Abstract
Background: The optimal treatment for correcting or preventing vitamin D insufficiency in cystic fibrosis (CF) patients has not been established.
Objective: The aim of the study was to assess the relative efficacy of three modes of vitamin D therapy: cholecalciferol (D3), ergocalciferol (D2), and UV light in raising or maintaining 25(OH)D levels above 30 ng/ml.
Design: Thirty adult CF subjects with vitamin D insufficiency were randomized into one of three treatment arms: D3, D2, or UV light. Subjects randomized to D3 or D2 ingested 50,000 IU of vitamin D weekly, and those randomized to UV exposed their skin to UV light from a lamp five times a week. Serum was collected for 25(OH)D and PTH at baseline and at 12 wk.
Results: Treatment with D3 and D2 raised 25(OH)D levels significantly, from a mean of 21.2 ± 10.18 to 47.1 ± 20.5 ng/ml (P < 0.001) and 24.4 ± 10.3 to 32.7± 9.7 ng/ml (P = 0.01), with 100% and 60% reaching 25(OH)D levels above 30 ng/ml, respectively. Treatment with UV did not raise 25(OH)D levels significantly; however, only 55% of subjects were adherent with UV therapy.
Conclusion: This study demonstrates that CF subjects are able to achieve or maintain optimal vitamin D status (>30 ng/ml) with two oral regimens of either D3 or D2 treatment, the former being more efficacious. A confounding variable for this observation is the fact that the D3 and D2 capsules contained different carriers, powder-based vs. oil-based, respectively. UV therapy did not alter vitamin D status, possibly due to poor adherence to UV therapy.
Adult cystic fibrosis patients can be given an oral regimen of vitamin D to improve vitamin D status during the winter months.
The prevalence of vitamin D insufficiency in patients with cystic fibrosis (CF) has been reported to be as high as 90% (1,2). This is significantly higher than healthy controls from a similar geographic area (2) or when compared with 25-hydroxyvitamin D [25(OH)D] levels from the Third National Health and Nutrition Examination Survey (NHANES III) (3). This high prevalence occurs despite the routine use of vitamin D supplementation in CF patients (1,4). Poor absorption of fat-soluble vitamins due to pancreatic insufficiency and diminished exposure to sunlight are thought to be among the causes for low vitamin D in this population (5).
Vitamin D insufficiency is associated with decreased bone mineral density (6,7) in patients with CF as well as in other disease states. Low bone mineral density, in turn, is associated with diminished lung function [as measured by forced expiratory volume in the first second (FEV1)] in these patients (6). A direct association between vitamin D insufficiency and poor lung function has also been reported in non-CF adults (8,9). Furthermore, vitamin D is postulated to enhance the innate immune system (10) and improve insulin sensitivity (11), all issues of clinical relevance in the CF population who suffer from CF-related diabetes, frequent pulmonary infections, and progressive respiratory failure (12).
The best way to overcome vitamin D insufficiency in CF patients has not been established. Current guidelines by the Cystic Fibrosis Foundation Consensus Conference for vitamin D supplementation with high-dose ergocalciferol (vitamin D2) appear to be inadequate (1). Previous studies in healthy adults (13,14) have suggested that high-dose cholecalciferol (vitamin D3) is more effective in raising 25(OH)D levels compared with ergocalciferol. In contrast, recent studies have shown that at lower daily replacement doses, ergocalciferol and cholecalciferol are equally efficacious in raising 25(OH)D levels in non-CF children and adults (15,16). An alternative to oral vitamin D supplementation in CF patients is UV light therapy to produce vitamin D3 in the skin. UV light therapy circumvents the problem of malabsorption in these patients and has been demonstrated to be effective in small pilot studies to improve vitamin D status in subjects with CF (17,18). A portable UV table lamp can provide the UVB radiation that sunlight cannot provide efficiently in northern latitudes during the winter months (19).
The relative efficacy of ergocalciferol, cholecalciferol, and limited UV light therapy in vitamin D replacement of CF patients has not yet been studied. The primary aim of this study was to compare the relative efficacy of these three modes of vitamin D replacement in clinically stable adults with CF.
Subjects and Methods
Subjects with CF were recruited from the Emory Cystic Fibrosis Center from November 2006 to March 2008. Subjects were recruited in the late fall and winter months during these 2 yr to minimize the contribution of endogenous vitamin D3 production from the sun. The study was approved by the Emory Institutional Review Board, and all subjects gave written informed consent. The study was registered at clinicaltrials.gov under study no. NCT00450073.
