Abstract
Context: Impairments in the pituitary-gonadal axis with aging are associated with loss of muscle mass and function and accumulation of upper body fat.
Objectives: We tested the hypothesis that physiological supplementation with testosterone and GH together improves body composition and muscle performance in older men.
Design, Setting, and Participants: One hundred twenty-two community-dwelling men 70.8 ± 4.2 yr of age with body mass index of 27.4 ± 3.4 kg/m2, testosterone of 550 ng/dl or less, and IGF-I in lower adult tertile (≤167 ng/dl) were randomized to receive transdermal testosterone (5 or 10 g/d) during a Leydig cell clamp plus GH (0, 3, or 5 μg/kg · d) for 16 wk.
Main Outcome Measures: Body composition by dual-energy x-ray absorptiometry, muscle performance, and safety tests were conducted.
Results: Total lean body mass increased (1.0 ± 1.7 to 3.0 ± 2.2 kg) as did appendicular lean tissue (0.4 ± 1.4 to 1.5 ± 1.3 kg), whereas total fat mass decreased by 0.4 ± 0.9 to 2.3 ± 1.7 kg as did trunk fat (0.5 ± 0.9 to 1.5 ± 1.0 kg) across the six treatment groups and by dose levels for each parameter (P ≤ 0.0004 for linear trend). Composite maximum voluntary strength of upper and lower body muscles increased by 14 ± 34 to 35 ± 31% (P < 0.003 in the three highest dose groups) that correlated with changes in appendicular lean mass. Aerobic endurance increased in all six groups (average 96 ± 137sec longer). Systolic and diastolic blood pressure increased similarly in each group with mean increases of 12 ± 14 and 8 ± 8 mm Hg, respectively. Other predictable adverse events were modest and reversible.
Conclusions: Supplemental testosterone produced significant gains in total and appendicular lean mass, muscle strength, and aerobic endurance with significant reductions in whole-body and trunk fat. Outcomes appeared to be further enhanced with GH supplementation.
Supplemental testosterone produces significant gains in total and appendicular lean mass, muscle strength, and aerobic endurance with significant reductions in whole body and trunk fat that are enhanced with growth hormone supplementation.
Alterations in body composition, physical function, and substrate metabolism occur with advancing age. Loss of skeletal muscle mass (sarcopenia) (1,2) contributes to declines in muscle strength and function along with diminished quality of life (3). In the Baltimore Longitudinal Aging Study, quadriceps strength decreased about 30% between 50–70 yr of age (4). In the Copenhagen Heart Study, leg strength in 80-yr-olds was 20–30% lower than in 70-yr-olds (5,6). Substantial losses in strength may result in difficulty rising from a chair, climbing stairs, generating gait speed, and maintaining balance (7), eventually resulting in frailty. These changes contribute to loss of independence, social isolation, depression, and inactivity, thereby increasing the risk for disability, osteoporosis, and bone fractures. Advancing age is also associated with upper body obesity and insulin resistance, both risk factors for accelerated atherogenesis (8).
Coincident with these age-related deteriorations in clinical status, endogenous production of anabolic hormones declines (9). Approximately 25–30% of men over 60 yr of age have hypogonadal testosterone levels (10) that may be associated with sarcopenia, muscle weakness, and upper body obesity (9,11,12). Restoring testosterone to youthful levels has increased synthesis of myofibrillar proteins (13), total body cell mass (14), muscle strength (13,15), and reduced trunk and visceral fat; blood pressure; lipids; and improved insulin sensitivity (16,17). It is unclear whether these benefits translate to enhanced functional performance (18). Declines in GH and IGF-I may also contribute to these age-related comorbidities in persons with normal testosterone levels (9,19). After puberty, 24-h GH production decreases progressively by about 14% per decade and up to 70% by the eighth decade of life (20,21,22). Similarly, circulating levels of IGF-I, a mediator of several but not all anabolic effects of GH, decline through the eighth to ninth decades with levels below the 2.5 percentile in 85–90% of older men (9) along with losses of lean tissue and increases in adiposity (23,24). In obese adults, GH supplementation may reduce abdominal fat (25,26,27,28).
Better understanding of the relative contributions of the testosterone and GH/IGF-I axes to sarcopenia, impaired muscle performance, and obesity could have therapeutic implications (29). Only two single-site studies investigated the effects of administering these hormones in combination but both used supraphysiological doses of recombinant human (rh) GH and failed to demonstrate substantive improvements in muscle performance (30,31). Our hypothesis was that endogenous testosterone and GH are important independent but complementary regulators of skeletal muscle mass and function, central obesity, and substrate metabolism throughout life into advanced age. To test this hypothesis, we conducted a multicenter study in older, community-dwelling men with levels of testosterone and IGF-I typical of their age to determine the effects of augmenting testosterone with a transdermal gel on muscle mass, physical performance, and adiposity and whether these effects could be augmented by increasing GH-IGF-I status with physiological doses of recombinant human GH.
Subjects and Methods
Study design
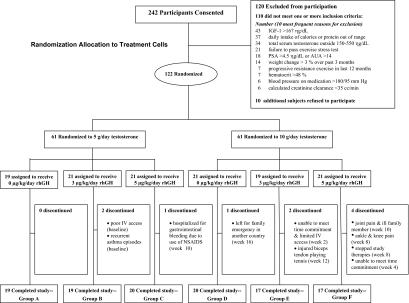
The Hormonal Regulators of Muscle and Metabolism in Aging study was a randomized, controlled, double-masked investigation of physiologic supplementation with testosterone and rhGH in older community-dwelling men who had levels of testosterone and IGF-I typical of older men. Randomization was two tiered (Fig. 1). Eligible participants were randomized first to either low or high eugonadal levels of testosterone using a Leydig cell clamp to fully suppress endogenous testosterone, thereby minimizing potential confounding. Treatment with exogenous testosterone alone often leads to variable inhibition of LH and endogenous testosterone production, resulting in substantial heterogeneity of serum testosterone levels during therapy. Participants were further randomized (second tier) to placebo or one of two doses of rhGH. Treatment duration was 16 wk; postintervention outcomes were determined during wk 16 and 17.
Figure 1.
Schema depicts the subjects screened for study, most common reasons for exclusion, numbers of eligible subjects enrolled and how they were randomized to study therapies, reasons for study discontinuation during the 16 wk of treatment interventions, and final numbers of evaluable subjects in the six allocation groups. NSAID, nonsteroidal antiinflammatory drug.
Study participants
Subjects providing local institutional review board-approved informed consent were screened at the University of Southern California (USC), Tufts University, and Washington University to enroll participants from different geographic areas to assure generalizability of outcomes. Eligibility required that men 65–90 yr of age have serum IGF-I in the lower tertile for adults (<167 ng/ml; 21.9 nmol/liter) and morning total serum testosterone in the lower half (150–550 ng/dl; 5.21–19.1 nmol/liter) of the adult male range. Other eligibility criteria included prostate-specific antigen (PSA) 4.0 ng/ml or less, hematocrit 50% or less, and fasting blood glucose less than 126 mg/dl (6.99 mmol/liter).
