Abstract
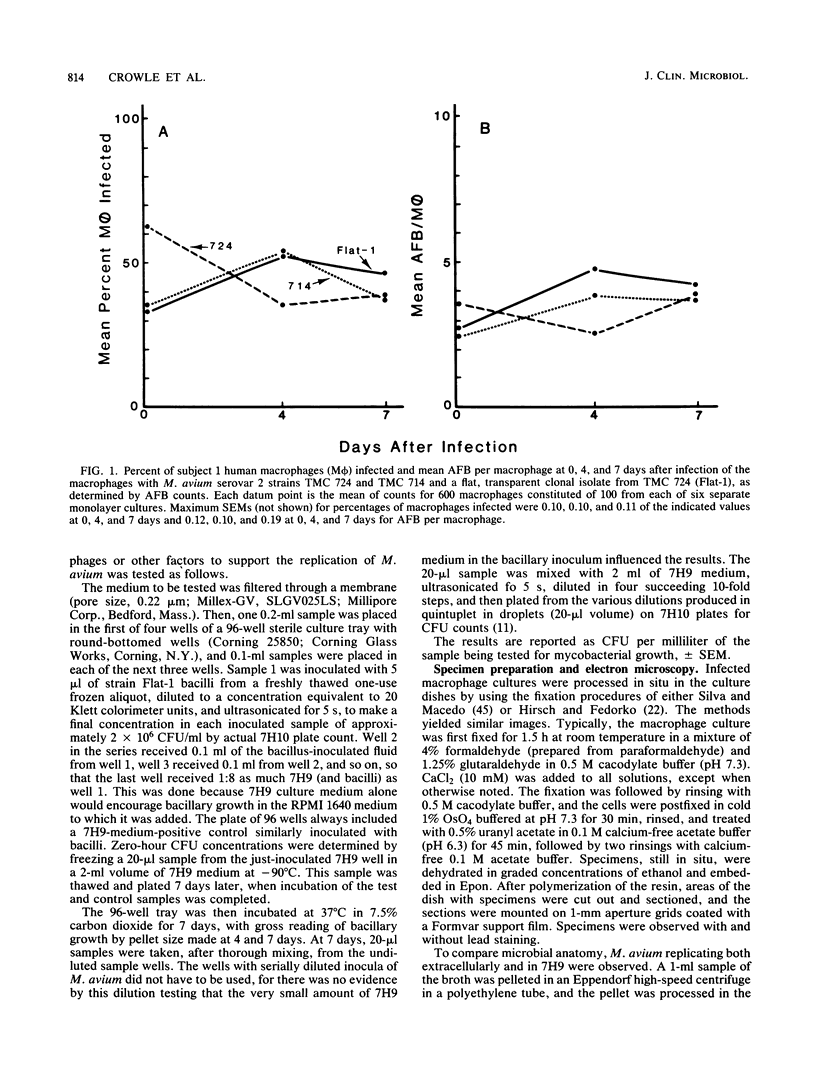
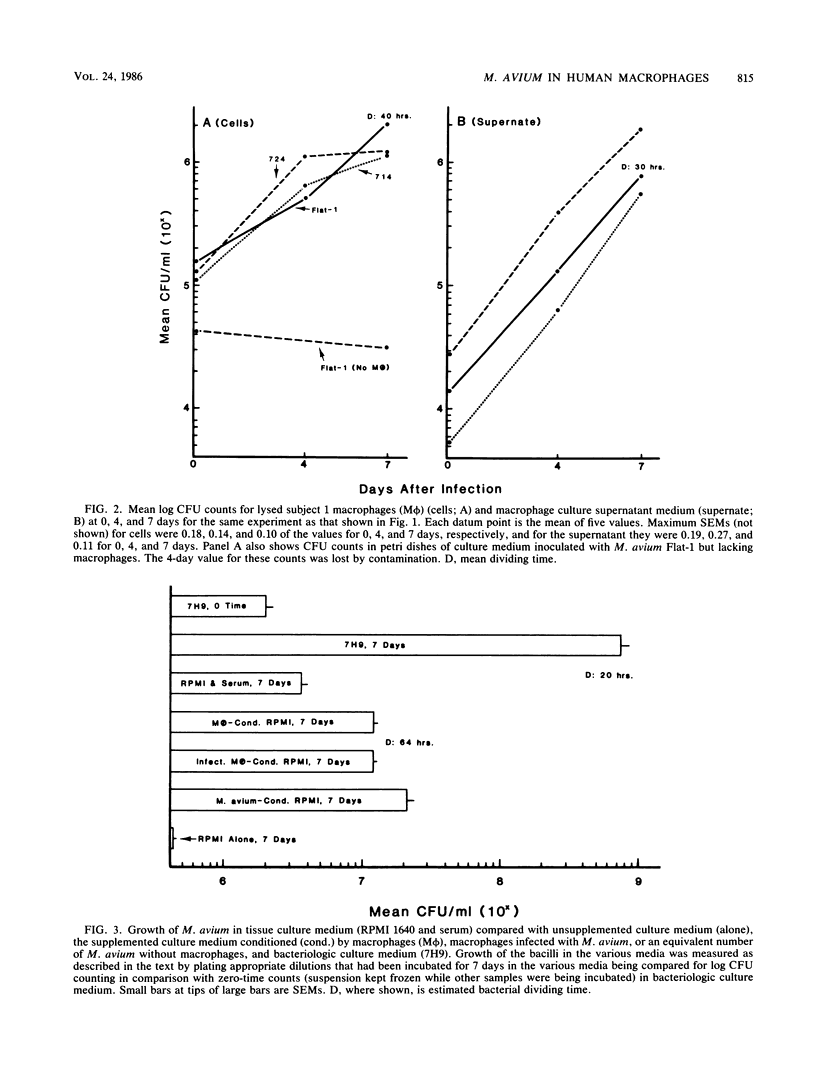
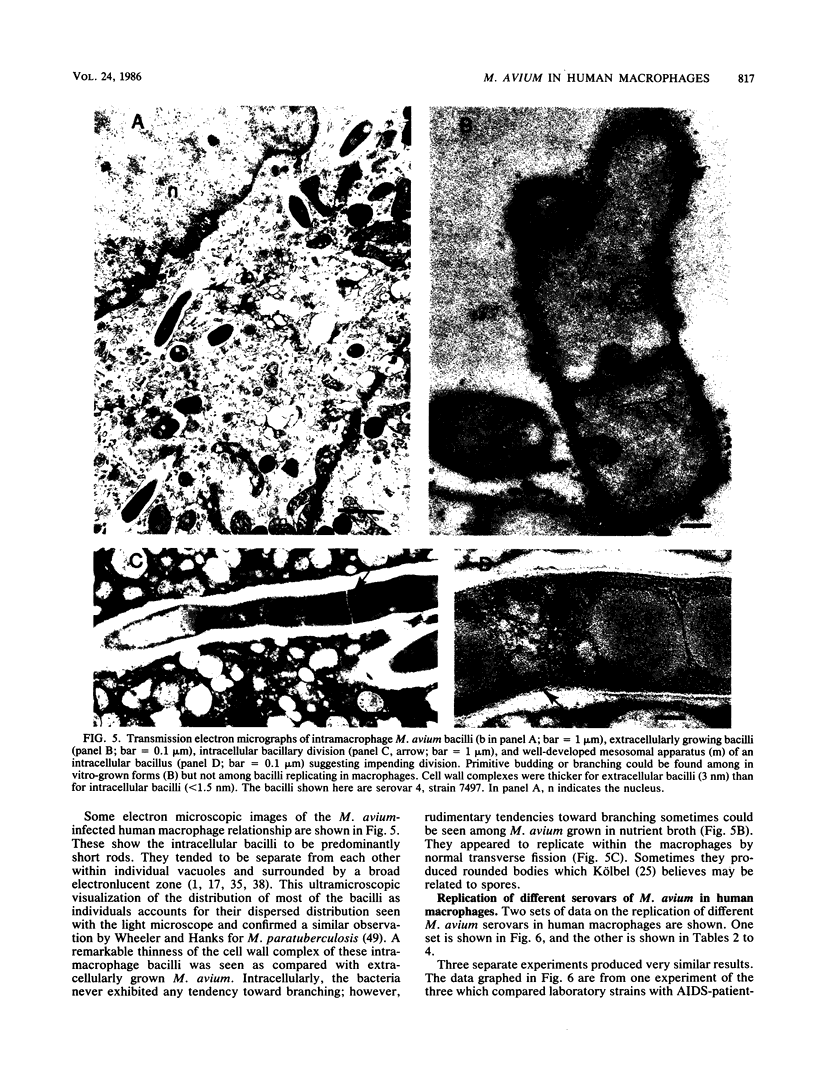
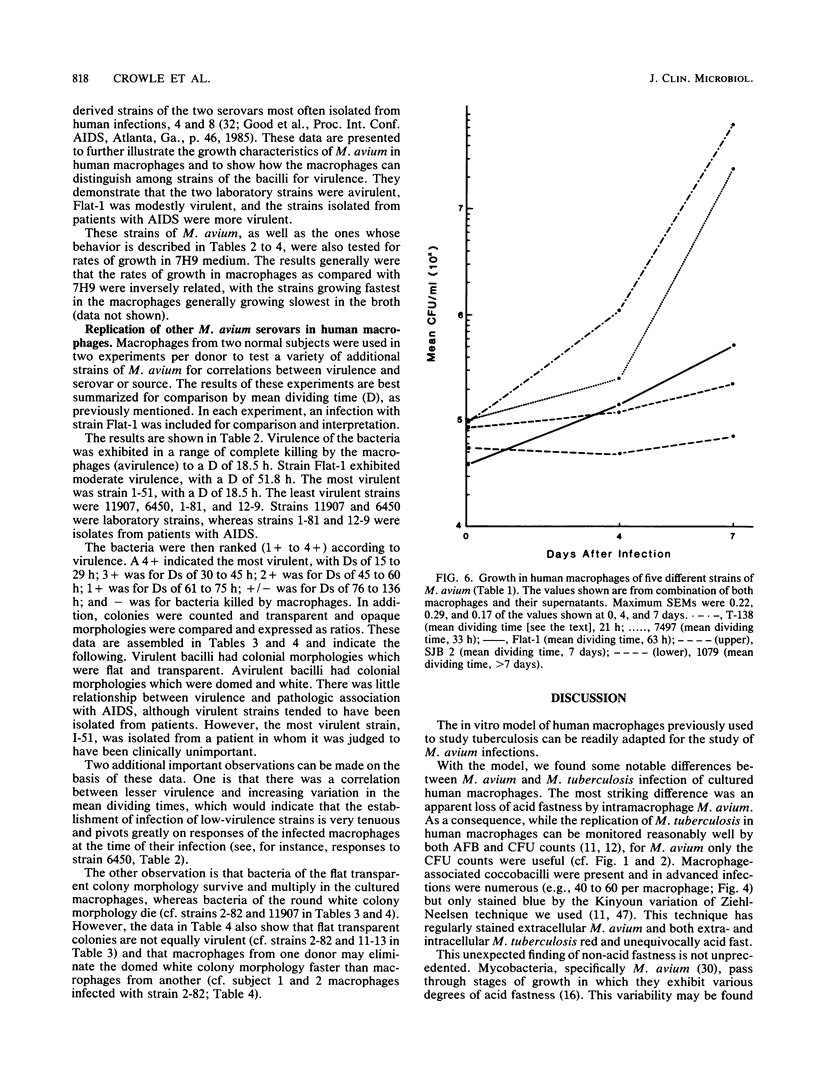
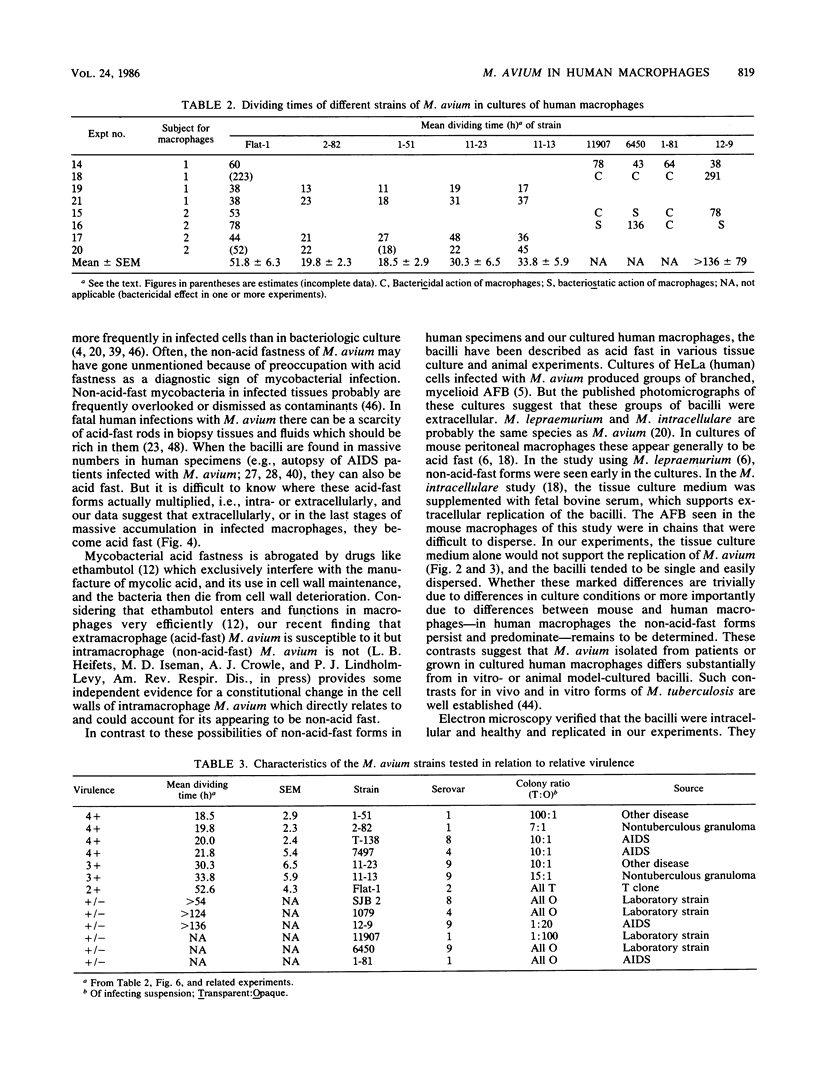
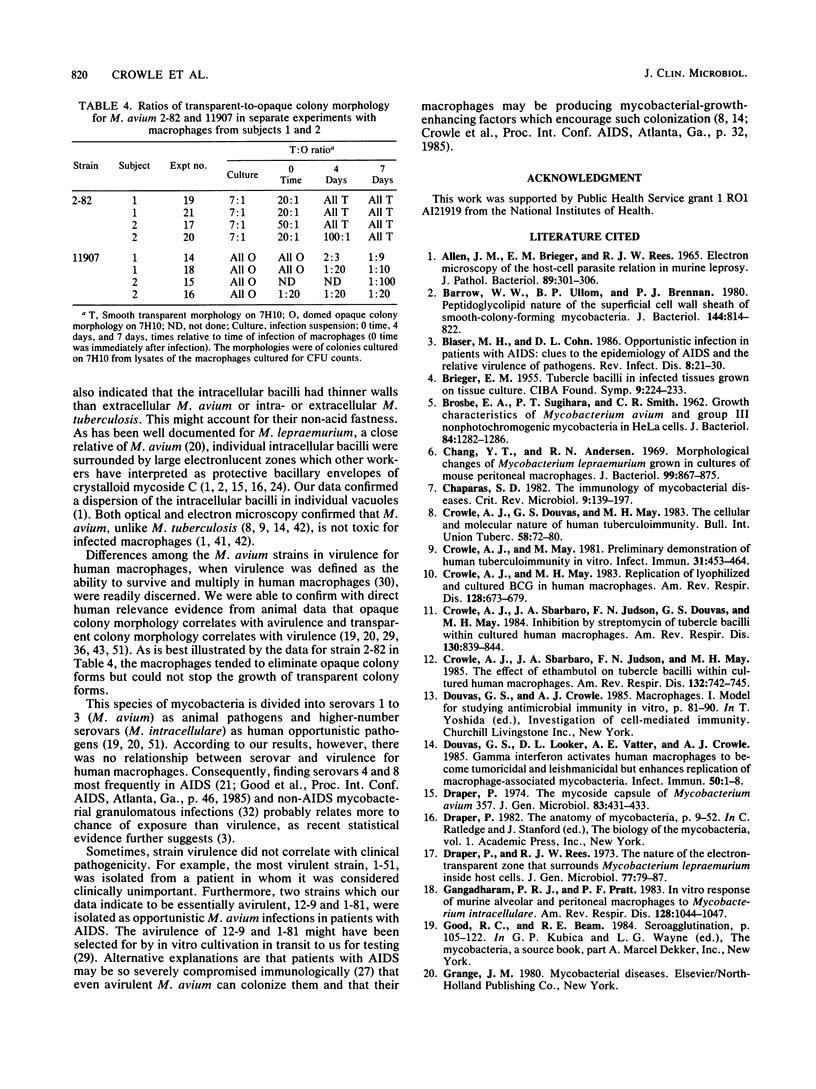
Mycobacterium avium is a cause of nontuberculous chronic granulomatous infections which is attracting increased attention as a frequent opportunistic pathogen in acquired immunodeficiency syndrome. Some important aspects of its human pathogenicity were investigated by using cultured human macrophages infected with it. The uptake and replication of various strains of M. avium in the macrophages could be measured by CFU counts of the bacteria in samples of lysed, sonicated macrophages. Microscopic counts of acid-fast bacilli were not useful because the bacteria multiplying in the macrophages were usually not acid fast. Electron microscopy showed the intracellular bacilli to multiply by transverse fission, to be surrounded in individual vacuoles by a broad electronlucent zone, and to have thinner cell walls than extracellularly grown M. avium. Fifteen strains, including examples of serovars 1, 2, 4, 8, and 9, were studied for uptake and rate of replication in cultured macrophages from three normal subjects. The strains were isolates from patients with nontuberculous granulomatous infection, acquired immunodeficiency syndrome, or unrelated problems, or they were laboratory reference cultures. There were no differences among them in phagocytosis, but there