oldest age groups. Stearns and Norton (2004) used U.S. Medicare data to directly measure the effect of the interaction between age and time-to-death. They found that the positive effect on expenditures in any of the last eight quarters preceding death significantly diminished with age. A similar negative effect of the interaction between age and time-to-death was estimated in the Swiss sickness fund model of Werblow, Felder, and Zweifel (2005).
The effect of age on health care expenditures after controlling for time-to-death is of particular interest to researchers and is germane to the policy questions about the future health care demands of an aging population. In particular, researchers have been asking whether the positive relationship between age and health expenditure is simply an artifact of higher mortality rates at older ages. Grossman's (1972) highly influential model of health care as an investment in human capital indicated that for a given health status, the decreasing value of healthy time and the decreasing expected length of life could reduce the equilibrium level of desired health as age rises. If this is so, any model that includes both time-to-death and age should estimate a negligible, or even negative, effect for age.
The time-to-death literature is mixed on the question of the effect of age after controlling for time-to-death. In one of the first models of this type, Zweifel, Felder, and Meiers (1999) used Swiss sickness fund data from the years 1981 to 1994 and found that the effect of age was negative and statistically insignificant for the cohort of all persons sixty-five and older. This result is the catalyst for their often-cited assertion that age is a “red herring” and is not, in fact, an important determinant of expenditures beyond the effect of increasing mortality. However, when controlled for time-to-death the result of age-neutral expenditures does not hold in other, similar studies. The estimated effect of age is positive and significant in time-to-death models of Oxford hospital expenditures (Seshamani and Gray 2004a, 2004b), in Medicare expenditures (Stearns and Norton 2004), and for long-term care and physicians' expenditures from the same Swiss sickness fund originally studied by Zweifel, Felder, and Meiers (data from a later time period) (Breyer and Felder 2006; Werblow, Felder, and Zweifel 2005). The authors of the last study did confirm the red herring result for persons who do not use long-term care services, thereby effectively amending their thesis to a “school of red herrings” consisting of selected sectors of the health care system.
It is not entirely surprising that the evidence on age as a red herring is mixed. Our review of the cost-of-dying literature has identified several age trends that should be captured in the single coefficient of age in the time-to-death models. On one hand, decedents' costs rise with age for some services and fall with age for others and, in some cases, rise to a certain age and then fall thereafter. On the other hand, survivors' costs generally rise with age for all services. Typically we might expect that because survivors make up the majority of any population and that costs rise with age, age would still have a positive effect on expenditures even after controlling for proximity to death. The “red herring” studies of Zweifel, Felder, and Meiers and of Werblow, Felder, and Zweifel show that after controlling for death, expenditures are age neutral in populations within five years of death. It is possible, however, that the inclusion of longer-surviving populations in the analyses could alter this result.
Time Trends and Future Predictions
The greater detail of the statistical models in the time-to-death literature offers an opportunity for more specificity in both identifying time trends in the relationship between expenditures and death and adjusting forecasts of future health expenditures. For data sets covering a number of years, calendar time itself can be included as a variable in the model specification, as the Zweifel, Felder, and Meiers and the Seshamani and Gray studies do. Coefficients of the calendar year dummy variables steadily rise with time, capturing the generally rising trend in per capita health care expenditures that has been the rule in the developed world.
If calendar time variables interacted with time-to-death variables in the models, the coefficients of these interaction terms would represent statistical estimates of the time trend in the effect of time-to-death. Unfortunately, the models reviewed here have not taken this step. But two of the studies do provide some evidence on time trends in other ways. Zweifel, Felder, and Meiers (1999) separated their data into two time periods, 1981 to 1992 and 1991 to 1994. Using the eighth quarter before death as a benchmark, they found that expenditures in the last six quarters were significantly different in the first sample, whereas only the last three quarters (all ages) or last quarter (65+) were significantly different in the second, indicating that the duration of end-of-life morbidity—the hatched bars of figure 2—may be shrinking. It is important to note, however, that this shorter duration could be due as much to rises in costs in the eighth quarter before death as to declines in the following quarters and that the analysis was limited to just the last two years of life, so we cannot make any firm conclusions about compression versus expansion of morbidity. Using a longer period for their analysis, Seshamani and Gray (2004b) plotted time-to-death and expenditure curves for the last ten years of life for cohorts dying in 1970, 1980, and 1990, which showed that the slope of the curve progressively flattened over time. The results of these two studies expand the findings from the cost-of-dying literature that health care expenditures during time periods close to death are growing more slowly than those further from death. While the evidence to date is fairly limited, the potential for time-to-death studies to provide more detailed confirmation of these trends, and to expand on them by examining how they change with age, presents an opportunity for future research.
