Abstract
Double or multiple antigen labeling in IHC classically relies on the existence of primary antibodies raised in different species or of different IgG isotypes to ensure the specific labeling with the secondary detection systems. However, suitable pairs of primary antibodies are not always available or the best choice (e.g., as diagnostic tools). During the last few years, several methods have been proposed to overcome this, but none of them offers the flexibility needed for reliable double or multiple enzymatic or fluorescent IHC. We present here a procedure that elutes the antibodies after a first round of immunolabeling, which, in combination with precipitation-based detection systems, allows multiple IHC rounds even for primary antibodies raised in the same species and IgG isotype. Compared with other proposed methods, this procedure ensures a reliable enzymatic or fluorescent staining without cross-reactivity and without loss of tissue antigenicity, thus offering a flexible tool for colocalization studies and pathological diagnosis. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 57:567–575, 2009)
Keywords: fluorescence microscopy, multiple enzymatic labeling, multiple immunofluorescent labeling, stripping buffer, precipitating substrates
IHC detection of two or more antigens on the same tissue section is already established as an important technique, both in fundamental research and in diagnostic pathology. Technically, sequential or simultaneous multiple labeling IHC requires the primary antibodies to be raised in different species or at least to be of different IgG isotypes if developed in the same species (Kumar-Singh et al. 2002; Buchwalow et al. 2005) to avoid any cross-reactivity between the detection systems used to visualize the signals. However, not infrequently, the best-suited antibodies for a study are only available as the same IgG isotype of the same species, and the paucity of the antigen makes a direct labeling approach impractical.
Different methods have been proposed to overcome this quandary, but a simple and universal system for both light microscopic and fluorescent detection remains to be identified. A heat-mediated antibody stripping procedure such as boiling in citrate buffer has been proposed to remove the antibodies after a first round of IHC (Lan et al. 1995; Tornehave et al. 2000; Toth and Mezey 2007), but in our experience, it is prone to remove some delicate biopsies from poly-lysine slides, especially if they have already been antigen retrieved by a previous heat-mediated procedure. Precipitation of peroxidase reaction products around tissue-bound antigen–antibody pairs has also been reported to shield them from interaction with subsequent pairs of the same species/IgG isotope primary–secondary antibodies (Butterworth et al. 1985; Sternberger and Sternberger 1986). Although this method had not been yet tested together with more sensitive detection procedures to evaluate the degree of actual shielding, it is also limited by the fact that it cannot be applied in fluorescent labeling IHC. In yet another approach, polyclonal monovalent Fab secondary antibodies have been proposed to opsonize the first primary antibodies, rendering them unavailable for subsequent secondary antibodies (Negoescu et al. 1994); however, given the general low affinity of the Fab secondary antibodies, this approach has not been very successful (Tornehave et al. 2000).
Interestingly, the procedure of detecting multiple antigens in independent sequential protocols is not new in protein Western blotting methodology. In this approach, an intermediate elution buffer is used that removes the previous pairs of primary–secondary antibodies (Harlow and Lane 1999). Such “stripping” buffers use pH, heat, osmolarity, detergents, or denaturing agents to interfere with the non-covalent binding of the antibodies to their epitopes, thus eluting the previous rounds of immunodetection. On the other hand, it is also known that some detection substrates used in IHC, such as DAB and tyramide, precipitate, forming stronger, covalent bonds on the tissue, and theoretically should not be eluted by such a stripping buffer.
In this study, we tested and compared a number of antibody elution methods and precipitation-based reporter systems for IHC using multiple antibodies. We report here an effective and universal method of multiple immunolabeling that allows the use of primary antibodies raised in the same species and IgG isotype for both enzymatic and fluorescent detections.
