Abstract
Cancer is the second leading cause of death in the United States. Despite the estimated 565,650 deaths in 2008 of Americans as a result of cancer, it is mostly a preventable disease. Simply by modification of diet, maintenance of optimum body weight, and regular physical activity, 30% to 40% of all instances of cancer could be prevented. Modification of diet alone by increasing vegetable and fruit intake could prevent 20% or more of all cases of cancer and may potentially prevent approximately 200,000 cancer-related deaths annually. Because of their safety, low toxicity, antioxidant properties, and general acceptance as dietary supplements, fruits, vegetables, and other dietary elements (phytochemicals and minerals) are being investigated for the prevention of cancer. Extensive research over the past several decades has identified numerous dietary and botanical natural compounds that have chemopreventive potential. In this review, we discuss promising natural chemopreventive compounds, their molecular targets, and their mechanisms, which may help the further design and conduct of preclinical and clinical trials.
INTRODUCTION
Cancer is the second leading cause of death in the United States with 565,650 projected cancer deaths and 1,437,180 new cases of cancer in 2008.1 In 2005, the global incidence of cancer was 11 million with more than 7.6 million deaths, and is expected to increase to an incidence of 15.5 million with 11.5 million deaths by 2030.2 The lifetime probability of being diagnosed with an invasive cancer is more than 40%.1 However, cancer is mostly a preventable disease.3 The two most important ways to reduce cancer risk are the avoidance of cancer-causing biologic, chemical, and physical agents and the habitual consumption of diets high in foods that protect against cancer. Approximately, 30% to 40% of cancer incidents are preventable by consuming a healthy diet, regular physical activity, and maintenance of optimum body weight, and more than 20% by consuming vegetables and fruits.3
Chemoprevention, by definition, is a means of cancer control by which the occurrence of the disease can be entirely prevented, slowed down, or reversed by the administration of one or more naturally occurring and/or synthetic agents. The concept of chemoprevention is gaining increasing attention because it is a cost-effective alternative to cancer treatment. The involvement of multiple factors and developmental stages and our increased understanding of cancer at the epigenetic, genetic, molecular, and cellular levels is opening up enormous opportunities to interrupt and reverse the initiation and progression of the disease and provide scientists with numerous targets to arrest by physiological and pharmacologic mechanisms, with the goal of preventing end-stage, invasive disease and impeding or delaying the development of cancer.4
WHY NATURAL COMPOUNDS?
Chemoprevention began in the 1920s with Berenblum5 and, after a period of relative dormancy, re-entered the cancer research mainstream in the 1970s through the work of Sporn.6 Because of their excessive toxicity and inadequate biodistribution, natural retinoids were replaced with more potent and less toxic synthetic retinoids. The first limited clinical trial with the vitamin A analog 13-cis-retinoic acid (13-cRA) showed a significant decrease in the size of oral premalignant lesions and reversal of dysplasia.7 The follow-up phase III trial in patients with leukoplakia using initial high-dose 13-cRA followed by either low-dose 13-cRA or β-carotene suggested that low-dose 13-cRA was better than β-carotene as maintenance therapy.8 Another phase III trial with high-doses of 13-cRA showed significant reduction in the incidence of second primary tumor (SPT) after 1 year of treatment and the protection lasted for 2 to 3 years.9,10 A number of retinoid trials with mixed results followed.11–13 The combination of 13-cRA, α-interferon, and α-tocopherol appeared to be very effective in delaying SPT,14 and a phase III trial with this combination versus no treatment has been initiated. However, because of patient accrual issues, this trial is currently delayed.
The identification of several biomarkers, including epidermal growth factor receptor (EGFR), cyclo-oxygenase-2 (COX-2), and Ras, which are associated with disease progression, and the discovery of novel targeted inhibitors for these biomarkers has opened new opportunities for chemoprevention.4 Subsequently, several targeted agents, such as the COX-2 inhibitors celecoxib and refecoxib, the EGFR inhibitors erlotinib and gefitinib, and farnesyltransferase inhibitors have been discovered.15–18 However, because of a lack of long-term safety data in patients without the evidence of active cancer, two proposed clinical trials to examine gefitinib and tipifarnib in the reversal of premalignant lesions of the lungs have been discontinued.
Safety is always a primary consideration in studies involving human subjects, particularly patients without evidence for overt cancer. An ideal chemopreventive agent should be nontoxic, effective at lower doses, economical, and easily available. Patient accrual to chemoprevention trials is sometimes a challenge, partly due to the toxicity of the pharmaceuticals investigated. In recent years, natural dietary agents have drawn a great deal of attention both from researchers and the general public because of their potential ability to suppress cancers as well as reduce the risk of cancer development. From multiple epidemiological and animal studies, it was clear that consumption of food rich in fruits and vegetables decreases the occurrence of cancers.19–23 Clinicians are also paying increasing attention to diet-derived chemopreventive agents as a result of the willingness of patients to use over-the-counter diet-derived agents. Cell culture and animal studies over the past several decades have suggested the cancer-preventive potential of several nutritional compounds, including those found in green and yellow vegetables, citrus fruits, and spices. Since the first primary tumors and SPTs share common host factors (genetic abnormalities, immune function, and hormone imbalances), environmental and/or occupational exposures, lifestyle factors, and gene-environment interactions, these agents should also be effective in the prevention of SPT. However, clinical trials have only recently started to investigate these compounds (Table 1). The chemopreventive properties and molecular targets of selected promising natural compounds are discussed later and summarized in Table 2.
Table 1.
Information About Ongoing Clinical Trials With Natural Compounds
| Agent and Trial No. | Trial Type | Cancer Type | Location/Site | Status | Phase |
|---|---|---|---|---|---|
| Green tea | |||||
| NCT00363805 | Prevention | Lung | University of Arizona | Recruiting | II |
| NCT00134381 | Prevention | Skin | University of Medicine and Dentistry New Jersey and Rutgers University | Recruiting | II |
| NCT00721890 | Maintenance | Ovarian | Centre hospitalier universitaire de Québec | Recruiting | II |
| NCT00685516 | Therapy | Prostate | Jonsson Comprehensive Cancer Center | Recruiting | II |
| NCT00003197 | Therapy | Solid tumors | Memorial Sloan-Kettering Cancer Center | Active | I |
| NCT00005828 | Therapy | Prostate | North Central Cancer Treatment Group | Completed | II |
| NCT00253643 | Prevention | Prostate | Oregon Health and Science University Cancer Institute | Recruiting | |
| NCT00303823 | Prevention | Cervical | University of Arizona | Recruiting | II |
| NCT00516243 | Therapy | Breast | M. D. Anderson Cancer Center | Recruiting | I |
| NCT00459407 | Therapy | Prostate | University of Arizona | Recruiting | I |
| NCT00176566 | Therapy | Oral leukoplakia | University of Medicine and Dentistry New Jersey | Completed | II |
| NCT00666562 | Therapy | Bladder | University of Wisconsin, Madison | Recruiting | II |
| NCT00088946 | Therapy | Bladder | Jonsson Comprehensive Cancer Center | Active | II |
| NCT00091325 | Prevention | Solid tumors | University of Arizona | Completed | I |
| NCT00573885 | Prevention | Lung | British Columbia Cancer Agency | Recruiting | II |
| NCT00611650 | Prevention | Lung | British Columbia Cancer Agency | Recruiting | II |
| NCT00233935 | Prevention | Osophageal | M. D. Anderson Cancer Center | Recruiting | I |
| NCT00262743 | Therapy | Leukemia | Mayo Clinic | Recruiting | I/II |
| NCT00003367 | Therapy | Prostate | Memorial Sloan-Kettering Cancer Center | Active | III |
| NCT00676780 | Basic science | Prostate | Louisiana State University | Active | II |
| NCT00455416 | Therapy | Lymphoma | Rikshospitalet HF | Recruiting | II |
| NCT00676793 | Basic science | Breast | Louisiana State University | Recruiting | II |
| NCT00744549 | Therapy | Prostate | University Health Network, Toronto | Recruiting | II |
| NCT00707252 | Therapy | Lung | Louisiana State University | Recruiting | I/II |
| Curcumin | |||||
| NCT00113841 | Therapy | Multiple myeloma | M. D. Anderson Cancer Center | Active | Pilot |
| NCT00745134 | Therapy | Rectal | M. D. Anderson Cancer Center | Recruiting | II |
| NCT00365209 | Prevention | Colon | Chao Family Comprehensive Cancer Center | Active | II |
| NCT00689195 | Therapy | Osteosarcoma | Tata Memorial Hospital | Recruiting | I |
| NCT00192842 | Therapy | Pancreatic | Rambam Health Care Campus | Recruiting | II |
| NCT00094445 | Therapy | Pancreatic | M. D. Anderson Cancer Center | Completed | II |
| NCT00295035 | Therapy | Colon | Tel-Aviv Sourasky Medical Center | III | |
| NCT00641147 | Therapy | FAP | Johns Hopkins University | Recruiting | II |
| NCT00248053 | FAP | Johns Hopkins University | Terminated | II | |
| NCT00486460 | Therapy | Pancreatic | Tel-Aviv Sourasky Medical Center | Recruiting | III |
| NCT00027495 | Prevention | Colon | University of Michigan Cancer Center | Completed | I |
