Abstract
Oral mucosal wounds heal with reduced scar formation compared with skin. The epithelial integrin αvβ6 is induced during wound healing, and it can activate fibrogenic transforming growth factor β1 (TGF-β1) and anti-fibrogenic TGF-β3 that play key roles in scar formation. In this study, expression of β6 integrin and members of the TGF-β pathway were studied in experimental wounds of human gingiva and both gingiva and skin of red Duroc pigs using real-time PCR, gene microarrays, and immunostaining. Similar to human wounds, the expression of β6 integrin was induced in the pig wounds 7 days after wounding and remained upregulated >49 days. The αvβ6 integrin was colocalized with both TGF-β isoforms in the wound epithelium. Significantly higher expression levels of β6 integrin and TGF-β1 were observed in the pig gingival wounds compared with skin. Early gingival wounds also expressed higher levels of TGF-β3 compared with skin. The spatio-temporal colocalization of αvβ6 integrin with TGF-β1 and TGF-β3 in the wound epithelium suggests that αvβ6 integrin may activate both isoforms during wound healing. Prolonged expression of αvβ6 integrin along with TGF-β3 in the gingival wound epithelium may be important in protection of gingiva from scar formation. (J Histochem Cytochem 57:543–557, 2009)
Keywords: scarless wound healing, transforming growth factor β, αvβ6 integrin
Wound healing is a complex process that restores tissue integrity. It is composed of coordinated functions and interactions of different cell types, extracellular matrix (ECM), cytokines, and growth factors. Adult cutaneous healing results in accumulation of collagen-rich scar tissue that is slowly remodeled over time (Singer and Clark 1999; Midwood et al. 2004). Dysregulation of skin wound healing after burn wounds, traumatic injuries, and certain surgical procedures can result in serious wound complications, such as hypertrophic scars (Ghahary et al. 1993; Clark 1996). Interestingly, however, oral mucosal wounds show accelerated healing with no or minimal scarring compared with skin (Häkkinen et al. 2000a; Szpaderska et al. 2003). The mechanisms underlying scarless oral wound healing have not been fully studied. However, the absence of scar in oral mucosal wounds may in part be because of the presence of saliva and specific microflora in the oral cavity or the fetal-like phenotype of the cells in the oral mucosa (Sciubba et al. 1978; Schor et al. 1996; Häkkinen et al. 2000a; Szpaderska et al. 2003; Schrementi et al. 2008). Therefore, comparing the biological mechanisms regulating scarless oral mucosal vs scar-forming dermal wound healing could provide valuable information about the pathophysiology of scar formation.
Transforming growth factor β (TGF-β) is a multifunctional growth factor with several crucial roles during normal wound healing. TGF-β regulates wound re-epithelialization and inflammation and promotes connective tissue regeneration (Verrecchia and Mauviel 2002). On the other hand, dysregulation of TGF-β plays a key role in abnormal wound healing, including scar formation. There are three mammalian isoforms; TGF-β1, -β2, and -β3 have distinct roles in wound repair. TGF-β1 overactivity is considered the primary pro-fibrotic factor that causes excessive ECM accumulation and scar formation, and suppression of its activity by pharmacological or genetic approaches results in markedly reduced scarring (McCallion and Ferguson 1996; O'Kane and Ferguson 1997; Shah et al. 2000). On the other hand, exogenous application of the anti-fibrogenic TGF-β3 or intrinsic abundance of TGF-β3 relative to TGF-β1 has been associated with improved wound healing outcomes (Shah et al. 1995; Hsu et al. 2001; Ferguson and O'Kane 2004; Schrementi et al. 2008). TGF-β isoforms are synthesized as latent molecules, consisting of mature TGF-β that is covalently bound to the latency-associated peptide (LAP). This latent complex associates with a family member of the latent TGF-β binding proteins (LTBPs) that facilitates TGF-β storage in the ECM. To be functional, TGF-β must be activated. There are several activators of TGF-β that can dissociate the mature TGF-β from LAP, allowing it to interact with its cell surface signaling receptors (Annes et al. 2003). Integrin-mediated activation seems to be the main mechanism of TGF-β activation in vivo (Yang et al. 2007). Integrin αvβ6 can activate both fibrogenic TGF-β1 and anti-fibrogenic TGF-β3 in vitro through a mechanism that requires LTBP-1 (Annes et al. 2002,2004; Keski-Oja et al. 2004). The β6 integrin knockout mouse share similarities in phenotype with the TGF-β1 knockout animals, including exaggerated lung and skin inflammation, suggesting a role for αvβ6 integrin in the activation of TGF-β1 in vivo (Huang et al. 1996). Moreover, the β6 knockout mice are protected from TGF-β1–mediated lung fibrosis induced by bleomycin (Munger et al. 1999). Furthermore, TGF-β1 activation through the αvβ6 integrin contributes to renal and radiation-induced fibrosis (Hahm et al. 2007; Puthawala et al. 2008).
Integrin αvβ6 is an epithelial cell surface receptor that is not normally present in adult tissue, but its expression is strongly induced in keratinocytes during wound healing (Breuss et al. 1993,1995; Häkkinen et al. 2000b). We have previously shown that the expression of αvβ6 integrin is induced at the wound basal epithelium of both human gingival and skin wounds (Haapasalmi et al. 1996; Häkkinen et al. 2000b). However, the function of αvβ6 integrin during wound healing, especially in regard to the regulation of TGF-β1 and TGF-β3 activity, remains largely unclear. To test the hypothesis that αvβ6 integrin may interact with TGF-β in the wound epithelium, we studied the spatio-temporal colocalization of these and other key molecules involved in TGF-β activation in the human oral mucosal wound epithelium. In addition, we studied the activity of the TGF-β pathway in the early stages of wound healing by gene expression profiling. We also hypothesized that the expression and localization of αvβ6 integrin and TGF-β1 and TGF-β3 are different in scarless gingival wounds and scar-forming skin wounds. To this end, we compared expression and localization of these molecules in skin and oral mucosal wounds over time using a well-established pig model.
Materials and Methods
Tissue Samples
Human Wounds
The experimental protocol for human wounding was approved by the Research Ethics Board of the University of British Columbia and complies with the ethical rules for human experimentation that are stated in the 1975 Declaration of Helsinki. Full-thickness excisional wounds (2 × 12 mm) were created in the gingiva of four healthy volunteers (males and females, 22–35 years of age) at least 3 mm away from the teeth margins. The wounds were left uncovered to heal for up to 60 days. Three biopsies (4 mm diameter) from different individuals were collected per time point. The tissue harvested from the initial wound (unwounded tissue), and the wound biopsies were rinsed in PBS and either placed in cryotubes or embedded in Tissue-Tek Optimal Cutting Temperature compound (OCT; Sakura Finetek, Torrance, CA), snap frozen in liquid nitrogen, and stored at −86C for later use. Transcriptional profiling of day 1, 3, and 7 wound samples was compared with their individual 0-day (non-wounded) sample using Affymetrix protocols (Affymetrix; Santa Clara, CA). Tissue samples embedded in Tissue-Tek O.C.T. were cut to 6-μm frozen sections with a 2800 Frigocut Cryostat Microtome (Leica; Nussloch, Germany) and transferred to 3-aminopropyltriethoxysillane-coated slides, fixed with cold acetone, air-dried, and kept at −86C until use.