Materials and Methods
Preparation of Tissue
Formalin-fixed, paraffin-embedded tonsil tissue blocks were collected from the archives in the Departments of Histology and Pathology (University of Medicine and Pharmacy Craiova, Craiova, Romania; Emergency County Hospital 1, Craiova, Romania). Four-μm-thick sections were cut, and based on routine hematoxylin staining, we selected tissue with a normal appearance or minimal inflammatory changes. Although tonsil was the primary tissue used because of a homogenous structure easily recognized in serial sections, we also included in the study formalin-fixed skin and hepatic biopsies, as well as breast, oral, and gastric carcinomas. Fresh-frozen liver, lymph node, and breast tissue sections were also collected on poly-lysine–charged slides, air dried, postfixed in 4% ice-cold buffered neutral formalin or acetone, and included as test slides to asses the aggressivity of different elution buffers.
General IHC Protocol
A sequential approach was followed for all immunolabelings, including the double, triple, and quadruple rounds of IHC. We used a panel of diagnosis-certified primary antibodies targeting membrane (CD20cy for B lymphocytes, CD45Ro and CD3 for T lymphocytes, CD31, CD34, and CD105 for endothelial cells), cytoplasmic (FVIII for endothelial cells, CD68 for macrophages, S100 for antigen presenting cells), and nuclear antigens [Ki-67 and proliferating cell nuclear antigen (PCNA) as proliferation markers] (Redox; Bucharest, Romania) (Table 1). Briefly, after an antigen retrieval step, sections were washed and blocked in 0.3% H2O2 and 1% skim milk for 30 min each, and the primary antibodies were added for overnight incubations at 4C. Sections were washed the next day and incubated with amplification kits such as alkaline phosphatase (AP) and horseradish peroxidase (HRP)-polymeric conjugates (EnVision; Dako, Redox) for enzymatic detection and Alexa 488-tyramide conjugate or directly labeled Alexa secondaries (Molecular Probes; Medist, Bucharest, Romania) for fluorescent detection. In addition, for enzymatic visualization of the signals, we used DAB (Dako), 3-amino-9-ethylcarbazole (AEC; Sigma-Aldrich, Cheminkpress, Craiova, Romania), Vector-Blue (Vector; Cheminkpress), and SigmaFast DAB with metal enhancer (Sigma-Aldrich) for HRP visualization and Nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP; Roche, Cheminkpress), Fast Red TR/Naphthol (Sigma-Aldrich), and Vector-VIP (Vector) for AP visualization (Table 2). After signal visualization, the slides were processed for different antibody elution methods (see below) and for further similar immunodetections. All intermediate washing steps were done in 0.1 M PBS, 0.5% Tween 20, pH 7.2, and all antibodies were diluted in PBS with 1% BSA (Sigma-Aldrich). Light microscopy slides were coverslipped without counterstaining or were lightly counterstained with hematoxylin. Fluorescence slides were coverslipped with antifading mounting medium (Dako). All experiments were repeated twice to ensure the consistency of the results.
Table 1.
Primary antibodies used in this study
| Antigen | IgG isotype, species | Specificity | Working dilution | Antigen retrieval | Source |
|---|---|---|---|---|---|
| CD20cy | IgG2a, Ms | B lymphocytes | 1:100 | 10 mM citrate buffer, pH 6 | Dako |
| CD45Ro | IgG2a, Ms | T lymphocytes | 1:50 | 10 mM citrate buffer, pH 6 | Dako |
| CD3 | Pab, Rbb | T lymphocytes | 1:100 | 10 mM citrate buffer, pH 6 | Dako |
| CD31 | IgG1, Ms | Endothelial cells | 1:50 | 10 mM citrate buffer, pH 6 | Dako |
| CD34 | IgG1, Ms | Endothelial cells | 1:50 | 10 mM citrate buffer, pH 6 | Dako |
| CD105 | Pab, Rbb | Endothelial cells | Ready to use | 10 mM citrate buffer, pH 6 | Abcam |
| CD105 | IgG1, Ms | Endothelial cells | 1:1000 | – | Dako |
| Collagen IV | IgG1, Ms | Basal membranes | 1:25 | 10 mM citrate buffer, pH 6 | Dako |
| F VIII | IgG1, Ms | Endothelial cells | 1:50 | 10 mM citrate buffer, pH 6 | Dako |
| CD68 | IgG1, Ms | Macrophages | 1:100 | 10 mM citrate buffer, pH 6 | Dako |
| S100 | Pab, Rbb | Antigen presenting cells | 1:2000 | 10 mM citrate buffer, pH 6 | Dako |
| PCNA | IgG2a, Ms | Cell proliferation marker | 1:50 | 10 mM citrate buffer, pH 6 | Dako |
| Ki67 | Pab, Rbb | Cell proliferation marker | 1:250 | 10 mM citrate buffer, pH 6 | Abcam |
| Ki67 | IgG1, Ms | Cell proliferation marker | 1:100 | 10 mM citrate buffer, pH 6 | Dako |
Abcam; Cheminkpress, Craiova, Romania.