were differences in intracellular replication. Laboratory strains tended to be avirulent, that is, they did not replicate in the macrophages. Patient isolates usually were virulent and could be compared for virulence by intracellular replication rates. Virulence correlated with flat, transparent bacterial colony morphology on nutrient agar but not with serovar or kind of patient from whom the bacteria were isolated. However, among strains of transparent colony morphology there were wide differences in virulence. A virulent bacilli generally produced domed, opalescent colonies on nutrient agar. A virulent bacilli predominated in populations of M. avium conditioned to growth in bacteriologic culture medium. Bacilli of virulent colony morphology predominated in populations passaged through cultured macrophages. The model described here presents a new approach to the investigation of the pathogenicity of M. avium for human subjects and may be more patient relevant than animal models.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN J. M., BRIEGER E. M., REES R. J. ELECTRON MICROSCOPY OF THE HOST-CELL PARASITE RELATION IN MURINE LEPROSY. J Pathol Bacteriol. 1965 Jan;89:301–306. doi: 10.1002/path.1700890131. [DOI] [PubMed] [Google Scholar]
- BROSBE E. A., SUGIHARA P. T., SMITH C. R. Growth characteristics of Mycobacterium avium and group III nonphotochromogenic mycobacteria in HeLa cells. J Bacteriol. 1962 Dec;84:1282–1286. doi: 10.1128/jb.84.6.1282-1286.