Subjects
Patients were included in the study if they were between the ages of 16 and 70 yr, had a screening serum 25(OH)D level between 10 and 40 ng/ml, and had a confirmed diagnosis of CF by genetic analysis or sweat chloride testing. Patients were considered to have pancreatic insufficiency if they were on pancreatic enzyme replacements. Exclusion criteria were history of skin disease or cancer, presence of renal or hepatic disease, recent treatment with high-dose vitamin D, treatment with prednisone, or a history of more than six hospitalizations within the past year. Patients were also excluded if they were planning any trips during the study to sunny climates lasting longer than 2–3 d. Our study included patients with serum 25(OH)D levels up to 40 ng/ml at screening, expecting that CF subjects would experience a decrease in 25(OH)D in the winter (20).
Design
Thirty CF subjects were randomized in six-member blocks to one of three treatment arms: vitamin D3, vitamin D2, or UV light therapy. The vitamin D2 50,000 IU and vitamin D3 50,000 IU capsules were obtained from Pliva, Inc. (East Hanover, NJ) and Tishcon Corp. (Westbury, NY), respectively. Vitamin D2 was contained in a hard gelatin capsule and embedded in refined soybean oil, glycerin, purified water, D&C yellow no. 10, and FD&C blue no. 1. Vitamin D3 came in the form of a powder comprised of lactose, microcrystalline cellulose, and magnesium stearate that was contained in a capsule made of hard gelatin. A sample of each vitamin D capsule was assayed by an independent laboratory using HPLC with a reversed phase column. The vitamin D3 capsules were found to contain 49,300 (± 900) IU of vitamin D3, and the vitamin D2 gel capsules were found to contain 47,100 (± 1100) IU of vitamin D2.
For all subjects, a nonfasting serum was collected at baseline. Subjects randomized to D2 or D3 were instructed to take one pill each week for 12 wk. CF subjects randomized to UV light therapy exposed their lower backs in a seated position to a portable UV indoor tanning lamp (Sperti sunlamp “Del Sol”; Sperti, Crescent Springs, KY) at a distance of 14 in. for 3–10 min depending on their skin type five times a week according to a UV light protocol developed to enhance vitamin D status in CF patients (Table 1) (18). The Sperti sunlamp mimics natural sunlight in that it emits 2–5% UVB between the wavelengths of 290 and 320 nm (18). Subjects were contacted on a weekly basis to ensure compliance with their respective treatment regimens. Subject recall was used to assess compliance. Subjects returned for their final nonfasting serum collection within 4 wk of study completion. A focused food frequency questionnaire was used to determine mean daily vitamin D consumption by subjects. For the majority of subjects, this questionnaire was administered at the end of the study. The questionnaire we used was adapted from a previously published version (21). On their final visit, those subjects randomized to UV light therapy had their backs examined for the presence of any burns.
Table 1.
Skin sensitivity types to UVB radiation and the recommended tanning times per session
| Skin type scale | Target minutes/session |
|---|---|
| Type I: always burns, never tans | |
| Type II: always burns easily, tans minimally | 3 |
| Type III: burns moderately, tans gradually | 6 |
| Type VI: burns minimally, always tans well | 9 |
| Type V: rarely burns, tans profusely | 12 |
| Type VI: never burns, deeply pigmented | 15 |
Adapted with modification from the U.S. Food and Drug Administration skin type scale (18).
Analytical methods
Vitamin D insufficiency was defined as a 25(OH)D level below 30 ng/ml. Efficacy for each treatment modality was defined as its ability to raise or maintain 25(OH)D levels above 30 ng/ml during the winter months. Serum 25(OH)D2, 25(OH)D3, and total 25(OH)D were determined using liquid chromatography-mass spectrometry by Quest Diagnostics Incorporated (San Juan Capistrano, CA). PTH was done using ELISA (Immutopics International, LLC, San Clemente, CA). Mean daily intake of vitamin D by subjects was estimated using an 11-item food frequency questionnaire. A serving size for each food item was described to the subjects. Subjects were then asked to recall the number of serving sizes they consumed for each food item within a given day, or week, or month. For each food item, the amount of vitamin D contained in each serving size was multiplied by the frequency of intake for each subject. Total vitamin D intake was then calculated and averaged to represent mean intake per day.