Study interventions
All subjects were treated monthly from baseline to wk 12 using a Leydig cell clamp with a long acting GnRH agonist (leuprolide acetate depot, 7.5 mg im; TAP Pharmaceutical Products Inc., Deerfield, IL) and either 5 g (groups A–C) or 10 g (groups D–F) of 1% testosterone transdermal gel (Solvay Pharmaceuticals Inc., Marietta, GA) was applied each morning for 16 wk. Participants also self-administered 0, 3, or 5 μg/kg rhGH (Genentech Inc., South San Francisco, CA; groups A/D, B/E, and C/F, respectively) as sc injections 2–3 h after dinner each evening (Fig. 1).
The 5- and 10-g doses of testosterone were chosen to produce a spectrum of serum levels via the Leydig cell clamp that were in the low normal range typical of older men or mid- to high-normal levels typical of younger men, respectively (32). The 3 μg/kg dose of rhGH was chosen because 3.3 but not 2.0 μg/kg · d increased whole-body protein synthesis in GH-deficient adults (33). The 5 μg/kg · d dose was chosen to produce a greater anabolic stimulus but was expected to be low enough to minimize adverse effects that have occurred with higher doses (34,35).
Safety
Study participants were evaluated for adverse events at wk 4, 8, 12, and 16. At each visit, blood pressure was measured thrice in each arm with 5-min intervals between readings; the lowest value was used for analysis. An independent Data Safety Monitoring Board held prescribed interim safety analyses after the first 30 and 70 participants had completed study therapies and recommended that the study continue. Adverse events were monitored until resolution and sex hormones were measured 12 wk after completion of study therapy in all participants.
Outcome measures
Body composition
Whole-body and regional lean and fat mass were quantified by dual-energy x-ray absorptiometry (DEXA), calibrated using a soft tissue phantom. Scans were analyzed at the USC Reading Center by an experienced DEXA-certified bionutritionist blinded to study assignment.
Muscle performance
Maximal voluntary muscle strength was assessed using the one-repetition maximum (1-RM) method (36) twice before randomization to minimize learning effects and after completion of study therapies (wk 17) for the bilateral leg press, leg extension, leg flexion, latissimus pull-down, and chest press (37). To normalize and consolidate whole-body strength assessments, results are presented as percentage change from baseline for the composite sum of 1-RM values for the five strength exercises.
Aerobic capacity
At baseline and wk 16, peak O2 consumption (VO2) was assessed by cycle ergometry having subjects pedal at 60 rpm with 15 or 20 W/min ramp protocols. Peak VO2 was the highest O2 consumption when subjects could not maintain a pedaling rate of 55 rpm or greater. After a 45-min rest period, aerobic endurance was determined as the length of time participants could cycle at 60 rpm at a constant workload of 80% of peak work (watts) achieved during the baseline peak VO2 test.
Hormone assays
For screening, total testosterone was measured using immunoassays in the local clinical university laboratories and IGF-I at Quest Diagnostics (San Juan Capistrano, CA). Testosterone, IGF-I, and insulin levels were determined after completion of the study by batch testing serum samples obtained at baseline and wk 16. Testosterone levels were quantified using a validated liquid chromatography-tandem mass spectrometry assay (38) at Boston Medical Center [interassay coefficients of variation (CVs) at 250 and 500 ng/dl (8.68 and 17.4 nmol/liter) were 5 and 3%, and intraassay CV was 3 and 2%, respectively]. IGF-I and insulin levels were determined in the USC GCRC Endocrine Core Laboratory. For IGF-I, samples were analyzed using an automated immunoassay analyzer [Immulite 1000; Siemens Healthcare Diagnostics, Deerfield, IL; sensitivity 20 ng/ml (2.6 nmol/liter), interassay CV 3.6% and intraassay CV 6.6%]. Insulin levels were analyzed using an automated enzyme immunoassay (Tosoh AIA 600 II analyzer; Tosoh Bioscience, Inc., South San Francisco, CA; sensitivity 0.31 μIU/ml, interassay CV 6.1%, intraassay CV 4.8%). homeostasis model assessment insulin resistance index (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were used to assess insulin resistance and sensitivity, respectively (39,40).
Statistical considerations
Sample size considerations
Sample size calculations were determined for changes in body composition based on effect sizes demonstrated in several of our prior studies of anabolic androgens in similar older populations (41,42,43). With 18 subjects per group (total n = 108), this provided greater than 95% power to detect: 1) a mean increase in total lean body mass (LBM) of 1.5 kg or decrease in total fat of 1.5 kg within each of the six treatment interventions,(paired t test with Bonferroni adjusted significance level of 0.05/6 = 0.0083), and 2) a between-group mean difference in total LBM or fat mass of 1.0 kg, (using a two group t test with an adjustment to account for all 15 pairwise comparisons). For the secondary outcomes including appendicular LBM, trunk fat, measures of muscle performance, and safety parameters, these analyses were exploratory without a priori power calculations. To account for an anticipated 10% dropout rate, 122 subjects were enrolled. Using one-way ANOVA with pairwise comparisons (adjusting for multiple comparisons), a sample size of 18 per group provided 80% power to detect at the 0.05 level small effect sizes of 0.19 or greater for the other outcomes.
Statistical analysis
Analyses were conducted for evaluable subjects (n = 112) who completed 16 wk of hormone treatments. Baseline characteristics were compared across the six intervention groups using one-way ANOVA for continuous variables and chi-square or Fisher’s exact test for discrete variables. The primary analyses were directed at comparisons of DEXA and muscle performance outcomes across and within the six groups. One-way ANOVA was used to compare baseline and wk 17 values across the six treatment groups, and one-way analysis of covariance (ANCOVA) was used to compare the primary outcome change score (wk 17 minus baseline) values adjusted for the baseline value as a covariate across the six treatment groups. Tukey pairwise comparisons (n = 15) were used to assess differences in the baseline adjusted change scores between the six treatment interventions.
Linear trend was assessed using the Wald test to examine dose responses across the six groups, and a two-way ANCOVA was used to determine whether there were interactions between testosterone (two levels) and GH (three levels) interventions. In addition, we performed paired t tests for each variable within each of the six treatment group to test whether the change scores were significant at the 0.05/6 = 0.0083 alpha level using a Bonferoni adjustment for multiple analyses. Finally, adverse events were contrasted across groups using Fisher’s exact test. Changes in safety parameters were tested using the paired t test or signed rank test. Statistical analyses were carried out using the Statistical Analysis System 9.1 (SAS Institute Inc., Cary, NC).