Table 2.
Amplification systems used in this study
| System | Source |
|---|---|
| Dual link anti-Ms/anti-Rbb, EnVision-AP conjugated | Dako |
| Dual link anti-Ms/anti-Rbb, EnVision-HRP conjugated | Dako |
| Anti-Ms Tyramide-Alexa 488 | Molecular Probes |
| Goat anti-Ms Alexa 488 | Molecular Probes |
| Goat anti-Ms Alexa 594 | Molecular Probes |
AP, alkaline phosphatase; HRP, horseradish peroxidase.
Antibody Elution Techniques
A variety of antibody elution (stripping) buffers have been described in the literature, especially for use in Western blotting (Harlow and Lane 1999). We tested different mechanisms, such as high osmolarity, extreme pH, and denaturing buffers, as reflected in our choice of studied reagents/buffers (Table 3). Because it was the most efficient elution buffer (see below), we describe here in detail the preparation procedure for the glycine SDS pH 2 buffer. This was done by first mixing 0.94 g glycine in 25 ml 20% SDS. The volume was increased to 500 ml with distilled water, and last, the pH was adjusted to 2 with HCl under a hood. The jars containing all the buffers were preheated at 50C, and the slides were incubated in these solutions on a platform shaker in an incubator. Tested incubation times were 5, 15, 30, and 60 min. After elution, the slides were washed abundantly in PBS-Tween and processed for the next immunodetection procedure. In addition, we also chose to compare other methods such as microwaving in citrate buffer to test the efficiency of enzymatic precipitation of the substrates as an antibody shielding procedure (Butterworth et al. 1985; Sternberger and Sternberger 1986; Lewis Carl et al. 1993; Lan et al. 1995; Tornehave et al. 2000; Toth and Mezey 2007).
Table 3.
Elution buffers tested in this study
| Condition | Buffer |
|---|---|
| Ionic strength | 3.5 M KCl |
| Low pH | 50 mM glycine-HCl, pH 2.2 |
| High pH | 100 mM glycine, NaOH, pH 10 |
| Denaturing | 25 mM glycine-HCl, 10% SDS, pH 2 |
| 62 mM Tris, 2% SDS, pH 6.75 | |
| 62 mM Tris, 2% SDS, 100 mM β-mercaptoethanol, pH 6.75 |
Reactivity Controls
The efficacy of elutions was controlled by performing the stripping procedure after primary antibody incubations and continuing with the secondary detection systems. Negative controls were obtained by skipping the primary antibodies. In addition, we also checked if the precipitation of different chromogens might trap inside the primary antibodies by redetecting these primary antibodies after the first round of IHC and elution, with a more sensitive detection method (such as tyramide fluorescent detection) than used in the first round of immunolabeling.