Pig Wounds
The experimental protocol was performed in accordance with Canadian Council on Animal Care guidelines and with a protocol approved by the Animal Care Committee of the Faculty of Medicine at the University of Calgary. Six juvenile (20–25 kg) female red Duroc pigs were obtained from the Neufeld farm (Acme, AB, Canada) and were housed at the University of Calgary Life Sciences Research Station. Six identically sized wounds as created in human gingiva were made in both the palatal gingiva and dorsal skin of each animal. Briefly, the animals were premedicated with IM ketamine (15 mg/kg) and acepromazine (0.4 mg/kg), and general anesthesia was induced by the administration of 1–2% isofluorane by mask. The gingival wounds were created as described above for the human wounds. Identical size full-thickness skin wounds were created on the dorsal skin as described previously (Gallant et al. 2004). A fentanyl transdermal patch (75 mg/hr) was used for long-term pain control (72 hr), and butorphanol (0.1 mg/kg, IM) was administered for break-through pain for the first 8 hr. Tissue biopsies (4 mm in diameter) harvested from gingiva and skin of red Duroc pigs were collected from unwounded tissue (day 0 samples) and from the wounds 1, 3, 7, 14, 21, 35, and 49 days after wounding (n=6 pigs per time point for skin; n=3 pigs per time point for gingiva). The tissue biopsies were snap frozen and used for either RNA extraction or frozen sectioning (see above).
Evaluation of Wound Healing
Clinical evaluation of the wounds in various time points was performed for the signs of scar formation based on clinical scar assessment scale by Wong et al. (unpublished data) and recorded by taking standardized photographs using a digital camera. In addition, histological evaluation of the wounds was performed based on the histological scar assessment scale (Wong et al., unpublished data) of hematoxylin–eosin–stained wound sections. The results of scar assessment scales are shown elsewhere (Wong et al., unpublished data), and only representative clinical and histological images from the last time point studied (49 days after wounding) are presented here.
DNA Microarray
DNA microarray was performed using the Affymetrix standard protocol (Affymetrix User's Guide). Total RNA was extracted from seven unwounded (day 0) and three wounded human gingival tissue samples per time point (days 1, 3, and 7) using TRIZOL reagent (Invitrogen Life Technologies; Carlsbad, CA) followed by a clean up process using RNeasy kits (QIAGEN; Valencia, CA) according to the manufacturer's protocols. Poly(A) mRNA was reverse transcribed to generate double-stranded cDNA using a 24-mer oligodeoxythymidylic acid primer with a T7 RNA polymerase prNSomoter site added to the 3′ end T7-oligo (dT) (Superscript cDNA Synthesis System; Life Technologies, Rockville, MD) (Dumur et al. 2004). The cDNA was used as a template for in vitro transcription (IVT) to yield biotin-labeled cRNA. To obtain gene expression profiles, the biotinylated cRNA was heat-fragmented and hybridized to oligonucleotide GeneChips (U95Av2; Affymetrix) that contained different probe sets representing different genes. After hybridization (16 hr, 45C), the probe arrays were washed in non-stringent buffer and stained with streptavidin phycoerythrin followed by an antibody amplification step. Arrays were scanned for fluorescence intensities using an Affymetrix scanner. Thereafter, the scanned image was analyzed with Affymetrix Micro-array Suite 5.0. Results are presented as the fold changes between pooled control samples (unwounded gingiva) and pooled test samples (1-, 3-, and 7-day-old wounds). Genes whose expression did not exceed the background level were omitted. ANOVA was used to determine the significantly upregulated and downregulated genes for all patients at each wound time point (p≤0.01). In this study, the genes that are related to TGF-β activation or signaling are presented.
Histology and IHC
Frozen sections from different time points of human and pig wounds were thawed at room temperature before fixation in acetone (−20C) for 5 min. Thereafter, they were stained with hematoxylin–eosin, mounted using Entellan (Merck; Whitehouse Station, NJ), and photographed using a digital camera (Coolpix 995; Nikon, Tokyo, Japan) attached to an Axiolab E light microscope (Carl Zeiss; Jena, Germany). For immuno-localization studies using single or double immunofluorescence staining, the following antibodies were used: αv (monoclonal antibody MAb L230, purified in our laboratory; Houghton et al. 1982), β6 (β6-B1; Huang et al. 1996), TGF-β1 (Promega; Madison, WI), TGF-β3 (PAb; Santa Cruz Biotechnology), LTBP-1 (Pharmingen; SanDiego, CA), connective tissue growth factor (CTGF; Abcam, Cambridge, UK), procollagen type-I (Chemicon International; Temecula, CA), matrix metalloproteinase (MMP)-9 (Chemicon International), and thrombospondin (TSP)-1 (Medi Corp.; Montreal, QC, Canada). For immunofluorescence staining, sections were first incubated with PBS containing BSA (10 mg/ml) and Triton X-100 (0.1%) at room temperature for 30 min. The tissue sections were incubated with a primary antibody in PBS containing BSA (1 mg/ml) and Triton X-100 (0.01%) at 4C overnight. Thereafter, for double immunofluorescence staining, the sections were washed and incubated with a second primary antibody at room temperature for 1 hr. After washings, the samples from both single and double immunofluorescence stainings were incubated with appropriate Alexa-conjugated secondary antibodies (Alexa 488 and Alexa 546; Molecular Probes, Eugene, OR) against primary antibodies at room temperature in the dark for 1 hr. The control samples were exposed to either secondary antibodies only or non-immune IgG and gave negligible immunoreactivity (data not shown). Subsequently, the slides were mounted using Immuno-Mount solution (Thermo Shadon; Pittsburgh, PA), examined by a Zeiss Laser Confocal Scanning Microscope 10 (LSM 10; Carl Zeiss), and images were captured using Northern Eclipse software (Empix Imaging; Mississauga, ON, Canada).
Extraction of RNA From Pig Skin and Gingiva
Triplicate frozen tissue samples from pig gingiva and skin (unwounded tissue and different time points of the wounds) were placed on dry ice, cut into small pieces using a razor blade, and transferred into the lysis buffer provided in the applied RNA extraction kit (Qiagen RNeasy Fibrous Tissue Mini Kit; Qiagen, Mississauga, ON, Canada). The samples were homogenized using hard-tissue Omni-tip plastic probes (Omni International; Marietta, GA) attached to a rotor stator (Power Gen 1000; Fisher Scientific, Ottawa, ON, Canada). To eliminate the cross-contamination between the samples, the plastic probes were washed in RNase-free water three times with the rotor stator operating. Subsequently, RNA was extracted from homogenized samples according to the manufacturer's protocol (Qiagen RNeasy Fibrous Tissue Mini Kit). The concentration of RNA in each sample was determined by spectrophotometry (GeneQuant; Biochrom, Cambridge, UK) at 260-nm wavelength. The RNA samples were considered acceptable if the 260:280 nm was >1.8. Extracted RNA was stored at −80C for later use.
PCR
The synthesis of cDNA was performed by reverse transcription of RNA (1 μg) using iScript Select cDNA Synthesis Kit (Bio-Rad; Hercules, CA) according to the manufacturer's standard protocol. Amplification of β6 integrin, TGF-β1, TGF-β3, and β-actin (as a housekeeping gene) cDNA was performed using the MiniOpticon Real-Time PCR Detection System (Bio-Rad). Primer sequences used for cDNA amplification were as follows: 5-ATA GTT CCA GCA TCG TTC AG-3 (forward) and 5-CGA ACT TGA ACT TGC AGA G-3 (reverse) for β6 integrin; 5-TTT CGC CTC AGT GCC CA-3 (forward) and 5-GCCAGAATTGAACCCGTTAA-3 (reverse) for TGF-β1; 5-GGA CAC CAA TTA CTG CTT CC-3 (forward) and 5-CCA GAT CCT GTC GGA AGT C-3 (reverse) for TGF-β3; and 5-CTG TGG CAT CCA CGA AAC-3 (forward) and 5-CAG ACA GCA CTG TGT TGG-3 (reverse) for β-actin. The β6, TGF-β1, and TGF-β3 levels were normalized to β-actin mRNA levels using the comparative CT method and reported as ratio of gingival to skin values in unwounded samples and different wound time points. Melting curve analyses were performed for all amplifications to verify that only single products were generated from the reactions. The gene expression levels of β6 integrin, TGF-β1, and TGF-β3 were reported from day 0 to day 49 in both gingival and skin wounds of red Duroc pigs. Moreover, the ratio of the gene expression in each time point in gingiva relative to skin is presented. Each set of PCR was repeated at least three times to check the reproducibility of the data. The differences between the gene expression levels in all wound time points were compared with unwounded samples using Student's t-test. Analysis was performed using Microsoft Excel software, and p≤0.05 was considered statistically significant.