Results
Study population
The first study participant was enrolled in June 2003 and the final participant completed evaluation in May 2007. Two hundred forty-two subjects were consented and screened for eligibility (Fig. 1). Of these, 122 eligible subjects were randomized to study interventions; 10 dropped out of study after randomization (three from the 5 g/d testosterone arm and seven from the 10 g/d testosterone arm); one of the dropouts was randomized to rhGH placebo, four to 3 μg/kg · d rhGH and five to 5 μg/kg · d rhGH. Thus, 112 participants completed all assessments at wk 17 and serve as the primary data set for analysis. Baseline characteristics before study interventions, including average body mass index of 27.4 ± 3.4 kg/m2, testosterone of 360 ± 98 ng/dl (12.5 ± 3.4 nmol/liter), and IGF-I (an indirect measure of GH status) of 111 ± 29 ng/dl (14.5 ± 3.8 nmol/liter) were similar among the six groups (Table 1). Participants were ambulatory, free-living men with peak VO2 typical of older persons without functional limitations (44).
Table 1.
Baseline characteristics for treatment groups
| Daily dose by study group | Testosterone, 5 g
|
Testosterone, 10 g
|
P valuea | ||||
|---|---|---|---|---|---|---|---|
| GH 0 (n = 19) Group A | GH 3 μg (n = 19) Group B | GH 5 μg (n = 20) Group C | GH 0 (n = 20) Group D | GH 3 μg (n = 17) Group E | GH 5 μg (n = 17) Group F | ||
| Age, yr | 72.7 ± 5.1b | 71.3 ± 3.9 | 70.0 ± 4.1 | 70.2 ± 4.6 | 69.9 ± 3.2 | 70.5 ± 3.9 | 0.32 |
| Ethnicity/race | |||||||
| Caucasian | 14 (74%) | 18 (95%) | 18 (90%) | 15 (75%) | 15 (88%) | 16 (94%) | 0.24 |
| Minority | 5 (26%) | 1 (5%) | 2 (10%) | 5 (25%) | 2 (12%) | 1 (6%) | |
| Medical history | |||||||
| Hypertension | 9 (47%) | 4 (21%) | 7 (35%) | 3 (15%) | 3 (18%) | 4 (24%) | 0.20 |
| History of cardiovascular disease | 2 (11%) | 1 (5%) | 5 (25%) | 2 (10%) | 0 (0%) | 3 (18%) | 0.21 |
| Elevated cholesterolc | 5 (26%) | 7 (37%) | 6 (35%) | 8 (45%) | 5 (29%) | 6 (35%) | 0.95 |
| History of smoking | 7 (37%) | 7 (37%) | 8 (40%) | 6 (30%) | 9 (53%) | 4 (24%) | 0.61 |
| Blood pressure | |||||||
| Systolic blood pressure, mm Hg | 121 ± 15 | 117 ± 13 | 113 ± 13 | 118 ± 15 | 119 ± 13 | 116 ± 12 | 0.57 |
| Diastolic blood pressure, mm Hg | 70 ± 8 | 68 ± 7 | 66 ± 7 | 68 ± 5 | 68 ± 6 | 69 ± 9 | 0.57 |
| Laboratory values | |||||||
| Hematocrit, % | 43.3 ± 3.5 | 43.3 ± 1.6 | 44.1 ± 2.5 | 42.1 ± 2.9 | 43.2 ± 2.7 | 43.2 ± 2.2 | 0.34 |
| Creatinine clearance, ml/mind | 79.8 ± 9.3 | 76.3 ± 21.5 | 88.3 ± 22.1 | 81.0 ± 15.5 | 87.4 ± 16.9 | 82.6 ± 22.2 | 0.33 |
| Albumin, g/dl | 4.0 ± 0.3 | 4.2 ± 0.4 | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.3 | 0.43 |
| Alanine aminotransferase, U/liter | 29.1 ± 8.4 | 30.4 ± 6.9 | 31.1 ± 10.7 | 30.0 ± 10.0 | 31.6 ± 12.3 | 32.0 ± 10.3 | 0.95 |
| Prostate | |||||||
| AUA score | 4.1 ± 3.7)c | 4.1 ± 2.8 | 5.2 ± 4.0 | 4.8 ± 4.0 | 4.6 ± 5.2 | 5.1 ± 4.0 | 0.91 |
| PSA ng/ml | 1.8 ± 1.0 | 1.3 ± 0.7 | 1.5 ± 1.0 | 1.4 ± 0.8 | 1.7 ± 0.8 | 1.4 ± 0.9 | 0.45 |
| Hormones | |||||||
| TSH, mIU/liter | 2.1 ± 1.0 | 2.2 ± 1.3 | 2.1 ± 1.3 | 1.9 ± 1.1 | 2.3 ± 1.3 | 2.6 ± 1.7 | 0.71 |
| Total testosterone, ng/dl | 385 ± 106 | 377 ± 103 | 373 ± 89 | 350 ± 98 | 359 ± 89 | 311 ± 94 | 0.24 |
| IGF-I, ng/ml | 101 ± 23 | 109 ± 24 | 115 ± 31 | 105 ± 32 | 127 ± 30 | 114 ± 32 | 0.11 |
| Metabolic measurements | |||||||
| Fasting glucose, mg/dl | 92 ± 9 | 93 ± 8 | 93 ± 10 | 89 ± 9 | 92 ± 18 | 94 ± 9 | 0.78 |
| HOMA-IRe | 1.52 ± 0.83 | 1.53 ± 1.48 | 1.81 ± 1.05 | 1.33 ± 0.61 | 1.54 ± 1.15 | 1.73 ± 0.93 | 0.76 |
| QUICKIe | 0.16 ± 0.01 | 0.16 ± 0.02 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.02 | 0.16 ± 0.02 | 0.55 |
| Total cholesterol, mg/dl | 172 ± 24 | 174 ± 29 | 171 ± 33 | 180 ± 28 | 179 ± 27 | 174 ± 33 | 0.91 |
| HDL cholesterol, mg/dl | 43 ± 8 | 46 ± 17 | 40 ± 11 | 45 ± 9 | 44 ± 14 | 42 ± 12 | 0.65 |
| LDL cholesterol, mg/dl | 104 ± 30 | 105 ± 28 | 101 ± 26 | 110 ± 25 | 112 ± 24 | 105 ± 27 | 0.86 |
| Fasting triglycerides, mg/dl | 127 ± 65 | 113 ± 41 | 142 ± 69 | 126 ± 63 | 115 ± 50 | 131 ± 73 | 0.72 |
| Body composition | |||||||
| Weight, kg | 79.1 ± 10.4 | 80.0 ± 13.2 | 86.0 ± 11.2 | 85.9 ± 14.0 | 86.1 ± 13.9 | 83.7 ± 11.4 | 0.31 |
| Body mass index, kg/m2 | 26.8 ± 3.5 | 25.9 ± 3.0 | 28.2 ± 3.2 | 28.5 ± 3.8 | 27.6 ± 3.2 | 27.3 ± 3.2 | 0.19 |
| Muscle performance | |||||||
| Peak VO2, ml/kg · min | 23.9 ± 6.2 | 26.2 ± 4.0 | 24.1 ± 3.5 | 24.1 ± 6.8 | 23.8 ± 3.7 | 25.4 ± 4.1 | 0.68 |
SI conversions: alanine aminotransferase (μkat per liter = units per liter × 0.0167); glucose (millimoles per liter = milligrams per deciliter × 0.0555); testosterone (nanomoles per liter = nanograms per deciliter × 0.0347); IGF-I (nanomoles per liter = nanograms per milliliter × 0.131); cholesterol (total, LDL, HDL; millimoles per liter = milligrams per deciliter × 0.0259); triglycercides (millimoles per liter = milligrams per deciliter × 0.0113). AUA, American Urological Association.