| NCT00176618 | Prevention | Aberrant crypt foci | University of Medicine and Dentistry New Jersey | Completed | |
| NCT00003365 | Prevention | Colorectal | Rockefeller University | Suspended | |
| NCT00118989 | Prevention | Adenomatous polyps | University of Pennsylvania | Recruiting | II |
| Resveratrol | |||||
| NCT00256334 | Therapy | Colon | University of California, Irvine | Recruiting | I/II |
| NCT00098969 | Prevention | Solid tumors | University of Michigan Cancer Center | Completed | I |
| NCT00433576 | Therapy | Colorectal | University of Michigan Cancer Center | Recruiting | I |
| NCT00578396 | Prevention | Colon | University of California, Irvine | Recruiting | I |
| NCT00455416 | Therapy | Follicular lymphoma | Rikshospitalet HF | Recruiting | II |
| Genistein | |||||
| NCT00244933 | Therapy | Breast | Barbara Ann Karmanos Cancer Institute | Active | II |
| NCT00290758 | Prevention | Breast | Robert H. Lurie Cancer Center | Active | II |
| NCT00546039 | Basic science | Prostate | University Hospital, Aker | Active | II |
| NCT00005827 | Therapy | Prostate | University of North Carolina Lineberger Comprehensive Cancer Center | Completed | I |
| NCT00001696 | PK | Cancer | National Cancer Institute | Completed | I |
| NCT00276835 | Therapy | Kidney cancer | Robert H. Lurie Cancer Center | Active | II |
| Melanoma (skin) | |||||
| NCT00118040 | Therapy | Bladder | University of Wisconsin, Madison | Active | II |
| NCT00058266 | Therapy | Prostate | Robert H. Lurie Cancer Center | Active | II |
| NCT00769990 | Therapy | Cancers | Masonic Cancer Center, University of Minnesota | Suspended | I/II |
| NCT00584532 | Therapy | Prostate | University of California, Davis | Completed | II/III |
| NCT00099008 | Prevention | Breast and endometrial pancreatic | University of Nortd Carolina Lineberger Comprehensive Cancer Center | Completed | I |
| NCT00376948 | Tderapy | Prostate | Barbara Ann Karmanos Cancer Institute | Suspended | II |
| NCT00269555 | Therapy | Leukemia | University of California, Davis | Active | |
| NCT00004858 | Therapy | Lymphoma | Parker Hughes Cancer Center | Active | I |
| NCT00499408 | Therapy | Prostate | Wake Forest University | Recruiting | II |
| Pomegranate | |||||
| NCT00413530 | Therapy | Prostate | M. D. Anderson Cancer Center | Recruiting | |
| NCT00719030 | Prevention | Prostate | University of California, Los Angeles | Recruiting | |
| NCT00732043 | Prevention | Prostate | Radiant Research | Recruiting | II |
| NCT00731848 | Therapy | Prostate | Radiant Research | Recruiting | II |
| NCT00336934 | Therapy | Prostate | Roll International Corporation | Recruiting | III |
| NCT00381108 | Therapy | BPH | University of California, Irvine | Recruiting | I |
| NCT00060086 | Therapy | Prostate | Jonsson Comprehensive Cancer Center | Active | II |
| NCT00455416 | Therapy | Follicular lymphoma | Rikshospitalet HF | Recruiting | II |
| NCT00433797 | Therapy | Prostate | University of Oslo | Recruiting | I/II |
| Lycopene | |||||
| NCT00042731 | Therapy | Prostate | H. Lee Moffitt Cancer Center and Research Institute | Completed | |
| NCT00416325 | Prevention | Prostate | University of Illinois | Completed | I |
| NCT00178113 | Prevention | Prostatic intraepithelial neoplasia | University of Pittsburgh | Completed | I |
| NCT00093561 | Prevention | Prostate | University of Illinois | Completed | I |
| NCT00416390 | Therapy | Precancerous/nonmalignant condition | University of Illinois | Active | |
| NCT00450749 | Therapy | Prostate | M. D. Anderson Cancer Center | Recruiting | II |
| NCT00006078 | Prevention | Prostate | University of Illinois | Completed | I |
| NCT00322114 | Prevention | Prostate | University of Illinois | Recruiting | II |
| NCT00402285 | Therapy | Prostate | University of California San Francisco Helen Diller Family Comprehensive Cancer Center | Active | |
| NCT00450957 | Prevention | Prostate | University of Illinois | Active | I |
| NCT00068731 | Therapy | Prostate | North Central Cancer Treatment Group | Active | II |
| NCT00744549 | Therapy | Prostate | University Health Network, Toronto | Recruiting | II |
| NCT00501371 | Therapy | BPH | Health Ever Bio-Tech Ltd | Recruiting | III |
| NCT00669656 | Therapy | Prostate | Norris Comprehensive Cancer Center | Recruiting | II |
| n-3 poly unsaturated fatty acids | |||||
| NCT00402285 | Therapy | Prostate | University of California San Francisco Helen Diller Family Comprehensive Cancer Center | Active | |
| NCT00114296 | Prevention | Breast | Cedars-Sinai Medical Center | Active | |
| NCT00003077 | Supportive | Soft tissue | Cancer and Leukemia Group B | Completed | I/II |
| NCT00627276 | Therapy | Breast | Oregon Health and Science University Cancer Institute | Recruiting | II |
| NCT00559156 | Therapy | Head and neck | Centre Regional de Lutte Contre le Cancer-Centre Val d'Aurelle | Active | II |
| NCT00723398 | Prevention | Breast | Penn State University | ||
| NCT00458549 | Treatment | Prostate | Dana-Farber Cancer Institute | Recruiting | |
| NCT00488904 | Prevention | Colorectal | Aalborg Hospital | Recruiting | IV |
| NCT00253643 | Prevention | Precancerous/nonmalignant condition, prostate | Oregon Health and Science University Cancer Institute | Recruiting | |
| NCT00168987 | Therapy | Colorectal neoplasm | Charite University, Berlin, Germany | Completed | IV |
| Hepatocellular carcinoma | |||||
| Cholangiocarcinoma | |||||
| NCT00798876 | Diagnostic | Prostate | University of California, Los Angeles | Recruiting | |
| NCT00533078 | Prevention | Colitis, mucositis, AML | University Hospital Inselspital, Berne | Recruiting | II |
| NCT00398333 | Supportive | Colorectal neoplasm | Hospital Clinic of Barcelona | Terminated | IV |
| NCT00145015 | Diagnostic | Colorectal neoplasm, ulcerative colitis, polyps | Institute of Food Research | Completed | |
| NCT00455416 | Therapy | Follicular lymphoma | Rikshospitalet HF | Recruiting | II |
| NCT00790140 | Therapy | Esophageal | University of Dublin, Trinity College | Recruiting | IV |
| NCT00510692 | Prevention | Familial adenomatous polyposis coli, FAP | S.L.A. Pharma AG | Completed | II/III |
Abbreviations: FAP, familial adenomatous polyposis; PK, pharmacokinetics; BPH, benign prostate hyperplasia; AML, acute myelocytic leukemia.
Table 2.
Source, Mechanism of Action, Synergistic Interactions With Other Drugs, and Molecular Targets of Promising Natural Compounds
| Agent | Natural Source | Mechanism of Action | Organ Site | Synergistic Interaction | Molecular Targets |
|---|---|---|---|---|---|
| Green tea polyphenols and EGCG | Camellia sinensis (green tea) | Antioxidant, anti-mutagenesis, anti-proliferation (cell cycle arrest, apoptosis), anti-inflammation, anti-angiogenesis, immunomodulation | Skin, lung, oral cavity, head and neck, esophagus, stomach, liver, pancreas, small intestine, colon, bladder, prostate, mammary glands | Curcumin, erlotinib, luteolin, genistein, atorvastin, TRAIL, tamoxifen, celecoxib, cisplatin, sulindac, dacarbazine, adriamycin | p53, p73, p21, Bax, EGFR, AKT, NF-κB, Bcl-2, cyclin D1, COX-2, VEGF, MMP-2/9, STAT3, ERK1/2, AP-1, IL-12, CD8+ T-cell |
| Curcumin | Curcuma longa (turmeric powder) | Anti-oxidant, antiproliferation (cell cycle arrest, apoptosis), anti-inflammation, anti-angiogenesis, immunomodulation | Skin, lung, oral cavity, head and neck, esophagus, stomach, liver, pancreas, small intestine, colon, bladder, prostate, mammary glands, lymphoma, soft palate, cervix | Genistein, green tea, embelin, FU, vinca alkaloid, vinorelbine, gemcitabine, soy isoflavone, oxaliplatin, paclitaxel, TRAIL, celecoxib, retinoic acid | EGFR, IGF-1R, AKT, NF-κB, Bcl-2, COX-2, ERK, AP-1, Sp, VEGF, VEGFR1, MMP-2/9, p53, p21, Bax, STAT3/5 |
| Luteolin | Artichoke, broccoli, celery, cabbage, spinach, green pepper, pomegranate leaves, peppermint, tamarind, and cauliflower | anti-inflammation, anti-allergy, anti-proliferation (G1 and G2/M arrest, apoptosis), antioxidant, pro-oxidant | Ovarian, gastric, liver, colon, breast, oral, oesophageal adenocarcinoma, prostate, lung, nasopharyngeal, cervix, leukemia, skin, and pancreatic | Cisplatin, doxorubicin, TRAIL, TNF-α | JNK, p53, DR5, BAX, p21, PUMA, EGFR, IGF-1R, AKT, NF-κB, Bcl-2, CDK, ERK, STAT3 |
| Resveratrol | Red wine, grapes (mainly in the skin), mulberries, peanuts, vines, pines | Antioxidant, antiproliferation (cell cycle arrest and apoptosis), antiangiogenesis, antiinflammation | Ovarian, breast, prostate, liver, uterine, leukemia, lung, gastric | EGCG, indole-3-carbinol, vitamin E analogue, methylseleninic acid, quercetin, genistein, TRAIL, cisplatin, doxorubicin, ellagic acid, platinum compounds, FU, paclitaxel | SOD, catalase, glutathione, ↑glutathione, AKT, NF-κB, iNOS, COX-2, STAT3, survivin, p53, p21, BAX, BAK, DR |
| Genistein | Soybeans and soy products, red clover (Trifolium pretense), sicilian pistachio (Pistacia vera) | Antioxidant, antiproliferation (growth inhibition, cell cycle arrest, apoptosis), anti-angiogenesis, anti-inflammation | Prostate, breast, skin, colon, stomach, liver, ovary, pancreas, oesophagus, head and neck | EGCG, letrozole, docetaxel, arsenic trioxide, resveratrol, lycopene, vitamin D, tamoxifen, paclitaxel, cisplatin, erlotinib, gemcitabine, doxorubicin, FU, camptothecins, adriamycin, bleomycin | AKT, NF-κB, Bcl-2, survivin, cyclin D1, COX-2, MMP-2/9, p53, p21, GADD153, Bax, STAT3/5, ERK1/2, CDK1, AP-1, IGF-1R |
| Pomegranate | Punica granatum (pomegranate fruit, pomegranate juice, pomegranate seed and seed oil) | Antioxidant, antiproliferation (growth inhibition, cell cycle disruption and apoptosis), antiangiogenesis, anti-inflammation | Prostate, skin, breast, lung, colon, oral, leukemia | NF-κB, Bcl-2, COX-2, VEGF, ERK, JNK, p38, AKT, mTOR, iNOS, cyclin, CDK, p21, p27, BAX, BAK | |