Results
Transcriptional Profiling of Gene Expression in Scarless Human Gingival Wounds Depicts High Remodeling and Matrix Production Activity and Involvement of Molecules in the TGF-β Pathway
Molecular events in the early wound healing process may dictate whether a wound forms a scar or not (Ferguson and O'Kane 2004). Therefore, we performed transcriptional profiling using RNA isolated from the whole tissue biopsies obtained from unwounded human gingival tissue and from early gingival wounds (1, 3, and 7 days after wounding). The known genes regulated by TGF-β or partners of the TGF-β signaling pathway were selected from the microarray data for closer analysis (Table 1). Interestingly, the results did not show any changes in the expression levels of TGF-β1 or TGF-β receptor I at any time points relative to unwounded tissue, likely because of low transcript levels of these genes. However, TGF-β receptor II (TGF-βRII) and TGF-βRIII were initially moderately downregulated in 1-day-old wounds and remained unchanged compared with unwounded tissue (Table 1). On the other hand, the expression of integrin αv and anti-fibrogenic TGF-β3, as well as LTBP-1, were upregulated in 7-day-old wounds (Table 1). In addition, the expression of CTGF, which is one of the targets of TGF-β signaling and mediates many of biological effects of TGF-β (Perbal 2004), was highly upregulated already in 1-day-old wounds and remained high also in 3- and 7-day-old wounds (Table 1). The expression of ECM molecules that can be induced by TGF-β (Varga et al. 1987), including type I and III collagens and fibronectin, was strongly downregulated in 1-day-old wounds and then upregulated in 7-day-old wounds. The expression of MMP-1 and MMP-9, expression of which can be regulated by TGF-β and which are also able to activate TGF-β (Salo et al. 1994; Yu and Stamenkovic 2000; Mu et al. 2002), was strongly increased at 1 and 7 days after wounding (Table 1). Taken together, we found that the gene expression level of αv integrin along with TGF-β3 and LTBP-1 and TGF-β–regulated genes (type I and III collagens, fibronectin, CTGF, MMP-1, and MMP-9) were upregulated in 7-day-old human gingival wounds.
Table 1.
Transcriptional profiling of TGF-β–related genes at different time points of human gingival wounds (1, 3, and 7 days old) compared with unwounded tissue (day 0)
| Molecule | Day 1 | Day 3 | Day 7 |
|---|---|---|---|
| TGF-β signaling and activation molecules | |||
| Transforming growth factor β1 (TGF-β1) | NC | NC | NC |
| Transforming growth factor β receptor I (TGF-βRI) | NC | NC | NC |
| Transforming growth factor β receptor II (TGF-βRII) | −1.60 | NC | NC |
| Transforming growth factor β receptor III (betaglycan, TGF-βRIII) | −2.31 | NC | NC |
| Transforming growth factor β3 (TGF-β3) | NC | NC | 2.60 |
| Latent transforming growth factor β binding protein-1 (LTBP-1) | NC | NC | 1.85 |
| Integrin αv (ITGαv) | NC | NC | 1.82 |
| Connective tissue growth factor (CTGF) | 2.71 | 4.15 | 4.74 |
| ECM molecules | |||
| Collagen type I, α2 (CoL1α2) | −21.13 | −1.84 | 4.22 |
| Collagen type III, α1 (Col3α1) | −6.01 | NC | 2.50 |
| Fibronectin-1 (FN-1) | −2.51 | NC | 12.67 |
| Tissue remodeling molecules | |||
| Matrix metalloproteinase-1 (MMP-1) | 40.11 | 21.72 | 17.07 |
| Matrix metalloproteinase-9 (MMP-9) | 16.71 | NC | 14.48 |
Results are presented as the fold changes between pooled samples (n=3; each wound sample was compared with its individual day 0 sample) analyzed by ANOVA to determine the significant changes over unwounded tissue (p<0.01). TGF, transforming growth factor; NC, no change; CTGF, connective tissue growth factor; ECM, extracellular matrix.
TGF-β1 and TGF-β3 Colocalize With αvβ6 Integrin in 7-Day-old Gingival Wounds
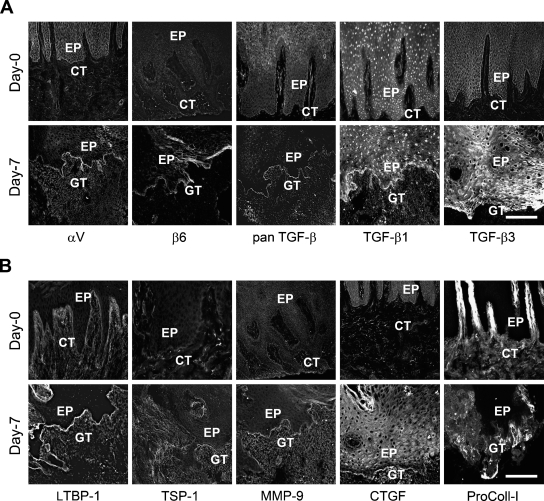
To validate the microarray gene expression data and to find out which cells express these molecules, the localization and relative staining intensity level of selected proteins in unwounded gingiva and 7-day-old human gingival wounds was studied. TGF-β1 and TGF-β3 were almost undetectable in the unwounded epithelium or connective tissue (Figure 1A). However, in 7-day-old wounds, TGF-β1 staining was very intense and localized at the basal aspect of wound epithelium. TGF-β3 was also localized abundantly to the basal aspect of wound keratinocytes but was also present in the suprabasal cell layers. Expression of both TGF-β1 and -β3 was weak in the granulation tissue. The gene array data showed upregulation of the αv integrin subunit expression in the 7-day-old wounds. Accordingly, there was strongly increased immunostaining of this integrin subunit in the 7-day-old wound epithelium compared with unwounded tissue (Figure 1A). Our previous observations have also shown high expression of αv integrin subunit in 7-day-old human gingival wounds and have shown that in this location the αv subunit forms heterodimers with the β6 integrin and probably also with the β1 subunit (Haapasalmi et al. 1996; Koivisto et al. 1999; Häkkinen et al. 2000b; Larjava et al. 2002). Our immunostaining confirmed colocalization of the αv and β6 integrins in the basal epithelium of the 7-day-old wounds (Figure 1A). Integrin αv was also localized into wound granulation tissue (Figure 1A).
Figure 1.
Immunostaining of representative samples from unwounded human gingival tissue (day 0) and a 7-day-old human gingival wound. Higher staining intensity of αv, β6, pan transforming growth factor (TGF)-β (TGF-β1, 2, 3), TGF-β1, TGF-β3 (A), and latent TGF-β binding protein 1 (LTBP-1), TSP-1, matrix metalloproteinase (MMP)-9, connective tissue growth factor (CTGF), and pro-collagen-I (B) was found in the 7-day-old human gingival wound basal epithelium compared with unwounded gingiva. EP, epithelium; CT, connective tissue; GT, granulation tissue. Bar = 200 μm.