ANOVA for continuous variables; χ2 or Fisher’s exact test for discrete variables.
Mean ± 1 sd for continuous variables and frequency (percent) for discrete variables.
Elevated cholesterol is use of cholesterol-lowering medication.
Creatinine clearance = [(140 − age) × wt]/72 × serum creatinine.
HOMA-IR = [(If) × (Gf)]/22.5; QUICKI = 1/[log (If) + log (Gf)], where (If) is the fasting insulin level (microunits per milliliter) and (Gf) is the fasting glucose level (millimoles per liter) for HOMA-IR.
Changes in testosterone and IGF-I levels
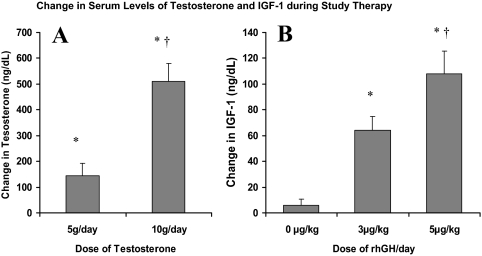
As expected, testosterone and rhGH administration produced dose-related increments in serum testosterone and IGF-I concentrations (Fig. 2). Testosterone levels increased in 58 subjects receiving 5 g/d by 143 ± 379 ng/dl (4.96 ± 13.2 nmol/liter; P < 0.001), which was lower (P < 0.001) than the increase of 510 ± 503 ng/dl (17.7 ± 17.5 nmol/liter; P < 0.001) in 54 subjects receiving 10 g/d. Changes in serum testosterone concentrations did not differ significantly among the three groups (A–C) receiving testosterone gel at 5 g/d or the three groups (D–F) receiving 10 g/d. Treatment with rhGH at 0, 3, and 5 μg/kg · d increased serum IGF-I levels by 6 ± 28 ng/dl (0.79 ± 3.7 nmol/liter; n = 39; P = 0.15), 64 ± 44 ng/dl (8.4 ± 5.8 nmol/liter; n = 36, P < 0.001), and 108 ± 51 ng/dl (14.1 ± 6.7 nmol/; n = 37, P < 0.001), respectively, with a significant trend across the rhGH dose groups (P < 0.001). The higher testosterone dose alone was associated with a small but significant increase in IGF-I in group D that did not receive rhGH (14 ± 28 ng/dl, 1.8 ± 3.7 nmol/liter; P = 0.03), consistent with the reported enhanced hepatic synthesis of IGF-I in response to the higher dose of testosterone (45).
Figure 2.
Changes (mean ± 1 se) in serum testosterone (A) and IGF-I (B) from baseline to wk 16 according to dose. *, Within group significant changes (P < 0.05); †, significant difference in change (P < 0.001) between 5 and 10 g/d testosterone dose groups and significant differences in change (P < 0.05) for IFG-1 between the 3 or 5 μg/kg · d and the 0 μg/kg · d rhGH dose groups.
Changes in body composition
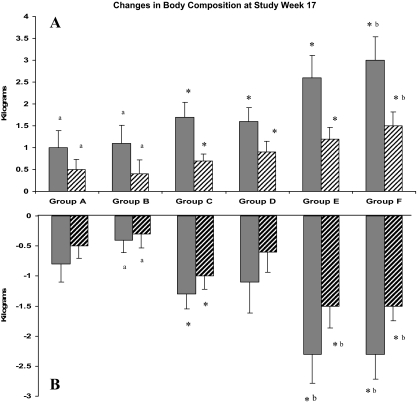
The mean increases in total LBM at wk 17 ranged from 1.0 ± 1.7 kg in group A to 3.0 ± 2.2 kg in group F (P = 0.0002, linear trend) with maximum gains in groups E and F of 6.9 and 7.5 kg, respectively. Using the Bonferoni adjustment (P < 0.008), significant changes occurred in groups C–F. The mean decrease in total fat at wk 17 ranged from −0.8 ± 1.3 kg in group A to −2.3 ± 1.7 kg in group F (P = 0.0002, linear trend; Table 2 and Fig. 3) with maximal losses of −6.4 kg and −7.1 kg in groups E and F, respectively. Bonferoni-adjusted significant changes occurred in groups C, E, and F. Changes in regional mass (appendicular lean and trunk fat) at wk 17 followed similar patterns and levels of significance (Table 2 and Fig. 3). At wk 17, total LBM increased more for the 54 subjects receiving 10 g/d than for the 58 subjects receiving 5 g/d (2.3 ± 2.0 vs. 1.3 ± 1.7 kg, P = 0.003). Total fat decreased more for subjects receiving 10 g/d than for those receiving 5 g/d (−1.8 ± 2.1 vs. −0.9 ± 1.2 kg, P = 0.003). There was also a linear trend across the placebo, 3 μg/kg · d, and 5 μg/kg · d rhGH doses for increases in total LBM (1.3 ± 1.6, 1.8 ± 2.1, and 2.3 ± 2.0 kg, respectively, P = 0.02) and decreases in total fat (−1.0 ± 1.9, − 1.3 ± 1.8, and − 1.7 ± 1.5 kg, respectively, P = 0.05). Two-way ANCOVA showed no interactions of the two hormones on body composition changes (Table 2).
Table 2.