The application of these results to modify forecasts of future health care expenditures is also in its early stages. Typically, two different methods of assigning future expenditures are applied to one or more demographic projection scenarios. The first method assumes constant age-specific spending rates, and the second incorporates the effects of time-to-death. By adjusting for the effects of time-to-death, the second method effectively takes into account the possible health expenditure effects of a change in future mortality. In the case of a demographic scenario in which mortality rates do not change, little difference would be expected between the two methods.
The results of these projections are fairly consistent. As a rule, greater projected mortality gains and longer projection periods lead to larger differences between projection methods. Stearns and Norton (2004) applied their Medicare time-to-death model to expenditure forecasts for cohorts aged sixty-six to seventy in 1998 and 2020 and projected lower expenditures than a strictly age-based model, by 9 percent and 15 percent, respectively. Miller (2001) also used a Medicare model to test the effect of time-to-death on different mortality scenarios through 2070. Under the most moderate mortality gains (life expectancy in 2070 equal to 82.0), the reduction in forecast expenditures is 15 percent, and under the most aggressive scenario (life expectancy of 93.5), the reduction grows to 57 percent. The difference between the time-to-death and age-based models using Seshamani and Gray's (2002) Oxford hospital data for projections to 2026 is estimated at 12 percent.
Similar exercises using broader data sets—including social care such as nursing homes and home care—from continental Europe estimate lower differences among projection methods. Madsen, Serup-Hansen, and Kristiansen (2002) reduced projections of Danish health care costs in 2020 by 3.4 percent using time-to-death methods, and Breyer and Felder (2006) obtained a reduction of 3.6 percent using Swiss sickness fund data and moderate mortality improvement through the year 2050. Since social care costs depend relatively more on age and associated frailty and less on time-to-death than do medical services like hospital treatment, the effect of including social care in projections of health care expenditures in an environment of falling mortality is to increase the positive influence of growing elderly cohorts and to decrease the importance of expense reductions due to fewer deaths.
Breyer and Felder added two interesting dimensions to their projection method. First, they introduced changes in end-of-life morbidity by shifting the age-expenditure curve by the amount of the expected increase in life expectancy. This adjustment reflects figure 2's postponement of morbidity scenario 2b and has the effect of lowering future expenditure projections by another 4.4 percent, for a total reduction of 8.0 percent when the effect of time-to-death also is included. If the compression of morbidity scenario in 2c were to occur, the reduction would be greater still.
A reduction of 8 percent in expenditure forecasts, though relatively small, still may be important given the size of the health care sector in developed countries. But the second added dimension of the Breyer and Felder model helps put the reduction in context and points to other, possibly more important, sources of health care cost increases. The authors introduced an age-independent growth factor of 1 percent in per capita expenditures, attributed to technological changes in medicine and based on a calculation for such changes from 1970 to 1995. Stretched out through 2050, this 1 percent of external growth adds 77 percent to the projected expenditures for that year, dwarfing the impact of different mortality and time-to-death scenarios. If the future is anything like the past in terms of technology and other drivers of health care cost inflation, attributions of rising health care expenditures to population aging—even as the percentage of the population over sixty-five rises significantly—may be missing the true culprits.
Conclusions
The literature reviewed here creates a reasonably detailed picture of the role of age, morbidity, and death in health care expenditures. The picture is not a uniform one, as there is considerable variation across categories of expenditures, as well as across age groups, and the trends are not always stable across jurisdictions or time periods. Morbidity appears to have been reduced in recent years for all ages, but the duration and severity of morbidity rise at older ages, so an aging population may experience more morbidity in aggregate. To age ninety, the lifetime use of hospital care grows relatively slowly with age at death and not at all thereafter, whereas the lifetime use of nursing home and home care services accelerates to age ninety and beyond.