Tissue Reactivity Under the Elution Buffer
After optimizing the elution protocol, we also tested whether it was not interfering with the reactivity of the tissue for further antigen detection rounds. Reactivity loss was evaluated for both monoclonal and polyclonal antibodies during these elution steps. This was done by comparing the reactivity achieved on single IHC detection with the reactivity after multiple rounds of immunolabelings, performed on serial sections. For the same purpose, we also performed a double fluorescent labeling, first using tyramide-Alexa 488 detection followed by elution, a second round of primary antibody, and detection with a directly conjugated Alexa-594 secondary. Given the broad variations in pH and osmolarity of the different elution methods tested, we used only Alexa-based detection systems, because they are known to maintain their properties over a broad spectrum of pH and osmolarity (Panchuk-Voloshina et al. 1999). For fluorescent studies, we used both 4- and 10-μm-thick sections. To study how two isolated antigens can be visualized using this approach, we labeled thick tonsil sections first for vessels (CD31, Ms, IgG1), detected this with tyramide-Alexa 488, which was followed by a 30-min elution in glycine SDS and detection for B lymphocytes (CD20cy, Ms, IgG1) with anti-Ms Alexa 594. A deconvolution analysis was also performed on these thick sections to test the actual degree of separation for the two signals (see below). More, to assess the reproducibility of the method, the same methodology was applied for CD105 (Ms, IgG1-tyramide-Alexa 488) and collagen IV (Ms, IgG1-Alexa 594) primary antibodies on oral squamous cell carcinoma tissue.
Image Analysis
The sections were imaged with an Eclipse 90i microscope (Nikon; Apidrag, Romania) equipped with a 5-megapixel cooled CCD camera and with narrow-band fluorescent filters centered for Alexa 594 and Alexa 488 excitation and emission wavelengths. Both light and fluorescent images were captured and archived using a Nikon frame grabber and the Nikon NIS-Elements software. For fluorescent-labeled slides, images were obtained by sequential scanning each channel with the specific pair of filters to eliminate the cross-talk of chromophores and to ensure a reliable quantification. To ensure that the analysis was done on signals coming from the same optical planes, the fluorescent images were subjected to a blind deconvolution algorithm based on a multi-pass, adaptative point spread function (PSF) subtraction of diffracted light (AutoQuantX2 demo version; Media Cybernetics, Bethesda, MD).
Results
Glycine SDS pH 2 Is the Optimal Antibody Elution Buffer
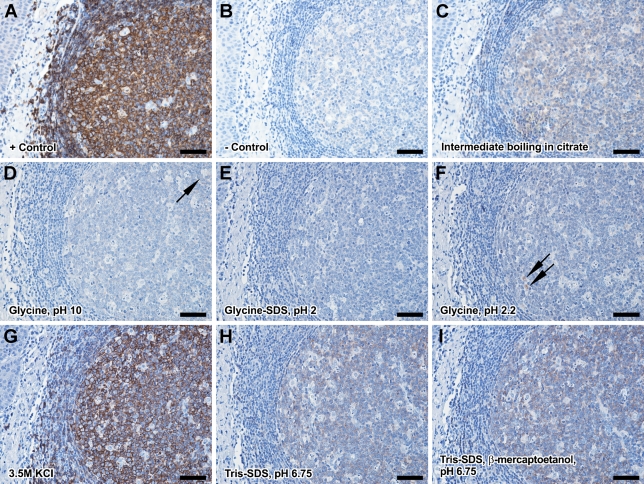
We first assessed the efficacy of the proposed elution methods to remove the primary antibodies after overnight incubation. All reaction controls were obtained by continuing the IHC sequence with an elution step and detecting the remaining signal as described in Materials and Methods. From all the tested methods, the best choice was incubation in glycine SDS pH 2 buffer that resulted in a complete elution of the primary antibodies as indicated by no remnant signal after incubation with sensitive secondary-detection systems and prolonged DAB incubation times (Figure 1). Glycine pH 2.2 and glycine pH 10 buffers offered a partial antibody elution because some faint signal could be still identified on the slides. Tris-SDS pH 6.75 and Tris-SDS + β-mercaptoetanol pH 6.75 buffers resulted in even lower elution rates, whereas 3.5 M KCl high-osmolarity solution and intermediate boiling in citrate had almost no influence on reducing the intensity of the signal. All elution incubations were initially done for 60 min at 50C on a platform shaker. After narrowing down the list of effective buffers, we also tested other incubation conditions and found that a minimum incubation time of 30 min and agitation are prerequisites for an effective elution in glycine SDS pH 2 buffer (Supplementary Figure 1).
Figure 1.