Change in body composition by treatment group
| Body composition | Testosterone, 5 g/d
|
Testosterone, 10 g/d
|
P values | ||||
|---|---|---|---|---|---|---|---|
| rhGH 0 (n = 19) Group A | rhGH 3 μg (n = 19) Group B | rhGH 5 μg (n = 20) Group C | rhGH 0 (n = 20) Group D | rhGH 3 μg (n = 17) Group E | rhGH 5 μg (n = 17) Group F | ||
| Total LBM, kg | |||||||
| Baseline | 55.6 ± 5.0a* | 57.7 ± 8.3 | 59.2 ± 8.1 | 59.0 ± 6.0 | 58.9 ± 7.4 | 59.1 ± 5.8 | 0.56b |
| Week 17 | 56.6 ± 4.7 | 58.8 ± 8.7 | 60.9 ± 8.3 | 60.6 ± 6.3 | 61.5 ± 7.8 | 62.1 ± 6.1 | 0.20b |
| Change (wk 17-baseline) | 1.0 ± 1.7* | 1.1 ± 1.8* | 1.7 ± 1.5 | 1.6 ± 1.4 | 2.6 ± 2.1 | 3.0 ± 2.2** | 0.01c |
| 0.7 (−2.0, 4.5)d* | 0.7 (−1.4, 4.8) | 1.5 (−2.4, 4.4) | 1.5 (−0.8, 4.7) | 1.9 (0.2, 7.5) | 2.6 (−0.7, 6.9) | 0.0002e | |
| P valuef | 0.02 | 0.02 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | 0.52g |
| Appendicular LBM, kg | |||||||
| Baseline | 24.2 ± 2.4 | 25.2 ± 3.8 | 26.0 ± 3.7 | 25.8 ± 2.7 | 25.8 ± 3.7 | 26.1 ± 3.1 | 0.49 |
| Week 17 | 24.6 ± 2.4 | 25.6 ± 3.9 | 26.7 ± 3.8 | 26.7 ± 2.5 | 27.0 ± 3.8 | 27.6 ± 3.2 | 0.10 |
| Change (wk 17-baseline) | 0.5 ± 1.0* | 0.4 ± 1.4* | 0.7 ± 0.7 | 0.9 ± 1.1 | 1.2 ± 1.1 | 1.5 ± 1.3** | 0.01 |
| 0.6 (−1.2, 2.9) | 0.0 (−2.0, 3.5) | 0.9 (−0.8, 1.8) | 0.8 (−0.6, 2.8) | 1.1 (−0.8, 3.5) | 1.6 (−0.5, 4.4) | 0.0002 | |
| P value | 0.06 | 0.25 | 0.0002 | 0.002 | 0.0003 | <0.0002 | 0.77 |
| Total fat mass, kg | |||||||
| Baseline | 20.9 ± 6.5 | 19.5 ± 5.8 | 23.8 ± 4.6 | 24.2 ± 9.2 | 24.3 ± 7.7 | 21.7 ± 8.1 | 0.21 |
| Week 17 | 20.1 ± 6.5 | 19.1 ± 6.0 | 22.5 ± 4.3 | 23.1 ± 8.4 | 22.0 ± 7.3 | 19.4 ± 7.8 | 0.31 |
| Change (wk 17-baseline) | −0.8 ± 1.3 | −0.4 ± 0.9* | −1.3 ± 1.1 | −1.1 ± 2.3 | −2.3 ± 2.0** | −2.3 ± 1.7** | 0.002 |
| −0.5 (−4.1, 1.0) | −0.2 (−2.0, 1.0) | −1.3 (−4.3, 1.3) | −0.7 (−7.0, 2.6) | −1.7 (−6.4, 0.9) | −2.1 (−7.1, 0.1) | 0.0004 | |
| P value | 0.02 | 0.06 | <0.0001 | 0.048 | 0.0003 | <0.0001 | 0.11 |
| Trunk fat, kg | |||||||
| Baseline | 12.2 ± 4.1 | 10.8 ± 3.5 | 13.9 ± 2.5 | 13.7 ± 5.1 | 13.9 ± 4.8) | 11.9 ± 4.8 | 0.12 |
| Week 17 | 11.7 ± 4.0 | 10.5 ± 3.6 | 12.9 ± 2.4 | 13.1 ± 4.7 | 12.3 ± 4.5 | 10.4 ± 4.7 | 0.16 |
| Change (wk 17-baseline) | −0.5 ± 0.9 | −0.3 ± 1.0* | −1.0 ± 1.0* | −0.6 ± 1.5 | −1.5 ± 1.3** | −1.5 ± 1.0** | 0.004 |
| −0.5 (−2.4, 0.8) | −0.4 (−1.8, 2.8) | −1.1 (−3.2, 0.5) | −0.5 (−4.6, 1.7) | −1.2 (−4.6, 0.2) | −1.6 (−4.5, 0.1) | 0.0003 | |
| P value | 0.02 | 0.08 | 0.0003* | 0.08 | <0.0001 | <0.0001 | 0.15 |
Data are means ± 1 sd.
One-way ANOVA across treatment groups at baseline and wk 17.
One way ANCOVA for change (wk 17-baseline) across treatment groups, adjusting for baseline.
Median (range).
Wald test for trend across treatment group.
Paired t test for mean change from baseline to wk 17.
Two-way ANCOVA test for interaction.
*, **Pairs of groups with different characters (* vs. **) are significantly different using the Tukey pairwise comparison procedure (P < 0.05).
Figure 3.
DEXA-derived changes (mean ± 1 se) in LBM and fat mass for each treatment group from baseline to wk 17. A, Increases in total LBM (solid bars) and appendicular lean mass (hatched bars). Changes across groups are significant for linear trend for total lean mass (P = 0.0002) and appendicular lean (P = 0.0002). B, Decreases in total body fat mass (solid bars) and trunk fat (hatched bars). Changes across groups are significant for linear trend for total fat mass (P = 0.0004) and trunk fat (P = 0.0003). *, Bonferoni adjusted within group changes (P < 0.008). Pairs of treatment groups with different letters (e.g. a vs. b) are significantly different by one-way ANCOVA with pairwise comparison (Tukey adjusted; P < 0.05).
In pairwise analyses at wk 17, increase in total LBM in group F (3.0 ± 2.2 kg) was greater than in groups A and B (1.0 ± 1.7 and 1.1 ± 1.8 kg, P = 0.02 and P = 0.03, respectively). Fat losses in groups E and F (−2.3 ± 2.0 and −2.3 ± 1.7 kg) were greater than losses in group B (−0.4 ± 0.9 kg, P = 0.01 for both).
Changes in muscle performance
Maximal voluntary muscle strength
By wk 17, three interventions (groups D–F) produced significant (P < 0.008) improvements ranging from 23 ± 27% up to 35 ± 31% (P = 0.08 for linear trend) for composite maximal voluntary strength with the greatest increases in groups receiving combined treatment with 10 g testosterone gel plus rhGH (groups E and F, Table 3). Pairwise comparisons showed no differences between the groups (P ≥ 0.50). For 95 subjects with paired data, improvements in composite strength for the 58 subjects receiving 5 g/d testosterone (18 ± 38%) and 54 subjects receiving 10 g/d (30 ± 27%) were similar (P = 0.09) as were changes for those receiving placebo vs. any dose of rhGH (22 ± 39 vs. 25 ± 30%, P = 0.76).
Table 3.