| Lycopene | Tomatoes, guava, rosehip, watermelon, papaya, apricot and pink grapefruit; most abundant in red tomatoes and processed tomato products | Antioxidant, antiproliferation (growth inhibition, cell cycle arrest, apoptosis), anti-angiogenesis, anti-inflammation, immunomodulator | Prostate, lung, breast, gastric, liver, pancreas, colotectal, head and neck, skin | Genistein, adriamycin, cisplatin | Cyclin D1, Bcl-2, Bcl-xL, AKT, BAD, NF-κB, MMP-9, Sp-1, IGF-BP3 |
| Ellagic acid | Pomegranate juice, and seed oil, different nuts, blue honeysuckle (Lonicera caerulea), strawberries and other berries, bark of arjun (Terminalia arjuna), leaves and fruits of T. bellerica and bark, leaves and fruits of T. muelleri | Antioxidant, anti-proliferation (growth inhibition, cell cycle arrest, apoptosis), anti-inflammation | Neuroblastoma, skin, pancreas, breast, prostate, colon, intestine, esophagus, bladder, oral, leukemia, liver | Cisplatin, vinorelbine, quercetin, resveratrol, cyclosporine A, 6-gingerol, seleno-methionine | p53, p21, JNK, p38, ↓CDK2, glutathione, glutathione-peroxidase, catalase, SOD, NF-κB, COX-2, PDGF, VEGF, MMP-2/9 |
| Lupeol | Mango, olive, fig, strawberry, red grapes | Antioxidant, anti-mutagenesis, anti-inflammation, antiproliferation (cell cycle arrest, apoptosis, induction of differentiation | Skin, lung, leukemia, pancreas, prostate, colon, liver, head and neck | Cisplatin | 14-3-3-σ, BAX, p21, Fas, Bcl-2, cyclin D1/2, Ras, NF-κB, COX-2, NOS, AKT |
| Betulinic acid | Widely distributed in plant kingdom; most abundant sources are Betula spp (birch tree), Ziziphus spp, Syzigium spp, Diospyros spp, and Paeonia spp | Anti-inflammation, apoptosis, immunomodulation | Skin, ovary, colon, brain, renal cell carcinoma, cervix, prostate, leukemia, lung, breast, head and neck | Bleomycin, FU, irinotecan, oxaliplatin, doxorubicin, cisplatin, taxol, dactinomycin, TRAIL, vincristine | PPAR-γ, p21, p38, JNK, topoisomerase I, NF-κB, COX-2, Bcl-2, cyclin D1/3, ↓Sp1,3 and 4 |
| n-3 polyunsaturated fatty acids | Corn oil, sunflower oil, safflower oil, and olive oil, soybeans, walnuts, dark green leafy vegetables such as kale, spinach, broccoli, and brussels sprouts, and seeds or their oils such as flaxseed, mustard seed, and rapeseed (canola) | Anti-inflammation, apoptosis, cell cycle arrest, lipid peroxidation | Colorectal, prostate, breast, colon, gastric, pancreatic, head and neck, esophageal, hematologic malignancies | Sodium butyrate | NF-κB, Bcl-2, STAT3, p53, Bax, p21, Fas/FasL, PPAR-γ, RXR, Ras, ERK 1/2 |
| Ginkolide B | Ginko biloba | Antioxidant, anti-angiogenic, apoptosis | Ovary, breast, brain | Ethanol | PAFR, NO, iNOS, eNOS, JNK |
Abbreviations: EGCG, epigallocatechin-3-gallate; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; EGFR, epidermal growth factor receptor; NF-κB, nuclear factor-κB; COX-2, cyclo-oxygenase-2; VEGF, vascular endothelial growth factor; MMP-2/9, matrix metalloproteinases; IL-12, interleukin 12; FU, fluorouracil; IGF-1R, insulin-like growth factor-1 receptor; Sp, stimulating protein; VEGFR1, vascular endothelial growth factor receptor 1; TNF-α, tumor necrosis factor α; JNK, Jun-N-terminal kinase; CDK, cyclin-dependent kinase; ERK, extracelluar signal–regulated kinase; SOD, superoxide dismutase; mTOR, mammalian target of rapanycin; iNOS, inducible nitric oxide synthase; DR, death receptor; IGF-BP3, insulin-like growth factor binding protein 3; PPAR-γ, peroxisome proliferator-activated receptor-γ; PAFR, platelet activating factor receptor; NO, nitric oxide; eNOS, endothelial nitric oxide synthase.
Tea Polyphenols
Tea is one of the most widely consumed beverages and is rich in substances with antioxidant properties. Different processing techniques yield different types of tea. Although both black tea and green tea have been studied for their chemopreventive potential, green tea showed higher promise and greater efficacy against multiple types of cancer. Epigallocatechin-3-gallate (EGCG) (Fig 1A) is the most abundant polyphenol in green tea and has gained the most attention with respect to anticarcinogenic activity.
Fig 1.
Chemical structures of natural chemopreventive agents. Most of these compounds are polyphenols containing multiple phenol rings in their structures.
Epidemiological studies from different countries and many animal model studies have yielded promising results of green tea and its constituents in reducing human cancer risk in multiple organ sites.24–29 In xenograft models, green tea polyphenols (GTP) inhibited tumor growth and suppressed metastasis of metastasis-specific mouse mammary carcinoma 4T1 cells30 and reduced tumor blood vessel formation in estrogen receptor–negative breast cancer.31 GTP extract (PPE) reduced the risk of colon carcinogenesis after azoxymethane insult in rats.32 These results are consistent with previously published results.33 A case control study at Mayo Clinic in patients with chronic lymphocytic leukemia (CLL) and other low-grade lymphomas who used over-the-counter products containing tea polyphenols showed that four of these patients had evidence of clinical benefit from these products.34 On the basis of these findings, Mayo Clinic has initiated an National Cancer Institute–sponsored phase I/II clinical trial of decaffeinated green tea extracts for patients with asymptomatic, early-stage CLL.34 Several phase I trials of healthy volunteers have also been conducted to define the basic biodistribution patterns, pharmacokinetic parameters, and preliminary safety profiles for short-term oral administration of various green tea preparations.35–37 The consumption of green tea appears to be relatively safe. A phase I study suggested that up to 1 g of green tea solids (equivalent of approximately 900 mL of green tea) could be safely consumed by patients with solid tumors.38
EGCG was also found to synergistically increase the efficacy of other drugs in cell culture and animal models. Our own study showed that EGCG synergistically increased the efficacy of erlotinib in head and neck cancer models39 and could “resensitize” erlotinib-resistant lung cancer cells to erlotinib (J. Cardelli, personal communication, August 2008). Accordingly, a phase I/II trial has been opened exploring the possibility that GTP together with erlotinib will be more effective than erlotinib alone as a second-line treatment approach for patients with non–small-cell lung cancer (NSCLC). EGCG also sensitized the tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) –resistant prostate cancer cell line LnCaP to TRAIL-mediated apoptosis.40 Because of these promising in vitro and in vivo results, several clinical trials are currently ongoing involving green tea alone or in combination with other drugs (www.clinicaltrials.gov).
Curcumin
Curcumin (Fig 1B), isolated from the roots (rhizomes) of the plant Curcuma longa, is the major yellow pigment present in turmeric, widely used as a spice. Although turmeric and its chemical components have been used in traditional medicine for thousands of years, it was not until the 1980s that curcumin attracted much attention because of its antitumorigenic activity. Kuttan et al41 reported that turmeric extract inhibited the growth of Chinese hamster ovary cells, and was cytotoxic to lymphocytes and Dalton's lymphoma cells in vivo. The same group used an ethanol extract of turmeric and curcumin ointment in patients with external cancerous lesions with promising results.42 Curcumin was shown to interrupt the carcinogenesis process by inhibiting the initiation step or suppressing the promotion and progression stages in animal models.43,44 Several studies in rodent models demonstrated the inhibitory effects of curcumin in colon carcinogenesis.45,46 Curcumin has been shown to inhibit the initiation and promotion of chemically induced skin cancer47 and DMBA-induced oral carcinogenesis.48 Curcumin also inhibits the growth of cancer cells from multiple organ sites in vitro and in xenograft models by inducing cell cycle arrest and apoptosis.28,49–51
Curcumin was also reported to exhibit synergistic chemopreventive effects with other diet-derived polyphenols, such as genistein,52 green tea,53 and embelin,54 and increased the efficacy of many anticancer drugs including fluorouracil (FU),55 vinca alkaloid,56 vinorelbine, and gemcitabine.57 Encouraged by the results of these cell culture and animal model studies, curcumin was brought to clinical trials. In a pilot study, 100% of patients showed a decreased polyp number and size after a mean of 6 months of treatment with curcumin and quercetin.58 Another phase I clinical trial conducted in patients with high risk or premalignant lesions showed that curcumin was safe up to 8 g/day.59 A pharmacodynamic and pharmacokinetic study of oral Curcuma extract was also carried out in patients with colorectal cancer.60 Several phase I and phase II clinical trials are now ongoing in multiple centers to study the chemopreventive efficacy of curcumin (www.clinicaltrials.gov).
Resveratrol
Resveratrol (Fig 1C) is a phytoalexin, a major constituent of red wine, and abundant in the grape skin. Table 3 shows the resveratrol content in different wines, juices, and foods. The cardioprotective and chemopreventive activities have brought resveratrol to public and scientific attention. Resveratrol prevented skin cancer development in mice treated with carcinogen and was effective in all three major stages of cancer development.61 Topical application of resveratrol in mice, both before and after UVB exposure, inhibited skin damage and decreased skin cancer incidence.62 Prophylactic use of resveratrol reduced the number and size of esophageal, intestinal, and colon tumors.62,63 Resveratrol prevented the development of DMBA-induced mammary carcinogenesis, inhibited the growth of MDA-MB-231 xenografts, induced apoptosis of prostate cancer cell lines PC-3, DU145, and LNCaP, and suppressed the progression of prostate cancer in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice.64–68
Table 3.
