Abstract
Structural chromosome aberrations are hallmarks of many human genetic diseases. The precise mapping of translocation breakpoints in tumors is important for identification of genes with altered levels of expression, prediction of tumor progression, therapy response, or length of disease-free survival, as well as the preparation of probes for detection of tumor cells in peripheral blood. Similarly, in vitro fertilization (IVF) and preimplantation genetic diagnosis (PGD) for carriers of balanced, reciprocal translocations benefit from accurate breakpoint maps in the preparation of patient-specific DNA probes followed by a selection of normal or balanced oocytes or embryos. We expedited the process of breakpoint mapping and preparation of case-specific probes by utilizing physically mapped bacterial artificial chromosome clones. Historically, breakpoint mapping is based on the definition of the smallest interval between proximal and distal probes. Thus, many of the DNA probes prepared for multiclone and multicolor mapping experiments do not generate additional information. Our pooling protocol, described here with examples from thyroid cancer research and PGD, accelerates the delineation of translocation breakpoints without sacrificing resolution. The turnaround time from clone selection to mapping results using tumor or IVF patient samples can be as short as 3 to 4 days. (J Histochem Cytochem 57:587–597, 2009)
Keywords: translocation, chromosome aberration, cytogenetics, thyroid cancer, IVF, PGD, fluorescence in situ hybridization, bacterial artificial chromosome, DNA probes
Congenital anomalies, including balanced and Robertsonian translocations and chromosomal inversions, occur in as much as 1.4% of the general population and have been observed at even higher rates among infertile couples and patients with recurrent abortions (Subrt 1980; Peng et al. 2006). For example, Stern and colleagues reported balanced translocations in 0.6% of all infertile couples, 3.2% of couples that failed over 10 in vitro fertilization (IVF) cycles, and 9.2% among infertile couples experiencing three or more consecutive first-trimester abortions (Stern et al. 1999).
The most commonly observed consequence of balanced reciprocal translocations in carriers without clinical disease symptoms is an increased fraction of germ cells with an abnormal chromosome complement. This has been attributed to disturbed homolog pairing during meiosis or precocious chromatid separation (Srb et al. 1965; Kalousek 2000). When translocations alter the expression of genes relevant to early human development, disturbed embryogenesis may also lead to primary infertility or repeated miscarriages (Subrt 1980; Munné 2002).
During the course of IVF, preimplantation genetic diagnosis (PGD) can now be offered to affected couples as an alternative to prenatal diagnosis and medically indicated termination of pregnancies with chromosomally unbalanced fetuses. If there is a sufficient number of fertilized normal embryos available for transfer, PGD also provides an efficient option to put an end to a familial disease (Munné 2002). However, the greatest benefit of PGD is the reduction of spontaneous abortions (Verlinsky et al. 2004). On the other hand, the observed increases in pregnancy rates after PGD among couples carrying non-Robertsonian translocations lag behind expectations (Munné et al. 2000; Munné 2002).
Precise localization of chromosomal breakpoints is also an important milestone in the identification of tumor-related genes and preparation of tumor-specific DNA probes. Work in our laboratories focuses on the activation of proto-oncogenes, among them receptor-type tyrosine kinase (rtk) genes, and their aberrant pattern of expression in tumors of the thyroid gland. In the papillary type of thyroid cancer (PTC), for example, the activation of the rtk genes ret or NTRK-1 is often the consequence of a chromosomal translocation in which the 3′ end of the gene containing the catalytic domain is fused in-frame to the 5′ end of a constitutively expressed gene (Herrmann et al. 1991; Pierotti et al. 1992; Jossart et al. 1995,1996; Beimfohr et al. 1999; Greco et al. 2004).
To determine translocation breakpoints, the conventional cytogenetic methods, i.e., chromosome banding procedures, are challenged when delineating subtle chromosome rearrangements, particularly for de novo abnormalities in newborns. Fortunately, fluorescence in situ hybridization (FISH), a technique for the analysis of chromosomal aberrations, is sensitive and specific enough to elaborate these objectives. To meet the needs of PGD or tumor research, an increase in the number of recombinant DNA libraries, such as FISH-mapped, large-insert clones, now allows almost every laboratory to prepare individualized FISH probes in-house and to circumvent the limitations of commercial probe availability (Stumm et al. 2006).
Initially, we used yeast artificial chromosome (YAC) probes spaced more or less evenly in 8–15-megabasepair (Mbp) intervals along the target chromosomes (Cassel et al. 1997; Fung et al. 1998,2001; Liehr et al. 2002; Zitzelsberger et al. 2002). The target interval was narrowed through repeated cycles of clone selection and hybridizations until a clone had been found that spanned the breakpoint (Fung et al. 1998). Although this proved to be a straightforward approach for breakpoint mapping in some patients (Cassel et al. 1997; Munné et al. 1998; Weier et al. 1999), the precise determination of breakpoint locations often became a time-consuming process plagued by YAC clone chimerisms (Selleri et al. 1992; Shizuya et al. 1992) or errors in the published physical maps (Fung et al. 1999).
The bacterial artificial chromosome (BAC) clones, on the other hand, show a much-reduced fraction of chimeric clones and have been used to maintain DNA fragments of several hundred kilobases (Shizuya et al. 1992; Thorsen et al. 2005). The popularity of BACs as probes in cytogenetic analyses or for generation of high-resolution physical maps and preparation of DNA sequencing templates can be attributed to their relative stability, ease of handling, and large DNA insert-to-vector size ratio (Kim et al. 1996; Osoegawa et al. 2001; Liehr et al. 2002; Carreira et al. 2007).
Our present study took advantage of a further advantage of BAC clones over YACs for breakpoint mapping: once retrieved from the −80C freezer, BAC clones grow much faster than YACs. This should reduce the length of each mapping cycle, compared with the use of YACs, thus accelerating the in situ delineation of chromosomal translocation breakpoints and preparation of breakpoint-specific DNA probes (Fung et al. 1998). Furthermore, we decided to use sets of overlapping BAC clones forming “contigs” or “pools” instead of single recombinant clones, because this minimizes the rates of so-called FISH failures (Munné et al. 1994; D'Alton et al. 1997; Plastira et al. 2006) or uninformative results (Sampson et al. 2004). The present article describes the strengths of BAC clone pooling strategies expediting probe preparation for PGD and the identification of candidate regions for gene expression studies in PTC tumors.
Materials and Methods
Preparation of Metaphase Cells
Metaphase spreads were made from short-term cultures of an anonymous normal male donor's white blood cells as described (Fung et al. 2002). Lymphocytes from a PGD patient (T-0512) were grown for 72 hr in RPMI 1640 (Invitrogen; Carlsbad, CA) supplemented with 10% FBS, 1% penicillin-streptomycin, and 2% phytohemagglutinin (HA-15; Abbott Molecular, Inc., Des Plaines, IL). Cells were blocked in mitosis during a 30-min treatment with colcemid (0.12 μg/ml, Invitrogen), harvested, and incubated in 75 mM KCl for 15 min at 37C (Bayani and Squire 2004). The cells were then spun down, and ∼107 cells were incubated in 5 ml of freshly prepared fixative [acetic acid-methanol (1:3; v/v)]. The fixation step was repeated twice before the cells were dropped on ethanol-cleaned microscope slides. Slides were aged for a minimum of 1 week in ambient air at 20C, then sealed in plastic bags and stored at −20C until used.