Change in composite maximum voluntary strength and aerobic endurance
| Testosterone, 5 g/d
|
Testosterone, 10 g/d
|
P value | |||||
|---|---|---|---|---|---|---|---|
| rhGH 0 (n = 19) Group A | rhGH 3 μg (n = 19) Group B | rhGH 5 μg (n = 20) Group C | rhGH 0 (n = 20) Group D | rhGH 3 μg (n = 17) Group E | rhGH 5 μg (n = 17) Group F | ||
| Composite strength (1-RM) | |||||||
| Number of paired subjects | 16 | 16 | 17 | 17 | 14 | 15 | |
| Change at wk 17, % | 22 ± 50a | 14 ± 34 | 19 ± 28 | 23 ± 27 | 32 ± 22 | 35 ± 31 | 0.50b |
| 15 (−104, 117)c | 10 (−35, 90) | 18 (−24, 79) | 22 (−17, 83) | 34 (−3, 73) | 29 (−22, 91) | 0.08d | |
| P valuee | 0.10 | 0.12 | 0.01 | 0.003 | 0.001 | 0.001 | 0.55f |
| Aerobic endurance, sec | |||||||
| Number of paired subjects | 14 | 15 | 16 | 14 | 13 | 14 | |
| Baseline | 318 ± 90 | 411 ± 157* | 332 ± 122 | 336 ± 119 | 260 ± 69** | 320 ± 81 | 0.02g |
| Week 16 | 457 ± 204 | 498 ± 235 | 399 ± 169 | 390 ± 144 | 355 ± 88 | 510 ± 219 | 0.16g |
| Change at wk 16 | 143 ± 166 | 69 ± 122 | 67 ± 114 | 51 ± 77 | 95 ± 90 | 160 ± 200 | 0.17b |
| 102 (−38, 600) | 78 (−112, 349) | 45 (−76, 422) | 51 (−91, 246) | 75 (−53, 287) | 140 (−56, 730) | 0.03d | |
| P value | 0.007 | 0.045 | 0.03 | 0.03 | 0.003 | 0.01 | 0.57f |
Data are means ± 1 sd.
One-way ANCOVA for change (wk 17-baseline) across treatment groups, adjusting for baseline.
Median (range).
Wald test for trend across treatment group.
Paired t test for mean change from baseline to wk 17.
Two-way ANCOVA test for interaction.
One-way ANOVA across treatment groups at baseline and wk 17.
*, **Pairs of groups with different characters (* vs. **) are significantly different using the Tukey pairwise comparison procedure (P < 0.05).
For the 95 subjects with paired DEXA and composite strength tests at baseline and wk 17, increases in strength were correlated with increases in total LBM (r = 0.32, P = 0.001). Increases in strength and appendicular lean mass were also correlated (r = 0.30, P = 0.003). There were no significant treatment interactions for testosterone and rhGH group assignments by two-way ANCOVA for composite strength at wk 17 compared with baseline.
Aerobic endurance
For 86 subjects undergoing paired testing, endurance times increased in each of the six groups (averaging 96 ± 137 sec longer) by study wk 16 and reaching Bonferroni-adjusted significance (P < 0.008) in groups A and E (Table 3). Improvements in aerobic endurance at wk 16 were unrelated to the dose of testosterone or rhGH. In pairwise analyses, improvement in aerobic endurance was greater for group F than D (P = 0.03). There was no linear trend across the six treatment cells.
Safety measures and adverse events
Gonadal function as measured by testosterone and LH returned to baseline levels in all participants within 12 wk of discontinuing study therapy (data not shown). New adverse symptoms or physical findings occurring in greater than 5% of subjects were generally similar for the interventions (P > 0.05, Table 4). Aching or muscle pains occurred in 24 of 73 subjects receiving rhGH and 13 of 39 subjects receiving no rhGH (P = 0.28) and could not be related to dose levels. However, breast engorgement or nipple pain occurred transiently during study therapy but more often in the 54 subjects receiving 10 g of testosterone compared with the 58 receiving 5 g of testosterone daily (P = 0.006). Changes in American Urological Association scores were similar among five of the groups after 16 wk of therapy but increased minimally by 2 ± 4 in group D (P = 0.04).
Table 4.
Emergent adverse events during study therapies
| New symptom or finding | Testosterone, 5 g/d
|
Testosterone, 10 g/d
|
P value* | |||||
|---|---|---|---|---|---|---|---|---|
| GH 0 (n = 19) Group A | GH 3 μg (n = 19) Group B | GH 5 μg (n = 20) Group C | GH 0 (n = 20) Group D | GH 3 μg (n = 17) Group E | GH 5 μg (n = 17) Group F | |||
| Large joint pain or knee swelling | 40 (36%)a | 4 | 6 | 8 | 12 | 6 | 4 | 0.16 |
| Pretibial or ankle edema | 39 (35%) | 6 | 7 | 10 | 6 | 4 | 6 | 0.68 |
| General aching or muscle pains | 33 (29%) | 6 | 7 | 2 | 3 | 7 | 8 | 0.07 |
| New skin rash (local/general) or bruises | 26 (19%) | 4 | 3 | 3 | 4 | 6 | 6 | 0.54 |
| Transient new cough/nasal congestion | 17 (15%) | 0 | 5 | 3 | 3 | 2 | 4 | 0.21 |
| Back pain | 14 (13%) | 0 | 3 | 1 | 3 | 5 | 3 | 0.11 |
| Transient hot flashes after treatment | 13 (12%) | 1 | 2 | 4 | 3 | 1 | 2 | 0.77 |
| Breast engorgement or nipple pain | 16 (14%) | 2 | 1 | 0 | 5 | 3 | 5 | 0.051b |
| Hand or wrist stiffness or pain | 8 (7%) | 1 | 4 | 1 | 1 | 1 | 0 | 0.32 |
| Mean ± sd | ||||||||
| Change in AUA score | n/a | 0.3 ± 3.4 | 1.0 ± 5.0 | 0.2 ± 2.2 | 2.2 ± 4.4 | −0.2 ± 3.6 | 1.2 ± 5.0 | 0.50 |
AUA, American Urological Association.
Total number with symptom.
P = 0.006 by Fisher’s exact test comparing 58 subjects receiving 5 g vs. 54 subjects receiving 10 g testosterone daily.
Table 5 shows the changes in blood pressure, laboratory tests, and metabolic measures for the 112 subjects (for complete details of changes in individual treatment groups see supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org). Similar but significant increases in systolic and diastolic blood pressure occurred with each of the six interventions (average change 12 ± 14 and 8 ± 8 mm Hg, respectively). At follow-up over the ensuing 12 wk after discontinuation of study therapies, the average increases in systolic and blood pressure were lower but still elevated by 9 ± 14 and 6 ± 10 mm Hg, respectively (P < 0.001 for both compared with baseline). Hematocrit increased significantly in four of the six groups; eight subjects had increases to 50–52%, one to 53% and none to 54% or greater. After discontinuation of study interventions, hematocrit returned to less than 50% in all subjects. Although PSA increased in subjects by 0.2 ± 0.8 ng/ml, it increased significantly only in group F (from 1.1 ± 0.9 to1.8 ± 1.1 ng/ml, P = 0.003); no subject had a PSA increment greater than 1.4 ng/ml and values returned to baseline on repeated testing.
Table 5.