Resveratrol Content in Different Beverages and Foods
| Food and Beverage | Serving Size | Total Resveratrol (mg) |
|---|---|---|
| Muscadine wine | 5 ounce glass | 2.12-6 |
| Red wine | ||
| Global | 5 ounce glass | 0.30-1.07 |
| Spanish | 5 ounce glass | 0.29-1.89 |
| Red grape juice (Spanish) | 5 ounce glass | 0.17-1.30 |
| Rose wine (Spanish) | 5 ounce glass | 0.06-0.53 |
| Pinot noir | 5 ounce glass | 0.06-0.30 |
| White wine (Spanish) | 5 ounce glass | 0.01-0.27 |
| Peanuts | ||
| Raw | 146 g | 0.01-0.26 |
| Boiled | 180 g | 0.32-1.28 |
| Peanut butter | 258 g | 0.04-0.13 |
| Red grapes | 160 g | 0.24-1.25 |
In preclinical studies, resveratrol was also effective against a number of other cancer types, including liver, pancreatic, gastrointestinal, lung, and some soft tissue tumors.28,69–73 Besides its in vitro effects, resveratrol also exerts antitumor activity in vivo and enhances the therapeutic effects of FU in a murine model of liver cancer.74 It also significantly abrogated benzo[a]pyrene diol epoxide–DNA adduct induction by Benzo[a]pyrene (B[a]P) in the lungs of BALB/c mice.75 A phase I study showed that even 5 g resveratrol was safe after oral administration.76 Several clinical trials to study the chemopreventive potential of resveratrol are now ongoing (www.linicaltrials.gov).
Lycopene
Lycopene (Fig 1D) is a natural antioxidant that imparts red color to tomatoes, guava, rosehip, watermelon, and pink grapefruit, and is found abundantly in red tomatoes and processed tomato products. Table 4 shows the lycopene content of several dietary sources. Because of its strong antioxidant properties, lycopene has drawn much attention as a cancer preventing agent. Epidemiological studies have shown that high intake of lycopene-containing vegetables is inversely associated with the incidence of certain types of cancer including digestive tract, prostate, and cervix.77–81 Initial evidence suggests that tomato products may help to prevent disease progression in benign prostate hyperplasia, and increases apoptosis in benign prostate hyperplasia and carcinoma.82,83 A combination of vitamin E, selenium, and lycopene dramatically inhibited prostate cancer development and increased disease-free survival.84 A reduction in Dunning R-3327H prostate cancer growth rate was observed in rats fed with diets containing broccoli, tomato, lycopene, and a combination of tomato plus broccoli.85 A phase II randomized clinical trial of lycopene supplementation before radical prostatectomy suggested that lycopene supplementation may decrease the growth of prostate cancer.86 Another phase II trial suggested that the combination of lycopene with soy isoflavones more strongly stabilized serum prostate-specific antigen (PSA) levels than lycopene alone in men with prostate cancer.87 In a cell culture model, lycopene strongly inhibited proliferation and induced apoptosis of prostate and breast cancer cell lines.88,89
Table 4.
Lycopene Content of Different Foods
| Source | Lycopene Content (μg/g) |
|---|---|
| Raw tomato | 8.8-42 |
| Tomato juice | 86-100 |
| Tomato sauce | 63-131 |
| Tomato ketchup | 124 |
| Watermelon | 23-72 |
| Pink grapefruit | 3.6-34 |
| Pink guava | 54 |
| Papaya | 20-53 |
| Rosehip puree | 7.8 |
| Apricot | < 0.1 |
A reduction in the incidence of aberrant crypt foci after lycopene treatment suggested its role in colon cancer prevention.90 Lycopene also strongly suppressed the growth of lung cancer cells and was found to be more potent than either α-carotene or β-carotene.91 Administration of lycopene during the postinitiation stage reduces the incidence of lung adenocarcinoma in mice.92,93 In two large cohort studies, α-carotene and lycopene intake were found to be significantly associated with a lower risk of lung cancer.94 Dietary tomato powder and lycopene supplementations were also found to prevent leiomyoma of the oviduct in the Japanese quail.95
Pomegranate
Pomegranate is widely consumed as both fresh fruit and juice. Although pomegranate fruit was used for various medicinal purposes in ancient times, its chemopreventive property was reported only at the beginning of the current century and has drawn much attention thereafter. The polyphenol-rich fractions from fermented juice, aqueous pericarp extract, or supercritical CO2-extracted seed oil inhibited growth of breast cancer cells,96 and decreased new blood vessel formation in the chicken chorioallantoic membrane model in vivo.97 It has also been shown that the pomegranate constituents cyanidin, delphinidin, and petunidin can inhibit the growth of MCF-7 breast cancer cells.98
Pomegranate seed oil significantly inhibited skin tumor development and promotion in CD1 mice.99 Treatment with pomegranate fruit extract was shown to induce cell cycle arrest and apoptosis of human lung carcinoma A549 cells, significantly inhibited A549 tumor growth in nude mice after oral administration,100 and protected A/J mice from carcinogen-induced lung carcinogenesis.101
A number of in vitro and in vivo studies suggest that pomegranate has strong potential for prostate cancer chemoprevention. Pomegranate fruit extract dose dependently inhibited the growth of PC-3 prostate cancer cell lines with the induction of apoptosis, and inhibited CWR22Ru1 xenografts with concomitant decrease in serum PSA levels.102 Pomegranate extract was found to significantly inhibit the proliferation of LNCaP and human umbilical vein endothelial cells and decrease xenografted prostate cancer size, tumor vessel density, vascular endothelial growth factor (VEGF) peptide levels, and HIF-1α expression in severe combined immunodeficiency mice.103 A phase II clinical trial conducted to assess the effects of pomegranate juice consumption on PSA progression in men with rising PSA after primary surgery or radiation showed significant increase in mean PSA doubling time.104 The statistically significant prolongation of PSA doubling time, coupled with corresponding laboratory effects on prostate cancer cell proliferation and apoptosis, warrant further testing in a placebo-controlled study.
Luteolin
Luteolin (Fig 1E) is a flavonoid abundant in several green vegetables, such as broccoli, celery, cabbage, spinach, green pepper, and cauliflower, that exhibits a wide array of pharmacologic properties ranging from anti-inflammation to anticancer effects.105 Luteolin is capable of inducing anticancer effects by inducing cell cycle arrest, senescence, or apoptosis in oral squamous cancer cells,106 human esophageal adenocarcinoma cells,107 lung carcinoma cells,108 human colon cancer cells,109 and human hepatoma cells.110 Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells in vitro and in xenografts111 and increased the efficacy of cisplatin in gastric cancer cells.112 In an animal model, the flavonoid also inhibited tumor promotion against DMBA-induced mammary tumors.113 Luteolin was also found to significantly decrease colon cancer incidence and the number of tumors per rat when administered at the initiation and the postinitiation stages of carcinogenesis.114 Well-controlled clinical trials are now warranted to evaluate the chemopreventive potential of luteolin in human subjects.
Genistein
Genistein is a phytoestrogen (Fig 1F) abundant in soybeans and soy products. Multiple lines of compelling evidence from a number of epidemiological studies support an inverse correlation between dietary soy consumption and the risk of prostate,115 breast,116,117 and endometrial118 cancer. The consumption of dietary genistein decreased tumor multiplicity and diminished the incidence of adenocarcinoma in the DMBA model of mammary cancer in rats.119 The soybean isoflavone mixture consisting of 74% genistein and 21% daidzein was found to inhibit DMBA-induced adenocarcinoma in the prostate and seminal vesicles in rats.120 Genistein inhibited PCa cell growth in culture by inducing G2/M arrest and apoptosis, inhibited the secretion of PSA, and increased the radiation effect against prostate cancer in cell culture and in orthotopic and metastatic in vivo models.121,122 Genistein also potentiated the antitumor activity of cisplatin in BxPC-3 tumor xenografts.123 On the basis of these observations, several early clinical trials either with genistein or soy products have been completed. A pilot study conducted in patients with prostate cancer and rising serum PSA levels suggested that soy isoflavones may benefit some patients with prostate cancer.124 Another phase II trial was carried out in PSA-recurrent prostate cancer after previous local therapy, which showed a decrease in serum PSA level from 56% to 20%.125 Other clinical trials are ongoing to study the efficacy of soy products and genistein in cancer prevention (www.clinicaltrials.gov).
Other Promising Natural Agents
Besides the aforementioned dietary agents, other natural compounds are being actively investigated for their chemopreventive potential, many of which show strong promise. These include ellagic acid, some triterpenes (such as lupeol, betulinic acid, ginsenosides, oleanolic acid), polyunsaturated fatty acids (PUFAs), and ginkolide B. Ellagic acid is an antioxidant polyphenol present in many fruits and vegetables including grapes, strawberries, raspberries, pomegranates, and nuts, that exhibited chemopreventive activity against skin, lung, esophageal, colon, bladder, prostate, and breast cancers.126,127 Among the triterpenes, lupeol128 and betulinic acid129 have been extensively investigated for their chemopreventive activities and showed a broad spectrum of activity against multiple cancer types in both cell culture and animal models. Among the PUFAs, the n-3 PUFAs (linoleic acid and its derivatives) have been extensively studied and exhibited chemopreventive potential in animal models of prostate, breast and colon carcinogenesis, and currently several preventive trials are ongoing against these cancers.130 Ginko biloba extracts and its constitutent ginkolide B have also been studied for their chemopreventive activities and showed some promise against several cancer types.131,132
MOLECULAR TARGETS FOR NATURAL CHEMOPREVENTIVE AGENTS
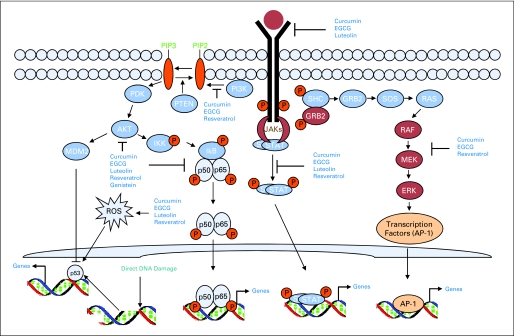
The cell signaling pathways activated by natural dietary agents are numerous and different for different agents. Moreover, the same compound activates different signaling pathways depending on the cell types. The main signaling pathways activated by dietary chemopreventive agents are illustrated in Figure 2 and Table 1, and described in the following part of this article.
Fig 2.