Next, we examined the localization of LTBP-1, the TGF-β–binding protein that is necessary for αvβ6 integrin–mediated activation of TGFβ, and MMP-9 (that can activate TGF-β), which showed upregulation in the 7-day-old wounds in our gene array analysis (Figure 1B). In normal tissue, LTBP-1 was localized in the gingival connective tissue with the most distinct staining at the epithelial basement membrane zone (BMZ). In 7-day-old wounds, the intensity of the LTBP-1 staining was increased, especially at the wound epithelium BMZ (Figure 1B). The immunoreactivity of MMP-9 was almost negligible in the unwounded tissue but showed strongly increased immunostaining in the granulation tissue and BMZ of the wound epithelium of 7-day-old wounds (Figure 1B). Next, we evaluated the accumulation and localization of other potential non-integrin activators of TGF-β in 7-day-old wounds. Expression of TSP-1 was almost negligible in the normal tissue. In the wounds, TSP-1 was localized diffusely throughout the granulation tissue with no clear preference to the BMZ (Figure 1B). We examined CTGF and type I procollagen, two molecules that can be induced by TGF-β and that showed strongly increased expression in the 7-day-old wounds. CTGF was localized in cells, likely fibroblasts, of the gingival connective tissue of unwounded tissue. In 7-day-old wounds, CTGF was present in the granulation tissue, BMZ, and suprabasal cell layers of the wound epithelium (Figure 1B). Type I procollagen was expressed around the blood vessels in the connective tissue papillae of the normal tissue (Figure 1B). In the wounds, expression of type I procollagen was observed in the granulation tissue, especially immediately underneath the fused wound epithelium (Figure 1B).
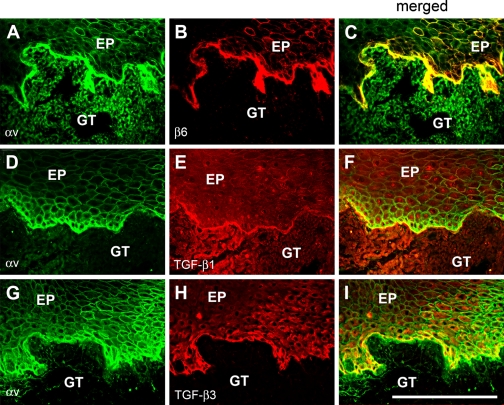
To assess whether it is possible that αvβ6 integrin and TGF-β interact in the wound epithelium, we performed double immunofluorescence staining for these molecules in the 7-day-old wounds (Figure 2). TGF-β1 colocalized with αvβ6 integrin at the BMZ (Figure 2). TGF-β3 also colocalized with αvβ6 integrin around the basal keratinocytes of the fused wound epithelium (Figure 2). In summary, we observed that αvβ6 integrin along with the key molecules involved in αvβ6 integrin-mediated activation of TGF-β and certain downstream targets of TGF-β signaling were coordinately upregulated and spatio-temporally colocalized in 7-day-old human gingival wounds.
Figure 2.
The colocalization of αv integrin with its binding partner β6 (A–C), TGF-β1 (D–F), and TGF-β3 (G–I) at the wound basal epithelium. Immunostaining of a representative sample from a 7-day-old human gingival wound is shown. Note that colocalization of different molecules is shown by yellow color in the merged images. EP, epithelium; GT, granulation tissue. Bar = 200 μm.
Localization of αvβ6 Integrin, TGF-β1, and TGF-β3 in Scarless Human Gingival Wounds up to 60 Days After Wounding
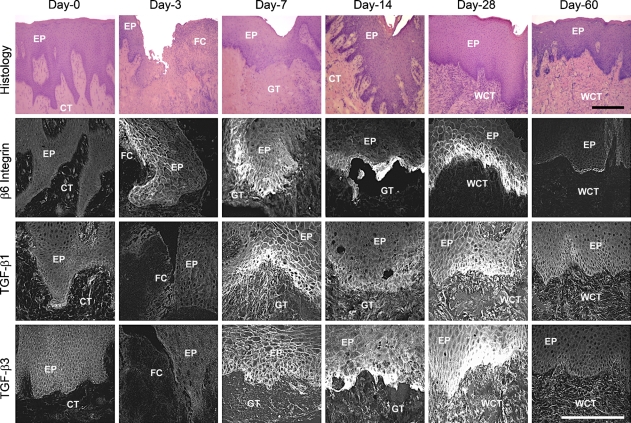
Having established that αvβ6 integrin is colocalized with two isoforms of TGF-β, suggesting that it may participate in TGF-β activation, we studied the localization of these proteins at later stages of gingival wound healing. Human gingival wounds showed minimal scarring at both clinical and histological levels after 60 days of healing (Figure 3; Wong et al., unpublished data). Immunoreactivity for β6 integrin was first detected in the basal and suprabasal cell layers of the migrating gingival epithelium of 3-day-old wounds (Figure 3). Staining intensity of β6 integrin increased in the basal keratinocytes of the fused wound epithelium in the 7-day-old wounds, remained high until day 28, and declined but was still detectable in the 60-day-old wounds (Figure 3). Immunostaining intensity of TGF-β1 was increased in the wound keratinocytes and granulation tissue in the 7-day-old wounds and remained high in wound keratinocytes up to 28 days, and then declined to unwounded tissue level by day 60 (Figure 3). The staining pattern of TGF-β3 followed that of TGF-β1 and was mainly confined to the basal keratinocytes of the wound area (Figure 3). Taken together, αvβ6 integrin is spatio-temporally coordinately colocalized with both of its ligands, TGF-β1 and -β3, during scarless gingival wound healing.
Figure 3.
Immunolocalization of β6 integrin, TGF-β1, and TGF-β3 in 0- to 60-day-old human gingival wounds. Representative histological images were taken from hematoxylin–eosin stainings of the non-wounded and wounded samples. EP, epithelium; CT, connective tissue; FC, fibrin clot; GT, granulation tissue; WCT, wound connective tissue. Bar = 200 μm.
Comparison of Wound Healing in the Gingiva and Skin of Red Duroc Pigs
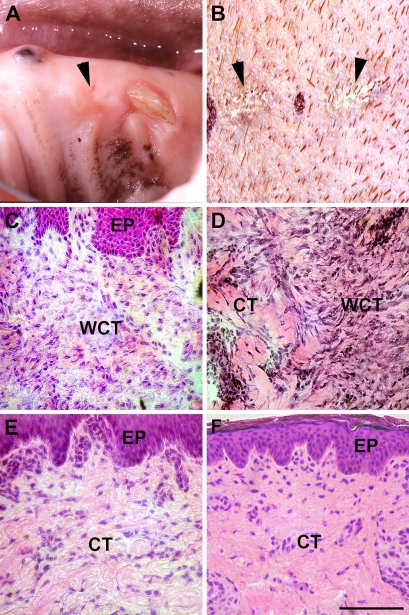
To determine whether the expression of αvβ6 integrin and TGF-βs show differential distribution in scar-forming wound healing, we chose the red Duroc pig model, a model of hypercontracted, hypertrophic-like healing (Gallant et al. 2004). We have shown that gingival wounds in red Duroc pigs heal similarly to human gingiva with minimal scarring (Wong et al., unpublished data). However, even small dermal wounds produce scarring similar to human skin (Zhu et al. 2007). Consistent with our previous findings, 49-day-old gingival wounds showed minimal scarring both at the clinical and histological levels (Figure 4). Dermal healing, however, produced clinically visible scarring, and histological changes were consistent with scar formation and showed areas with hypercellularity and disorganized collagen fibers (Figure 4).
Figure 4.