Change in safety measures during study therapy
| Safety parameter
|
n
|
Baseline Mean ± sd
|
n
|
Wk 16 Mean ± sd
|
n
|
Wk 16 from baseline Mean ± sd
|
P valuea
|
Groups with changes at P < 0.008
|
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure, mm Hg | 112 | 117 ± 14 | 112 | 130 ± 18 | 112 | 12 ± 14 | <0.0001 | A, D, F |
| Diastolic blood pressure, mm Hg | 112 | 68 ± 7 | 112 | 76 ± 9 | 112 | 8 ± 8 | <0.0001 | A, B, C, D, E, F |
| Hematocrit, % | 112 | 43.2 ± 2.7 | 112 | 45.2 ± 3.6 | 112 | 2.0 ± 3.2 | <0.0001 | D, E, F |
| PSA, ng/ml | 112 | 1.5 ± 0.9 | 112 | 1.7 ± 1.2 | 112 | 0.2 ± 0.8 | 0.0002 | F |
| Alanine aminotransferase, U/liter | 112 | 31 ± 10 | 112 | 31 ± 11 | 111 | 0.3 ± 10.2 | 0.43 | None |
| Fasting blood glucose, mg/dl | 111 | 92 ± 11 | 112 | 95 ± 13 | 111 | 3.1 ± 10.2 | 0.002 | None |
| HOMA-IRb | 111 | 1.57 ± 1.03 | 112 | 2.15 ± 2.18 | 111 | 0.6 ± 2.1 | 0.01 | None |
| QUICKIb | 111 | 0.16 ± 0.02 | 112 | 0.16 ± 0.02 | 111 | −0.004 ± 0.02 | 0.003 | None |
| Total cholesterol, mg/dl | 112 | 175 ± 29 | 112 | 177 ± 30 | 112 | 2.2 ± 27.3 | 0.20 | None |
| HDL cholesterol, mg/dl | 112 | 43 ± 12 | 112 | 47 ± 12 | 112 | 3.5 ± 6.7 | <0.0001 | E |
| LDL cholesterol, mg/dl | 112 | 106 ± 27 | 112 | 110 ± 29 | 112 | 3.8 ± 23.2 | 0.08 | None |
| Fasting triglycerides, mg/dl | 112 | 126 ± 61 | 112 | 108 ± 46 | 112 | −18.2 ± 57.0 | 0.0002 | F |
P values from paired t test or Wilcoxon signed rank test.
HOMA-IR = [(If) X (Gf)]/22.5; QUICKI = 1/[log (If) + log (Gf)], where (If) is the fasting insulin level (microunits per milliliter) and (Gf) is the fasting glucose level (millimoles per liter) for HOMA-IR.
Metabolic effects
Fasting blood sugar increased by 3 ± 10 mg/dl (0.17 ± 0.56 mmol/liter; P = 0.002) across the entire study population but did not reach Bonferoni-adjusted significance (P < 0.008) in any of the six groups (supplemental Table 1). HOMA-IR and QUICKI, indices of insulin resistance, changed minimally but were likewise unchanged in each of the six groups. Total and low-density lipoprotein (LDL) cholesterol were unchanged in the entire cohort or any of the six groups. High-density lipoprotein (HDL) cholesterol increased by 3.5 ± 6.7 mg/dl (0.09 ± 0.17 mmol/liter; P < 0.0001) for the 112 participants but only increased significantly in group E by 4 ± 6 mg/dl (0.10 ± 0.16 mmol/dl; P = 0.004). Fasting triglycerides decreased by −18 ± 57 mg/dl (0.20 ± 0.64 mmol/liter; P = 0.0002) but only significantly in group F by −40 ± 77 (0.45 ± 0.87 mmol/liter; P = 0.003).
Discussion
This is the first study to investigate the combined effects of 16 wk of physiological transdermal testosterone during a Leydig cell clamp and GH administration in older community-dwelling men. There were demonstrable benefits with the six different interventions with greater gains in whole-body and appendicular skeletal muscle mass, reductions in whole-body and trunk fat, and improvements in global measures of muscle performance with the higher dose levels. In particular, combined supplementation with testosterone and GH produced mean increases in total lean mass of up to about 3 kg (maximum increase of 7.5 kg) and mean decreases in fat of up to about 2.3 kg (maximum decrease of 7.1 kg) by wk 17 that were in the upper range of changes reported with physiological testosterone (14,18,30,46,47,48,49,50,51,52) or GH (30,31,53,54,55,56,57,58,59) administered to older men for 3–12 months. These changes occurred in the context of a relatively low frequency of largely expected adverse outcomes (60,61).
By wk 17, maximal voluntary strength of the major muscle groups of the upper and lower body increased significantly by 23 ± 27% up to 35 ± 31% in the three highest dose groups (D–F). However, paired muscle strength data were obtained for only 95 subjects. Intermittent musculoskeletal symptoms (e.g. flare of unilateral knee osteoarthritis) prevented some participants from performing all five strength tests, and other subjects showed different levels of motivation during testing sessions, despite coaching efforts to achieve maximal performance. These factors or insufficient sample size per treatment cell may have limited our ability to demonstrate statistical interactions or dose effects of the two hormones on muscle strength. Nevertheless, increases in muscle strength validated that the increases in lean tissue demonstrated by DEXA were due to accretion of myofibrillar protein and not just hydration effects. Furthermore, the increases in voluntary strength were of a similar magnitude to the losses reported in longitudinal studies of aging through the eighth to ninth decades of life (4,5,6), suggesting that the treatment effects were physiologically relevant.
There were also sizable improvements in aerobic endurance for all six groups that ranged from 51 ± 77 to 160 ± 200 sec at wk 17. Collectively, the global improvements in skeletal muscle strength and aerobic endurance were more substantial than previously reported in studies of testosterone, rhGH, or combination of the two hormones during treatment in older men, which showed minimal if any benefits (13,18,30,31,46,47,48,49,50,51,52,53,54,55,56,57,58,59). These improvements in muscle performance for our subjects with relatively intact functional status will be important if such effects can be translated to allow more functionally impaired individuals with sarcopenia or frailty to perform physical tasks and activities of daily living with less effort.
Analysis of the 2 × 3 factorial design showed no statistical interactions among the treatment interventions with the changes in body composition or skeletal muscle strength, consistent with our a priori hypothesis that these hormones would have important independent but complementary effects likely reflecting different mechanisms of action. However, there were apparent dose-related effects for some parameters and possibly additive effects when these hormones were coadministered. Indeed, accrual of total and appendicular LBM was highly significant by linear trend analysis as was the loss of total and trunk fat mass with benefits increasing in magnitude from lower to higher dose combinations (i.e. groups A–F). Furthermore, total LBM increased significantly more for subjects randomized to 10 g than 5 g of testosterone per day, and the improvements were greater for subjects who received any dose of rhGH vs. placebo. Similarly, loss in total and trunk fat was greater with the higher dose of testosterone.