For breakpoint delineation in cancer cells, we used the childhood PTC cell line S48TK6 (Zitzelsberger et al. 1999; Weier et al. 2006). This tumorigenic cell line is a subclone of a primary culture of cancer cells (S48TK) prepared from a thyroid cancer that arose following the 1986 nuclear accident in Chernobyl, Ukraine. Because the line S48TK6 continued to undergo karyotype changes, the cells were once more cloned using limiting dilution. This gave rise to the tumorigenic cell line S48TK6A4. Colcemid-arrested tumor cell metaphase spreads were prepared from cell lines S48TK6 and its derivative, S48TK6A4, as described (Zitzelsberger et al. 1999). Gross chromosomal changes in the S48TK6 metaphases were characterized by means of G banding, comparative genomic hybridization (CGH), and spectral karyotyping (SKY) (Zitzelsberger et al. 1999; Weier et al. 2006).
Cytogenetic Analysis of PGD Patient Metaphase Cells
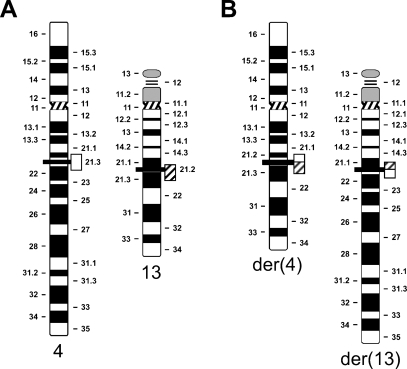
Prior to our study, cells from the 31-year-old female IVF patient T-0512, who was suspected to carry a translocation, were analyzed by G banding to define the chromosomes involved in the translocation and the approximate location of the breakpoints. The karyotype information 46,XX, t(4;13)(q21.3;q21.2), suggesting a balanced, reciprocal t(4;13)(q21.3;q21.2), was available before probe preparation commenced.
Preparation of DNA Probes and DNA Labeling
The general scheme for the selection and optimization of breakpoint-specific probes for PGD has been described previously (Cassel et al. 1997). Using information in publicly available databases (http://genome.ucsc.edu/ and http://www.ncbi.nlm.nih.gov/gquery/gquery.fcgi), we selected BAC clones that map to the estimated breakpoint interval as well as to adjacent chromosome bands. The BACs were provided by the Human Genome Center, California Institute of Technology, Pasadena, CA. For initial mapping of clones, BAC DNA was isolated using an alkaline lysis DNA extraction protocol (Birnboim and Doly 1979; Weier et al. 1995). The isolation of DNA from individual clones was done from 10-ml bacterial cultures grown overnight in Luria-Bertani (LB) medium (Sambrook et al. 1989) containing 12.5 μg/ml chloramphenicol (Sigma; St. Louis, MO) (Lu et al. 2008). Briefly, cell pellets resuspended in 10 ml PBS were treated with 50 μg/ml lysozyme (Sigma; stock is 50 mg/ml in 10 mM Tris, pH 7.5) and lyzed in sodium hydroxide (0.2 N NaOH, 1% SDS). After neutralization by addition of 3 M NaOAc and pelleting of bacterial DNA, the BAC DNA was precipitated in isopropanol, washed once in 70% cold ethanol, and resuspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). Finally, the DNA was extracted once with phenol-chloroform, precipitated with isopropanol, and resuspended in 20–40 μl sterile water. The DNA concentrations were determined by staining with Hoechst 33342 and fluorometry using a TKO100 fluorometer (Hoefer; San Francisco, CA) (Weier et al. 1995).
The BAC-derived probe DNA (typically 1–2 μl of DNA in a 10-μl reaction) was labeled via random priming following the instructions of the kit manufacturer (BioPrime Kit; Invitrogen, San Diego, CA) (Jossart et al. 1996; Fung et al. 2000). For non-isotopic, indirect labeling, biotin-14-dCTP or digoxigenin-11-2′-deoxyuridine, 5′-triphosphate was incorporated into the DNA.
The preparation of DNA probes for BAC pools was performed in essentially the same way, with the following modification: individual BACs were grown overnight in 10 ml of LB broth containing 20 μg/ml chloramphenicol. Then 5 ml of each culture was combined in the desired pool, the cells were spun down and resuspended in 10 ml PBS containing 50 μg/ml lysozyme, and DNA was isolated and labeled as described above.
Probes for SKY analyses were purchased from Applied Spectral Imaging (Carlsbad, CA) and used according to the manufacturer's instructions (Zitzelsberger et al. 1999,2002).
In Situ Hybridization
For FISH, 1 μl of each probe, 1 μl of human COT-1 DNA (1 mg/ml; Invitrogen), 1 μl of salmon sperm DNA (10 mg/ml; Invitrogen), and 7 μl of the hybridization master mix [78.6% formamide (Invitrogen), 14.3% dextran sulfate in 1.43× SSC, pH 7.0 (20× SSC is 3 M sodium chloride, 300 mM trisodium citrate)] (Lu et al. 2008) were thoroughly mixed and denatured at 76C for 10 min. Next, the hybridization mix was incubated at 37C for 30 min, allowing the COT-1 DNA to preanneal with the probes. In parallel, the slides were denatured for 4 min at 76C in 70% formamide/2× SSC, pH 7.0, dehydrated in 70%, 85%, and 100% ethanol for 2 min each step, and allowed to air dry. The hybridization mix was then pipetted onto the slides, covered with a 22 × 22-mm2 coverslip, and sealed with rubber cement. The slides were incubated overnight in a moisture chamber at 37C. After the rubber cement was removed, the slides were immersed in 2× SSC at 21C until the coverslips slid off. Subsequently, the slides were washed twice in 50% formamide/2× SSC at 45C for 10 min each, followed by two washes in 2× SSC at 21C. Slides were then incubated in PNM {5% non-fat dry milk (Carnation), 1% sodium azide in PN buffer [0.1 M sodium phosphate buffer, pH 8.0, 1% Nonidet-P40 (Sigma)]} for 10 min at 21C. Bound probes were detected with either fluorescein-conjugated avidin (cat #A-2011; Vector, Burlingame, CA) or anti–digoxigenin-rhodamine (Roche Molecular Systems; Indianapolis, IN) as described (Weier et al. 1995). Finally, the slides were mounted in 4′,6-diamidino-2-phenylindole (DAPI) (0.5 μg/ml; Calbiochem, La Jolla, CA) in antifade solution (Weier et al. 1995).
Image Acquisition and Analysis
Fluorescence microscopy was performed on a Zeiss Axioskop microscope equipped with a filter set for simultaneous observation of Texas Red-rhodamine and FITC, and a separate filter for DAPI detection (ChromaTechnology; Brattleboro, VT). Images were collected using a cooled CCD camera (VHS Vosskuehler; Osnabruck, Germany). Further processing of the images was done using Adobe Photoshop software (Adobe, Inc.; Mountain View, CA).
This article was prepared as an account of work sponsored by the United States government. Although this article is believed to contain correct information, neither the United States government nor any agency thereof, nor the regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe on privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof, or the regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof, or the regents of the University of California.
Results
Probe Mapping
Prior to the hybridization of DNA probes to patient samples or tumor cells, all probes were tested on normal male metaphase spreads to ensure sufficient signal strength, correct cytogenetic map positions, and absence of chimerism (Selleri et al. 1992).