Representative clinical and histological (hematoxylin–eosin staining) images of 49-day-old gingival (A,C) and skin (B,D) wounds and of unwounded gingiva (E) and skin (F) of red Duroc pigs. Arrowheads indicate the healing wound site in the gingiva (A) and scar in the skin (B). EP, epithelium; WCT, wound connective tissue; CT, connective tissue. Bar = 200 μm.
Localization of αvβ6 Integrin, TGF-β1, and TGF-β3 in Scarless vs Scar-forming Wounds of Red Duroc Pigs
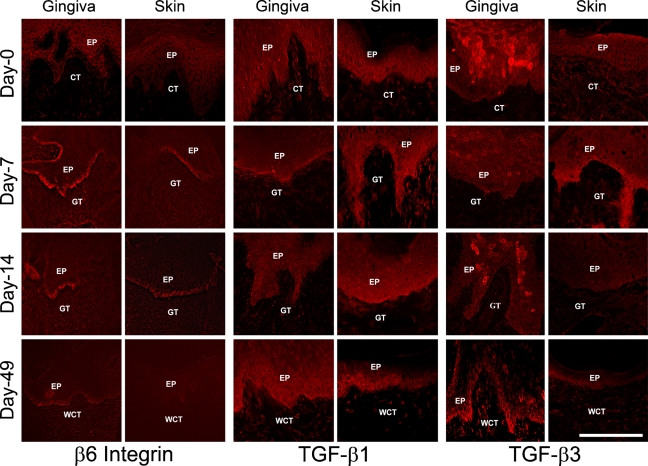
Immunostaining showed that integrin β6 protein was not detectable in unwounded gingiva and skin of red Duroc pigs (day 0). However, β6 integrin immunostaining was strongly increased in the basal wound keratinocytes of the fused epithelium of 7- and 14-day-old gingival and skin wounds (Figure 5). In the 49-day-old wounds, β6 integrin was still detectable in the basal keratinocytes of the wounded gingiva but not in the skin (Figure 5). Species specificity of the TGF-β antibodies to pig specimens was tested in Western blotting of the frozen sections of 7-day-old wounds. Specific band corresponding to the latent TGF-β complex was detected with both TGF-β1 and -β3 antibodies, indicating that these antibodies were reactive to pig TGF-βs (data not shown). Immunostaining for TGF-β1 was detected with the same intensity in non-wounded tissue and in all wounds in the gingival and skin wound basal epithelium and some connective tissue cells (Figure 5). Immunostaining for TGF-β3 was mostly confined in the suprabasal keratinocytes of gingival epithelium of the normal gingiva and 7- and 14-day-old gingival wounds (Figure 5). In the 49-day-old wounds, TGF-β3 was predominantly localized to the gingival wound basal epithelium and some connective tissue cells (Figure 5). In the skin, however, TGF-β3 was only detected on day 7 at the wound basal keratinocytes and in association with some stromal cells in unwounded tissue and in 49-day-old wounds (Figure 5). In summary, in red Duroc pig wounds, β6 integrin was detected in the wound epithelium at all time points in the gingiva, but its accumulation was downregulated by day 49 postwounding in the skin. TGF-β3 localized to the basal epithelium in the later stages of healing in the gingival wounds compared with skin wounds. The immunostaining level of TGF-β1 protein did not differ significantly in the gingival vs skin wounds.
Figure 5.
Representative images of immunolocalization of β6 integrin, TGF-β1, and TGF-β3 in 0- to 49-day-old gingival and skin wounds in red Duroc pigs. EP, epithelium; CT, connective tissue; GT, granulation tissue; WCT, wound connective tissue. Bar = 200 μm.
Gene Expression Level of β6 Integrin and Its Ligands, TGF-β1 and TGF-β3, in the Scarless vs Scar-forming Wound Healing of Red Duroc Pigs From Day 0 (Unwounded) to Day 49
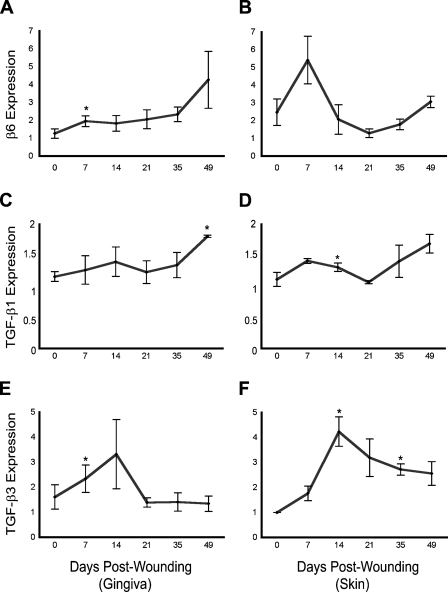
To evaluate the gene expression pattern of β6 integrin, TGF-β1, and TGF-β3 during scarless gingival and scar-forming skin wound healing of red Duroc pigs, we isolated total RNA from the wounds from day 0 (unwounded) to day 49 for real-time PCR. Compared with the unwounded gingiva, β6 integrin expression was significantly upregulated by day 7 postwounding, remained steady until day 35, and further increased by day 49 (Figure 6). In the skin wounds, the expression of β6 integrin increased by ∼2-fold in the 7-day-old wounds compared with unwounded tissue and then returned to the level in unwounded tissue by day 14 and remained the same through the later wound time points (Figure 6). The expression of TGF-β1 in gingiva did not change until day 49 postwounding, when an ∼50% increase in TGF-β1 expression compared with unwounded tissue was detected (Figure 6). The expression of TGF-β1 in the skin samples showed only moderate changes over the course of healing and followed almost the same pattern as the gingival samples (Figure 6). The expression of TGF-β3 was upregulated at day 7 and day 14 in the gingival wounds, returning to the level of unwounded tissue at the later time points of healing (Figure 6). The expression of TGF-β3 in the skin wounds also increased up to 4-fold by day 14 and gradually decreased but still remained somewhat higher than unwounded tissue at day 49 (Figure 6).
Figure 6.
Real-time PCR analysis of the expression of mRNA for β6 integrin and its ligands, TGF-β1 and TGF-β3, at different stages of wound healing relative to unwounded tissue in red Duroc pigs. n=3; *p≤0.05.
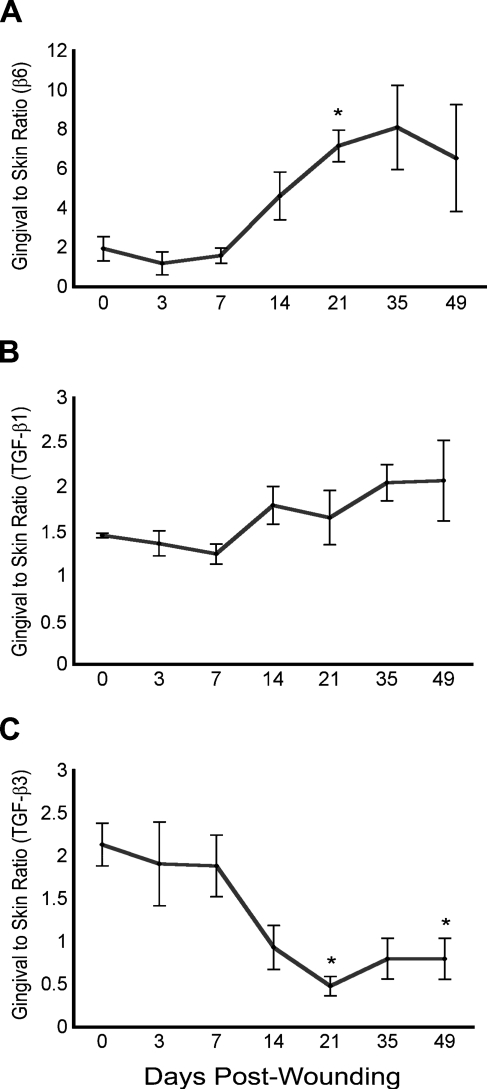
To directly compare the expression level of β6 integrin and its ligands in the gingival and skin wounds, we analyzed the RNA isolated from the gingival and skin samples again in the same real-time PCR experiment. The results showed that unwounded gingiva expressed about twice as much β6 integrin mRNA compared with skin (Figure 7). After day 7 postwounding, the expression of β6 integrin mRNA in the gingival wounds became ∼8-fold higher than in the parallel wounds in the skin (Figure 7). The expression of TGF-β1 mRNA was ∼50% higher in unwounded gingiva vs skin, and this difference was also apparent during different time points of wound healing (Figure 7). The expression level of TGF-β3 mRNA was ∼2-fold in the unwounded gingiva compared with skin (Figure 7). This difference decreased over time, and after 21 days of healing, the skin wounds expressed relatively more TGF-β3 mRNA than the gingival specimens (Figure 7). Taken together, gingival tissue compared with skin responded to wounding with a larger increase in β6 integrin expression and a gradual decrease of initially higher TGF-β3 expression, whereas TGF-β1 levels did not significantly change.
