Abstract
Inhibitor of DNA-binding-1 (ID1) negatively regulates cell differentiation and senescence, and enhances cellular proliferation and angiogenesis. Elevated levels of ID1 have been found in a variety of cancers, including prostate cancer, but whether ID1 has a tumourigenic role remains to be established. We established heterozygous and homozygous ID1-transgenic mouse lines driven by the prostate-specific probasin promoter (−426 to +28 bp). Although elevated levels of ID1 were confirmed by RT-PCR, immunohistochemistry, and Western blot analysis, there were no morphological changes identified in the prostate of transgenic mice at 26 and 52 weeks. Thus, overexpression of ID1 alone is not sufficient to drive neoplastic change in mouse prostate. (J Histochem Cytochem 57:599–604, 2009)
Keywords: inhibitor of DNA-binding-1, ID1, probasin, prostate, cancer, transgenic
Prostate cancer predominately affects older men, and although mortality rates are low, the widespread incidence of the disease—at least in Western countries—makes it the second most lethal form of cancer in men. Despite this, little is known about how to effectively combat this disease in all of its many stages.
Inhibitor of DNA binding-1(ID1) regulates the transcription of a number of genes by interacting with their specific transcription factors. ID1 works by binding to the basic helix-loop-helix (bHLH) region of these transcription factors to form heterodimers. Because ID1 lacks a DNA binding domain, the formed heterodimers cannot bind to DNA and thus are non-functional (Sikder et al. 2003). Because some bHLH proteins are necessary for cell differentiation, ID1 is able to inhibit cellular differentiation by binding to them and blocking their regulatory function. ID1 has also been shown to enhance cellular proliferation and angiogenesis (Benezra et al. 2001), and to function as a negative regulator of cellular senescence (Zebedee and Hara 2001). Observations linking high ID expression to proliferating cells and low ID levels to terminally differentiated cells (Norton 2000) further support these roles.
These studies suggest that ID1 may play a role in the neoplastic transformation of cells. In support of this, ID1 levels have been found to be elevated in a variety of cancers, including breast (Lin et al. 2000; Fong et al. 2003), thyroid (Kebebew et al. 2000), endometrial (Takai et al. 2001), cervical (Schindl et al. 2001), ovarian (Schindl et al. 2003), renal cell (Li et al. 2007), and prostate cancers (Ouyang et al. 2002), and the elevated ID1 levels are correlated with the severity of malignancy (Lin et al. 2000; Ouyang et al. 2001,2002; Fong et al. 2003; Schindl et al. 2003; Li et al. 2007).
Although normal prostate and benign prostatic hyperplasia exhibit only very weak levels of ID1, poorly differentiated prostate adenocarcinomas show dramatically increased levels of this protein (Ouyang et al. 2001,2002). Furthermore, the levels of ID1 in cancer cells are negatively correlated with the degree of differentiation (Ouyang et al. 2001,2002). Although ID1 appears to play a crucial role in carcinogenesis, it was unclear prior to this study whether ID1 could initiate prostate carcinogenesis in vivo. On the basis of our data from transgenic mice with probasin-driven expression of ID1, we can now conclude that ID1 alone is unlikely to be able to induce prostate carcinogenesis.
Materials and Methods
Id1 Transgene Construction
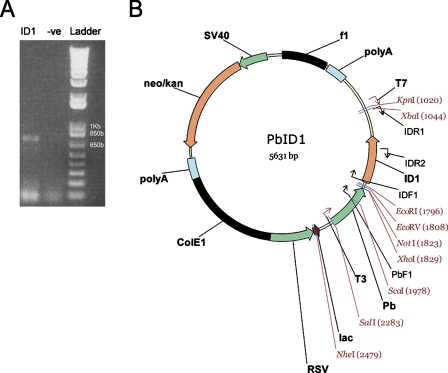
Total RNA from normal mouse skin was extracted and reversely transcribed. Id1 cDNA was amplified by PCR using primer IDF1 (5′cggatccaggatcatgaaggtcgccagt3′) and IDR1 (5′tctagaaatccgagaagcacgaaa3′) with Taq polymerase (Sigma; Sydney, Australia). The Id1 PCR product was separated on a 1% agarose gel, excised, and purified with Wizard SV Gel and PCR Clean-up system (Promega; Sydney, Australia). The purified product was ligated into a pGEM-T Easy cloning vector (Promega) with T4 DNA ligase (Promega) and transformed into JM109-competent cells. Insert sequence was confirmed by T7 or SP6 primers. Id1 from a clone with the correct sequence was digested with EcoRI and XbaI and subcloned into a probasin (Pb) promoter (−426 to +28 bp) containing expression vector (Pb-RSV). The Pb-ID1 vector was then digested with SalI and MluI. The purified product was injected into a C57BL/6 fertilized oocyte (Ozgene; Perth, Australia).