These results point to the likely source of future age-driven pressures on expenditures. Social care services to maintain health and lifestyle are likely to grow much faster as a consequence of the population's aging than are medical treatments provided by hospitals and doctors. Prescription drugs, which are used for both curative and maintenance purposes, could fall somewhere in the middle. Yet in recent years, prescription drugs have been one of the fastest-growing areas of the health care system, with the costs growing for survivors and decedents alike. This discrepancy highlights the point made in the forecasts by Breyer and Felder (also see, e.g., Mayhew 2000) that other inflationary forces are at work, particularly technology, which certainly applies to the case of pharmaceuticals. Even as the proportion of the elderly population rises, it may not be decisive in determining how health care expenditures change.
The existence of factors potentially more important than age in contributing to future health spending growth should not discourage further exploration of the relationship among aging, mortality, and health care expenditures. Incorporating both age and proximity-to-death in expenditure models represents an important advance over simple age-based models, especially for the elderly, for whom mortality rates are high. Taking into account forecasts of lower mortality rates in both predicted per capita expenditures and population counts lowers the forecast of total expenditures. Even though the reduction in total future expenditures may be fairly small, the mix of services that account for these expenditures could change significantly, with important implications for policy and system management.
If the drop in expenditures because of falling death rates and lower costs of dying appears to be relatively small, the end-of-life morbidity literature offers another potential contributor to future cost reductions. The compression of morbidity at the end of life could further ease the pressure of aging on health care services by enabling persons of a given age and proximity to death to remain healthier and more independent than they were in the past. But even though the morbidity prevalence data broadly support the compression of morbidity, health expenditures have not experienced a corresponding relative decline in the populations where this is taking place. In fact, the evidence points in the opposite direction, in which the costs further from death have been growing faster and the time-to-death/expenditure curve has flattened. Among the possible explanations for this apparent discrepancy are that improvements in morbidity have been obtained through the use of more services. More evidence is needed for time trends in both morbidity and health expenditures relative to proximity to death to confirm these or other explanations.
The discrepancy between lower morbidity and higher expenditures also highlights the role of cohort effects. Comparing the expenditure/time-to-death profiles of different ages at any point in time compares cohorts that may be socioeconomically very different and may use the health care system in very different ways. If the experience of the first sixty years of the baby boom generation is any indication, the cohort effect could be most dramatic for this age group, which is wealthier, better educated, and more inclined to demand preventive and personalized health care services. While the baby boom is not yet part of the elderly population, the recent growth in per capita health expenditures in percentage terms has been higher for its age group, forty-five to sixty-four, than for the current elderly (CMS 2006). As cohorts with currently higher expenditures age, the overall costs may begin to accelerate even if mortality and morbidity continue to drop.
The potential policy responses to these complex dynamics are many. The shift in health care utilization from end-of-life care to prevention, chronic care, and symptom management has significant implications for resource and infrastructure planning for future system needs, implying less use of hospital care and more use of prescription drugs, nursing homes, and home care. In all cases, decision makers may wish to ensure that the costs of these services are justified by improvements in future life expectancy, quality of life, and/or future system costs. In addition, costs paid with public funds appear to be growing more slowly than costs financed through a public-private mix, thereby raising questions about who will be paying for what, whether the escalating costs are due partly to relatively less cost control in these mixed sectors, and whether issues related to access to needed services will emerge. To the extent that aging, by providing a seemingly simple explanation for health care expenditure growth, has diverted attention from other cost drivers, a better understanding of the role of aging should help focus the debate.
Acknowledgments
The authors wish to thank three anonymous referees and the editor for helpful comments and suggestions, which greatly improved the exposition of the paper. Dr. Coyte acknowledges funding support provided by the Canadian Health Services Research Foundation and the Canadian Institutes for Health Research.