Statistical methods
Data were first examined to ensure normality. All recorded variables were normally distributed. We used SAS 9.2 (SAS Institute, Cary, NC) to perform all analyses. The study was originally powered to detect a difference of 7.5 ng/ml in 25(OH)D between groups. Simple ANOVA was used to compare differences in mean covariates by treatment groups and analysis of covariance was used to compare mean differences when controlling for covariates (PROC GLM and PROC MIXED). Least squares means were calculated for all means reported using a Bonferroni correction whenever repeated measures were present. We used Fisher’s Exact Test (PROC FREQ) to calculate P value, comparing count data between treatment arms to examine heterogeneity of treatment groups. To determine whether initial 25(OH)D status effected change in 25(OH)D, we used Pearson’s correlation (PROC CORR). When considering our final models, we used initial 25(OH)D status, age, type of CF mutation, serum calcium, vitamin D intake, and use of vitamin supplement as potential covariates. We tested for interaction with CF mutation, and use of vitamins; however, significant interaction was not present. We conducted analyses using intent to treat. Mixed linear models using random intercept and random slope were used to model data.
Results
Subject demographics
We screened 33 individuals. Of those, 30 entered the study, and one patient each was lost to follow-up in the UV and D3 groups. The mean age, BMI, FEV1 percent predicted, and use of pancreatic enzyme replacement (a marker of pancreatic insufficiency) were not significantly different among treatment groups (Table 2). The mean age and distribution of mutations of our subjects were typical of adult patients with CF (5,22).
Table 2.
Baseline characteristics of adult CF patients
| UV | D3 | D2 | P value | |
|---|---|---|---|---|
| Total (n) | 9 | 9 | 10 | |
| Age (yr) | 33.3 ± 15.7 | 35.7 ± 12.5 | 27.9 ± 11.9 | 0.43a |
| Sex (n) | ||||
| Male | 4 | 4 | 5 | |
| Female | 5 | 5 | 5 | 0.52 |
| BMI (kg/m2) | 23.7 ± 3.9 | 22.5 ± 4.2 | 22.7 ± 2.9 | 0.76a |
| Pretreatment predicted %FEV1 | 72.4 ± 28.4 | 65.5 ± 24.5 | 76.5 ± 25.8 | 0.66a |
| Reported MVI/ADEK/Ca+D use, n (%) | 8 (88.9) | 8 (88.9) | 8 (80) | 0.68 |
| Average daily vitamin D intake (IU) | 370 ± 218 | 456 ± 290 | 587 ± 321 | 0.26a |
| Mutations (n) | ||||
| Homo 508 | 1 | 2 | 4 | |
| Hetero 508 | 6 | 3 | 3 | |
| Neither | 2 | 3 | 3 | 0.56 |
| Reported pancreatic enzyme use, n (%) | 9 (100) | 8 (88.9) | 10 (100) | 0.24 |
Values are reported as means ± sd, number, or number (percent). MVI, Multi-vitamins.
P − ANOVA.
Baseline vitamin D status
Sixty-eight percent of our study population were vitamin D insufficient [25(OH)D <30 ng/ml] at baseline. All groups had similar dietary intakes of vitamin D, between 369 and 587 IU (Table 2). Greater than 80% of the subjects reported daily use of a multivitamin, which did not differ among the three groups. The mean initial 25(OH)D was 22.6 ± 10.8, 21.2 ± 10.1, and 24.4 ± 10.3 ng/ml for UV, D3, and D2, respectively, and was not different among the three treatment groups (P = 0.8). Twenty-two percent of the subjects in the D3 and UV groups were vitamin D sufficient [25(OH)D >30 ng/ml], whereas 40% of subjects in the D2 group were sufficient (Table 3).
Table 3.
Vitamin D, calcium, and PTH levels before and after intervention
| UV | D3 | D2 | Pa | |
|---|---|---|---|---|
| Initial 25(OH)D (ng/dl) | 22.6 ± 10.8 | 21.2 ± 10.18 | 24.4 ± 10.3 | 0.8 |
| Final 25(OH)D (ng/dl) | 28.3 ± 9.2 | 47.1 ± 20.5 | 32.7 ± 9.7 | 0.03 |
| % Sufficient (>30 ng/ml) | ||||
| Initial (%) | 22 | 22 | 40 | 0.09 |
| Final (%) | 22 | 100 | 60 | 0.003 |
| Initial Ca (mg/dl) | 9.01 ± 0.45 | 9.06 ± 0.28 | 9.44 ± 0.42 | 0.04 |
| Final Ca (mg/dl) | 8.97 ± 0.38 | 8.87 ± 0.50 | 9.40 ± 0.43 | 0.24 |
| Initial PTH (pg/ml) | 111.1 ± 45.0 | 49.8 ± 46.7 | 129.9 ± 47.3 | 0.01 |
| Final PTH (pg/ml) | 110.0 ± 37.6 | 40.0 ± 47.6 | 86.1 ± 40.8 | 0.02 |
Values are reported as means ± sd, unless otherwise indicated.