Choosing the optimum antibody-elution protocol. The procedure involved incubation with a primary antibody (follicular B-cell marker CD20cy here), applying the elution procedure, and detecting the remaining primary antibody. Positive and negative controls for this reaction were obtained by skipping the elution step (A) or not adding the primary antibody (B). Boiling in citrate buffer (C), incubating in glycine pH 10 (D), glycine-HCl, pH 2.2 (F), 3.5 M KCl (G), Tris-SDS, pH 6.75 (H), and Tris-SDS β-mercaptoethanol, pH 6.75 (I) showed insufficient signal reduction, whereas the glycine-SDS pH 2 protocol (E) showed a complete antibody elution. All elutions involved a 30-min incubation under agitation at 50C. Arrows indicate sites with faint remnant signal. Bar = 50 μm.
Considering that acidic buffers should also block endogenous or exogenous peroxidase, we wanted to see how this buffer interacts with the detection enzymes themselves. The sequence primary antibody, 5 min blocking, secondary system, and enzymatic detection showed an incomplete elution as expected. When we moved the blocking step after the secondary system, both AP- and HRP-based detections showed no signal, suggesting that this elution medium can effectively inhibit the activity of exogenous AP and HRP, independently of antibody elution.
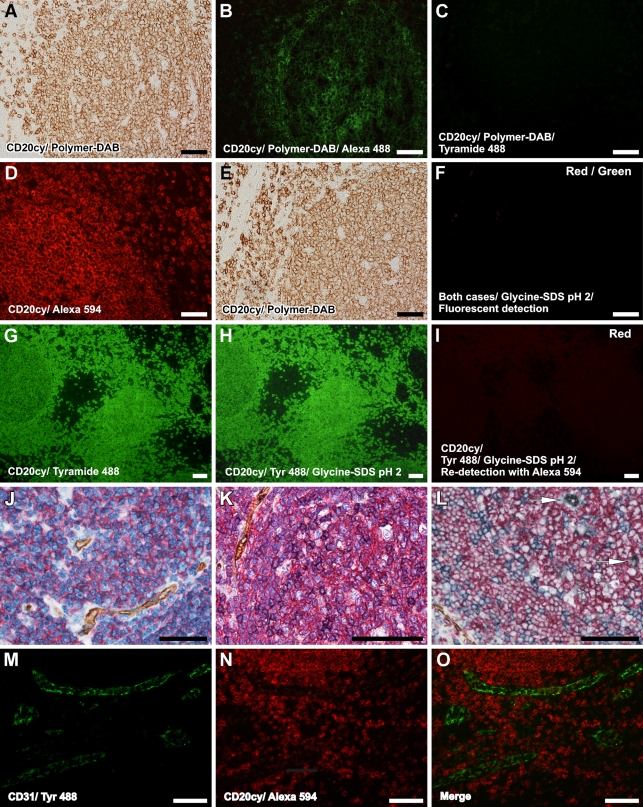
To test the possibility that precipitating substrates from previous immunolabeling rounds did not interfere with the next ones, or in other words, to test the efficacy of the elution buffer in removing the primary antibodies that might be trapped under the precipitated substrate, we performed complete IHC sequences with DAB and NBT-BCIP detections and tried to redetect the primary antibodies using Alexa 488 anti-primary and tyramide-Alexa 488 secondaries, with or without an intermediate elution step. Our results showed that, although with an intermediate elution step, no signal could be detected, skipping the elution buffer led to redetection of the primary antibodies colocalizing with the prior enzymatic signal (Figure 2). Surprisingly, Alexa secondary-labeled antibody showed a more intense signal compared with the more sensitive tyramide detection, an observation that might be explained by the loss of necessary space and electron-rich sites needed for tyramide to precipitate on the tissue.
Figure 2.