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Cohn D. L. Opportunistic infections in patients with AIDS: clues to the epidemiology of AIDS and the relative virulence of pathogens. Rev Infect Dis. 1986 Jan-Feb;8(1):21–30. doi: 10.1093/clinids/8.1.21. [DOI] [PubMed] [Google Scholar]
- Chang Y. T., Andersen R. N. Morphological changes of Mycobacterium lepraemurium grown in cultures of mouse peritoneal macrophages. J Bacteriol. 1969 Sep;99(3):867–875. doi: 10.1128/jb.99.3.867-875.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparas S. D. The immunology of mycobacterial infections. Crit Rev Microbiol. 1982;9(2):139–197. doi: 10.3109/10408418209104488. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., May M. H. Replication of lyophilized and cultured BCG in human macrophages. Am Rev Respir Dis. 1983 Oct;128(4):673–679. doi: 10.1164/arrd.1983.128.4.673. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., May M. Preliminary demonstration of human tuberculoimmunity in vitro. Infect Immun. 1981 Jan;31(1):453–464. doi: 10.1128/iai.31.1.453-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Sbarbaro J. A., Judson F. N., Douvas G. S., May M. H. Inhibition by streptomycin of tubercle bacilli within cultured human macrophages. Am Rev Respir Dis. 1984 Nov;130(5):839–844. doi: 10.1164/arrd.1984.130.5.839. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Sbarbaro J. A., Judson F. N., May M. H. The effect of ethambutol on tubercle bacilli within cultured human macrophages. Am Rev Respir Dis. 1985 Oct;132(4):742–745. doi: 10.1164/arrd.1985.132.4.742. [DOI] [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Draper P. The mycoside capsule of Mycobacterium Avium 357. J Gen Microbiol. 