P − ANOVA.
Factors associated with increases in 25(OH)D
The initial 25(OH)D level was associated with the rate of change in 25(OH)D (Table 4). The negative correlation coefficient indicates that those who start out with the highest serum 25(OH)D levels exhibited the smallest increase in 25(OH)D throughout the study period in response to oral vitamin D. We therefore controlled for initial 25(OH)D level when examining the mean rates of change. When controlling for initial serum 25(OH)D status, those receiving D3 had the largest increase in serum 25(OH)D when compared with the other two treatments (P < 0.01) (Table 4). Those receiving D3 also had the largest increase in serum 25(OH)D3 (P < 0.001) (Table 4). Serum 25(OH)D2 concentrations also increased in those receiving D2 (P = 0.02) and decreased, but not significantly, in those receiving D3 or UV. We examined age, BMI, sex, CF mutation, skin type, and vitamin D intake as potential covariates, although none was significant at the 5% level.
Table 4.
Changes in 25(OH)D levels
| Least squares means for total 25(OH)D, 25(OH)D3, and 25(OH)D2 using mixed linear model to account for initial levelsa
|
Pearson’s correlation between initial 25(OH)D levels and changes in 25(OH)D levels
|
|||||
|---|---|---|---|---|---|---|
| UV | D3 | D2 | Δ 25(OH)D2 | Δ 25(OH)D3 | Δ 25(OH)D | |
| Initial 25(OH)D | 23.1 | 21.2 | 24.4 | −0.38c | 0.24 | 0.02 |
| Final 25(OH)D | 28.2 | 47.1 | 32.7 | |||
| Pb | 0.25 | <0.001 | 0.04 | |||
| Initial 25(OH)D2 | 1.9 | 4.3 | 2.2 | 0.34 | −0.42c | −0.29 |
| Final 25(OH)D2 | 0.6 | 1.1 | 20.4 | |||
| Pb | 0.39 | 0.38 | <0.001 | |||
| Initial 25(OH)D3 | 20.2 | 16.9 | 22.2 | 20.2 | 16.9 | 22.2 |
| Final 25(OH)D3 | 27.1 | 46.0 | 12.3 | |||
| Pb | 0.11 | <0.001 | 0.03 | |||
Values are reported as means and were calculated using PROC GLM with a repeated statement.
P compares initial and final levels using least squares means regression.
Indicates significance with Pearson’s correlation.
Total serum 25(OH)D concentrations after D2, D3, or UV light
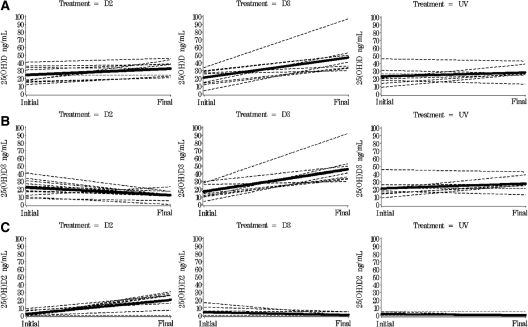
Subjects randomized to oral forms of vitamin D (D2 or D3) exhibited significant increases in total serum 25(OH)D concentrations (Table 4). The most robust increase in total serum 25(OH)D occurred in the D3-treated group, where the final total serum 25(OH)D was 47.1 ± 3.1 ng/ml, resulting in a significant total net increase of 25(OH)D of 25.3 ± 2.8 ng/ml (P < 0.001). In the D2-treated group, the total increase in 25(OH)D concentration was less impressive at 8.8 ± 2.8 ng/ml (P = 0.04) (Fig. 1A). In the D3-treated group, 100% of the subjects reached vitamin D sufficiency [25(OH)D > 30 ng/ml] compared with only 60% of those treated with D2 and 22% of those treated with UV (P < 0.001). In those randomized to receive UV treatment, the final total serum 25(OH)D was 28.2 ± 3.2 ng/ml for a nonsignificant change of 5.2 ± 3.4 ng/ml (P = 0.25) (Fig. 1A).
Figure 1.
Change in total 25(OH)D, 25(OH)D3, and 25(OH)D2 by treatment group. Bold line indicates predicted value for the group using PROC MIXED for mixed linear models and controls for initial levels. Dashed lines represent individual change. The total 25(OH)D concentrations were significantly higher in subjects receiving D3 and D2, but not in subjects receiving UV (A). The 25(OH)D3 concentrations were significantly higher in subjects receiving D3 and significantly lower in subjects receiving D2. There were no significant changes in 25(OH)D3 concentrations in those receiving UV (B). The 25(OH)D2 concentrations were significantly higher in subjects receiving D2, and not in subjects receiving D3 or UV (C).