BAC Contigs for PGD
Cells from an anonymized PGD case (T-0512) reported to carry a reciprocal t(4;13)(q21.3;q21.2) (Figure 1) were provided to us by the referring clinicians after standard G-banding karyotype analysis. Initially, we selected 60 BAC clones from the Roswell Park Cancer Institute (RPCI) RP11 library (Osoegawa et al. 2001) spread out over the following intervals: chromosome 4: 79.7 Mbp–91.3 Mbp (from clone RP11-57L13 at 4q21.2 to clone RP11-350B19 at 4q23) and chromosome 13: 57.4 Mbp–66.8 Mbp (from clone RP11-16M6 at 13q21 to clone RP11-21B13 at 13q23) (data not shown). Hybridization results to normal metaphase spreads or to patient metaphase cells were unusually poor: 20 of 60 clones failed to produce informative hybridization signals. Clones that gave analyzable signals allowed us to narrow the breakpoint region on 4q22.1 to an interval distal to clone RP11-2I7 at 89.6 Mbp–89.8 Mbp, but proximal to the map position of BAC clone RP11-115D19 at 90.7 Mbp–90.9 Mbp (data not shown). The FISH mapping experiments in individual BAC clones for chromosome 13 were plagued by hybridization failures, but allowed us to narrow the breakpoint region to between the proximal clone RP11-16M6 (at ∼57.4 Mbp) (data not shown) and the three BAC clones RP11-10M21, RP11-138D23, and RP11-346A3, which map into the interval 66.165 Mbp–66.753 Mbp (Table 1).
Figure 1.
Schematic diagram of the karyotypic abnormalities in a case reported as t(4;13)(q22.1;q21.3). The normal homologs of chromosomes 4 and 13 are shown in A. Breakpoints are indicated by thick horizontal lines at the approximate breakpoint position. The two derivative chromosomes der(4) and der(13) are shown in B. The open and hatched boxes represent the breakpoint-spanning probe contigs for chromosomes 4 and 13, respectively. In our experiments, the chromosome 4– and 13–specific probes were detected in red and green, respectively. Please note that the translocation separates proximal and distal parts of the chromosome 13–specific DNA probe contig of different sizes.
Table 1.
BAC pools for breakpoint delineation on chromosome 13q
| Clone | Band | Pool | Position (Mbp)a | BAC size (bp) |
|---|---|---|---|---|
| RP11-524F1 | 13q21.2 | 13-1 | 58.618–58.785 | 166,436 |
| RP11-26P21 | 13q21.2 | 13-1 | 59.004–59.210 | 206,407 |
| RP11-218B22 | 13q21.2 | 13-1 | 59.241–59.395 | 154,212 |
| RP11-442F12 | 13q21.2 | 13-1 | 59.389–59.599 | 209,794 |
| RP11-430I3 | 13q21.2 | 13-1 | 59.599–59.662 | 63,441 |
| RP11-350G11 | 13q21.31 | 13-2 | 60.619–60.721 | 102,564 |
| RP11-310K10 | 13q21.31 | 13-2 | 60.719–60.882 | 163,231 |
| RP11-432J3 | 13q21.31 | 13-2 | 60.881–60.940 | 59,272 |
| RP11-210L5 | 13q21.31 | 13-2 | 60.938–61.113 | 176,085 |
| RP11-543A19 | 13q21.31 | 13-2 | 61.112–61.178 | 66,386 |
| RP11-179D6 | 13q2131 | 13-2 | 61.179–61.264 | 85,563 |
| RP11-429G17 | 13q21.31 | 13-2 | 61.262–61.426 | 164,831 |
| RP11-71L7 | 13q21.31 | 13-2 | 61.426–61.530 | 103,728 |
| RP11-527N12 | 13q21.31 | 13-3 | 62.520–62.700 | 178,323 |
| RP11-282D7 | 13q21.31 | 13-3 | 62.699–62.806 | 106,534 |
| RP11-320N6 | 13q21.31 | 13-3 | 62.806–62.945 | 139,649 |
| RP11-67L17 | 13q21.31 | 13-3 | 62.945–63.070 | 125,693 |
| RP11-473M10 | 13q21.31 | 13-3 | 63.070–63.233 | 163,218 |
| RP11-394A14 | 13q2131 | 13-3 | 63.235–63.409 | 174,801 |
| RP11-520F9 | 13q21.31 | 13-3 | 63.408–63.481 | 73,811 |
| RP11-205B18 | 13q21.31 | 13-3 | 63.480–63.638 | 158,460 |
| RP11-261A1 | 13q21.31 | 13-4 | 64.362–64.528 | 166,490 |
| RP11-211D10 | 13q21.31-q21.32 | 13-4 | 64.560–64.742 | 182,321 |
| RP11-379K8 | 13q21.32 | 13-4 | 64.787–64.966 | 179,790 |
| RP11-229I7 | 13q21.32 | 13-4 | 64.967–65.062 | 96,045 |
| RP11-326D19 | 13q21.32 | 13-4 | 65.063–65.222 | 159,919 |
| RP11-223F20 | 13q21.32 | 13-4 | 65.232–65.400 | 168,897 |
| RP11-298H15 | 13q21.32 | 13-4 | 65.416–65.575 | 160,015 |
| RP11-10M21 | 13q21.32 | 13-5 | 66.165–66.378 | 114,290 |
| RP11-138D23 | 13q21.32 | 13-5 | 66.378–66.543 | 164,604 |
| RP11-576O3 | 13q21.32 | 13-5 | 66.544–66.683 | 139,881 |
| RP11-346A3 | 13q21.32 | 13-5 | 66.683–66.753 | 71,774 |
| RP11-531B22 | 13q21.32 | 13-5 | 66.751–66.834 | 82,944 |
| RP11-164E20 | 13q21.32 | 13-5.5 | 66.883–67.067 | 184,374 |
| RP11-520F22 | 13q21.32 | 13-5.5 | 67.006–67.147 | 141,913 |
| RP11-51P14 | 13q21.32 | 13-5.5 | 67.144–67.174 | 28,761 |
| RP11-562L19 | 13q21.32 | 13-5.5 | 67.174–67.254 | 81,586 |
| RP11-248N6 | 13q21.32 | 13-5.5 | 67.255–67.416 | 162,695 |
| RP11-338L17 | 13q21.33 | 13-6 | 67.492–67.560 | 68,556 |
| RP11-157F14 | 13q21.33 | 13-6 | 67.558–67.682 | 123,887 |
| RP11-520F24 | 13q21.33 | 13-6 | 67.682–67.841 | 159,235 |
| RP11-318G21 | 13q22.3 | 13-7 | 77.297–77.480 | 183,337 |
| RP11-122N18 | 13q22.3 | 13-7 | 77.314–77.489 | 175282 |
The Human Genome Reference DNA Sequence was completed in April 2003. Unique position information is estimated from Mapviewer build 35.1 at http://www.ncbi.nlm.nih.gov/mapview/.
BAC, bacterial artificial chromosome; Mbp, megabasepair.