Figure 7.
Real-time PCR analysis of the expression of β6 integrin, TGF-β1, and TGF-β3 mRNA in unwounded tissue and in the wounds in gingiva relative to skin in red Duroc pigs. n=3; *p<0.05.
Discussion
Oral mucosal wounds heal with minimal scarring unlike scar-forming dermal wounds (Häkkinen et al. 2000b; Szpaderska et al. 2003). Integrin αvβ6 is induced during wound healing in both tissues and is an in vitro activator of both fibrogenic TGF-β1 and anti-fibrogenic TGF-β3 (Breuss et al. 1995; Häkkinen et al. 2000b; Keski-Oja et al. 2004). Integrin αvβ6 localizes TGF-β to the cell surface (Munger et al. 1999), suggesting that it can potentially be a local activator of TGF-β isoforms during wound healing. Activation of TGF-β1 by αvβ6 integrin has been shown to play an important role in some fibrotic conditions, such as kidney and pulmonary fibrosis (Munger et al. 1999; Kaminski et al. 2000; Ma et al. 2003; Hahm et al. 2007). Furthermore, increased abundance of TGF-β3 relative to TGF-β1 protects healing wounds from scar formation, suggesting that there may be a cross-regulation of TGF-β1 and TGF-β3 during wound healing (Shah et al. 1995; Hsu et al. 2001; Ferguson and O'Kane 2004; Schrementi et al. 2008). In addition to αvβ6 integrin, there are other members of the αv family that can bind to and activate TGF-β (Wipff and Hinz 2008). However, most of them are not expressed in epithelial cells (e.g., αvβ3, αvβ8) (Larjava et al. 1996) or have shown to be expressed just temporarily in migratory wound epithelium in early time points of wound healing (e.g., αvβ5) (Clark et al. 1996). In this study, we showed that αvβ6 integrin could potentially regulate the activity of its ligands, TGF-β1 and TGF-β3, during wound healing as shown by the spatio-temporal colocalization of those molecules at the wound site. Furthermore, integrin αvβ6 and anti-fibrogenic TGF-β3 showed extended accumulation in the scarless gingival wound epithelium compared with scar-forming skin.
Previous interventional studies have shown that molecular events in very early wound healing may dictate whether tissue scars or not (Ferguson and O'Kane 2004). For that reason, we first performed transcriptional profiling of molecules involved in TGF-β activation and signaling at the early time points of human gingival wound healing. Our findings showed that the gene expression level of αv integrin, along with TGF-β3–, LTBP-1–, and TGF-β–induced target genes during wound healing (type I and III collagens, fibronectin, CTGF, MMP-1, and MMP-9), were upregulated in 7-day-old human gingival wounds compared with unwounded tissue. With the immunolocalization studies, the gene microarray data were confirmed, and a strong accumulation of αv and β6 integrins in the wound epithelium was detected. We did not detect expression of β6 integrin by transcriptional profiling, most likely because its expression was very low relative to the other transcripts because it was only present in the basal wound keratinocytes at the wound site. Expression of αv integrin was more widespread in the epithelium and accounted most likely for the expression of αvβ1 integrin in addition to αvβ6 integrin by the wound keratinocytes (Larjava et al. 2002). Interestingly, αvβ6 integrin colocalized with TGF-β1, TGF-β3, and LTBP-1 at or immediately underneath 7-day-old human gingival wound basal epithelium but not in unwounded tissue. Spatio-temporal colocalization of these molecules suggests a potential for local activation of TGF-βs by αvβ6 integrin during wound healing. Obviously, more biochemical and functional evidence is needed to confirm this possibility. However, based on our findings, CTGF and type I procollagen, molecules that are upregulated by TGF-β signaling, showed both increased immunostaining at the same location where αvβ6 integrin and TGF-βs were present at the basal epithelial cells or immediately underneath. Thus, in this location, TGF-β may have been activated by αvβ6 integrin.
We also studied the accumulation and localization of MMP-9 and TSP-1 as other potential activators of TGF-β during wound healing in the 7-day-old human gingival wounds. MMP-9 is both induced by TGF-β and activates it (Salo et al. 1994; Yu and Stamenkovic 2000). Its upregulation in gene microarray analysis and high accumulation at the wound basal membrane zone (BMZ) of the 7-day-old gingival wounds detected by immunostaining suggests a role for MMP-9 in the local activation of TGF-β during wound healing along with other possible activators. Interestingly, high MMP activity has been also linked to scarless fetal wound healing (Dang et al. 2003). TSP-1 did not show, however, apparent changes in the gene array data or in the immunostaining of the 7-day-old wounds compared with unwounded samples. TSP-1 has been associated with optimal healing in animal models (DiPietro et al. 1996) and collaborates with αvβ6 integrin in TGF-β activation in vivo (Ludlow et al. 2005), but it may be more important for regulation of angiogenesis and granulation tissue formation than in the epithelium (DiPietro et al. 1994).
We have shown previously that, during wound healing, the expression of αvβ6 integrin is induced in the basal wound epithelium and stays upregulated at least up to day 14 after wounding (Haapasalmi et al. 1996; Häkkinen et al. 2000b). In this study, we used another more sensitive antibody against β6 integrin (Huang et al. 1996) that showed extended expression of β6 integrin in human gingival wounds up to 60 days. High staining intensity of β6 integrin in the fused epithelium of human gingival wounds from day 7 to day 28 was concurrent with maximal accumulation of both TGF-β1 and TGF-β3 in the epithelium. A previous short-term study of rat skin wound healing also showed peak activation of TGF-β1 that occurred before day 7 postwounding (Yang et al. 1999). Higher accumulation of β6 integrin and TGF-β3 at the later time points in human gingival wound epithelium compared with skin may suggest a preferential activity for TGF-β3 over TGF-β1 in those time points.
We chose red Duroc pigs to compare the expression pattern and localization of β6 integrin, TGF-β1, and TGF-β3 during early and late stages of gingival and skin wound healing. Red Duroc pigs are among tight-skinned animals that share many common wound healing characteristics with humans in terms of scar formation in the skin (Gallant et al. 2004; Gallant-Behm et al. 2005; Zhu et al. 2007). We showed that, similar to humans, gingival wounds in red Duroc pigs heal with minimal scarring (Wong et al., unpublished data). The protein and gene expression pattern of αvβ6 integrin has not been previously compared during gingival and skin wound healing in a long-term study. Interestingly, the localization of αvβ6 integrin in gingival wounds in red Duroc pigs was similar to humans. Integrin αvβ6 was accumulated with high staining intensity in the basal wound epithelium of both gingival and skin wounds from day 7 to day 14. Its accumulation, however, extended to the latest time point studied (49 days after wounding) in the gingival wounds but not in the skin. Persistent accumulation of αvβ6 integrin in the gingival compared with skin wounds was also observed in another study that was performed with larger sized gingival and skin wounds in red Duroc pigs (unpublished data). A significantly higher level of αvβ6 integrin mRNA expression at different time points of gingival wound healing may explain its extended presence in the gingival wounds but not in those of skin.