One subject in the vitamin D3-treated group had a much greater increase in 25(OH)D compared with others in this group (Fig. 1A). This subject was also the only one in the group that had pancreatic sufficiency. We performed sensitivity analysis after excluding this subject, and our outcomes remained the same.
25(OH)D2 and 25(OH)D3 concentrations after D2, D3, and UV light
As expected, subjects who received D3 had significant increases in serum 25(OH) D3 concentrations from 16.9 to 46.0 ng/ml (P < 0.001). In the D2-treated group, the serum 25(OH)D2 level increased by 17.7 ± 3.3 ng/ml, but the 25(OH)D3 level decreased by 8.9 ± 3.3 ng/ml. This decrease in serum 25(OH)D3 concentrations was significant compared with those in the D3-treated group (P < 0.001) (Fig. 1B). Those subjects who received UV light did not have a significant increase in 25(OH)D3 (P = 0.09).
The 25(OH)D2 concentrations in subjects receiving D3 and UV started low initially (over half of the sample in both groups had levels that were not detectable at the beginning of the intervention) at 4.3 and 1.9 ng/ml and did not change significantly with therapy (P = 0.12 and 0.17, respectively).
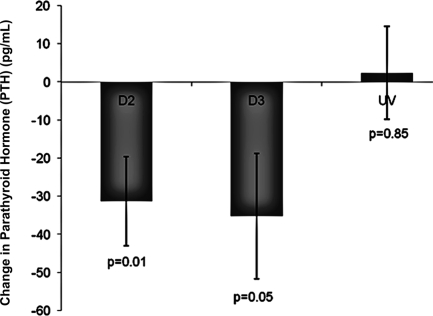
PTH concentrations after D2, D3, and UV light
Initial PTH levels in D3-treated subjects were nearly half that of D2- and UV-treated subjects (P = 0.01) (Table 3). When controlling for initial PTH values, there was a significant change in PTH from baseline to final measurements in the D3 and D2 treatment groups compared with the UV treatment group (Fig. 2). There was no significant difference in the change in PTH between the two treatment groups D2 and D3 (P = 0.86).
Figure 2.
Change in serum PTH concentrations in response to treatment with ergocalciferol 50,0000 IU once a week for 12 wk (D2), cholecalciferol 50,000 IU once a week for 12 wk (D3), or UV light with a Sperti lamp five times a week for 12 wk (UV). Change in PTH were controlled for initial PTH level. Mean change was calculated using least squared means. Subjects receiving both D2 and D3 had significantly reduced PTH levels (P = 0.01 and P = 0.05, respectively). There was no significant difference in the change in PTH between the two oral vitamin D treatment groups, D2 and D3 (P = 0.86).
Safety
There were no reported adverse events, nor were there any cases of hypercalcemia. All of the individuals randomized to a pill (D3 or D2) were 80% compliant or better. Only 55% of those on UV therapy were compliant with therapy more than 80% of the time. When we only considered five of nine subjects who were adherent with UV therapy, then the rise in 25(OH)D was similar to those treated with D2 (P = 0.93).
Discussion
In this prospective, randomized study of three different regimens to correct vitamin D insufficiency, we found that both oral vitamin D2 and vitamin D3 were effective in raising 25(OH)D concentrations, with vitamin D3 demonstrating the greater response. UV therapy was limited by poor adherence to the study protocol but was equally as effective as oral vitamin D2 after exclusion of nonadherent subjects. As expected, PTH levels followed the same trend, with the greatest decrease in PTH level occurring in the vitamin D3-treated group. Our results are in agreement with previous studies in healthy subjects, demonstrating superiority of vitamin D3 over vitamin D2 in raising 25(OH)D levels (13,14). Trang et al. (14) showed that when subjects were given 4000 IU vitamin D3 for 14 d, they were able to raise their 25(OH)D levels 1.7 times more than subjects given the same dose and duration of vitamin D2 (increase of 9.3 vs. 5.5 ng/ml). Armas et al. (13) compared vitamin D2 to vitamin D3 when given as a single dose of 50,000 IU. Despite a similar rise in 25(OH)D levels over the first 3 d for both vitamin D2 and vitamin D3, suggesting equal absorption, 25(OH)D levels in the vitamin D2 group rapidly fell back to baseline by the second week. The area under the curve by d 28 was 204.7 ng · d/ml for vitamin D3 compared with 60.2 ng · d/ml for vitamin D2.