Genotyping and Breeding
All animals used in this study were maintained and used in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. DNA was extracted from mouse toes and amplified with PCR primers PbF1 (5′gacactgcccatgccaatca3′) and IDR2 (5′ccagctccttgaggcgtgag3′). Heterozygous animals were maintained from founder animals crossed with wild-type C57BL/6 mice. Heterozygous animals (F1) were then crossed to produce litters containing homozygous, heterozygous, and wild-type offspring. F2 animals testing positive for the transgene were then crossed with wild-type mice. Animals from the F2 generation, which produce all heterozygous animals when crossed with wild-type mice, were regarded as being homozygous.
RT-PCR
The anterior, ventral, and dorso-lateral prostatic lobes were isolated, and RNA was extracted using Tri-Zol as described (Singh et al. 2002,2005). RNA was further purified with RNAeasy minicolumns (Qiagen; Melbourne,Australia) and subjected to reverse transcription after DNase I treatment (Invitrogen; Melbourne, Australia).
Immunohistochemistry
Five-μm paraffin-embedded sections were antigen retrieved as described previously (Sved et al. 2004) and blocked in 3% H2O2 (10 min), 10% normal horse serum (20 min), ID1 primary antibody (1:200, 60 min) (C-20; Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-rabbit secondary antibody (1:200, 30 min), avidin-biotin complex peroxidase (30 min), and DAB (5 min), and counterstained with hematoxylin (2.5 min). All sections were then photographed under identical conditions.
Western Blot Analysis
Individual prostatic lobes were isolated, and protein was extracted in lysis buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitor cocktail (Sigma). Proteins (60 μg) were loaded onto a NuPAGE Novex 12% Bis-Tris gel and separated in MES buffer (Invitrogen), and transferred to nitrocellulose membranes. ID1 was detected using the C-20 anti-ID1 antibody (Santa Cruz Biotechnology).
Histological Assessment
Prostates from both the heterozygous and homozygous animals were isolated, sectioned to 5-μm thickness, and stained with hematoxylin and eosin. Sections were analyzed for morphological changes by a board-certified pathologist. No mouse genetic information was given to the pathologist.
Results
Generation of Id1-transgenic Mice
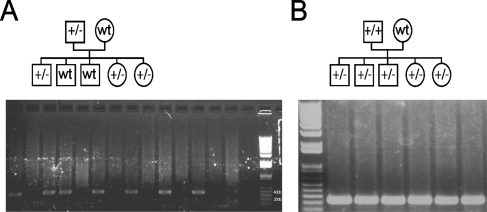
For generation of transgenic mice, Id1 cDNA was amplified by PCR (Figure 1A) and ligated into a probasin promoter (−426 to +28 bp) containing expression vector (Figure 1B). The transgene, including the probasin promoter, the Id1 open reading frame, and the transcription termination region, was injected into a fertilized oocyte (C57BL/6 mice) and screened for transgene incorporation. Southern blot analysis revealed nine transgenic founders (data not shown). Genotyping of the offspring allowed the development of heterozygous (Figure 2A) and homozygous animals (Figure 2B) from three lines. Of these, two were used for further studies.
Figure 1.
Id1 transgene construction. (A) The Id1 coding region was isolated from mouse skin, sequenced, and used in the construction of the Pb-ID1 vector. (B) This vector was then digested with the restriction enzymes SalI and MluI to produce the transgene.
Figure 2.
A breeding diagram and confirmation of heterozygous and homozygous status with PCR analysis using transgene-specific primers. (A) When heterozygous animals were crossed with wild-type animals, only half of the offspring incorporated the transgene. (B) When homozygous animals were crossed with wild-type animals, all offspring incorporated the transgene.