Efficiency of the proposed elution method and enzymatic- and fluorescent-based applications. After a classical detection procedure with a precipitation reporter system (A), the primary antibody can be still redetected with species-specific fluorescent secondary (B) but not with a tyramide-based system (C). After a fluorescent detection with a species-specific fluorescent secondary (D) or with an enzymatic procedure (E), applying the elution method and trying to redetect the signal shows a complete loss of primary antibody and fluorescent-labeled secondary, respectively (F). On the other hand, a tyramide-based green fluorescent detection (G) is not affected by the elution buffer (H), but the primary antibodies that linked the tyramide reporter are also eluted and cannot be redetected with a species-specific red fluorescent secondary (I). Eluting the antibodies does not disrupt tissue antigenicity. One, two, and three elution steps are used to perform double, triple, and quadruple immunolabelings. Ms CD31/Polymer-HRP/DAB, Ms CD20cy/Polymer-AP/Fast Red (J); Ms CD31/Polymer-HRP/DAB, Ms CD20cy/Polymer-AP/Fast Red, Ms CD45Ro/Polymer-HRP/Vector VIP (K); Ms CD31/Polymer-HRP/DAB, Ms CD20cy/Polymer-AP/Fast Red, Ms CD45Ro/Polymer-AP/NBT, Ms CD68/Polymer-HRP/Co enhanced DAB (L); Ms CD31/tyramide Alexa 488 (M) combined with Ms CD20cy/Alexa 594 (N) in O. For J and K, the slides have been counterstained with hematoxylin. Arrows in L represent macrophages. Bar = 50 μm.
Most Detection Substrates Are Stable in Glycine SDS pH 2 Buffer
We also tested the stability of substrates in the selected elution buffer. Our data showed that among all AP and HRP substrates mentioned in the Materials and Methods, except Vector blue and Vector-VIP substrates that were completely washed in the elution buffer and metal-enhanced DAB that changed its color from gray-black to brownish, all other substrates maintained their properties even after multiple elution times.
For fluorescent detections, in accordance with our hypothesis, tyramide-Alexa amplification showed no signal loss even after prolonged elution times, whereas direct Alexa anti-primary labeling was completely washed in the glycine SDS buffer (Figure 2).
No Antigenicity Loss Occurs Even After Multiple Antibody Elution Steps
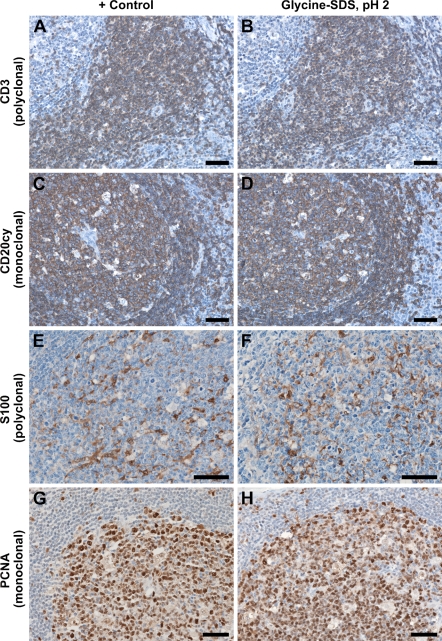
To study whether the treatment with glycine SDS pH 2 buffer did not interfere with the antigenicity of the tissue, we first tested the reactivity of all primary antibodies used in this study in the presence or absence of an initial elution step. For all tested monoclonal and polyclonal antibodies, there was no reactivity loss even after 1 hr of elution treatment (Figure 3). We further tested the reactivity of the primary antibodies after up to four rounds of immunodetections and elutions. No abnormal staining or decreased reactivity was observed for all antibody sequence combinations and for antibodies recognizing membranar, cytoplasmic, or nuclear antigens, such as the CD subclass lymphocyte markers, S100 cytoplasmic staining in antigen-presenting cells, and Ki-67 and PCNA proliferation markers, respectively. Similarly, the tissue adhesiveness on poly-lysine–charged slides was not affected even after such prolonged or repeated elution times for formalin-fixed tonsil, skin, and hepatic biopsies, breast, oral, and gastric carcinomatous tissue, and fresh-frozen postfixed liver, lymph node, and breast tissue. In fact, some gastric carcinomatous fixed tissue was so fragile that it could not be processed for antigen retrieval by boiling in citrate buffer. In these cases, we routinely performed an overnight incubation in citrate buffer at 80C. It is worth mentioning that treating these tissues with glycine SDS buffer after this overnight retrieval also did not alter their adhesiveness on poly-lysine–coated slides. For the formalin-fixed, paraffin-embedded tissue, we tested other retrieval methods such as 5-min incubation in 90% formic acid, proteinase K digestion, and boiling in EDTA pH 9; after all these procedures, continuing with glycine SDS pH 2 treatment did not alter tissue integrity.