References
- Bains M, Oxley H. Towards High-Performing Health Systems. Policy Studies. Geneva: Organisation for Economic Co-operation and Development; 2004. Aging-Related Spending Projections on Health and Long-Term Care. [Google Scholar]
- Barendregt JJ, Bonneux L, Van Der Maas PJ. Health Expectancy: An Indicator for Change? Technology Assessment Methods Project Team. Journal of Epidemiology and Community Health. 1994;48(5):482–87. doi: 10.1136/jech.48.5.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barer ML, Evans RG, McGrail KM, Green B, Hertzman C, Sheps SB. Beneath the Calm Surface: The Changing Face of Physician-Service Use in British Columbia, 1985/86 versus 1996/97. Canadian Medical Association Journal. 2004;170(5):803–7. doi: 10.1503/cmaj.1020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barer ML, Pulcins IR, Evans RG, Hertzman C, Lomas J, Anderson GM. Trends in Use of Medical Services by the Elderly in British Columbia. Canadian Medical Association Journal. 1989;141(1):39–45. [PMC free article] [PubMed] [Google Scholar]
- BC (British Columbia) Government Statistics. Population and Demography. British Columbia, Other Provincial and Territorial Populations: 1971–2006. BC Stats. [accessed January 10, 2007]. Available at, http://www.bcstats.gov.bc.ca/DATA/pop/popstart.asp.
- Breyer F, Felder S. Life Expectancy and Health Care Expenditures: A New Calculation for Germany Using the Costs of Dying. Health Policy. 2006;75(2):178–86. doi: 10.1016/j.healthpol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Busse R, Krauth C, Schwartz FW. Use of Acute Hospital Beds Does Not Increase as the Population Ages: Results from a Seven-Year Cohort Study in Germany. Journal of Epidemiology and Community Health. 2002;56(4):289–93. doi: 10.1136/jech.56.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) National Vital Statistics Reports. Deaths: Final Data for 2003. Washington, D.C.: U.S. Department of Health and Human Services; 2006. [Google Scholar]
- CDC (Centers for Disease Control and Prevention) National Center for Health Statistics: Trends in Health and Aging. Washington, D.C.: U.S. Department of Health and Human Services; 2007. [accessed January 10, 2007]. Available at, http://www.cdc.gov/nchs/agingact.htm. [Google Scholar]
- CMS (Centers for Medicare and Medicaid Services) Personal Health Care Expenditures by Age: 1987–1999. Washington, D.C.: U.S. Department of Health and Human Services; 2006. [accessed May 15, 2006]. Available at, http://www.cms.hhs.gov/NationalHealthExpendData/downloads/agetables.pdf. [Google Scholar]
- Crimmins EM. Trends in the Health of the Elderly. Annual Review of Public Health. 2004;25:79–98. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Hayward MD, Saito Y. Changing Mortality and Morbidity Rates and the Health Status and Life Expectancy of the Older Population. Demography. 1994;31(1):159–75. [PubMed] [Google Scholar]
- Crimmins EM, Saito Y. Trends in Healthy Life Expectancy in the United States, 1970–1990: Gender, Racial and Educational Differences. Social Science and Medicine. 2001;52(11):1629–41. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Saito Y, Ingegneri D. Trends in Disability-Free Life Expectancy in the United States: 1970–90. Population and Development Review. 1997;23(3):555–62. [Google Scholar]
- Crimmins EM, Saito Y, Reynolds SL. Further Evidence on Recent Trends in the Prevalence and Incidence of Disability among Older Americans from Two Sources: The LSOA and the NHIS. Journals of Gerontology Series B: Psychological and Social Sciences. 1997;52(2):S59–71. doi: 10.1093/geronb/52b.2.s59. [DOI] [PubMed] [Google Scholar]
- Cutler DM, McClellan M. Is Technological Change in Medicine Worth It? Health Affairs. 2001;20(5):11–29. doi: 10.1377/hlthaff.20.5.11. [DOI] [PubMed] [Google Scholar]