These results prompted us to change our mapping strategy, replace individual clones with selected contiguous sets of BACs, and prepare pools of labeled DNA probes. For the long arm of chromosome 13, we prepared eight BAC pools termed pool 13-1 to pool 13-7, composed entirely of clones from the RPCI RP11 library (Table 1). The clone pools 13-1 to 13-6 cover part of the long arm of chromosome 13 more or less evenly, from band q21.2 to band q21.33, whereas pool 13-7 is a distal reference probe comprising two clones that map in band 13q22.3 between 77.3 Mbp and 77.5 Mbp (Table 1).
In our first BAC pool FISH experiment, we combined biotin-labeled DNA from pools 13-1, 13-3, 13-5, and 13-7 with digoxigenin-labeled probe made from pools 13-2, 13-4, and 13-6. Hybridization of these “superpool” DNA probes to normal male metaphase spreads and detection with avidin-FITC and rhodamine-conjugated anti-digoxigenin antibodies showed strong, specific signals on both homologs of chromosome 13 without noticeable cross-hybridization to other chromosomes (Figure 2A). Owing to the close proximity of biotin- and digoxigenin-labeled probes, which were detected with green- and red-fluorescent reagents, respectively, the superimposed FISH signals appear yellow in the pseudo-RGB images in Figure 2.
Figure 2.
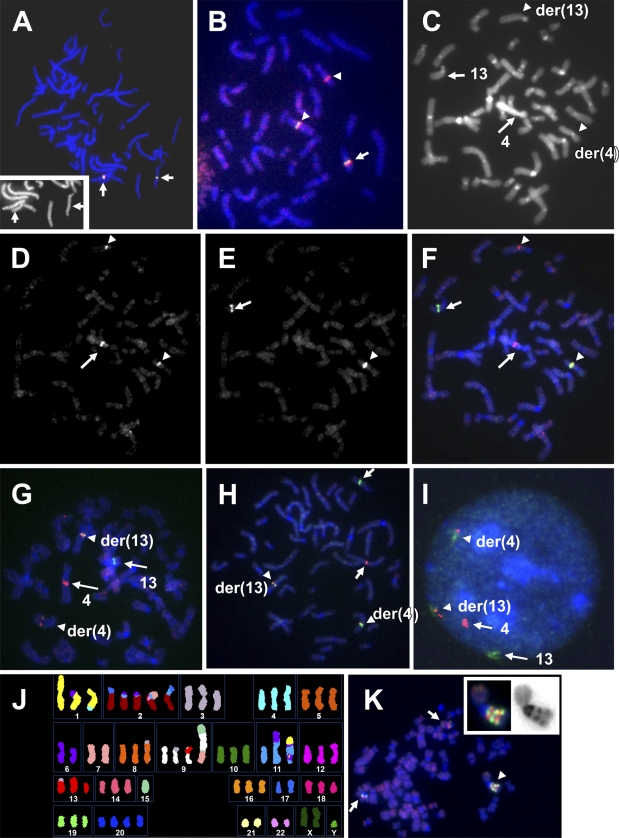
Hybridization of bacterial artificial chromosome (BAC) pools for delineation of chromosome breakpoints in human cells. (A) Hybridization of chromosome 13–specific BAC pools to metaphase cells from a normal male donor demonstrates exclusive binding to the target region on the long arm of chromosome 13. The image shows a pseudo-RGB picture of bound probes on 4′,6-diamidino-2-phenylindole (DAPI)–counterstained metaphase chromosomes. The insert shows the DAPI channel (blue fluorescence with arrows indicating the two homologs of chromosome 13). (B–I) Hybridization of BAC pool–derived DNA probes to patient T-0512 cells carrying a t(4;13)(q22.1;q21.3). Arrows in these panels indicate normal chromosomes, whereas arrowheads indicate derivative chromosomes. (B) Hybridization of seven pools for chromosome 13 generates signals on the normal homolog and the der(13) (center) as well as on the der(4) (in the upper right), indicating that probes bind proximal and distal to the breakpoint on chromosome 13. (C–F) Combined hybridization of a chromosome 4–specific BAC pool (red) and pools 13-4 to 13-6 (green) shows the hybridization pattern expected for a red probe pool spanning the chromosome 4 breakpoint, but the green probe pool binds distal to the breakpoint on chromosome 13. DAPI, red, and green fluorescence images are shown in C, D, and E, respectively. Panel F shows the pseudo-RGB picture. (G) Fluorescence in situ hybridization (FISH) results showing the pattern generated by hybridization of the chromosome 4–specific probe pool (red) in combination with the breakpoint-spanning pool 13-3 (green). (H,I) Hybridization of the extended probe set for chromosome 13 (green) and chromosome 4 (red) to metaphase and interphase cells. Arrows in H indicate normal chromosomes, and arrowheads indicate the red+green–labeled derivative chromosomes. (J) Spectral karyotyping analysis of metaphase chromosomes from the post-Chernobyl childhood thyroid cancer cell line S48TK indicates several abnormal chromosomes carrying genetic material derived from chromosome 1 (yellow). (K) Hybridization of chromosome 1q–specific BAC probe pools indicates a marker chromosome (arrowhead) with complex rearrangements including amplification of the proximal part of the long arm of chromosome 1. Arrows indicate metaphase chromosomes that show the normal order of signals, i.e., pool P1-1 (green) bind proximal to pool P1-2 (red). The insert shows an enlarged image of the abnormal chromosome (left: FISH results; right: inverted DAPI image).
Hybridization of the same combination of chromosome 13–specific probe pools to metaphase cells from T-0512 showed strong hybridization signals on the normal chromosome 13 and the der(13) as well as on the der(4) (Figure 2B). All three hybridization domains showed green as well as red fluorescence. Thus, the first BAC pool hybridization confirmed the results obtained with individual clones, i.e., the interval covered by pools 13-1 to 13-6 extends onto both sides of the breakpoint region. Because both derivative chromosomes in Figure 2B showed red and green signals, the breakpoint must lie between pools 13-2 and 13-6, i.e., between 60.6 Mbp and 67.8 Mbp.
For delineation of the breakpoint on the long arm of chromosome 4 in patient T-0512, we chose nine BAC clones that cover the region between 89.5 Mbp and 90.7 Mbp (Table 2). These nine BAC probes were combined in two pools as shown in Table 2: Pool 4-1 is a five-BAC contig centered around the clone RP11-2I7, which was known to be proximal to the chromosome 4–specific breakpoint. Pool 4-2 binds distal to pool 4-1 and covers the interval from 90.3 Mbp to 90.7 Mbp on the long arm of chromosome 4, i.e., slightly proximal to the above-mentioned clone RP11-115D19 that was mapped distal to the breakpoint region. Pools 4-1 and 4-2 cover unique, non-overlapping chromosome regions of ∼796 kb and 399 kb, respectively. The chromosome 4–specific probe pools were labeled with digoxigenin.
Table 2.
BAC pools for breakpoint delineation on chromosome 4q22.1 clone
| Clone | Pool | Position (Mbp)a | BAC size (bp) |
|---|---|---|---|
| RP11-10L7 | 4-1 | 89.542–89.653 | 112,291 |
| RP11-466G12 | 4-1 | 89.652–89.841 | 189,402 |
| RP11-2I7 | 4-1 | 89.840–89.942 | 163,551 |
| RP11-496N17 | 4-1 | 89.941–90.052 | 111,908 |
| RP11-502A23 | 4-1 | 90.168–90.338 | 171,273 |
| RP11-84C13 | 4-2 | 90.337–90.448 | 112,003 |
| RP11-173C9 | 4-2 | 90.447–90.567 | 120,662 |
| RP11-549C16 | 4-2 | 90.566–90.737 | 172,195 |
| RP11-79M20 | 4-2 | 90.572–90.736 | 165,894b |
Unique positions were estimated from Mapviewer build 35.1 at http://www.ncbi.nlm.nih.gov/mapview/.