Molecular targets of natural chemopreventive agents. The cell signaling pathways activated by natural dietary agents are numerous and different for different agents. Multiple growth factor receptors (epidermal growth factor receptor, insulin-like growth factor 1 receptor, fibroblast growth factor, platelet-derived growth factor receptor) are activated at the cell surface in tumorigenesis. Activation of these receptors activates several downstream signaling pathways. Among these pathways, the Ras-MAPK (such as ERK and JNK) pathways, the JAK-STAT pathways, the PI3K-AKT pathways and the NF-κB pathways are important and are the targets of natural chemopreventive agents. Some natural agents inhibit the receptors at the cell surface either by dephosphorylating them or by inducing their degradation, which ultimately modulate the downstream signaling pathways important for proliferation, angiogenesis, and apoptosis. Inhibition of AKT and ERK signaling by natural agents is quite common, although in many cases this inhibition is the result of growth factor receptor inhibition. Inhibition of NF-κB signaling pathway by interfering with multiple targets of signaling is another common target of natural agents. Many natural compounds generate reactive oxygen species (ROS), which activate p53 family members and induce cell cycle arrest and apoptosis. EGCG, epigallocatechin-3-gallate; ERK, extracellular signal–regulated kinase.
p53 Family Members
The tumor suppressor p53 plays a pivotal role in controlling the cell cycle, apoptosis, genomic integrity, and DNA repair in response to various genotoxic stresses.133,134 After activation, p53 can bind to regulatory DNA sequences and activate the expression of target genes, which can be grouped into four categories: cell cycle inhibition (p21, reprimo, cyclin G1, GADD45, 14-3-3), apoptosis (PERP, NOXA, PUMA, p53AIP1, ASPP1/2, Fas, BAX, PIDD), genetic stability (p21, DDB2, MSH2, XPC) and inhibition of angiogenesis (TSP1, Maspin, BAI1, GD-AIF).135–137 In addition to its transactivation function, p53 can also act as a transrepressor.138,139 Because of these roles, p53 has been considered a molecular guardian of the genome.
Many natural chemopreventive agents induce cell cycle arrest or apoptosis by activating p53 and its target genes. EGCG induced the expression of p53 and its target p21 and BAX in prostate cancer cells with wild-type p53, but not with inactive p53.140 EGCG also activated p53 and BAX in breast carcinoma cells.141 Luteolin also induced cell cycle arrest and apoptosis and increased chemosensitization by activating p53 and its targets p21, BAX, and PUMA (unpublished data from Munna L. Agarwal).112 In human breast cancer and bladder cancer cells, curcumin was shown to induce apoptosis through p53-dependent BAX induction.142,143 Curcumin also induced p53-mediated apoptosis by activating its mitochondrial translocation.144 Huang et al145 reported that resveratrol induced apoptosis only in cells expressing wild-type p53, but not in p53-deficient cells. Resveratrol activates the expression of p21, p27, BAX, PUMA, MDM2, and cyclin G, all important downstream targets.146 The dietary chemopreventive agent genistein also activates p53 in multiple cell types. For example, genistein induced G2/M arrest and apoptosis in human malignant glioma cell lines by activating p53 and p21.147
Many natural compounds can also induce cell cycle arrest and apoptosis in cells lacking functional p53. In addition to p53, mammalian cells contain two closely related proteins, p63 and p73.148,149 We have previously reported that EGCG induces apoptosis by activating p73-dependent expression of a subset of p53 target genes including p21, reprimo, cyclin G1, PERP, MDM2, WIG1, and PIG11.150 p73 is also activated in response to EGCG in multiple myeloma cells.151 Our unpublished results also suggest that the dietary agents curcumin and luteolin activate p73.
Nuclear Factor-Kappa B
Nuclear factor-kappa B (NF-κB) is a master transcription factor consisting of closely related proteins that generally exist as dimers and bind to a common DNA sequence within the promoters/enhancers of target genes, called the κB site, to promote transcription of target genes through the recruitment of coactivators and corepressors.152 The NF-κB family of transcription factors consists of five members, p50, p52, p65 (Rel A), c-Rel, and Rel B, which share an N-terminal Rel homology domain responsible for DNA binding and homo- and heterodimerization. NF-κB is activated by free radicals, inflammatory stimuli, cytokines, carcinogens, tumor promoters, endotoxins, γ-radiation, ultraviolet (UV) light, and x-rays and induces NF-κB target genes important for cellular growth and transformation, suppression of apoptosis, invasion, metastasis, chemoresistance, radioresistance, and inflammation.
Most of the natural chemopreventive agents including curcumin,153,154 resveratrol,155 EGCG,156 lycopene,157 genistein,158 and luteolin108 act as potent inhibitors of NF-κB pathways. These compounds may block one or more steps in the NF-κB signaling pathway such as inhibition of the most upstream growth factor receptors that activate the NF-κB signaling cascade, translocation of NF-κB to the nucleus, DNA binding of the dimers, or interactions with the basal transcriptional machinery. The NF-κB target genes influenced by the natural chemopreventive agents include inhibition of Bcl-2 and Bcl-x(L), cyclin D1, matrix metalloproteinases (MMP), and VEGF.
Activator Protein 1
Activator protein 1 (AP-1) is a group of dimeric basic region-leucine zipper proteins consisting of Jun (c-Jun, JunB, JunD), Fos (c-Fos, FosB, Fra-1, and Fra-2), Maf (c-Maf, MafB, MafA, MafG/F/K, and Nrl), and ATF (ATF2, LRF1/ATF3, B-ATF, JDP1, JDP2) subfamilies.159 These proteins form either homo- or heterodimers and bind either to AP-1 DNA recognition elements (5‘-TGAG/CTCA-3‘) or to cAMP response elements (5‘-TGACGTCA-3‘) and activate their target genes. In addition to being transcriptional activators, an increasing body of evidence suggests that some of the biologic effects of AP-1 are mediated by gene repression.159 AP-1–regulated genes include important modulators of invasion and metastasis, angiogenesis, proliferation, differentiation, and survival.159
Several natural chemopreventive compounds such as green tea,160 resveratrol,161 and curcumin162 have been reported to suppress AP-1 activation and modulate AP-1 target genes, which is ultimately linked to their chemopreventive potential. Green tea polyphenols inhibit the transcriptional activity of AP-1 in multiple cells types, which is essential for their growth inhibitory effects.160,163 Pretreatment with resveratrol inhibits TPA-induced AP-1 DNA binding by inhibiting the nuclear expression of c-Jun and c-Fos.161 Multiple studies also suggest that suppression of AP-1 activity is important for mediating the proapoptotic function of curcumin. Curcumin reduced cell survival of human glioma cells, which was correlated with the inhibition of AP-1 and NF-κB signaling pathways.162
Signal Transducers and Activators of Transcription Pathway
A novel signal transduction pathway to the nucleus has been uncovered through the study of transcriptional activation in response to interferon.164 Activation of various tyrosine kinases leads to phosphorylation, dimerization, and nuclear localization of the signal transducers and activators of transcription (STAT) proteins, binding to specific DNA elements and direct transcription. So far, seven mammalian STAT family members (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6) have been cloned that share common structural elements. Constitutive activation of STAT3 and STAT5 has been implicated in multiple myelomas, lymphomas, leukemias, and several solid tumors.165 Several dietary agents, such as green tea,166 resveratrol,167 and curcumin,168 have been implicated to modulate STAT activation in tumor cells. Consumption of GTP substantially inhibited STAT3 expression in TRAMP mice, which may contribute to growth inhibition and apoptosis in this autochthonous mouse prostate cancer model.169 PPE also inhibits angiogenesis of breast cancer cells by inhibiting the expression of VEGF and MMP-9 via suppressing STAT3 activation.166 Resveratrol has been reported to modulate interleukin (IL)-6–induced ICAM-1 gene expression by attenuating STAT3 phosphorylation.167 Several studies have also demonstrated a role for STAT signaling pathways in curcumin-mediated chemoprevention. Prevention of tumor-induced T-cell apoptosis by curcumin was mediated via STAT5A-induced expression of Bcl-2.170 Selvendiran et al110 reported that luteolin inhibited phosphorylation of STAT3, which targeted it for proteosomal degradation and inhibited the expression of cyclin D1, survivin, Bcl-x(L), and VEGF.
Growth Factors and Their Receptors
Growth factors are proteins that bind to receptors on the cell surface, with the primary result of activating cellular proliferation and/or differentiation. Several growth factor signaling molecules, such as endothelial growth factor, platelet-derived growth factor, fibroblast growth factor, transforming growth factor, insulin-like growth factor, and colony-stimulating factor are implicated in carcinogenesis. Abnormal growth factor signaling pathways lead to increased cell proliferation, suppression of apoptotic signals, and invasion, contributing to metastasis. As a consequence of growth factor receptor activation, several downstream signaling pathways, most important of which are PI3K-AKT and Ras-MAPK, are activated. These signaling pathways have significant impacts on tumorigenesis and become targets for many natural chemopreventive and chemotherapeutic agents.