The expression and localization of TGF-β isoforms has been mostly studied in the earlier stages of normal wound healing. Many short-term studies in wound healing models (mostly rodents) have shown a peak in TGF-β1 level in the first 24 hr and, in some instances, a second peak on day 7 postwounding in skin wounds (O'Kane and Ferguson 1997). Only a few short-term studies have compared the expression of TGF-β isoforms in oral mucosal vs dermal wound healing (<3 days) using mouse models (Szpaderska et al. 2003; Schrementi et al. 2008). It was found that the steady-state expression of TGF-β1 was lower in early stages of gingival wounds compared with the skin. The level of TGF-β3, however, in the gingiva was three times higher than skin at 24 hr postwounding and declined afterward, resulting in a higher TGF-β3 to TGF-β1 ratio in earlier stages of gingival wound healing compared with the skin. Mice are loose-skinned animals with significantly different healing process than humans, and the results may not necessarily apply to tight-skinned species, including humans (Hayward and Robson 1991). In this study, we investigated the protein and gene expression levels of TGF-β1 and -β3 in unwounded tissues and in the wound time points later than 3 days, especially in relation to the expression of β6 integrin. The results showed only small changes in the relative immunostaining intensity and expression of TGF-β1 mRNA at all time points of gingival and skin wounds compared with unwounded tissue. Interestingly, however, the gene expression of TGF-β1 was higher in all gingival samples compared with the skin. Expression of TGF-β3 has been shown to peak later in dermal wound healing when TGF-β1 is decreasing (O'Kane and Ferguson,1997). A recent study showed peak TGF-β1 mRNA expression at 7 days after wounding in the pig skin wounds, whereas expression of TGF-β3 increased at later time points (Murphy-Ullrich and Poczatek 2000). Although considerable upregulation in TGF-β1 mRNA levels in either gingival or skin wounds was not detected, we did identify that peak mRNA expression of TGF-β3 was found to be around day 14 in samples of both tissues. The staining intensity of TGF-β3 was generally higher in the normal gingiva and gingival wounds compared with skin. Late colocalization of TGF-β3 to β6 integrin at the basal epithelium of 49-day-old gingival wounds was also in agreement with the results from human gingival wounds. This suggests that there may be a sustained local activation of the anti-fibrotic TGF-β3 by αvβ6 integrin in the later stages of gingival wound healing, resulting in reduced scarring.
Based on knocked out and transgenic animal studies performed in mice, the presence or absence of αvβ6 integrin does not change the normal wound healing process (Häkkinen et al. 2004; AlDahlawi et al. 2006). It is still not clear how αvβ6 integrin functions during normal and abnormal wound healing in tight-skinned species, including humans. It has been shown that in compromised wound healing conditions, such as in animals under stress and in chronic wounds that develop in β6 integrin overexpressing mice, the activation of TGF-β by αvβ6 integrin may play a role (Häkkinen et al. 2004; AlDahlawi et al. 2006). High levels of αvβ6 expression have also been detected in non-healing chronic wounds in humans (Häkkinen et al. 2004). Considering that TGF-β regulates re-epithelialization, connective tissue regeneration, and scar formation during wound healing (Kaminski et al. 2000), and the evidence that epithelial cells have a significant role in the development of inflammatory skin lesions (Carroll et al. 1995; Chan et al. 2002), it is possible that the local modulation of TGF-β isoforms by their activators, namely αvβ6 integrin, during wound healing may be important in the wound outcome: regeneration or scar formation.
In summary, we found that there was persistent accumulation of αvβ6 integrin in scarless gingival wounds of both humans and red Duroc pigs but not in the scar-forming skin wounds of the pigs. Peak accumulation of αvβ6 integrin between day 7 and day 28 in human gingival wounds was spatio-temporally concurrent with increased staining intensity of both TGF-β1 and TGF-β3. Late stages of gingival wound healing in the pigs also showed co-accumulation of αvβ6 integrin and TGF-β3 at the basal wound epithelium. TGF-β3 was only detected in 7-day skin wounds of the pigs. TGF-β1 showed steady accumulation in different wound healing time points of both skin and gingiva in red Duroc pigs. Persistent accumulation of αvβ6 integrin in the pig gingival wounds was supported by its long-lasting mRNA expression. Although there was higher local accumulation of TGF-β3 in later stages of wound healing in pig gingival wound epithelium, we found the peak mRNA expression of TGF-β3 to be around day 14 in both gingival and skin wounds. TGF-β1 showed steady expression in both gingival and skin wounds, with generally higher expression level in gingiva comparing to skin. Therefore, based on the findings in this study, we suggest that persistent expression of αvβ6 integrin along with higher local accumulation of TGF-β3 in the later stages of wound healing in the gingival wound basal epithelium may sustain the anti-scarring effects of this TGF-β3 isoform in the gingiva but not in the skin. Appropriate functional studies should be undertaken in the future to show the effectiveness of such local modulation.
Acknowledgments
These studies were supported by the Canadian Institutes of Health Research and by a New Emerging Team grant to D.A.H. from the Institute for Gender and Health.
We thank Dr. Dean Sheppard for his generous gift of the β6-B1 antibody. We also thank Dr. Leeni Koivisto, Dr. Tara Habijanac, and Mr. Cristian Sperantia for expert assistance.