ID1 Is Overexpressed in Transgenic Mouse Prostate
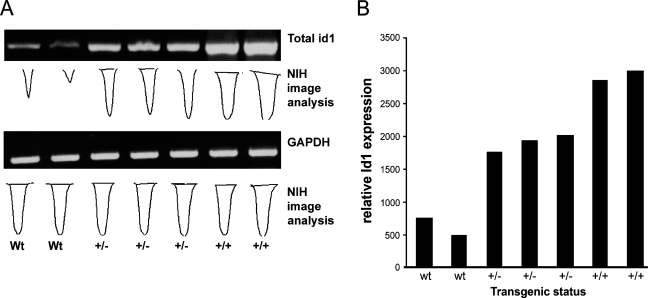
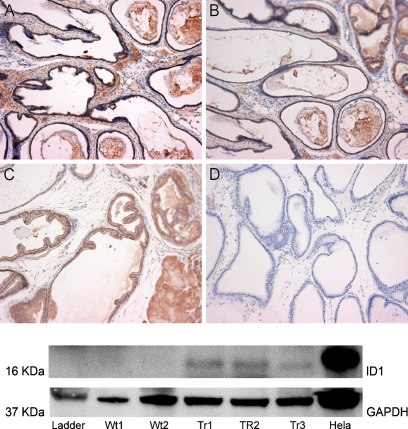
We confirmed expression of the transgene through RNA analysis, immunohistochemistry, and Western blot. RT-PCR showed that the transgenic animals overexpressed the Id1 mRNA, as compared with the wild-type control. The homozygous animals expressed higher levels of Id1 than did their heterozygous counterparts (Figures 3A and 3B). Consistent with the elevated mRNA levels, immunohistochemical detection of ID1 expression indicated that the Id1-transgenic animals overexpressed the ID1 protein, as compared with the wild-type control (Figures 4A–4D). The ID1 expression was primarily contained within the epithelial compartment. Western blot analyses of the individual lobes of transgenic and wild-type prostates revealed that ID1 is overexpressed in the ventral lobes of ID1-transgenic animals (Figure 4).
Figure 3.
Identification of Id1 mRNA expression in transgenic animals. (A) Heterozygous animals exhibited higher levels of total Id1 mRNA than did their wild-type counterparts, and the homozygous animals exhibited a further increase. National Institutes of Health image analysis was used to quantitate the intensity of the Id1 and housekeeping bands. (B) The ratio of Id1 band intensity to the intensity of the housekeeping band is depicted in the bar graph.
Figure 4.
Detection of ID1 protein in transgenic animals. Top: Immunohistochemistry was performed on homozygous transgenic animals (A,B) and wild-type animals (C). An isotype control shows no nonspecific binding to the tissue (D). The majority of stain is restricted to epithelial cells of the prostate. Bottom: Western blot for ID1 was performed with proteins taken from the ventral lobes of two wild-type (Wt1 and Wt2) and three homozygous transgenic animals (Tr1, Tr2, and Tr3). HeLa cell lysates were used as a positive control and GAPDH as a loading control.
Histological Assessment
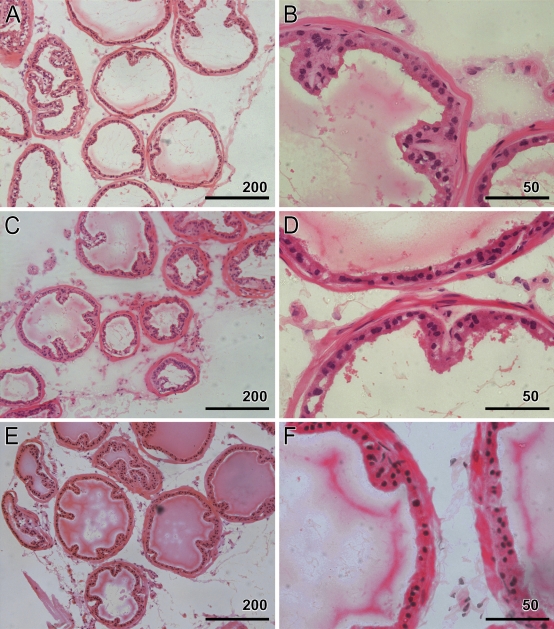
Heterozygous and homozygous animals from two lines were sacrificed at 26 and 52 weeks. Age-matched wild-type mice were used as the control. Prostates were isolated, sectioned, and stained with hematoxylin and eosin for morphologic assessment. Although the pathologist was informed about the nature of the project, he was not given any information to help in identifying the transgenic status. A total of 31 animals were analyzed. Of these, 12 were transgenic (6 homozygous and 6 heterozygous) and 3 were wild types at 26 weeks, and 13 were transgenic (6 homozygous and 7 heterozygous) and 3 were wild types at 52 weeks. Figure 5 shows that there was no difference between any of the sections between wild-type and transgenic mice at 52 weeks for morphological changes, including cancer, hyperplasia, or dysplasia.