The Cystic Fibrosis Foundation consensus statement from 2005 (23) recommends daily oral supplementation with ergocalciferol at a daily dose of 400 to 800 IU. Several studies in CF patients have demonstrated such doses to be inadequate at optimizing 25(OH)D levels. A prospective study of 20 CF patients demonstrated that after 1 yr of treatment with 800 IU vitamin D3 daily, none of the patients were able to raise their 25(OH)D levels above 30 ng/ml (70 nmol/liter) (24). Rovner et al. (2) showed that 90% of CF patients remained vitamin D insufficient despite routine supplementation with 800 IU/d. Furthermore, in a recent large retrospective study in CF patients (4), it was demonstrated that despite daily cholecalciferol supplementation (mean dose, 647 ± 339 IU), which increased 25(OH)D levels significantly (mean, 14.2 to 25 ng/ml), only 18% of CF patients were able to achieve 25(OH)D levels above 30 ng/ml.
For vitamin D-insufficient CF patients [25(OH)D levels <30 ng/ml], the Cystic Fibrosis Foundation consensus statement recommends treatment with ergocalciferol 50,000 IU either once or twice weekly. Lark et al. (25) demonstrated that CF subjects are only able to absorb 50% of ergocalciferol given as a single dose of 100,000 IU compared with healthy controls. They also demonstrated that CF subjects were less efficient at converting ergocalciferol to 25(OH)D compared with controls (25). Boyle et al. (1) demonstrated that only 8% of vitamin D-insufficient CF patients were able to reach 25(OH)D levels above 30 ng/ml after 8 wk of treatment with weekly 50,000 IU doses of ergocalciferol. Those that did not achieve vitamin D sufficiency were treated for another 8 wk, yet none was able to achieve vitamin D sufficiency. Green et al. (26) demonstrated that ergocalciferol administered once, twice, or three times a week for 8 wk was able to raise 25(OH)D levels above 30 ng/ml in only 33, 26, and 43%, respectively. Furthermore, the proportion of patients with follow-up 25(OH)D levels above 30 ng/ml were the same irrespective of whether or not they had been treated with ergocalciferol. Based on these data, the recommendations for maintenance of vitamin D status and correction of vitamin D insufficiency should be revised to higher levels, preferably with vitamin D3.
In our study, CF subjects taking vitamin D2 are able to raise their total 25(OH)D concentration levels; however, this is attenuated by the corresponding decrease in 25(OH)D3. Circulating vitamin D exists mostly as 25(OH)D3 representing vitamin D from cutaneous and most dietary sources of vitamin D. Less circulating vitamin D exists as 25(OH)D2, which represents vitamin D from dietary sources fortified with ergocalciferol only and is limited to very few foods. This finding of lowered 25(OH)D3 after vitamin D2 therapy is of concern because ergocalciferol is most commonly available by prescription to correct vitamin D insufficiency in the United States and is recommended by the Cystic Fibrosis Foundation for correction of vitamin D insufficiency. Based on the studies of Trang et al. (14) and Armas et al. (13), some have suggested that ergocalciferol not be used as a supplement to correct vitamin D insufficiency (27).
A recent study by Holick et al. (15) demonstrated equal efficacy of vitamin D2 and vitamin D3 in raising 25(OH)D levels. However, vitamin D2 and D3 were given daily at much lower doses (1000 IU/d) in that trial and to subjects without chronic malabsorption, as in the CF patients we studied. This suggests that there may be differences in vitamin D2 and D3 when given in pharmacological (>10,000 IU) doses rather than in more physiological doses (<2,000 IU), although such studies in CF have not been performed. A recent study (28) demonstrated that at vitamin D3 intake doses of less than 2000 IU/d there is rapid conversion of vitamin D3 to 25(OH)D3, but at doses of more than 2000 IU/d, vitamin D3 concentrations can be detected in the serum along with a rise in 25(OH)D3. It has been demonstrated that vitamin D3 binds vitamin D binding protein (DBP) more avidly than vitamin D2 (29), thus allowing vitamin D3 to have a longer circulating half-life. One could infer from these two studies that at lower intake levels, vitamin D’s metabolism becomes independent of how avidly it binds DBP. At higher intake doses, the differential avidity for DBP of vitamin D3 and vitamin D2 becomes more evident. Finally, vitamin D2 may activate the pregnane X receptor, which in turn up-regulates the CYP24, the enzyme that catabolizes vitamin D. The pregnane X receptor is a recently described nuclear receptor that is important for the detoxification of foreign substances in the body (30). Whether vitamin D2 up-regulates the catabolism of vitamin D metabolites greater than vitamin D3 is not known.