BAC size was determined via BLAST search at http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD = Web and PAGE_TYPE = BlastHome using the BAC end sequences.
The following experiments were designed to determine the breakpoint locations relative to the BAC pools, and to optimize probes. In a second hybridization of BAC pools to patient metaphase spreads, we combined the two digoxigenin-labeled pools for chromosome 4 (4-1 and 4-2) with a combination of four biotinylated DNA probes prepared from pools 13-4, 13-5, 13-5.5, and 13-6 (Table 1). The chromosome 4– and 13–specific probes were detected in red and green, respectively. The images in Figures 2C–2F summarize the results of this hybridization. All four chromosomes of interest [4, 13, der(4), and der(13)] can be identified by their DAPI banding pattern (Figure 2C) and the red, green, or yellow hybridization signal (Figures 2D–2F).
As a rule of thumb, in this hybridization scheme, the normal homologues show hybridization domains in a single color [either red (chromosome 4) or green (chromosome13)] (Munné et al. 1998; Weier et al. 1999). The color of signal domains on the derivative chromosomes depends on whether a probe binds proximal to the breakpoint (i.e., no translocation of probe target and signals are found on the normal chromosome and its derivative) or distal (probe target being translocated). If probe binding extends significantly on both sides of the breakpoint (i.e., it spans the breakpoint region), probe signals will be found on both derivative chromosomes. The image in Figure 2D shows three red signals: one on the normal chromosome 4 and two on derivative chromosomes der(4) and der(13), as expected for a probe pool that spans the breakpoint on chromosome 4. We also noted that the signal on the normal copy of chromosome 4 was very strong, and signals on the derivative chromosomes were approximately of equal strength (Figure 2D). The green fluorescent signals were found exclusively on the normal copy of chromosome 13 and der(4) (Figure 2E). Thus, all biotinylated probes from pool 13-4 to pool 13-6 bound distal to the breakpoint on chromosome 13.
Having learned that the breakpoint on chromosome 13 lies proximal to pool 13-4 (Figure 2E), but within or distal to pool 13-2 (Figure 2B), we decided to map pool 13-3. Dual-color FISH using a combination of biotinylated pool 13-3 DNA and the two digoxigenin-labeled pools for chromosome 4 showed the expected signals on the normal non-rearranged copies of chromosomes 4 and 13 (Figure 2G). Red and green signals found on both derivative chromosomes clearly indicated that pool 13-3 spans the breakpoint on chromosome 13 in T-0512. However, we noted that the green signal on the der(4) chromosome was very faint, whereas the green signal on the der(13) was strong. Thus, only a small fraction of probe contained in pool 13-3 bound distal to the breakpoint, and most of this pool bound proximally.
In summary, only three overnight FISH experiments with BAC pools and patient metaphase spreads allowed us to narrow the breakpoint position to a 1.1 Mbp interval between 62.5 Mbp and 63.6 Mbp on chromosome 13. The next step in the PGD probe preparation process was probe optimization: because the chromosome 4–specific DNA probe contig was split more or less evenly (Figure 2D), we decided to design a chromosome 13–specific BAC pool probe that would be split asymmetrically by the translocation, thus allowing unambiguous identification of derivative chromosomes in interphase cell nuclei. This was achieved easily by combining the previously prepared biotinylated probe from pool 13-3 with DNA probes prepared from pools 13-4, 13-5, 13-5.5, and 13-6. This set of probes covers an interval from 62.5 Mbp to 67.8 Mbp. The FISH result showed that signals from biotinylated chromosome 13 probes were split into two unequal parts: the signals derived from pool 13-3 BAC binding to the proximal long arm of chromosome 13 were weaker than those of probes that covered the distal part (Figure 2H). Thus, the der(4) chromosome showed stronger green signals than did the der(13) (Figure 2H).
This set of hybridization probes that extend unequally on the proximal and distal sides of the chromosome 13–specific breakpoint and a simple dual-color probe detection scheme allow classification of all chromosomes involved in this translocation in interphase cell nuclei (Figure 2I).
BAC Contigs for Characterization of Chromosome Rearrangements in the PTC Cell Line S48TK
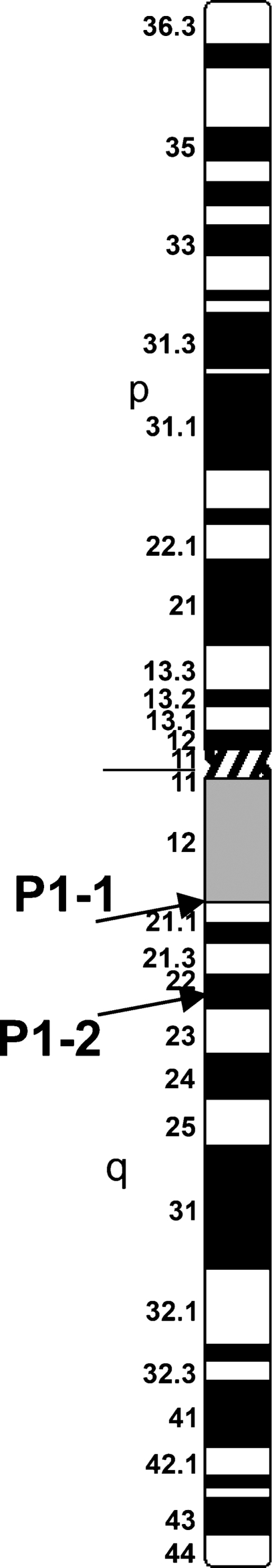
Conventional karyotyping using G banding and SKY analysis of cell line S48TK had indicated several derivative chromosomes carrying chromosome 1–derived material (Zitzelsberger et al. 1999) (Figure 2J). CGH indicated extra copies of the proximal long arm of chromosome 1 (Weier et al. 2006). To initiate the characterization of rearrangements involving the long arm of chromosome 1, we prepared BAC pools P1-1 and P1-2 (Table 3). Each of the two BAC pools is composed of three minimally overlapping BAC clones, which, according the NBCI Map Viewer (http://www.ncbi.nlm.nih.gov/projects/mapview/), bind to DNA sequences at the border between bands 1q12 and 1q21 or bands 1q12 and 1q22, respectively (Figure 3), i.e., proximal to the neurotrophic tyrosine kinase receptor type 1 gene (NTRK1) or TRK-A (Weier et al. 1995). According to the information provided by the Human Genome Reference DNA Sequence, Mapviewer build 35.1, pools P1-1 and P1-2 cover ∼516 kb and 354 kb, respectively, of unique sequence.
Table 3.