- Cutler DM, Richardson E. Measuring the Health of the US Population. In: Baily M, Reiss P, Winston C, editors. Brookings Papers on Economic Activity: Microeconomics. Washington, D.C.: Brookings Institution; 1997. pp. 214–82. [Google Scholar]
- Cutler DM, Sheiner L. Demographics and Medical Care Spending: Standard and Non-Standard Effects. 1998. National Bureau of Economic Research Working Paper Series 6866, no. 33. [PubMed] [Google Scholar]
- Dang TT, Antolin P, Oxley H. Geneva: OECD; Fiscal Implications of Ageing: Projections of Age-Related Spending. [Google Scholar]
- Deber R, Forget E, Roos L. Medical Savings Accounts in a Universal System: Wishful Thinking Meets Evidence. Health Policy. 2004;70(1):49–66. doi: 10.1016/j.healthpol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Doblhammer G, Kytir J. Compression or Expansion of Morbidity? Trends in Healthy-Life Expectancy in the Elderly Austrian Population between 1978 and 1998. Social Science and Medicine. 2001;52(3):385–91. doi: 10.1016/s0277-9536(00)00141-6. [DOI] [PubMed] [Google Scholar]
- EPC (Economic Policy Committee of the European Union) Brussels: EPC; [accessed May 8, 2006]. The Impact of Ageing Populations on Public Finances: Overview of Analysis Carried Out at EU Level and Proposals for a Future Work Programme. Available at, http://ec.europa.eu/economy_finance/epc/epc_sustainability_ageing_en.htm. [Google Scholar]
- Freedman VA, Crimmins E, Schoeni RF, Spillman BC, Aykan H, Kramarow E, Land K, Lubitz J, Manton K, Martin LG, Shinberg D, Waidmann T. Resolving Inconsistencies in Trends in Old-Age Disability: Report from a Technical Working Group. Demography. 2004;41(3):417–41. doi: 10.1353/dem.2004.0022. [DOI] [PubMed] [Google Scholar]
- Freund D, Smeeding TM. The Future Costs of Health Care in Aging Societies: Is the Glass Half Full or Half Empty? Prepared for the Seminar: “Ageing Societies: Responding to the Policy Challenges,” Monday, April 8, 2002, University of New South Wales, Sydney, Australia.
- Fries JF. Aging, Natural Death, and the Compression of Morbidity. New England Journal of Medicine. 1980;303(3):130–35. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- Fries JF. Measuring and Monitoring Success in Compressing Morbidity. Annals of Internal Medicine. 2003;139, part 2(5):455–59. doi: 10.7326/0003-4819-139-5_part_2-200309021-00015. [DOI] [PubMed] [Google Scholar]
- Gerdtham UG. Pooling International Health Care Expenditure Data. Health Economics. 1992;1(4):217–31. doi: 10.1002/hec.4730010404. [DOI] [PubMed] [Google Scholar]
- Gerdtham UG, Sogaard J, Andersson F, Jonsson B. An Econometric Analysis of Health Care Expenditure: A Cross-Section Study of the OECD Countries. Journal of Health Economics. 1992;11(1):63–84. doi: 10.1016/0167-6296(92)90025-v. [DOI] [PubMed] [Google Scholar]
- Getzen TE. Population Aging and the Growth of Health Expenditures. Journal of Gerontology. 1992;47(3):S98–104. doi: 10.1093/geronj/47.3.s98. [DOI] [PubMed] [Google Scholar]
- Goetghebeur MM, Forrest S, Hay JW. Understanding the Underlying Drivers of Inpatient Cost Growth: A Literature Review. American Journal of Managed Care. 2003;9(SP1):SP3–12. [PubMed] [Google Scholar]
- Gornick M, McMillan A, Lubitz J. A Longitudinal Perspective on Patterns of Medicare Payments. Health Affairs. 1993;12(2):140–50. doi: 10.1377/hlthaff.12.2.140. [DOI] [PubMed] [Google Scholar]
- Grossman M. On the Concept of Health Capital and the Demand for Health. Journal of Political Economy. 1972;80(2):223–55. [Google Scholar]
- Hay JW. Hospital Cost Drivers: An Evaluation of 1998–2001 State-Level Data. American Journal of Managed Care. 2003;9(SP1):SP13–24. [PubMed] [Google Scholar]