We found that UV light therapy did not significantly raise 25(OH)D levels. One explanation for the lack of a significant rise in 25(OH)D levels with UV light therapy could be attributed to the high nonadherence rate of subjects assigned to this treatment modality. Over half of the eligible subjects randomized to receive UV light therapy were not adherent with therapy per self-report. When we excluded those individuals, the mean 25(OH)D increase in the UV-treated group was 11 ng/ml (P = 0.04); however, only one of the individuals reached vitamin D sufficiency. In addition, our subjects exposed only their backs to the sunlamp, a much smaller surface area, when compared with a previous study in CF patients showing a change in 25(OH)D from 21 to 44 ng/ml after 8 wk of whole body tanning (17). Therefore, we cannot make definitive conclusions about whether UV light therapy is inferior to oral vitamin D replacement due to nonadherence of the study participants. Increased adherence as well as increased frequency and duration of UV light therapy with increased surface area exposure during therapy sessions would likely result in higher levels of 25(OH)D.
Our study has limitations. We studied adult CF patients with clinically stable disease, and UV light therapy was not supervised. Thus, adherence to UV light therapy was by subject report only. Another limitation of our study is the fact that two different formulations were used for the ergocalciferol and cholecalciferol pills. We cannot therefore rule out a difference in bioavailability of the two pills due to their different formulations. Another limitation is that the dose of vitamin D in the ergocalciferol pill was slightly lower than the cholecalciferol pill (approximately 4% less). However, this small difference in vitamin D cannot completely account for the large difference in 25(OH)D when comparing the cholecalciferol- and ergocalciferol-supplemented groups [cholecalciferol treatment resulted in 88% higher 25(OH)D than the ergocalciferol treatment]. Also, our study was a short-term study, and it does not evaluate long-term effects of vitamin D treatment on bone mineral density and efficiency of intestinal calcium absorption. It is not known what level of 25(OH)D is required to prevent bone loss and maximize calcium absorption in CF. Optimal levels of 25(OH)D have been extrapolated from non-CF populations. A recent study in pediatric CF patients suggests that these patients have normal baseline intestinal calcium absorption that did not improve further with daily 2000 IU D3 treatment (31).
We conclude that oral vitamin D3 given at a dose of 50,000 IU once a week for 12 wk is the most efficacious method to increase 25(OH)D and lower PTH values in CF subjects who are vitamin D insufficient. Ergocalciferol and UV light were not as effective in our study and should be reserved as alternative therapies. The optimal means to correct low vitamin D in patients with CF is not yet determined. However, this study along with others suggests that vitamin D3 should be considered over vitamin D2. Because vitamin D status is often low in CF patients, a more preventative approach to treatment of vitamin D status should be adopted, especially during the winter.
Acknowledgments
The authors are grateful to the staff at the Emory Cystic Fibrosis Center. We also thank Dr. Tai C. Chen of Boston University School of Medicine for his help confirming vitamin D2 and D3 content in the corresponding pills, Dr. Li Hao for her assistance with PTH assay, and Quest Diagnostics for 25(OH) vitamin D subtype analysis.
Footnotes
This work was supported by the UV Foundation, the University Research Committee of Emory University, National Institutes of Health Grant K23AR054334, and the Cystic Fibrosis Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 31, 2009
Abbreviations: CF, Cystic fibrosis; DBP, vitamin D binding protein; FEV1, forced expiratory volume in the first second; 25(OH)D, 25-hydroxyvitamin D.