BAC pools for breakpoint delineation on chromosome 1
| Clone | Band | Pool | Position (Mbp)a | BAC size (bp)b |
|---|---|---|---|---|
| RP11-37N10 | 1q12-q21 | P1-1 | 142.777–142.944 | 166,807 |
| RP11-315I20 | 1q12-q21 | P1-1 | 142.928–143.134 | 206,466 |
| RP11-71P2 | 1q12-q21 | P1-1 | 143.125–143.293 | 167,389 |
| RP11-262A11 | 1q22 | P1-2 | 152.356–152.532 | 176,285 |
| RP11-243J18 | 1q22 | P1-2 | 152.441–152.599 | 158,208 |
| RP11-299D6 | 1q22 | P1-2 | 152.522–152.710 | 188,752 |
Unique positions were estimated from Mapviewer build 35.1.
BAC sizes are from the clone database at http://genome.ucsc.edu/.
Figure 3.
Ideogram of human chromosome 1. Arrows indicate the map position of the two BAC pools for tumor cell analysis (P1-1, P1-2).
Hybridization of a biotinylated probe for pool P1-1, in combination with a digoxigenin-labeled probe for pool P1-2, onto normal chromosome 1 showed the green and red signals in the expected positions (not shown). Hybridization onto metaphase chromosomes prepared from line S48TK revealed a marker chromosome carrying approximately two to three copies of the target segment 1q21-1q22 (Figure 2K).
Discussion
Preimplantation genetic analysis is a laboratory procedure to identify chromosomally abnormal embryos among morphologically normal embryos, and thus increase the chances of nidation and successful pregnancy (Munné et al. 1994; Braude et al. 2002; Munné 2002; Verlinsky et al. 2004). Typically, no more than one or two blastomeres are removed for PGD from embryos on day 3 or 4 after insemination (Munné 2002; Sampson et al. 2004). Interphase cell analysis is an important component of PGD, because following biopsy, blastomeres can be found in any stage of the mitotic cell cycle. Chromosome-specific DNA repeat probes, which are commercially available for all human chromosomes, are suitable for detecting numerical chromosome aberrations in individual or single interphase nuclei (D'Alton et al. 1997; Weier et al. 2005; Stumm et al. 2006). The majority of these commercial DNA probes are alpha satellite DNA repeats, which bind at or near the chromosomal centromeres (Waye et al. 1987; Baumgartner et al. 2006). In the absence of structural chromosome aberrations, the DNA repeat probes are the first choice of probes for chromosome enumeration because of their ease of use, short hybridization times, and typically bright signals.
For reciprocal translocation carriers, however, the prevalence of unbalanced gametes carrying a partial aneusomy is estimated to range from 50% to 70% (Scriven et al. 1998; Braude et al. 2002; Sampson et al. 2004). Centromeric probes are likely to miss most partial aneusomies. A number of FISH approaches for the analysis of blastomeres have been proposed, such as translocation probe sets binding distal to the translocation breakpoints and allowing scoring of chromosome arms (Pehlivan et al. 2003; Sampson et al. 2004) or the multicolor banding of chromosomes in interphase nuclei using probes prepared by chromosome microdissection (Iourov et al. 2007).
Several years ago, we proposed to prepare DNA probes or probe contigs composed of YACs that span individual translocation breakpoints (Cassel et al. 1997). This was a rather time-consuming process, going through repeated cycles of clone selection and mapping. High-quality non-chimeric probes had to be selected, and every cycle took at least 7–10 days (Fung et al. 1998). With time constraints in IVF programs, often little time was left for probe optimization once a breakpoint had been mapped (Fung et al. 1999).
The aim of the present study was to expedite the process of mapping translocation breakpoints by using BACs and DNA probe pooling strategies. Developed initially as sequencing templates for the International Human Genome Project (Osoegawa et al. 2001), several BAC libraries are now available for the human genome, and these allow the rapid preparation of probes for virtually any region of the human genome. Thus, BACs are being used more and more frequently for preparation of DNA microarrays (Fiegler et al. 2003) or cytogenetics analyses (Liehr et al. 2002; Tönnies et al. 2007; Baldwin et al. 2008).
The probe preparation process for the t(4;13) case presented in this report used contigs or pools of BAC clones to minimize, if not eliminate, hybridization failures or so-called uninformative experiments (Pehlivan et al. 2003; Sampson et al. 2004). With translocation breakpoints roughly determined by G banding, large numbers of BACs can be selected from in-house libraries and assembled in probe pools even before initiation of the IVF cycle. As the t(4;13) example shows, only a few overnight hybridizations will be required to localize the breakpoint to a single pool and optimize the probe for single interphase cell analysis. The small number of cells and the brief time frame (i.e., hours) available for PGD requires probes that perform in FISH experiments with virtually 100% efficiency. Spanning probes can be designed to reach this benchmark, and they have the great advantage of being able to detect accurately all possible chromosome segregations as well as being able to distinguish normal and balanced constitutions. This is expected to lead to more reliable PDG procedures, reducing the number of failed embryo transfers and making interphase PGD more affordable for infertile couples.
A second objective of the present study was to evaluate the use of BAC pools in the cytogenetic analysis of tumor cells. We chose cell line S48TK, a cell line established from a papillary thyroid tumor that arose in a child following the 1986 nuclear accident in Chernobyl, USSR, because these cells carry a large number of unbalanced translocations, some of which involve chromosome 1 (Lehmann et al. 1996; Zitzelsberger et al. 1999). The long arm of chromosome 1 is of particular interest because it harbors the NTRK1 proto-oncogene, which is activated through recombination in a number of human solid tumor types, among them childhood PTC (Kozma et al. 1988; Beimfohr et al. 1999). For the example presented in Figure 2K, we chose two BAC contigs that bind proximal to NTRK1, because our CGH studies suggested extra copies of the proximal long arm of chromosome 1 in S48TK (Weier et al. 2006). Interestingly, recurrent amplifications of the region 1q21-1q22 have also been reported in panels of sarcomas and bladder and breast cancers, as well as hepatocellular carcinomas (Forus et al. 1998; Meza-Zepeda et al. 2002). The hybridization results (Figure 2K) confirm the complex nature of the chromosome 1q21-q22 amplicon in S48TK, which seems to comprise several potentially incomplete copies of the region flanked by our probe contigs, P1-1 and P1-2. Two other chromosomes in the metaphase spread shown in Figure 2K showed hybridization signals as expected, i.e., P1-1 binds proximal to P1-2. Given the different sizes of hybridization signal domains shown in Figure 2K, it is reasonable to speculate that hybridization of single BAC clones or even smaller probes such as cosmids might have missed a number of these copies.
In summary, the preparation and hybridization of pools of BAC clones requires slightly more up-front effort, but compared with FISH using single clones, it greatly increases hybridization efficiencies, minimizes hybridization failures, expedites breakpoint mapping without sacrificing resolution, and increases the sensitivity to detect small rearrangements.
Acknowledgments
This work was supported in part by National Institutes of Health Grants CA-80792, CA-88258, CA-123370, and HD-44313, and a grant from the Director, Office of Energy Research, Office of Health and Environmental Research, U.S. Department of Energy, under contract DE-AC02-05CH11231. J.F.W. was supported in part by Grant HD-45736 from the National Institutes of Health and a grant from the University of California Discovery Program, which also supported A.B.
We acknowledge editorial help provided by E. Lowe, and support from staff at Reprogenetics, who provided metaphase spreads and initial mapping data. Ideograms were kindly provided by D. Adler, PhD, Department of Pathology, University of Washington. We would like to express our thanks to the scientists at the Human Genome Center, California Institute of Technology, Pasadena, whose generosity has made these studies possible.