Curcumin inhibits the ligand-stimulated activation of EGFR and enhances the growth inhibitory effects of FU and oxaliplatin through EGFR and insulin-like growth factor receptor pathways.171,172 Decreased expression and activation (tyrosine phosphorylation) of EGFR, HER-2, HER-3, and IGF-1R as well as their downstream effectors such as AKT and COX-2 were observed after curcumin treatment together with FOLFOX (FOLFOX-FU plus oxaliplatin).172
Several studies have also demonstrated an essential role for the inhibition of growth factor signaling for the chemopreventive potential of GTP. EGCG treatment inhibited the phosphorylation of EGFR and its downstream targets AKT and ERK in head and neck cancer and potentiated the effects of the tyrosine kinase inhibitor erlotinib.39,173 EGCG induced internalization and ubiquitin-mediated degradation of EGFR, ultimately undermining EGFR signaling. Adachi et al174 reported that the inhibitory effect of EGCG on activation of EGFR was associated with altered lipid order in HT29 colon cancer cells. EGCG also inhibits the activation of IGF-1 receptor in human colon cancer cells.175
Inhibition of IGF-1-induced activation of IGF-1R and AKT were demonstrated in prostate cancer PC-3 and DU145 cells by luteolin. Inhibition of AKT by luteolin resulted in decreased phosphorylation of its downstream targets, including p70S6K1, GSK-3β, and FKHR/FKHRL1. Luteolin also inhibited IGF-1–induced activation of EGFR and MAPK/ERK signaling.176 Growth inhibition and apoptosis of pancreatic tumor cells by luteolin was also associated with the inhibition of EGFR tyrosine kinase activity.177
Host Factors/Immunoprevention
Immunoprevention is an approach to cancer prevention that aims to stimulate the host immune system to eliminate damaged cells before tumor onset. It is now established that the absence of functional T cells or T cell–derived cytokines, such as interferon (IFN)-γ, enhances the onset of spontaneous and carcinogen-induced tumor.178 Thus, activation of functional T cells or production of several cytokines, such as INF-γ or IL-12, might contribute to cancer prevention. Several natural agents have been found to modulate certain host factors, which were important for their chemopreventive potential. GTP prevented UV radiation–induced skin cancer by inducing IL-12–dependent DNA repair, activating cytotoxic (CD8+) T cells, inducing tumor cell apoptosis, and inhibiting angiogenic factors.179,180 EGCG also enhanced CD8+ T cell–mediated antitumor immunity induced by DNA vaccination.181 Tumor-induced immunodepletion caused apoptosis of thymic CD4+/CD8+ single/double positive cells as well as loss of circulating CD4+/CD8+ T cells. The administration of curcumin to tumor-bearing animals resulted in restoration of progenitor, effecter, and circulating T cells.170 Genistein modulated immune responses and increased host resistance to B16F10 tumor, which may be related to increases in the activities of cytotoxic T cells and natural killer cells.182 Resveratrol enhanced IFN-γ expression in CD8+ T cells, leading to immune stimulation, and suppressed the CD4+CD25+ cell population, rendering the peritumoral microenvironment unfavorable to tumors in tumor-bearing mice.183
In addition to the tumor cells themselves, host components, including the stroma, an expanding vasculature, and often chronic inflammation, contribute to tumor growth. Thus, targeting these host factors might delay tumor progression. Some natural agents target these host factors. EGCG inhibited viability, capillary tube formation, and migration of human umbilical vein endothelial cells and inhibited angiogenic and metastasis markers (von Willebrand factor, VEGF, CD31, MMP-2, MMP-7, MMP-9, and MMP-12) in a xenograft model of pancreatic cancer.184 Luteolin inhibited vascular VEGF-induced angiogenesis and tumor growth in vivo in a murine xenograft model.185 A number of studies also suggest that angiogenesis is the target of chemoprevention by curcumin.186,187
CONCLUSION AND FUTURE DIRECTIONS
Chemoprevention research has gained momentum through the US Food and Drug Administration approval of tamoxifen and raloxifene for breast cancer risk reduction. Various epidemiological and preclinical findings and the results of several early clinical studies convincingly argue for a definitive role of selected dietary products in the prevention and treatment of cancers. Many of these agents target multiple signal transduction pathways, which vary widely depending on cancer origin. The key challenge to researchers is how best to use this information for effective cancer prevention in populations with different cancer risks. Moreover, low potency and poor bioavailability of dietary agents pose further challenges to scientists. The introduction of synthetic analogs of natural compounds may be a solution for these potency and bioavailability limitations. For example, the synthetic curcumin analog EF24 exhibited approximately 10-fold greater potency than natural curcumin.154 Some natural compounds have exhibited synergism with established chemopreventive agents or with other natural compounds. Since drug-associated toxicity remains a significant barrier for currently available chemotherapeutic and chemopreventive drugs, using natural compounds (which have better safety profiles) as adjuvant therapy with current chemotherapeutic agents may help to mitigate drug-associated toxicities. For example, genistein was found to sensitize prostate cancer to radiation in animal studies122 and a recent clinical trial suggested that soy isoflavones could prevent radiation-induced bladder and bowel adverse effects and erectile dysfunction.188 Because of the advances in our understanding of multistep and field carcinogenesis, the introduction of new technologies for screening and early detection, and the emergence of promising molecularly targeted agents, prevention and therapy are beginning to converge at the level of early-phase clinical trials.189 The future full convergence of prevention-therapy drug development will open new avenues for natural compounds in reducing the public health impact of major cancers. However, more preclinical studies and clinical trials are certainly needed to validate the usefulness of these agents either alone or in combination with existing therapies.
Acknowledgment
We thank Anthea Hammond, PhD, for her critical and editorial review of this article.
Footnotes
Supported by Grants No. P50 CA128613, U01 CA101244, and R01 CA112643 from the National Institutes of Health. D.M.S. and F.R.K. are Distinguished Cancer Scholars of the Georgia Cancer Coalition.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Fadlo R. Khuri, sanofi-aventis (C) Stock Ownership: None Honoraria: Fadlo R. Khuri, sanofi-aventis; Dong M. Shin, Bristol Myers Squibb, sanofi-aventis Research Funding: Fadlo R. Khuri, sanofi-aventis, Novartis; Dong M. Shin, Eli Lily Oncology, Genentech, Domantis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: A.R.M. Ruhul Amin, Dong M. Shin
Collection and assembly of data: A.R.M. Ruhul Amin
Manuscript writing: A.R.M. Ruhul Amin, Omer Kucuk, Fadlo R. Khuri, Dong M. Shin
Final approval of manuscript: A.R.M. Ruhul Amin, Omer Kucuk, Fadlo R. Khuri, Dong M. Shin
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Strong K, Mathers C, Epping-Jordan J, et al. Preventing cancer through tobacco and infection control: How many lives can we save in the next 10 years? Eur J Cancer Prev. 2008;17:153–161. doi: 10.1097/CEJ.0b013e3282b6fda8. [DOI] [PubMed] [Google Scholar]
- 3.Glade MJ. Food, nutrition, and the prevention of cancer: A global perspective: American Institute for Cancer Res/World Cancer Res Fund, American Institute for Cancer Res, 1997. Nutrition. 1999;15:523–526. doi: 10.1016/s0899-9007(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 4.Haddad RI, Shin DM. Recent development in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 5.Berenblum I. The modifying influence of dichloroethyl sulphide on the induction of tumours in mice by tar. J Pathol Bacteriol. 1929;32:425–434. [Google Scholar]
- 6.Sporn MB, Dunlop NM, Newton DL. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 7.Hong WK, Endicott J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 8.Lippman SM, Batsakis JG, Toth BB, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 9.Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:825–827. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 10.Benner SE, Pajak TF, Lippman SM, et al. Prevention of second primary tumors with isotretinoin in patients with squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 1994;86:140–141. doi: 10.1093/jnci/86.2.140. [DOI] [PubMed] [Google Scholar]
- 11.Pastorino U, Chiesa E, Infante M, et al. Safety of high dose vitamin A. Randomized trial on lung cancer chemoprevention. Oncology. 1991;48:131–137. doi: 10.1159/000226912. [DOI] [PubMed] [Google Scholar]
- 12.Lippman SM, Lee JJ, Karp DD, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001;93:605–618. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 13.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:426–427. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 14.Shin DM, Khuri FR, Murphy B, et al. Combined interferon-alfa, 13-cis-retinoic acid and alfa-tocoferol in locally advanced head and neck squamous cell carcinoma: Novel bioadjuvant phase II trial. J Clin Oncol. 2001;19:3010–3017. doi: 10.1200/JCO.2001.19.12.3010. [DOI] [PubMed] [Google Scholar]
- 15.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 16.Khuri FR, Cohen V. Molecularly targeted approaches to the chemoprevention of lung cancer. Clin Cancer Res. 2004;10:4249s–4253s. doi: 10.1158/1078-0432.CCR-040019. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Wang Y, Lantry LE, et al. Farnesyltransferase inhibitors are potent lung cancer chemopreventive agents in A/J mice with a dominant-negative p53 and/or heterozygous deletion of Ink4a/Arf. Oncogene. 2003;22:6257–6265. doi: 10.1038/sj.onc.1206630. [DOI] [PubMed] [Google Scholar]
- 18.Choe MS, Zhang X, Shin HJC, et al. Interaction between epidermal growth factor receptor– and cyclooxygenase 2–mediated pathways and its implications for the chemoprevention of head and neck cancer. Mol Cancer Ther. 2005;4:1448–1455. doi: 10.1158/1535-7163.MCT-04-0251. [DOI] [PubMed] [Google Scholar]
- 19.Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: A global perspective. Pharmacol Ther. 2003;99:1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 20.Block G, Patterson B, Suber A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 21.Benetou V, Orfanos P, Lagiou P, et al. Vegetables and fruits in relation to cancer risk: Evidence from the Greek EPIC cohort study. Cancer Epidemiol Biomarkers Prev. 2008;17:387–392. doi: 10.1158/1055-9965.EPI-07-2665. [DOI] [PubMed] [Google Scholar]
- 22.Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122:2330–2336. doi: 10.1002/ijc.23319. [DOI] [PubMed] [Google Scholar]
- 23.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: A review. J Am Diet Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 24.Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769–775. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- 25.Nakachi K, Suemasu K, Suga K, et al. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89:254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jian L, Xie LP, Lee AH, et al. Protective effect of green tea against prostate cancer: A case-control study in southeast China. Int J Cancer. 2004;108:130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 27.Wu AH, Yu MC, Tseng CC, et al. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003;106:574–579. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- 28.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: Progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 29.Bushman JL. Green tea and cancer in humans: A review of the literature. Nutr Cancer. 1998;31:151–159. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- 30.Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clin Cancer Res. 2005;11:1918–1927. doi: 10.1158/1078-0432.CCR-04-1976. [DOI] [PubMed] [Google Scholar]
- 31.Sartippour MR, Heber D, Ma J, et al. Green tea and its catechins inhibit breast cancer xenografts. Nutr Cancer. 2001;40:149–156. doi: 10.1207/S15327914NC402_11. [DOI] [PubMed] [Google Scholar]