Figure 5.
Histological assessment of transgenic animals. Hematoxylin and eosin–stained sections from transgenic and wild-type animals at 52 weeks. A and B represent a wild-type animal, whereas C–F represent two lines of ID1-homozygous transgenic animals. There are no observable morphological differences in transgenic animals when compared with age-matched wild-type animals.
Discussion
ID1 has been implicated in various processes known to lead to cancer, such as the inhibition of differentiation, enhanced cellular proliferation and angiogenesis, and the negative regulation of cellular senescence. ID1 levels are elevated in a variety of cancers, including prostate cancer, and the elevated ID1 levels in prostate cancer are correlated with the severity of malignancy (Ouyang et al. 2002). In an attempt to investigate the effects of ID1 overexpression and to determine whether ID1 is sufficient to drive prostate cancer onset, we constructed transgenic mice in which ID1 expression was driven by the probasin (−426 to +28 bp) promoter.
Probasin protein is localized to the apical membrane of secretory epithelial cells in the ducts of the ventral and the dorsolateral prostate, with very low levels of expression detected in the anterior prostate and seminal vesicles (Matuo et al. 1985). Transcription of the probasin gene is subject to androgen regulation (Greenberg et al. 1994). The probasin (−426 to +28 bp) promoter is the androgen-responsive region of the promoter (Kasper et al. 1999), and can target gene expression specifically to the prostate in a developmentally and hormonally regulated fashion (Greenberg et al. 1994). The probasin promoter is now the most widely used prostate-specific promoter.
Despite achieving elevated expression levels of ID1 mRNA and proteins in the ventral lobe, we found no evidence of morphological changes related to prostate cancer onset in two independent transgenic lines at 26 and 52 weeks. The observation period was sufficiently long, inasmuch as probasin promoter–driven expression of SV40 (Greenberg et al. 1995) or mutant androgen receptor (Han et al. 2005) causes prostate cancer or prostate intraepithelial neoplasia at 12 and 10 weeks, respectively. The lack of even minor morphological changes despite the overexpression of ID1 may be due to a number of factors. First, except for very potent cancer-associated genes, cancer is the result of “multiple hits.” For this reason, even if ID1 alone is not able to induce prostate cancer onset, it is not excluded from playing a role in prostate cancer development or progression in a collective manner. Also, a tumor-promoting role cannot be excluded. Second, it is worth noting that although many researchers maintain the connection between elevated levels of ID1 and cancer, emerging data suggest that ID1 is more commonly expressed in the surrounding endothelium rather than in cancer cells, and that different cancers may utilize ID1 in different ways (Perk et al. 2006). Finally, we questioned whether the failure to achieve morphological changes could have been due to insufficiently high transgenic ID1 protein levels. Indeed, it could be argued that a dramatically elevated level comparable to those in HeLa cells (used as the positive control, shown in the Western blot analysis presented in Figure 4) was not achieved with the probasin promoter. We then compared the increment in ID1 levels from wild-type to transgenic mice with that from normal to cancer tissues. Based on immunohistochemistry, the increased transgenic expression level achieved in this study was very similar to that seen in human and rodent prostate cancer tissue (Ouyang et al. 2001,2002). The only ID1 Western blot analysis we can find was conducted in sex hormone–induced rat prostate cancer (Ouyang et al. 2001). ID1 was not detected in normal prostates, but was increased in cancerous prostates to a range overlapping with our study. Thus, the finding of no indication of morphological change in transgenic mouse prostate is unlikely to be explained by the expression levels of transgene.
In conclusion, probasin-driven expression of the Id1 gene in the ventral prostate does not induce morphological changes at 52 weeks. These results are important as reminders of the need to reappraise the role of ID1 in prostate cancer development.
Acknowledgments
This work was supported by the Cancer Institute NSW Fellowship (to QD) and the Cure Cancer Foundation (to QD and DM).