Presented in part at HCS2005, Nordwijkerhout, The Netherlands, April 27–30, 2005.
References
- Baldwin EL, May LF, Justice AN, Martin CL, Ledbetter DH (2008) Mechanisms and consequences of small supernumerary marker chromosomes: from Barbara McClintock to modern genetic-counseling issues. Am J Hum Genet 82:398–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner A, Weier JF, Weier H-UG (2006) Chromosome-specific DNA repeat probes. J Histochem Cytochem 54:1363–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayani J, Squire JA (2004) Preparation of cytogenetic specimens from tissue samples. In Bonifacino JS, ed. Current Protocols in Cell Biology, Suppl 23. New York, John Wiley & Sons, Inc., 22.2.1–22.2.15 [DOI] [PubMed]
- Beimfohr C, Klugbauer S, Demidchik EP, Lengfelder E, Rabes HM (1999) NTRK1 re-arrangement in papillary thyroid carcinomas of children after the Chernobyl reactor accident. Int J Cancer 80:842–847 [DOI] [PubMed] [Google Scholar]
- Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude P, Pickering S, Flinter F, Ogilvie CM (2002) Preimplantation genetic diagnosis. Nat Rev Genet 3:941–953 [DOI] [PubMed] [Google Scholar]
- Carreira IM, Mascarenhas A, Matoso E, Couceiro AB, Ramos L, Dufke A, Mazauric M, et al. (2007) Three unusual but cytogenetically similar cases with up to five different cell lines involving structural and numerical abnormalities of chromosome 18. J Histochem Cytochem 55:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel MJ, Munné S, Fung J, Weier H-UG (1997) Carrier-specific breakpoint-spanning DNA probes for pre-implantation genetic diagnosis [PGD] in interphase cells. Hum Reprod 12:101–109 [DOI] [PubMed] [Google Scholar]
- D'Alton ME, Malone FD, Chelmow D, Ward BE, Bianchi DW (1997) Defining the role of fluorescence in situ hybridization on uncultured amniocytes for prenatal diagnosis of aneuploidies. Am J Obstet Gynecol 176:769–776 [DOI] [PubMed] [Google Scholar]
- Fiegler H, Carr P, Douglas EJ, Burford DC, Hunt S, Scott CE, Smith J, et al. (2003) DNA microarrays for comparative genomic hybridization based on DOP-PCR amplification of BAC and PAC clones. Genes Chromosomes Cancer 36:361–374 [DOI] [PubMed] [Google Scholar]
- Forus A, Berner JM, Meza-Zepeda LA, Saeter G, Mischke D, Fodstad O, Myklebost O (1998) Molecular characterization of a novel amplicon at 1q21-q22 frequently observed in human sarcomas. Br J Cancer 78:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J, Munné S, Duell T, Weier H-UG (1998) Rapid cloning of translocation breakpoints: from blood to YAC in 50 days. J Biochem Mol Biol Biophys 1:181–192 [Google Scholar]
- Fung J, Munné S, Garcia J, Kim U-J, Weier H-UG (1999) Reciprocal translocations and infertility: molecular cloning of breakpoints in a case of constitutional translocation t(11;22)(q23;q11) and preparation of probes for preimplantation genetic diagnosis (PGD). Reprod Fertil Dev 11:17–23 [DOI] [PubMed] [Google Scholar]
- Fung J, Munné S, Weier H-UG (2001) Detection of chromosome translocation products in single interphase cell nuclei. In Darzynkiewicz Z, Chrissman HA, Robinson JP, eds. Methods in Cell Biology, vol. 64, part B, Cytometry. 3rd ed. San Diego, Academic Press, 98–117 [DOI] [PubMed]
- Fung J, Weier H-UG, Goldberg JD, Pedersen RA (2000) Multilocus genetic analysis of single interphase cells by spectral imaging. Hum Genet 107:615–622 [DOI] [PubMed] [Google Scholar]
- Fung J, Weier H-UG, Pedersen RA, Zitzelsberger HF (2002) Spectral imaging analysis of metaphase and interphase cells. In Rautenstrauss B, Liehr T, eds. FISH Technology. Heidelberg, Springer Verlag, 363–387
- Greco A, Roccato E, Pierotti MA (2004) TRK oncogenes in papillary thyroid carcinomas. In Farid NR, ed. Molecular Basis of Thyroid Cancer, Cancer Treatment and Research. Boston, Kluwer Academic Publishers, 207–219 [DOI] [PubMed]
- Herrmann MA, Hay ID, Bartelt DH, Ritland SR, Dahl RJ, Grant CS, Jenkins RB (1991) Cytogenetic and molecular genetic studies of follicular and papillary thyroid cancers. J Clin Invest 88:1596–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iourov IY, Liehr T, Vorsanova SG, Yurov YB (2007) Interphase chromosome-specific multicolor banding (ICS-MCB): a new tool for analysis of interphase chromosomes in their integrity. Biomol Eng 24:415–417 [DOI] [PubMed] [Google Scholar]
- Jossart GH, Greulich KM, Siperstein AE, Duh Q, Clark OH, Weier H-UG (1995) Molecular and cytogenetic characterization of a t(1;10;21) translocation in the human papillary thyroid cancer cell line TPC-1 expressing the ret/H4 chimeric transcript. Surgery 118:1018–1023 [DOI] [PubMed] [Google Scholar]
- Jossart GH, O'Brien B, Cheng J-F, Tong Q, Jhiang SM, Duh Q, Clark OH, et al. (1996) A novel multicolor hybridization scheme applied to localization of a transcribed sequence (D10S170/H4) and deletion mapping in the thyroid cancer cell line TPC-1. Cytogenet Cell Genet 75:254–257 [DOI] [PubMed] [Google Scholar]
- Kalousek DK (2000) Pathogenesis of chromosomal mosaicism and its effect on early human development. Am J Med Genet 91:39–45 [DOI] [PubMed] [Google Scholar]
- Kim UJ, Shizuya H, Kang HL, Choi SS, Garrett CL, Smink LJ, Birren BW, et al. (1996) A bacterial artificial chromosome-based framework contig map of human chromosome 22q. Proc Natl Acad Sci USA 93:6297–6301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma SC, Redmond SMS, Fu XC, Saurer SM, Groner B, Hynes NE (1988) Activation of the receptor kinase domain of the trk oncogene by recombination with two different cellular sequences. EMBO J 7:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann L, Zitzelsberger H, Kellerer AM, Braselmann H, Kulka U, Georgiadou-Schumacher V, Negele T, et al. (1996) Chromosome translocations in thyroid tissues from Belarussian children exposed to radioiodine from the Chernobyl accident, measured by FISH-painting. Int J Radiat Biol 70:513–516 [DOI] [PubMed] [Google Scholar]
- Liehr T, Weise A, Heller A, Starke H, Mrasek K, Kuechler A, Weier H-UG, et al. (2002) Multicolor chromosome banding (MCB) with YAC/BAC-based probes and region-specific microdissection DNA libraries. Cytogenet Genome Res 97:43–50 [DOI] [PubMed] [Google Scholar]
- Lu CM, Wang M, Greulich-Bode K, Weier JF, Weier HUG (2008) Quantitative DNA fiber mapping. In Liehr T, ed. Springer FISH Lab Manual. Heidelberg, Springer Verlag, 269–291