1974 Aug;83(2):431–433. doi: 10.1099/00221287-83-2-431. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Pratt P. F. In vitro response of murine alveolar and peritoneal macrophages to Mycobacterium intracellulare. Am Rev Respir Dis. 1983 Dec;128(6):1044–1047. doi: 10.1164/arrd.1983.128.6.1044. [DOI] [PubMed] [Google Scholar]
- Greene J. B., Sidhu G. S., Lewin S., Levine J. F., Masur H., Simberkoff M. S., Nicholas P., Good R. C., Zolla-Pazner S. B., Pollock A. A. Mycobacterium avium-intracellulare: a cause of disseminated life-threatening infection in homosexuals and drug abusers. Ann Intern Med. 1982 Oct;97(4):539–546. doi: 10.7326/0003-4819-97-4-539. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazda J., Vrubel F., Dornetzhuber V. Course of infection induced in man by inoculation with mycobacteria originating in water. Am Rev Respir Dis. 1967 May;95(5):848–853. doi: 10.1164/arrd.1967.95.5.848. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Salton M. R., Barksdale L. Ultrastructure of superficial mycosidic integuments of Mycobacterium sp. J Bacteriol. 1976 Feb;125(2):739–743. doi: 10.1128/jb.125.2.739-743.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C., Ashbaugh P. Factors that affect the cell cycle of Mycobacterium avium. Rev Infect Dis. 1981 Sep-Oct;3(5):914–925. doi: 10.1093/clinids/3.5.914. [DOI] [PubMed] [Google Scholar]