Figure 3.
Antigenicity of the tissue is not lost for both monoclonal and polyclonal antibodies targeting nuclear, cytoplasmic, and membranar antigens. Shown here are parallel stainings on serial sections for Rbb Pab CD3 without (A) and with an initial eluting step (B), for Ms IgG2a CD20cy (C,D), for Rbb Pab S100 (E,F), and for Ms IgG2a proliferating cell nuclear antigen (PCNA) (G,H), and show no reactivity loss as signal intensity and localization. CD3 is well characterized to label total T lymphocytes in the interfollicular spaces; CD20cy labels the B-cell population in the lymphoid follicles. S100 shows a cytoplasmic staining pattern in dendritic reticulum cells of the lymphoid follicles, and PCNA shows a nuclear staining pattern in the germinal centers. Bar = 50 μm.
Fluorescent Colocalization Studies With the Same Species of Primary Antibodies
We finally wanted to test our optimized protocol in another practical approach: the possibility of colocalization studies on fluorescent-labeled sections. On thick sections labeled for CD31/CD20cy and CD105/collagen IV antibody pairs, as described in the Materials and Methods, deconvolution showed, as expected, no colocalization for the respective two isolated antigens (Supplementary Figure 2). We also determined that, despite its accentuated precipitating effect, an optimized tyramide precipitation time does respect the morphology of the detected structure, and confocal or deconvolution-based studies and 3D renderings are perfectly possible using this technique.
Discussion
A frequent problem in multiple antigen labeling in IHC is that the best-suited antibodies can be raised in the same species and even belong to the same iso-species IgG isotype. Using the prior knowledge of stripping buffers frequently used in Western blotting, we tested the application of such a buffer in breaking the antibody–antigen non-covalent bonds at the tissue level (Figure 4). We further optimized conditions in which this elution buffer was most effective and with a minimal effect on tissue antigenicity and morphology. We thus found that indeed no detectable changes in tissue reactivity occur for a panel of monoclonal and polyclonal primary antibodies after prolonged elution times or after multiple applications of the glycine SDS pH 2 buffer. No reactivity loss occurred even when considering antibodies directed against cytoplasmic and nuclear antigens.
Figure 4.
Schematic diagram of the optimized general protocol for double and multiple antigen labeling in IHC, with the first primary antibodies raised in the same species and of the same species IgG isotype.
Other published methods such as microwaving in citrate buffer and enzymatic precipitation of the substrates as a shielding technique (Butterworth et al. 1985; Sternberger and Sternberger 1986; Lewis Carl et al. 1993; Lan et al. 1995; Tornehave et al. 2000) were also compared with the present procedure and were found less efficient, mainly because of their increased tendency of damaging the tissue, the appearance of more sensitive detection methods, or too laborious optimizations. In another recently published method, primary antibodies were non-covalently labeled in vitro with monovalent Fab fragments carrying the reporter molecules (Brown et al. 2004). Suitable but even more expensive kits exploiting this non-covalent labeling principle have also been developed commercially (van der Loos and Gobel 2000). However, the time needed to optimize each pair of primary–secondary antibodies or finding optimum high affinity–labeled Fab secondaries, as well as the costs of these kits, constitute the main drawbacks compared with the present approach.
Our method was also very efficient with both progressive and non-progressive precipitating substrates that could potentially trap antibodies within the precipitate, because no reactivity was observed after elutions times as little as 30 min. Because we observed that the glycine SDS elution buffer also offers an efficient inhibition of AP and HRP, this might extend its applicability to inhibiting endogenous enzymatic activity on difficult tissue, instead of separate incubations with hydrogen peroxide or levamisole.