- Meza-Zepeda LA, Forus A, Lygren B, Dahlberg AB, Godager LH, South AP, Marenholz I, et al. (2002) Positional cloning identifies a novel cyclophilin as a candidate amplified oncogene in 1q21. Oncogene 21:2261–2269 [DOI] [PubMed] [Google Scholar]
- Munné S (2002) Preimplantation genetic diagnosis of numerical and structural chromosome abnormalities. Reprod Biomed Online 4:183–196 [DOI] [PubMed] [Google Scholar]
- Munné S, Fung J, Cassel MJ, Márquez C, Weier H-UG (1998) Preimplantation genetic analysis of translocations: case-specific probes for interphase cell analysis. Hum Genet 102:663–674 [DOI] [PubMed] [Google Scholar]
- Munné S, Grifo J, Cohen J, Weier H-UG (1994) Mosaicism and aneuploidy in arrested human preimplantation embryos: a multiple probe fluorescence in situ hybridization (FISH) study. Am J Hum Genet 55:150–159 [PMC free article] [PubMed] [Google Scholar]
- Munné S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J (2000) Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril 73:1209–1218 [DOI] [PubMed] [Google Scholar]
- Osoegawa K, Mammoser AG, Wu C, Frengen E, Zeng C, Catanese JJ, de Jong PJ (2001) A bacterial artificial chromosome library for sequencing the complete human genome. Genome Res 11:483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehlivan T, Rubio C, Rodrigo L, Remohi J, Pellicer A, Simon C (2003) Preimplantation genetic diagnosis by fluorescence in situ hybridization: clinical possibilities and pitfalls. J Soc Gynecol Investig 10:315–322 [DOI] [PubMed] [Google Scholar]
- Peng HH, Chao AS, Wang TH, Chang YL, Chang SD (2006) Prenatally diagnosed balanced chromosome rearrangements: eight years' experience. J Reprod Med 51:699–703 [PubMed] [Google Scholar]
- Pierotti MA, Santoro M, Jenkins RB, Sozzi G, Bongarzone I, Grieco M, Monzini N, et al. (1992) Characterization of an inversion on the long arm of chromosome 10 juxtaposing D10S170 and RET and creating the oncogenic sequence RET/TPC. Proc Natl Acad Sci USA 89:1616–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastira K, Maher E, Fantes J, Ramsay J, Angelopoulou R (2006) Using BAC clones to characterize unbalanced chromosome abnormalities in interphase cells. Eur J Med Genet 49:235–246 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press
- Sampson JE, Ouhibi N, Lawce H, Patton PE, Battaglia DE, Burry KA, Olsen SB (2004) The role of preimplantation genetic diagnosis in balanced translocation carriers. Am J Obstet Gynecol 190:1707–1713 [DOI] [PubMed] [Google Scholar]
- Scriven PN, Handyside AH, Ogilvie CM (1998) Chromosome translocation: segregation modes and strategies for preimplantation genetic diagnosis. Prenat Diagn 18:1437–1449 [PubMed] [Google Scholar]
- Selleri L, Eubanks JH, Giovannini M, Hermanson GG, Romo A, Djabali M, Maurer S, et al. (1992) Detection and characterization of “chimeric” yeast artificial chromosome clones by fluorescent in situ suppression hybridization. Genomics 14:536–541 [DOI] [PubMed] [Google Scholar]
- Shizuya H, Birren B, Kim UJ, Mancino V, Slepak T, Tachiiri Y, Simon M (1992) Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc Natl Acad Sci USA 89:8794–8797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srb AM, Owen RD, Edgar RS (1965) General Genetics. 2nd ed. San Francisco, W.H. Freeman and Co
- Stern C, Pertile M, Norris H (1999) Chromosome translocations in couples with in vitro fertilisation implantation failure. Hum Reprod 14:2097–2101 [DOI] [PubMed] [Google Scholar]
- Stumm M, Wegner R-D, Bloechle M, Eckel H (2006) Interphase M-FISH applications using commercial probes in prenatal and PGD diagnostics. Cytogenet Genome Res 114:296–301 [DOI] [PubMed] [Google Scholar]
- Subrt I (1980) Reciprocal translocation with special reference to reproductive failure. Hum Genet 55:303–307 [DOI] [PubMed] [Google Scholar]
- Thorsen J, Zhu B, Frengen E, Osoegawa K, de Jong PJ, Koop BF, Davidson WS, et al. (2005) A highly redundant BAC library of Atlantic salmon (Salmo salar): an important tool for salmon projects. BMC Genomics 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönnies H, Pietrzak J, Bocian E, MacDermont K, Kuechler A, Belitz B, Trautmann U, et al. (2007) New immortalized cell lines of patients with small supernumerary marker chromosome: towards the etablishment of a cell bank. J Histochem Cytochem 55:651–660 [DOI] [PubMed] [Google Scholar]
- Verlinsky Y, Cohen J, Munné S, Gianaroli L, Simpson JL, Ferraretti AP, Kuliev A (2004) Over a decade of experience with preimplantation genetic diagnosis: a multicenter report. Fertil Steril 82:292–294 [DOI] [PubMed] [Google Scholar]
- Waye JS, Durfy SJ, Pinkel D, Kenwrick S, Patterson M, Davies KE, Willard HF (1987) Chromosome-specific alpha satellite DNA from human chromosome 1: hierarchical structure and genomic organization of a polymorphic domain spanning several hundred kilobase pairs of centromeric DNA. Genomics 1:43–51 [DOI] [PubMed] [Google Scholar]
- Weier HU, Rhein AP, Shadravan F, Collins C, Polikoff D (1995) Rapid physical mapping of the human trk protooncogene (NTRK1) to human chromosome 1q21-q22 by P1 clone selection, fluorescence in situ hybridization (FISH), and computer-assisted microscopy. Genomics 26:390–393 [DOI] [PubMed] [Google Scholar]
- Weier HUG, Munné S, Fung J (1999) Patient-specific probes for preimplantation genetic diagnosis (PGD) of structural and numerical aberrations in interphase cells. J Assist Reprod Genet 16:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier HUG, Tuton TB, Chu LW, Ito Y, Lu C-M, Baumgartner A, Zitzelsberger HF, et al. (2006) Molecular cytogenetic characterization of a human thyroid cancer cell line. Cytogenet Genome Res 114:284–291 [DOI] [PubMed] [Google Scholar]
- Weier JF, Weier H-UG, Jung CJ, Gormley M, Yan Zhou Y, Chu LW, Genbacev O, et al. (2005) Human cytotrophoblasts acquire aneuploidies as they differentiate to an invasive phenotype. Dev Biol 279:420–432 [DOI] [PubMed] [Google Scholar]
- Zitzelsberger H, Lehmann L, Hieber L, Weier H-UG, Janish C, Fung J, Negele T, et al. (1999) Cytogenetic changes in radiation-induced tumors of the thyroid. Cancer Res 59:135–140 [PubMed] [Google Scholar]
- Zitzelsberger HF, O'Brien B, Weier H-UG (2002) Multicolor FISH techniques for the detection of inter- and intrachromosomal rearrangements. In Rautenstrauss B, Liehr T, eds. FISH Technology. Heidelberg, Springer Verlag, 408–424