- Hoover DR, Crystal S, Kumar R, Sambamoorthi U, Cantor JC. Medical Expenditures during the Last Year of Life: Findings from the 1992–1996 Medicare Current Beneficiary Survey. Health Services Research. 2002;37(6):1625–42. doi: 10.1111/1475-6773.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S, MacDonald G. The Relationship between Health Expenditure and GDP in Australia: Evidence from a New Approach. Perth, Western Australia: Curtin University of Technology, School of Economics and Finance; 2000. [Google Scholar]
- Jacobzone S, Cambois E, Champlain E, Robine JM. OECD Labour Market and Social Policy, Occasional Papers. Vol. 37. Geneva: 1998. The Health of Older Persons in OECD Countries: Is It Improving Fast Enough to Compensate for Population Ageing? [Google Scholar]
- Jacobzone S, Cambois E, Robine JM. Is the Health of Older Persons in OECD Countries Improving Fast Enough to Compensate for Population Ageing? OECD Economic Studies. 2000;30:150–90. [Google Scholar]
- Jagger C, Barberger-Gateau P, Robine JM. Disability in Older People—Indicators, Process and Outcomes. Disability and Rehabilitation. 2005;27(5):209–12. doi: 10.1080/09638280400006416. [DOI] [PubMed] [Google Scholar]
- Koenig L, Siegel JM, Dobson A, Hearle K, Ho S, Rudowitz R. Drivers of Healthcare Expenditures Associated with Physician Services. American Journal of Managed Care. 2003;9(SP1):SP34–42. [PubMed] [Google Scholar]
- Levinsky NG, Yu W, Ash A, Moskowitz M, Gazelle G, Saynina O, Emanuel EJ. Influence of Age on Medicare Expenditures and Medical Care in the Last Year of Life. Journal of the American Medical Association. 2001;286(11):1349–55. doi: 10.1001/jama.286.11.1349. [DOI] [PubMed] [Google Scholar]
- Long MJ, Marshall BS. The Relationship of Impending Death and Age Category to Treatment Intensity in the Elderly. Journal of Evaluation in Clinical Practice. 2000;6(1):63–70. doi: 10.1046/j.1365-2753.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Lubitz JD, Beebe J, Baker C. Longevity and Medicare Expenditures. New England Journal of Medicine. 1995;332(15):999–1003. doi: 10.1056/NEJM199504133321506. [DOI] [PubMed] [Google Scholar]
- Lubitz JD, Prihoda R. The Use and Costs of Medicare Services in the Last 2 Years of Life. Health Care Financing Review. 1984;5(3):117–31. [PMC free article] [PubMed] [Google Scholar]
- Lubitz JD, Riley GF. Trends in Medicare Payments in the Last Year of Life. New England Journal of Medicine. 1993;328(15):1092–96. doi: 10.1056/NEJM199304153281506. [DOI] [PubMed] [Google Scholar]
- Madsen J, Serup-Hansen N, Kristiansen IS. Future Health Care Costs—Do Health Care Costs during the Last Year of Life Matter? Health Policy. 2002;62(2):161–72. doi: 10.1016/s0168-8510(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Manton KG. Changing Concepts of Morbidity and Mortality in the Elderly Population. The Milbank Memorial Fund Quarterly. 1982;60(2):183–244. [PubMed] [Google Scholar]
- Manton KG. A Longitudinal Study of Functional Change and Mortality in the United States. Journal of Gerontology. 1988;43(5):S153–61. doi: 10.1093/geronj/43.5.s153. [DOI] [PubMed] [Google Scholar]
- Manton KG, Stallard E. Changes in Health, Mortality, and Disability and Their Impact on Long-Term Care Needs. Journal of Aging and Social Policy. 1996;7(3–4):25–52. doi: 10.1300/J031v07n03_03. [DOI] [PubMed] [Google Scholar]
- Manton KG, Stallard E, Corder L. Changes in Morbidity and Chronic Disability in the U.S. Elderly Population: Evidence from the 1982, 1984, and 1989 National Long Term Care Surveys. Journals of Gerontology Series B: Psychological and Social Sciences. 1995;50(4):S194–204. doi: 10.1093/geronb/50b.4.s194. [DOI] [PubMed] [Google Scholar]