References
- Benezra R, Rafii S, Lyden D (2001) The Id proteins and angiogenesis. Oncogene 20:8334–8341 [DOI] [PubMed] [Google Scholar]
- Fong S, Itahana Y, Sumida T, Singh J, Coppe JP, Liu Y, Richards PC, et al. (2003) Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc Natl Acad Sci USA 100:13543–13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, Demayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, et al. (1995) Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 92:3439–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, et al. (1994) The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol 8:230–239 [DOI] [PubMed] [Google Scholar]
- Han G, Buchanan G, Ittmann M, Harris JM, Yu X, Demayo FJ, Tilley W, et al. (2005) Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci USA 102:1151–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Rennie PS, Bruchovsky N, Lin L, Cheng H, Snoek R, Dahlman-Wright K, et al. (1999) Selective activation of the probasin androgen-responsive region by steroid hormones. J Mol Endocrinol 22:313–325 [DOI] [PubMed] [Google Scholar]
- Kebebew E, Treseler PA, Duh QY, Clark OH (2000) The helix-loop-helix transcription factor, Id-1, is overexpressed in medullary thyroid cancer. Surgery 128:952–957 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Z, Xin D, Chua CW, Wong YC, Leung SC, Na Y, et al. (2007) Prognostic significance of Id-1 and its association with EGFR in renal cell cancer. Histopathology 50:484–490 [DOI] [PubMed] [Google Scholar]
- Lin CQ, Singh J, Murata K, Itahana Y, Parrinello S, Liang SH, Gillett CE, et al. (2000) A role for Id-1 in the aggressive phenotype and steroid hormone response of human breast cancer cells. Cancer Res 60:1332–1340 [PubMed] [Google Scholar]
- Matuo Y, Nishi N, Muguruma Y, Yoshitake Y, Kurata N, Wada F (1985) Localization of prostatic basic protein (“probasin”) in the rat prostates by use of monoclonal antibody. Biochem Biophys Res Commun 130:293–300 [DOI] [PubMed] [Google Scholar]
- Norton JD (2000) ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci 113:3897–3905 [DOI] [PubMed] [Google Scholar]
- Ouyang XS, Wang X, Lee DTW, Tsao SW, Wong YC (2001) Up-regulation of TRPM-2, MMP-7 and ID-1 during sex hormone-induced prostate carcinogenesis in the Noble rat. Carcinogenesis 22:965–973 [DOI] [PubMed] [Google Scholar]
- Ouyang XS, Wang X, Lee DTW, Tsao SW, Wong YC (2002) Over expression of Id-1 in prostate cancer. J Urol 167:2598–2602 [PubMed] [Google Scholar]
- Perk J, Gil-Bazo I, Chin Y, de Candia P, Chen JJ, Zhao Y, Chao S, et al. (2006) Reassessment of id1 protein expression in human mammary, prostate, and bladder cancers using a monospecific rabbit monoclonal anti-id1 antibody. Cancer Res 66:10870–10877 [DOI] [PubMed] [Google Scholar]
- Schindl M, Oberhuber G, Obermair A, Schoppmann SF, Karner B, Birner P (2001) Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res 61:5703–5706 [PubMed] [Google Scholar]
- Schindl M, Schoppmann SF, Strobel T, Heinzl H, Leisser C, Horvat R, Birner P, et al. (2003) Level of Id-1 protein expression correlates with poor differentiation, enhanced malignant potential, and more aggressive clinical behavior of epithelial ovarian tumors. Clin Cancer Res 9:779–785 [PubMed] [Google Scholar]
- Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM, Sikder HA, Devlin MK, et al. (2003) Id proteins in cell growth and tumorigenesis. Cancer Cell 3:525–530 [DOI] [PubMed] [Google Scholar]
- Singh J, Young L, Handelsman DJ, Dong Q, Singh J, Young L, Handelsman DJ, et al. (2005) Molecular cloning and characterization of a novel androgen repressible gene expressed in the prostate epithelium. Gene 348:55–63 [DOI] [PubMed] [Google Scholar]
- Singh J, Young L, Handelsman DJ, Dong QH (2002) Prostate epithelial expression of a novel androgen target gene. J Androl 23:652–660 [PubMed] [Google Scholar]
- Sved P, Scott KF, McLeod D, King N, Singh J, Tsatralis T, Nikolov B, et al. (2004) Oncogenic action of secreted phospholipase A2 in prostate cancer. Cancer Res 64:6934–6940 [DOI] [PubMed] [Google Scholar]
- Takai N, Miyazaki T, Fujisawa K, Nasu K, Miyakawa I (2001) Idl expression is associated with histological grade and invasive behavior in endometrial carcinoma. Cancer Lett 165:185–193 [DOI] [PubMed] [Google Scholar]
- Zebedee Z, Hara E (2001) Id proteins in cell cycle control and cellular senescence. Oncogene 20:8317–8325 [DOI] [PubMed] [Google Scholar]