References
- Boyle MP, Noschese ML, Watts SL, Davis ME, Stenner SE, Lechtzin N 2005 Failure of high-dose ergocalciferol to correct vitamin D deficiency in adults with cystic fibrosis. Am J Respir Crit Care Med 172:212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner AJ, Stallings VA, Schall JI, Leonard MB, Zemel BS 2007 Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Am J Clin Nutr 86:1694–1699 [DOI] [PubMed] [Google Scholar]
- Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR 2002 Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777 [DOI] [PubMed] [Google Scholar]
- Stephenson A, Brotherwood M, Robert R, Atenafu E, Corey M, Tullis E 2007 Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr 85:1307–1311 [DOI] [PubMed] [Google Scholar]
- Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V 2008 Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 69:374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth CS, Selby PL, Webb AK, Dodd ME, Musson H, McL Niven R, Economou G, Horrocks AW, Freemont AJ, Mawer EB, Adams JE 1999 Low bone mineral density in adults with cystic fibrosis. Thorax 54:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan Jr DS, Papadopoulos A, Staron RB, Addesso V, Schulman L, McGregor C, Cosman F, Lindsay RL, Shane E 1998 Bone mass and vitamin D deficiency in adults with advanced cystic fibrosis lung disease. Am J Respir Crit Care Med 157:1892–1899 [DOI] [PubMed] [Google Scholar]
- Black PN, Scragg R 2005 Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest 128:3792–3798 [DOI] [PubMed] [Google Scholar]
- Burns JS, Dockery DW, Neas LM, Schwartz J, Coull BA, Raizenne M, Speizer FE 2007 Low dietary nutrient intakes and respiratory health in adolescents. Chest 132:238–245 [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL 2006 Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Gysemans C, Giulietti A, Bouillon R 2005 Vitamin D and diabetes. Diabetologia 48:1247–1257 [DOI] [PubMed] [Google Scholar]
- Stenbit A, Flume PA 2008 Pulmonary complications in adult patients with cystic fibrosis. Am J Med Sci 335:55–59 [DOI] [PubMed] [Google Scholar]
- Armas LA, Hollis BW, Heaney RP 2004 Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89:5387–5391 [DOI] [PubMed] [Google Scholar]
- Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R 1998 Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 68:854–858 [DOI] [PubMed] [Google Scholar]
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD 2008 Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, Cox JE 2008 Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab 93:2716–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowitz E, Larkö O, Gilljam M, Hollsing A, Lindblad A, Mellström D, Strandvik B 2005 Ultraviolet B radiation improves serum levels of vitamin D in patients with cystic fibrosis. Acta Paediatr 94:547–552 [DOI] [PubMed] [Google Scholar]
- Chandra P, Wolfenden LL, Ziegler TR, Tian J, Luo M, Stecenko AA, Chen TC, Holick MF, Tangpricha V 2007 Treatment of vitamin D deficiency with UV light in patients with malabsorption syndromes: a case series. Photodermatol Photoimmunol Photomed 23:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AR, Kline L, Holick MF 1988 Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67:373–378 [DOI] [PubMed] [Google Scholar]
- Robberecht E, Vandewalle S 2008 Cholecalciferol and 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr 87:190; author reply, 190–191 [DOI] [PubMed] [Google Scholar]
- Brault Dubuc M, Caron Lahaie L 2003 Valeur nutritive des aliments. 9th ed. St-Lambert, Québec, Canada: Société Brault-Lahaie; 331 [Google Scholar]
- Tsui LC 1992 The spectrum of cystic fibrosis mutations. Trends Genet 8:392–398 [DOI] [PubMed] [Google Scholar]
- Aris RM, Merkel PA, Bachrach LK, Borowitz DS, Boyle MP, Elkin SL, Guise TA, Hardin DS, Haworth CS, Holick MF, Joseph PM, O'Brien K, Tullis E, Watts NB, White TB 2005 Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab 90:1888–1896 [DOI] [PubMed] [Google Scholar]
- Hanly JG, McKenna MJ, Quigley C, Freaney R, Muldowney FP, FitzGerald MX 1985 Hypovitaminosis D and response to supplementation in older patients with cystic fibrosis. Q J Med 56:377–385 [PubMed] [Google Scholar]
- Lark RK, Lester GE, Ontjes DA, Blackwood AD, Hollis BW, Hensler MM, Aris RM 2001 Diminished and erratic absorption of ergocalciferol in adult cystic fibrosis patients. Am J Clin Nutr 73:602–606 [DOI] [PubMed] [Google Scholar]
- Green D, Carson K, Leonard A, Davis JE, Rosenstein B, Zeitlin P, Mogayzel Jr P 2008 Current treatment recommendations for correcting vitamin D deficiency in pediatric patients with cystic fibrosis are inadequate. J Pediatr 153:554–559 [DOI] [PubMed] [Google Scholar]
- Houghton LA, Vieth R 2006 The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr 84:694–697 [DOI] [PubMed] [Google Scholar]
- Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW 2008 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr 87:1738–1742 [DOI] [PubMed] [Google Scholar]
- Nilsson SF, Ostberg L, Peterson PA 1972 Binding of vitamin D to its human carrier plasma protein. Biochem Biophys Res Commun 46:1380–1387 [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, Pineau T, Saric J, Navarro F, Maurel P, Vilarem MJ 2005 Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest 115:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman LS, Cassidy JT, Popescu MF, Hewett JE, Kyger J, Robertson JD 2008 Percent true calcium absorption, mineral metabolism, and bone mineralization in children with cystic fibrosis: effect of supplementation with vitamin D and calcium. Pediatr Pulmonol 43:772–780 [DOI] [PubMed] [Google Scholar]