- Manton KG, Stallard E, Liu K. Forecasts of Active Life Expectancy: Policy and Fiscal Implications. Journal of Gerontology. 1993;48 doi: 10.1093/geronj/48.special_issue.11. spec. no. 11–26. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Iburg KM, Begg S. Adjusting for Dependent Comorbidity in the Calculation of Healthy Life Expectancy. Population Health Metrics. 2006;4:4. doi: 10.1186/1478-7954-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Iburg KM, Salomon JA, Tandon A, Chatterji S, Ustun B, Murray CJ. Global Patterns of Healthy Life Expectancy in the Year 2002. BMC Public Health. 2004;4:66. doi: 10.1186/1471-2458-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Robine JM. How Good Is Sullivan's Method for Monitoring Changes in Population Health Expectancies? Journal of Epidemiology and Community Health. 1997;51(1):80–86. doi: 10.1136/jech.51.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Sadana R, Salomon JA, Murray CJ, Lopez AD. Healthy Life Expectancy in 191 Countries, 1999. Lancet. 2001;357(9269):1685–91. doi: 10.1016/S0140-6736(00)04824-8. [DOI] [PubMed] [Google Scholar]
- Mayhew L. Health and Elderly Care Expenditure in an Aging World. Laxenburg, Austria: International Institute for Applied Systems Analysis; 2000. [Google Scholar]
- McClellan M. Are the Returns to Technological Change in Health Care Declining? Proceedings of the National Academy of Sciences USA. 1996;93(23):12701–8. doi: 10.1073/pnas.93.23.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail K, Green B, Barer ML, Evans RG, Hertzman C, Normand C. Age, Costs of Acute and Long-Term Care and Proximity to Death: Evidence for 1987–88 and 1994–95 in British Columbia. Age and Ageing. 2000;29(3):249–53. doi: 10.1093/ageing/29.3.249. [DOI] [PubMed] [Google Scholar]
- Meara E, White C, Cutler DM. Trends in Medical Spending by Age, 1963–2000. Health Affairs. 2004;23(4):176–83. doi: 10.1377/hlthaff.23.4.176. [DOI] [PubMed] [Google Scholar]
- Menec V, Lix L, Steinbach C, Ekuma O, Sirski M, Dahl M, Soodeen R-A. Patterns of Health Care Use and Costs at the End of Life. Winnipeg: Manitoba Centre for Health Policy, Faculty of Medicine, University of Manitoba; 2004. [Google Scholar]
- Miller T. Increasing Longevity and Medicare Expenditures. Demography. 2001;38(2):215–26. doi: 10.1353/dem.2001.0018. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Salomon JA, Mathers C. A Critical Examination of Summary Measures of Population Health. Bulletin of the World Health Organization. 2000;78(8):981–94. [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co-operation and Development) OECD Tables and Figures on Aging. [accessed November 5, 2006]. Available at, http://www.oecd.org/dataoecd/27/44/2345400.pdf.
- Olshansky SJ, Carnes BA, Cassel C. In Search of Methuselah: Estimating the Upper Limits to Human Longevity. Science. 1990;250(4981):634–40. doi: 10.1126/science.2237414. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Rudberg MA, Carnes BA, Cassel CK, Brody JA. Trading off Longer Life for Worsening Health: The Expansion of Morbidity Hypothesis. Journal of Aging and Health. 1991;3(2):194–216. [Google Scholar]
- Omran AR. The Epidemiologic Transition: A Theory of the Epidemiology of Population Change. The Milbank Quarterly. 1971/2005;83(4):731–57. doi: 10.1111/j.1468-0009.2005.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt UE, Hussey PS, Anderson GF. Cross-National Comparisons of Health Systems Using OECD Data, 1999. Health Affairs. 2002;21(3):169–81. doi: 10.1377/hlthaff.21.3.169. [DOI] [PubMed] [Google Scholar]
- Robine JM, Michel JP. Looking Forward to a General Theory on Population Aging. Journals of Gerontology Series A: Biological and Medical Sciences. 2004;59(6):M590–97. doi: 10.1093/gerona/59.6.m590. [DOI] [PubMed] [Google Scholar]