- McCarthy C. Effect of palmitic acid utilization on cell division in Mycobacterium avium. Infect Immun. 1974 Feb;9(2):363–372. doi: 10.1128/iai.9.2.363-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Synthesis and release of sulfolipid by Mycobacterium avium during growth andcell division. Infect Immun. 1976 Nov;14(5):1241–1252. doi: 10.1128/iai.14.5.1241-1252.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchy J. K. The seroagglutination test in the study of nontuberculous mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):867–870. doi: 10.1093/clinids/3.5.867. [DOI] [PubMed] [Google Scholar]
- Meissner G. The value of animal models for study of infection due to atypical mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):953–959. doi: 10.1093/clinids/3.5.953. [DOI] [PubMed] [Google Scholar]
- Mizuguchi Y., Fukunaga M., Taniguchi H. Plasmid deoxyribonucleic acid and translucent-to-opaque variation in Mycobacterium intracellulare 103. J Bacteriol. 1981 May;146(2):656–659. doi: 10.1128/jb.146.2.656-659.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura M., Izumi S., Mori T., Takeo K., Nonaka T. Freeze-etching study of human and murine leprosy bacilli. Int J Lepr Other Mycobact Dis. 1977 Jul-Sep;45(3):248–254. [PubMed] [Google Scholar]
- Olitzki A. L., Davis C. L., Schaefer W. B., Cohn M. L. Colony variants of avian-Battey group Mycobacteria intracerebrally injected into mice. Pathol Microbiol (Basel) 1969;34(5):316–323. doi: 10.1159/000162176. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Crossprotection against nontuberculous mycobacterial infections by Mycobacterium tuberculosis memory immune T lymphocytes. J Exp Med. 1986 Jan 1;163(1):203–208. doi: 10.1084/jem.163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert C. M., O'Leary T. J., Levens D. L., Simrell C. R., Macher A. M. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983 Sep;112(3):357–382. [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. The interpretation of the ultrastructure of mycobacterial cells in transmission electron microscopy of ultrathin sections. Int J Lepr Other Mycobact Dis. 1983 Jun;51(2):225–234. [PubMed] [Google Scholar]
- VOLINI F., COLTON R., LESTER W. DISSEMINATED INFECTION CAUSED BY BATTEY TYPE MYCOBACTERIA. Am J Clin Pathol. 1965 Jan;43:39–46. doi: 10.1093/ajcp/43.1.39. [DOI] [PubMed] [Google Scholar]
- WHEELER W. C., HANKS J. H. UTILIZATION OF EXTERNAL GROWTH FACTORS BY INTRACELLULAR MICROBES: MYCOBACTERIUM PARATUBERCULOSIS AND WOOD PIGEON MYCOBACTERIA. J Bacteriol. 1965 Mar;89:889–896. doi: 10.1128/jb.89.3.889-896.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcock A. J. L'immunologie des maladies respiratoires non tuberculeuses: rôle des réactions d'hypersensibilité immédiate. Bull Int Union Tuberc. 1983 Mar;58(1):72–75. [PubMed] [Google Scholar]
- Zlotnik A., Crowle A. J. Lymphokine-induced mycobacteriostatic activity in mouse pleural macrophages. Infect Immun. 1982 Aug;37(2):786–793. doi: 10.1128/iai.37.2.786-793.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]