References
- AlDahlawi S, Eslami A, Häkkinen L, Larjava HS (2006) The alphavbeta6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen 14:289–297 [DOI] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB (2004) Integrin αVβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J Cell Biol 165:723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB (2003) Making sense of latent TGF-β activation. J Cell Sci 116:217–224 [DOI] [PubMed] [Google Scholar]
- Annes JP, Rifkin DB, Munger JS (2002) The integrin alphaVbeta6 binds and activates latent TGF-β3. FEBS Lett 511:65–68 [DOI] [PubMed] [Google Scholar]
- Breuss JM, Gallo J, DeLisser HM, Klimanska IV, Folkesson HG, Pittet JF, Nishimura SL, et al. (1995) Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 108:2241–2251 [DOI] [PubMed] [Google Scholar]
- Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R (1993) Restricted distribution of integrin β6 mRNA in primate epithelial tissues. J Histochem Cytochem 41:1521–1527 [DOI] [PubMed] [Google Scholar]
- Carroll JM, Romero MR, Watt FM (1995) Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 83:957–968 [DOI] [PubMed] [Google Scholar]
- Chan T, Ghahary A, Demare J, Yang L, Iwashina T, Scott PG, Tredget EE (2002) Development, characterization, and wound healing of the keratin 14 promoted transforming growth factor-beta1 transgenic mouse. Wound Repair Regen 10:177–187 [DOI] [PubMed] [Google Scholar]
- Clark RA, Tonnesen MG, Gailit J, Cheresh DA (1996) Transient functional expression of alphaVbeta 3 on vascular cells during wound repair. Am J Pathol 148:1407–1421 [PMC free article] [PubMed] [Google Scholar]
- Clark RAF (1996) The Molecular and Cellular Biology of Wound Repair. New York, Plenum Press
- Dang CM, Beanes SR, Lee H, Zhang X, Soo C, Ting K (2003) Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast Reconstr Surg 111:2273–2285 [DOI] [PubMed] [Google Scholar]
- DiPietro LA, Nebgen DR, Polverini PJ (1994) Downregulation of endothelial cell thrombospondin-1 enhances in vitro angiogenesis. J Vasc Res 31:178–185 [DOI] [PubMed] [Google Scholar]
- DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ (1996) Thrombospondin-1 synthesis and function in wound repair. Am J Pathol 148:1851–1860 [PMC free article] [PubMed] [Google Scholar]
- Dumur CI, Garrett CT, Archer KJ, Nasim S, Wilkinson DS, Ferreira-Gonzalez A (2004) Evaluation of a linear amplification method for small samples used on high-density oligonucletide microarray analysis. Anal Biochem 331:314–321 [DOI] [PubMed] [Google Scholar]
- Ferguson MW, O'Kane S (2004) Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci 359:839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant CL, Olson ME, Hart DA (2004) Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveals an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen 12:305–319 [DOI] [PubMed] [Google Scholar]
- Gallant-Behm CL, Olson ME, Hart DA (2005) Cytokine and growth factor mRNA expression patterns associated with the hypercontracted, hyperpigmented healing phenotype of red duroc pigs: a model of abnormal human scar development. J Cutan Med Surg 9:165–177 [DOI] [PubMed] [Google Scholar]
- Ghahary A, Shen YJ, Scott PG, Gong Y, Tredget EE (1993) Enhanced expression of mRNA for transforming growth factor-beta, type I and type III collagen in human post-burn hypertrophic scar tissue. J Lab Clin Med 122:465–473 [PubMed] [Google Scholar]
- Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, Kramer R, et al. (1996) Keratinocytes in human wounds express alpha v beta 6 integrin. J Invest Dermatol 106:42–48 [DOI] [PubMed] [Google Scholar]
- Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, et al. (2007) Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170:110–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häkkinen L, Hildebrand HC, Berndt A, Kosmehl H, Larjava H (2000a) Immunolocalization of tenascin-C, alpha9 integrin subunit, and alphavbeta6 integrin during wound healing in human oral mucosa. J Histochem Cytochem 48:985–998 [DOI] [PubMed] [Google Scholar]
- Häkkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, Rauvala H, et al. (2004) Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol 164:229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häkkinen L, Uitto VJ, Larjava H (2000b) Cell biology of gingival wound healing. Periodontol 2000 24:127–152 [PubMed] [Google Scholar]
- Hayward PG, Robson MC (1991) Animal models of wound contraction. Prog Clin Biol Res 365:301–312 [PubMed] [Google Scholar]
- Houghton AN, Eisinger M, Albino AP, Cairncross JG, Old LJ (1982) Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med 156:1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Peled ZM, Chin GS, Liu W, Longaker MT (2001) Ontology of transforming growth factor-β1, TGF-β3, and TGF-β receptors I and II in fetal rat fibroblasts and skin. Plast Reconstr Surg 107:1787–1794 [DOI] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV Jr, et al. (1996) Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 133:921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski N, Allard JD, Pittet JF, Fengrong Z, Griffiths MJD, Morris D, Huang X, et al. (2000) Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA 97:1778–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J, Koli K, Von Mechner H (2004) TGF-β activation by traction? Trends Cell Biol 14:657–659 [DOI] [PubMed] [Google Scholar]
- Koivisto L, Larjava K, Häkkinen L, Uitto V-J, Heino J, Larjava H (1999) Different integrins mediate cell spreading, haptotaxis and lateral migration of HaCaT keratinocytes on fibronectin. Cell Adhes Commun 7:245–257 [DOI] [PubMed] [Google Scholar]
- Larjava H, Haapasalmi K, Salo T, Wiebe C, Uitto VJ (1996) Keratinocyte integrins in wound healing and chronic inflammation of the human periodontium. Oral Dis 2:77–86 [DOI] [PubMed] [Google Scholar]
- Larjava H, Koivisto L, Häkkinen L (2002) Keratinocyte interactions with fibronectin during wound healing. In Heino J, Kähäri V-M, eds. Cell Invasion. Georgetown, TX, Landes Biosciences, Eurekah.com, 42–64
- Ludlow A, Yee KO, Lipman R, Bronson R, Weinreb P, Huang X, Sheppard D, et al. (2005) Characterization of integrin β6 and thrombospondin-1 double-null mice. J Cell Mol Med 9:421–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, et al. (2003) Transforming growth factor-β-dependent and independent pathways of induction of tubulointerstitial fibrosis in β6−/− mice. Am J Pathol 163:1261–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallion RL, Ferguson MWJ (1996) Fetal wound healing and the development of anti-scarring therapies for adult wound healing. In Clark RAF, ed. The Molecular and Cellular Biology of Wound Repair. 2nd ed. New York, Plenum Press, 561–600
- Midwood KS, Williams LV, Schwarzbauer JE (2004) Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 36:1031–1037 [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, et al. (2002) The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol 157:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, et al. (1999) The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96:319–328 [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Poczatek M (2000) Activation of latent TGF-β by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 11:59–69 [DOI] [PubMed] [Google Scholar]
- O'Kane S, Ferguson MWJ (1997) Transforming growth factor betas and wound healing. Int J Biochem Cell Biol 29:63–78 [DOI] [PubMed] [Google Scholar]
- Perbal B (2004) CCN proteins: multifunctional signalling regulators. Lancet 363:62–64 [DOI] [PubMed] [Google Scholar]
- Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, et al. (2008) Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo T, Mäkelä M, Kylmäniemi M, Autio-Harmainen H, Larjava H (1994) Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab Invest 70:176–182 [PubMed] [Google Scholar]
- Schor SL, Ellis I, Irwin CR, Banyard J, Seneviratne K, Dolman C, Gilbert AD, et al. (1996) Subpopulations of fetal-like gingival fibroblasts: characterization and potential significance for wound healing and the progression of periodontal disease. Oral Dis 2:155–166 [DOI] [PubMed] [Google Scholar]
- Schrementi ME, Ferreira AM, Zender C, DiPietro LA (2008) Site-specific production of TGF-β in oral mucosal and cutaneous wounds. Wound Repair Regen 16:80–86 [DOI] [PubMed] [Google Scholar]
- Sciubba JJ, Waterhouse JP, Meyer J (1978) A fine structural comparison of the healing of incisional wounds of mucosa and skin. J Oral Pathol 7:214–227 [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MWJ (1995) Neutralization of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J Cell Sci 108:985–1002 [DOI] [PubMed] [Google Scholar]
- Shah M, Rorison P, Ferguson MWJ (2000) The role of transforming growth factors beta in cutaneous scarring. In Garg HG, Longaker MT, eds. Scarless Wound Healing. New York, Mrcel Dekker, 213–226
- Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746 [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA (2003) Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res 82:621–626 [DOI] [PubMed] [Google Scholar]
- Varga J, Rosenbloom J, Jimenez SA (1987) Transforming growth factor β causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J 247:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A (2002) Transforming growth factor β signaling through the smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol 118:211–215 [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Hinz B (2008) Integrins and the activation of latent transforming growth factor beta1: an intimate relationship. Eur J Cell Biol 87:601–615 [DOI] [PubMed] [Google Scholar]
- Yang L, Qiu CX, Ludlow A, Ferguson MW, Brunner G (1999) Active transforming growth actor-beta in wound repair: determination using a new assay. Am J Pathol 154:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, et al. (2007) Absence of integrin-mediated TGF-β1 activation in vivo recapitulates the phenotype of TGF-β1-null mice. J Cell Biol 176:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I (2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 14:136–176 [PMC free article] [PubMed] [Google Scholar]
- Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH (2007) Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen 15(suppl 1):S32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]