Although the elution principle has been tried before in double IHC as a harsh oxidizing buffer (Tramu et al. 1978), the present methodology shows practically no side effects on tissue stability and antigenicity and is adequate for both enzymatic and close colocalization studies on fluorescence microscopy. For instance, we successfully used this technique in double immunolabelings for CD105/FVIII, CD105/collagen IV, and CD105/Ki67 mouse antibody pairs to describe tumoral vessels in oral squamous cell carcinomas (Margaritescu et al. 2008).
Altogether, we describe here a use of a simple, inexpensive, and effective stripping buffer that, in combination with precipitating-reporter systems, allows reliable multiple antigen labeling IHC, which could be a valuable tool for multiple colocalization studies and optimal use of limited biopsy tissue.
Acknowledgments
This study was supported by the Ministry of Education and Research, Programul Cercetare de Excelenta Contract 190/2006, project leader Prof. Laurentiu Mogoanta.
References
- Brown JK, Pemberton AD, Wright SH, Miller HR (2004) Primary antibody-Fab fragment complexes: a flexible alternative to traditional direct and indirect immunolabeling techniques. J Histochem Cytochem 52:1219–1230 [DOI] [PubMed] [Google Scholar]
- Buchwalow IB, Minin EA, Boecker W (2005) A multicolor fluorescence immunostaining technique for simultaneous antigen targeting. Acta Histochem 107:143–148 [DOI] [PubMed] [Google Scholar]
- Butterworth BH, Khong TY, Loke YW, Robertson WB (1985) Human cytotrophoblast populations studied by monoclonal antibodies using single and double biotin-avidin-peroxidase immunocytochemistry. J Histochem Cytochem 33:977–983 [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D (1999) Using Antibodies: A Laboratory Manual. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press.
- Kumar-Singh S, Cras P, Wang R, Kros JM, van SJ, Lubke U, Ceuterick C, et al. (2002) Dense-core senile plaques in the Flemish variant of Alzheimer's disease are vasocentric. Am J Pathol 161:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC (1995) A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem 43:97–102 [DOI] [PubMed] [Google Scholar]
- Lewis Carl SA, Gillete-Ferguson I, Ferguson DG (1993) An indirect immunofluorescence procedure for staining the same cryosection with two mouse monoclonal primary antibodies. J Histochem Cytochem 41:1273–1278 [DOI] [PubMed] [Google Scholar]
- Margaritescu C, Simionescu C, Pirici D, Mogoanta L, Ciurea R, Stepan A (2008) Immunohistochemical characterization of tumoral vessels in oral squamous cell carcinoma. Rom J Morphol Embryol 49:447–458 [PubMed] [Google Scholar]
- Negoescu A, Labat-Moleur F, Lorimier P, Lamarcq L, Guillermet C, Chambaz E, Brambilla E (1994) F(ab) secondary antibodies: a general method for double immunolabeling with primary antisera from the same species. Efficiency control by chemiluminescence. J Histochem Cytochem 42:433–437 [DOI] [PubMed] [Google Scholar]
- Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung WY, et al. (1999) Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem 47:1179–1188 [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH (1986) The unlabeled antibody method: comparison of peroxidase-antiperoxidase with avidin-biotin complex by a new method of quantification. J Histochem Cytochem 34:599–605 [DOI] [PubMed] [Google Scholar]
- Tornehave D, Hougaard DM, Larsson L (2000) Microwaving for double indirect immunofluorescence with primary antibodies from the same species and for staining of mouse tissues with mouse monoclonal antibodies. Histochem Cell Biol 113:19–23 [DOI] [PubMed] [Google Scholar]
- Toth ZE, Mezey E (2007) Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem 55:545–554 [DOI] [PubMed] [Google Scholar]
- Tramu G, Pillez A, Leonardelli J (1978) An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem 26:322–324 [DOI] [PubMed] [Google Scholar]
- van der Loos CM, Gobel H (2000) The animal research kit (ARK) can be used in a multistep double staining method for human tissue specimens. J Histochem Cytochem 48:1431–1438 [DOI] [PubMed] [Google Scholar]