- 32.Xiao H, Hao X, Simi B, et al. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis. 2008;29:113–119. doi: 10.1093/carcin/bgm204. [DOI] [PubMed] [Google Scholar]
- 33.Gao YT, McLaughlin JK, Blot WJ, et al. Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- 34.Shanafelt T, Lee Y, Call T, et al. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk Res. 2006;30:707–712. doi: 10.1016/j.leukres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 35.Yang CS, Chen L, Lee MJ, et al. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–354. [PubMed] [Google Scholar]
- 36.Chow HH, Cai Y, Alberts DS, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–58. [PubMed] [Google Scholar]
- 37.Chow HH, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- 38.Pisters KM, Newman RA, Coldman B, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19:1830–1838. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Zhang H, Tighiouart M, et al. Synergistic inhibition of head and neck tumor growth by green tea (-)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–1014. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siddiqui IA, Malik A, Adhami VM, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–2063. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 41.Kuttan R, Bhanumathy P, Nirmala K, et al. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 42.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 43.NCI D. Clinical development plan: Curcumin. J Cell Biochem. 1996;26(Suppl):72–85. [PubMed] [Google Scholar]
- 44.Rao CV, Rivenson A, Simi B, et al. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- 45.Huang MT, Lou YR, Ma W, et al. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–5847. [PubMed] [Google Scholar]
- 46.Kawamori T, Lubet R, Steele VE, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 47.Conney AH. Enzyme induction and dietary chemicals as approaches to cancer chemoprevention: The Seventh DeWitt S. Goodman Lecture. Cancer Res. 2003;63:7005–7031. [PubMed] [Google Scholar]
- 48.Li N, Chen X, Liao J, et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis. 2002;23:1307–1313. doi: 10.1093/carcin/23.8.1307. [DOI] [PubMed] [Google Scholar]
- 49.Li M, Zhang Z, Hill DL, et al. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 50.Kuttan G, Kumar KB, Guruvayoorappan C, et al. Antitumor, anti-invasion, and antimetastatic effects of curcumin. Adv Exp Med Biol. 2007;595:173–184. doi: 10.1007/978-0-387-46401-5_6. [DOI] [PubMed] [Google Scholar]
- 51.Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. 2007;595:149–172. doi: 10.1007/978-0-387-46401-5_5. [DOI] [PubMed] [Google Scholar]
- 52.Verma SP, Salamone E, Goldin B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem Biophys Res Commun. 1997;233:692–696. doi: 10.1006/bbrc.1997.6527. [DOI] [PubMed] [Google Scholar]
- 53.Khafif A, Schantz SP, Chou TC, et al. Quantitation of chemopreventive synergism between (−)-epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis. 1998;19:419–424. doi: 10.1093/carcin/19.3.419. [DOI] [PubMed] [Google Scholar]
- 54.Sreepriya M, Bali G. Effects of administration of embelin and curcumin on lipid peroxidation, hepatic glutathione antioxidant defense and hematopoietic system during N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Mol Cell Biochem. 2006;284:49–55. doi: 10.1007/s11010-005-9012-7. [DOI] [PubMed] [Google Scholar]
- 55.Koo JY, Kim HJ, Jung KO, et al. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J Med Food. 2004;7:117–121. doi: 10.1089/1096620041224229. [DOI] [PubMed] [Google Scholar]
- 56.Kunnumakkara AB, Guha S, Krishnan S, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 57.Sen S, Sharma H, Singh N. Curcumin enhances vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem Biophys Res Commun. 2005;331:1245–1252. doi: 10.1016/j.bbrc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 58.Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 59.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 60.Sharma RA, McLelland HR, Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 61.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 62.Athar M, Back JH, Tang X, et al. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li ZG, Hong T, Shimada Y, et al. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23:1531–1536. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- 64.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- 65.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 66.Gill C, Walsh SE, Morrissey C, et al. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67:1641–1653. doi: 10.1002/pros.20653. [DOI] [PubMed] [Google Scholar]
- 67.Narayanan BA, Narayanan NK, Re GG, et al. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int J Cancer. 2003;104:204–212. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- 68.Aziz MH, Nihal M, Fu VX, et al. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 69.Ma X, Tian X, Huang X, et al. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca2+ and mCICR-mediated MPT activation in HepG2 cells. Mol Cell Biochem. 2007;302:99–109. doi: 10.1007/s11010-007-9431-8. [DOI] [PubMed] [Google Scholar]
- 70.Sun ZJ, Pan CE, Liu HS, et al. Anti-hepatoma activity of resveratrol in vitro. World J Gastroenterol. 2002;8:79–81. doi: 10.3748/wjg.v8.i1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carbó N, Costelli P, Baccino FM, et al. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophys Res Commun. 1999;254:739–743. doi: 10.1006/bbrc.1998.9916. [DOI] [PubMed] [Google Scholar]
- 72.Ding XZ, Adrian TE. Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Pancreas. 2002;25:e71–e76. doi: 10.1097/00006676-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 73.Kubota T, Uemura Y, Kobayashi M, et al. Combined effects of resveratrol and paclitaxel on lung cancer cells. Anticancer Res. 2003;23:4039–4046. [PubMed] [Google Scholar]
- 74.Wu SL, Sun ZJ, Yu L, et al. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J Gastroenterol. 2004;10:3048–3052. doi: 10.3748/wjg.v10.i20.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Revel A, Raanani H, Younglai E, et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23:255–261. doi: 10.1002/jat.916. [DOI] [PubMed] [Google Scholar]
- 76.Boocock DJ, Faust GE, Patel KR, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 77.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 78.Seren S, Lieberman R, Bayraktar UD, et al. Lycopene in cancer prevention and treatment. Am J Ther. 2008;15:66–81. doi: 10.1097/MJT.0b013e31804c7120. [DOI] [PubMed] [Google Scholar]
- 79.Franceschi S, Bidoli E, La Vecchia C, et al. Tomatoes and risk of digestive-tract cancers. Int J Cancer. 1994;59:181–184. doi: 10.1002/ijc.2910590207. [DOI] [PubMed] [Google Scholar]
- 80.Giovannucci E, Rimm EB, Liu Y, et al. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 81.García-Closas R, Castellsagué X, Bosch X, et al. The role of diet and nutrition in cervical carcinogenesis: A review of recent evidence. Int J Cancer. 2005;117:629–637. doi: 10.1002/ijc.21193. [DOI] [PubMed] [Google Scholar]
- 82.Schwarz S, Obermüller-Jevic UC, Hellmis E, et al. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J Nutr. 2008;138:49–53. doi: 10.1093/jn/138.1.49. [DOI] [PubMed] [Google Scholar]
- 83.Kim HS, Bowen P, Chen L, et al. Effects of tomato sauce consumption on apoptotic cell death in prostate benign hyperplasia and carcinoma. Nutr Cancer. 2003;47:40–47. doi: 10.1207/s15327914nc4701_5. [DOI] [PubMed] [Google Scholar]
- 84.Venkateswaran V, Fleshner NE, Sugar LM, et al. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004;64:5891–5896. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- 85.Canene-Adams K, Lindshield BL, Wang S, et al. Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67:836–843. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 86.Kucuk O, Sarkar FH, Sakr W, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10:861–868. [PubMed] [Google Scholar]
- 87.Vaishampayan U, Hussain M, Banerjee M, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 88.Wang AH, Zhang LS. Effect of lycopene on the proliferation of MCF-7 and MDA-MB-231 cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:958–960. [PubMed] [Google Scholar]
- 89.Fornelli F, Leone A, Verdesca I, et al. The influence of lycopene on the proliferation of human breast cell line (MCF-7) Toxicol in Vitro. 2007;21:217–223. doi: 10.1016/j.tiv.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 90.Sengupta A, Ghosh S, Das RK, et al. Chemopreventive potential of diallylsulfide, lycopene and theaflavin during chemically induced colon carcinogenesis in rat colon through modulation of cyclooxygenase-2 and inducible nitric oxide synthase pathways. Eur J Cancer Prev. 2006;15:301–305. doi: 10.1097/00008469-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 91.Levy J, Bosin E, Feldman B, et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer. 1995;24:257–266. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- 92.Kim DJ, Takasuka N, Kim JM, et al. Chemoprevention by lycopene of mouse lung neoplasia after combined initiation treatment with DEN, MNU and DMH. Cancer Lett. 1997;120:15–22. doi: 10.1016/s0304-3835(97)00281-4. [DOI] [PubMed] [Google Scholar]
- 93.Kim DJ, Takasuka N, Nishino H, et al. Chemoprevention of lung cancer by lycopene. Biofactors. 2000;13:95–102. doi: 10.1002/biof.5520130116. [DOI] [PubMed] [Google Scholar]
- 94.Michaud DS, Feskanich D, Rimm EB, et al. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am J Clin Nutr. 2000;72:990–997. doi: 10.1093/ajcn/72.4.990. [DOI] [PubMed] [Google Scholar]
- 95.Sahin K, Ozercan R, Onderci M, et al. Lycopene supplementation prevents the development of spontaneous smooth muscle tumors of the oviduct in Japanese quail. Nutr Cancer. 2004;50:181–189. doi: 10.1207/s15327914nc5002_8. [DOI] [PubMed] [Google Scholar]
- 96.Kim ND, Mehta R, Yu W, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71:203–217. doi: 10.1023/a:1014405730585. [DOI] [PubMed] [Google Scholar]
- 97.Toi M, Bando H, Ramachandran C, et al. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis. 2003;6:121–128. doi: 10.1023/B:AGEN.0000011802.81320.e4. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Vareed SK, Nair MG. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005;76:1465–1472. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 99.Hora JJ, Maydew ER, Lansky EP, et al. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J Med Food. 2003;6:157–161. doi: 10.1089/10966200360716553. [DOI] [PubMed] [Google Scholar]
- 100.Khan N, Hadi N, Afaq F, et al. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28:163–173. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- 101.Khan N, Afaq F, Kweon MH, et al. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67:3475–3482. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- 102.Malik A, Afaq F, Sarfaraz S, et al. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sartippour MR, Seeram NP, Rao JY, et al. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int J Oncol. 2008;32:475–480. [PubMed] [Google Scholar]
- 104.Pantuck AJ, Leppert JT, Zomorodian N, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 105.Shimoi K, Saka N, Kaji K, et al. Metabolic fate of luteolin and its functional activity at focal site. Biofactors. 2000;12:181–186. doi: 10.1002/biof.5520120129. [DOI] [PubMed] [Google Scholar]
- 106.Yang SF, Yang WE, Chang HR, et al. Luteolin induces apoptosis in oral squamous cancer cells. J Dent Res. 2008;87:401–406. doi: 10.1177/154405910808700413. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46:2042–2053. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 108.Ju W, Wang X, Shi H, et al. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol. 2007;71:1381–1388. doi: 10.1124/mol.106.032185. [DOI] [PubMed] [Google Scholar]
- 109.Lim do Y, Jeong Y, Tyner AL, et al. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G66–G75. doi: 10.1152/ajpgi.00248.2006. [DOI] [PubMed] [Google Scholar]