- Robine JM, Mormiche P, Sermet C. Examination of the Causes and Mechanisms of the Increase in Disability-Free Life Expectancy. Journal of Aging and Health. 1998;10:171–91. [Google Scholar]
- Roos NP, Montgomery P, Roos LL. Health Care Utilization in the Years Prior to Death. The Milbank Memorial Fund Quarterly. 1987;65(2):231–54. [PubMed] [Google Scholar]
- Sagardui-Villamor J, Guallar-Castillon P, Garcia-Ferruelo M, Banegas JR, Rodriguez-Artalejo F. Trends in Disability and Disability-Free Life Expectancy among Elderly People in Spain: 1986–1999. Journals of Gerontology Series A: Biological and Medical Sciences. 2005;60(8):1028–34. doi: 10.1093/gerona/60.8.1028. [DOI] [PubMed] [Google Scholar]
- Scitovsky AA. “The High Cost of Dying” Revisited. The Milbank Memorial Fund Quarterly. 1994;72(4):561–91. [PubMed] [Google Scholar]
- Seshamani M, Gray A. The Impact of Ageing on Expenditures in the National Health Service. Age and Ageing. 2002;31(4):287–94. doi: 10.1093/ageing/31.4.287. [DOI] [PubMed] [Google Scholar]
- Seshamani M, Gray A. Ageing and Health-Care Expenditure: The Red Herring Argument Revisited. Health Economics. 2004a;13(4):303–14. doi: 10.1002/hec.826. [DOI] [PubMed] [Google Scholar]
- Seshamani M, Gray A. A Longitudinal Study of the Effects of Age and Time to Death on Hospital Costs. Journal of Health Economics. 2004b;23(2):217–35. doi: 10.1016/j.jhealeco.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Singer BH, Manton KG. The Effects of Health Changes on Projections of Health Service Needs for the Elderly Population of the United States. Proceedings of the National Academy of Sciences USA. 1998;95(26):15618–22. doi: 10.1073/pnas.95.26.15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillman BC. Changes in Elderly Disability Rates and the Implications for Health Care Utilization and Cost. The Milbank Quarterly. 2004;82(1):157–94. doi: 10.1111/j.0887-378X.2004.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillman BC, Lubitz JD. The Effect of Longevity on Spending for Acute and Long-Term Care. New England Journal of Medicine. 2000;342(19):1409–15. doi: 10.1056/NEJM200005113421906. [DOI] [PubMed] [Google Scholar]
- Spillman BC, Lubitz JD. New Estimates of Lifetime Nursing Home Use: Have Patterns of Use Changed? Medical Care. 2002;40(10):965–75. doi: 10.1097/00005650-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Stearns SC, Norton EC. Time to Include Time to Death? The Future of Health Care Expenditure Predictions. Health Economics. 2004;13(4):315–27. doi: 10.1002/hec.831. [DOI] [PubMed] [Google Scholar]
- van Weel C, Michels J. Dying, Not Old Age, to Blame for Costs of Health Care. Lancet. 1997;350(9085):1159–60. doi: 10.1016/S0140-6736(97)08312-8. [DOI] [PubMed] [Google Scholar]
- Werblow A, Felder S, Zweifel P. Population Ageing and Health Care Expenditure: A School of “Red Herrings”? Otto von Guericke University; 2005. Working Paper Series, 1–19. Magdeburg: Faculty of Economics and Management. [DOI] [PubMed] [Google Scholar]
- Wilmoth JR. Demography of Longevity: Past, Present, and Future Trends. Experimental Gerontology. 2000;35:1111–29. doi: 10.1016/s0531-5565(00)00194-7. 9–10. [DOI] [PubMed] [Google Scholar]
- Yang Z, Norton EC, Stearns SC. Longevity and Health Care Expenditures: The Real Reasons Older People Spend More. Journals of Gerontology Series B: Psychological and Social Sciences. 2003;58(1):S2–10. doi: 10.1093/geronb/58.1.s2. [DOI] [PubMed] [Google Scholar]
- Zweifel P, Felder S, Meiers M. Ageing of Population and Health Care Expenditure: A Red Herring? Health Economics. 1999;8(6):485–96. doi: 10.1002/(sici)1099-1050(199909)8:6<485::aid-hec461>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]