- 110.Selvendiran K, Koga H, Ueno T, et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: An implication for the antitumor potential of flavonoids. Cancer Res. 2006;66:4826–4834. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 111.Chiu FL, Lin JK. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate. 2008;68:61–71. doi: 10.1002/pros.20690. [DOI] [PubMed] [Google Scholar]
- 112.Wu B, Zhang Q, Shen W, et al. Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol Cell Biochem. 2008;313:125–132. doi: 10.1007/s11010-008-9749-x. [DOI] [PubMed] [Google Scholar]
- 113.Samy RP, Gopalakrishnakone P, Ignacimuthu S. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem Biol Interact. 2006;164:1–14. doi: 10.1016/j.cbi.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 114.Manju V, Nalini N. Protective role of luteolin in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Cell Biochem Funct. 2007;25:189–194. doi: 10.1002/cbf.1305. [DOI] [PubMed] [Google Scholar]
- 115.Hebert JR, Hurley TG, Olendzki BC, et al. Nutritional and socioeconomic factors in relation to prostate cancer mortality: A cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 116.Adlercreutz H, Honjo H, Higashi A, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54:1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 117.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 118.Goodman MT, Wilkens LR, Hankin JH, et al. Association of soy and fiber consumption with the risk of endometrial cancer. Am J Epidemiol. 1997;146:294–306. doi: 10.1093/oxfordjournals.aje.a009270. [DOI] [PubMed] [Google Scholar]
- 119.Hilakivi-Clarke L, Onojafe I, Raygada M, et al. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br J Cancer. 1999;80:1682–1688. doi: 10.1038/sj.bjc.6690584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Onozawa M, Kawamori T, Baba M, et al. Effects of a soybean isoflavone mixture on carcinogenesis in prostate and seminal vesicles of F344 rats. Jpn J Cancer Res. 1999;90:393–398. doi: 10.1111/j.1349-7006.1999.tb00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lakshman M, Xu L, Ananthanarayanan V, et al. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–2032. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, Raffoul JJ, Che M, et al. Prostate cancer treatment is enhanced by genistein in vitro and in vivo in a syngeneic orthotopic tumor model. Radiat Res. 2006;166:73–80. doi: 10.1667/RR3590.1. [DOI] [PubMed] [Google Scholar]
- 123.Mohammad RM, Banerjee S, Li Y, et al. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer. 2006;106:1260–1268. doi: 10.1002/cncr.21731. [DOI] [PubMed] [Google Scholar]
- 124.Hussain M, Banerjee M, Sarkar FH, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–117. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 125.Pendleton JM, Tan WW, Anai S, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132. doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 127.Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269:262–268. doi: 10.1016/j.canlet.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 128.Chaturvedi PK, Bhui K, Shukla Y. Lupeol: Connotations for chemoprevention. Cancer Lett. 2008;263:1–13. doi: 10.1016/j.canlet.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 129.Cichewicz RH, Kouzi SA. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev. 2004;24:90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- 130.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ye B, Aponte M, Dai Y, et al. Ginkgo biloba and ovarian cancer prevention: Epidemiological and biological evidence. Cancer Lett. 2007;251:43–52. doi: 10.1016/j.canlet.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 132.DeFeudis FV, Papadopoulos V, Drieu K. Ginkgo biloba extracts and cancer: A research area in its infancy. Fundam Clin Pharmacol. 2003;17:405–417. doi: 10.1046/j.1472-8206.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 133.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 134.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 135.Carr AM. Cell cycle: Piecing together the p53 puzzle. Science. 2000;287:1765–1766. doi: 10.1126/science.287.5459.1765. [DOI] [PubMed] [Google Scholar]
- 136.Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–99. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 137.Ko LJ, Prives C. p53: Puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 138.Agarwal MK, Amin AR, Agarwal ML. DNA replication licensing factor minichromosome maintenance deficient 5 rescues p53-mediated growth arrest. Cancer Res. 2007;67:116–121. doi: 10.1158/0008-5472.CAN-06-2835. [DOI] [PubMed] [Google Scholar]
- 139.Ho JS, Ma W, Mao DY, et al. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol. 2005;25:7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hastak K, Agarwal MK, Mukhtar H, et al. Ablation of either p21 or Bax prevent p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005;19:789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 141.Roy AM, Baliga MS, Katiyar SK. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor-negative human breast carcinoma cells via modulation in protein expression of p53 and Bax and caspase-3 activation. Mol Cancer Ther. 2005;4:81–90. [PubMed] [Google Scholar]
- 142.Choudhuri T, Pal S, Agwarwal ML, et al. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 143.Tian B, Wang Z, Zhao Y, et al. Effects of curcumin on bladder cancer cells and development of urothelial tumors in a rat bladder carcinogenesis model. Cancer Lett. 2008;264:299–308. doi: 10.1016/j.canlet.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 144.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- 145.Huang C, Ma WY, Goranson A, et al. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis. 1999;20:237–242. doi: 10.1093/carcin/20.2.237. [DOI] [PubMed] [Google Scholar]
- 146.Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007;304:273–285. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- 147.Schmidt F, Knobbe CB, Frank B, et al. The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines. Oncol Rep. 2008;19:1061–1066. [PubMed] [Google Scholar]
- 148.Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 149.Yang A, Kaghad M, Wang Y, et al. P63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 150.Amin AR, Thakur VS, Paul RK, et al. SHP-2 tyrosine phosphatase inhibits p73-dependent apoptosis and expression of a subset of p53 target genes induced by EGCG. Proc Natl Acad Sci U S A. 2007;104:5419–5424. doi: 10.1073/pnas.0700642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shammas MA, Neri P, Koley H, et al. Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: Biologic activity and therapeutic implications. Blood. 2006;108:2804–2810. doi: 10.1182/blood-2006-05-022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aggarwal BB. Nuclear factor-kappaB: The enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 153.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 154.Kasinski AL, Du Y, Thomas SL, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 156.Hastak K, Gupta S, Ahmad N, et al. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 157.Kim GY, Kim JH, Ahn SC, et al. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Immunology. 2004;113:203–211. doi: 10.1111/j.1365-2567.2004.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li Y, Kucuk O, Hussain M, et al. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-κB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66:4816–4825. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 159.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 160.Kim HS, Kim MH, Jeong M, et al. EGCG blocks tumor promoter-induced MMP-9 expression via suppression of MAPK and AP-1 activation in human gastric AGS cells. Anticancer Res. 2004;24:747–753. [PubMed] [Google Scholar]
- 161.Kundu JK, Shin YK, Surh YJ. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as prime targets. Biochem Pharmacol. 2006;72:1506–1515. doi: 10.1016/j.bcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 162.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 163.Shimizu M, Deguchi A, Joe AK, et al. EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J Exp Ther Oncol. 2005;5:69–78. [PubMed] [Google Scholar]
- 164.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 165.Grandis JR, Drenning SD, Chakraborty A, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth in vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leong H, Mathur PS, Greene GL. Green tea catechins inhibit angiogenesis through suppression of STAT3 activation. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0196-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wung BS, Hsu MC, Wu CC, et al. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: Effects on the inhibition of STAT3 phosphorylation. Life Sci 7. 2005;8:389–397. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 168.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 169.Siddiqui IA, Shukla Y, Adhami VM, et al. Suppression of NFkappaB and its regulated gene products by oral administration of green tea polyphenols in an autochthonous mouse prostate cancer model. Pharm Res. 2008;25:2135–2142. doi: 10.1007/s11095-008-9553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bhattacharyya S, Mandal D, Saha B, et al. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem. 2007;282:15954–15964. doi: 10.1074/jbc.M608189200. [DOI] [PubMed] [Google Scholar]
- 171.Korutla L, Cheung JY, Mendelsohn J, et al. Inhibition of ligand-induced activation of epidermal growth factor receptor tyrosine phosphorylation by curcumin. Carcinogenesis. 1995;16:1741–1745. doi: 10.1093/carcin/16.8.1741. [DOI] [PubMed] [Google Scholar]
- 172.Patel BB, Sengupta R, Qazi S, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 173.Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- 174.Adachi S, Nagao T, Ingolfsson HI, et al. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 175.Shimizu M, Deguchi A, Hara Y, et al. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem Biophys Res Commun. 2005;334:947–953. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- 176.Fang J, Zhou Q, Shi XL, et al. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2007;28:713–723. doi: 10.1093/carcin/bgl189. [DOI] [PubMed] [Google Scholar]
- 177.Lee LT, Huang YT, Hwang JJ, et al. Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res. 2002;22:1615–1627. [PubMed] [Google Scholar]
- 178.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 179.Meeran SM, Mantena SK, Elmets CA. (−)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- 180.Mantena SK, Roy AM, Katiyar SK. Epigallocatechin-3-gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of CD8+ T cells in tumors. Photochem Photobiol. 2005;81:1174–1179. doi: 10.1562/2005-04-11-RA-487. [DOI] [PubMed] [Google Scholar]
- 181.Kang TH, Lee JH, Song CK, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802–811. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guo TL, McCay JA, Zhang LX, et al. Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J Nutr. 2001;131:3251–3258. doi: 10.1093/jn/131.12.3251. [DOI] [PubMed] [Google Scholar]
- 183.Yang Y, Paik JH, Cho D, et al. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int Immunopharmacol. 2008;8:542–547. doi: 10.1016/j.intimp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 184.Shankar S, Ganapathy S, Hingorani SR, et al. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- 185.Bagli E, Stefaniotou M, Morbidelli L, et al. Luteolin inhibits vascular endothelial growth factor-induced angiogenesis: Inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3′-kinase activity. Cancer Res. 2004;64:7936–7946. doi: 10.1158/0008-5472.CAN-03-3104. [DOI] [PubMed] [Google Scholar]
- 186.Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 187.Gururaj AE, Belakavadi M, Venkatesh DA, et al. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun. 2002;297:934–942. doi: 10.1016/s0006-291x(02)02306-9. [DOI] [PubMed] [Google Scholar]
- 188.Ahmad IU, Forman JD, Sarkar FH, et al. Reduction of adverse events by soy isoflavones in patients undergoing external beam radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:S318. [Google Scholar]
- 189.Lippman SM, Heymach JV. The convergent development of molecular-targeted drugs for cancer treatment and prevention. Clin Cancer Res. 2007;13:4035–4041. doi: 10.1158/1078-0432.CCR-07-0063. [DOI] [PubMed] [Google Scholar]