Two recent studies of testosterone monotherapy (5 mg/d by patch for 2 yr or testosterone undecanoate 80 mg/d orally for 6 months) in older men failed to demonstrate improvements in LBM or muscle performance (51,52). In both studies, increments in testosterone levels during treatment, unlike the current study, were minimal or did not increase into the normal range, possibly explaining the absence of benefits in those studies. Only two other studies investigated combined therapy with these two hormones in older men (30,31). The first study involved 10 men who received serial treatment for 1 month with testosterone (5 mg/d by patch) alone, rhGH (6.25 μg/kg · d) alone and the combination of each with 3-month intervening washout periods (30). There were no changes in lean mass or strength with the three interventions. In the second study, 74 men were randomized to receive testosterone (100 mg im biweekly), rhGH (20–30 μg/kg three times per week), the combination, or placebos for 26 wk (31). Lean mass increased by 3.1 kg with testosterone alone and 4.3 kg with the combination. There was a marginal increase (P = 0.05) in composite 1-RM strength of six upper and lower body muscle strength tests and a modest 2.3 ml/kg · min increase in maximal O2 uptake only with the combination. The design in those trials differed substantially from the current investigation, including use of different formulations and routes of administration of testosterone, higher nonphysiological dosing with rhGH, and different durations of treatment (1 month to 2 yr). Thus, it is difficult to compare those studies with our investigation that used much smaller doses of rhGH approximating physiological replacement; neither of the previous studies showed the global increases in lean tissue and muscle performance demonstrated in the current trial.
Adverse events occurring during therapy were generally modest and included the small but reversible increases in hematocrit (2.0 ± 3.2%) and PSA (0.2 ± 0.8 ng/dl) at wk 16, both typical of testosterone therapy (60). However, the increases in systolic and diastolic blood pressure of 12 ± 14 and 8 ± 8 mm Hg, respectively, across the six groups that persisted, albeit at lower levels for up to 3 months after study therapies had been discontinued, were not anticipated. Previous testosterone and GH treatment studies generally showed no effect or decreases in blood pressure (17,26), although GH has been reported to increase blood pressure (62). It is possible that increases in blood pressure, which also occurred without rhGH, were related to expansion of intravascular volume (62) as reflected by the 35% occurrence of new lower extremity edema or due to an unexpected high frequency of certain polymorphisms of the androgen receptor CAG repeat (63). Regardless, this important outcome must be investigated further in future studies of these anabolic hormone therapies.
There were no worrisome metabolic changes and some improvements. Fasting blood glucose increased by about 3 mg/dl (∼0.17 mmol/liter), but mean levels remained well below the threshold for impaired fasting glucose (<110 mg/dl, <6.11 mmol/liter) in all groups. Similarly, insulin resistance as assessed by the HOMA-IR and QUICKI indices, worsened minimally across the study population but did not reach significance in any of the six groups. Total and LDL cholesterol were unchanged but HDL cholesterol improved by 3.5 ± 6.7 mg/dl (0.09 ± 0.17 nmol/liter) and fasting triglycerides decreased by −18 ± 57 mg/dl (−0.20 ± 0.64 mmol/liter) for the entire study cohort. We do not know whether more prolonged therapy would further reduce upper body fat or enhance physical activity and thereby improve metabolic parameters associated with cardiovascular disease risks or adversely affect these metabolic parameters.
In conclusion, combined administration of physiological doses of testosterone and rhGH resulted in substantial gains in lean mass, voluntary muscle strength, and aerobic endurance along with reductions in total and trunk fat that were of greater magnitude than treatment with testosterone alone. An Institute of Medicine Expert Panel has recommended conducting focused short-term efficacy trials of testosterone in older persons with symptomatic impairments before embarking on larger, long-term safety trials (64). In this context, our preliminary findings provide the basis to carefully evaluate the health benefits and safety of strategies that augment both androgen and GH/IGF-I status in future controlled studies before using these agents together in clinical practice to treat complications of aging. Future efficacy trials to evaluate such strategies should be conducted in older persons with functional limitations, especially those with sarcopenia or frailty.
Supplementary Material
Acknowledgments
We are grateful for the dedicated efforts of the volunteers to complete this complex metabolic investigation and for other members of the local research teams without whom the study would not have been successful. We appreciate the efforts of Mr. Michael Hutchinson and Mr. George Martinez (University of Southern California), who created the web portal and electronic database for distant entry of CRF data. Finally, we thank Mr. Chris Hahn and Mr. Jiaxiu He (University of Southern California), who provided additional statistical assistance, and Anqi Zhang, Ph.D., who performed the liquid chromatography-tandem mass spectroscopy assays for testosterone. F.R.S. (principal investigator and corresponding author) and S.P.A. (lead statistician) take responsibility for all aspects of the Hormonal Regulators of Muscle and Metabolism in Aging project and were responsible for the final study design, its conduct, and analysis of the data. Outcomes from the Hormonal Regulators of Muscle and Metabolism in Aging study have not been published previously and are not under consideration elsewhere.
Footnotes
The work for this trial was supported in part from the National Institutes of Health (NIH) Grant R01 AG18169 and local National Center for Research Resources (NCRR) General Clinical Research Center (GCRC) Grant M0I RR000043 at the University of Southern California; the U.S. Department of Agriculture (USDA) ARS Cooperative Agreement 58-1950-9-001; the NCRR GCRC Grant M01 RR000054 at Tufts University, where any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA; the NCRR GCRC at Washington University School of Medicine (Grant M01 RR000036); the Mass Spectrometry Research Resource at Washington University (NIH Grants RR000954, DK020579, and DK056341); and NIH Grants U01AG14369 and 1R01DK70534 at Boston Medical Center, Boston University School of Medicine. Study therapies were provided by Solvay Pharmaceuticals Inc., Genentech Inc., and Tap Pharmaceutical Products Inc.; industry sponsors provided no monetary support.
Disclosure Summary: F.R.S., S.B., K.E.Y., R.R., C.C.-S., and E.T.S. were responsible for the hypotheses, specific aims, and study design. C.C.-S., E.F.B., E.T.S., Y.S., R.R., P.C., J.U., and F.R.S. were responsible for acquisition of clinical data. S.P.A., Y.W., M.K., and Y.S. were responsible for creation of the study manual of operations, case report forms, and an electronic database for web-based data entry, and the manual and electronic screening of data for outliers, quality control, and audits of all data with verification from source documents, and statistical analyses., whereas batch testing for insulin and IGF-I levels was performed by the research staff in the University of Southern California General Clinical Research Center Core Endocrine Laboratory. All authors reviewed the entire database and analyses, contributed to consensus of data for presentation and its interpretation, and then reviewed and contributed to the writing of multiple iterations and final version of the manuscript.
First Published Online March 17, 2009
Abbreviations: ANCOVA, Analysis of covariance; CV, coefficient of variation; DEXA, dual-energy x-ray absorptiometry; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment insulin resistance index; LBM, lean body mass; LDL, low-density lipoprotein; QUICKI, quantitative insulin sensitivity check index; rh, recombinant human; 1-RM, one-repetition maximum; PSA, prostate-specific antigen; USC, University of Southern California; VO2, O2 consumption.
References
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD 1998 Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763 [DOI] [PubMed] [Google Scholar]
- Holloszy JO 1995 Workshop on sarcopenia: muscle atrophy in old age. J Gerontol Biol Med Sci 50A:1–161 [PubMed] [Google Scholar]
- Dutta C, Hadley E, Lexell J 1997 Sarcopenia and physical performance in old age: overview. Muscle Nerve Suppl 5:S5–S9 [PubMed] [Google Scholar]
- Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF 1997 Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol 83:1581–1587 [DOI] [PubMed] [Google Scholar]
- Danneskiold-Samsoe B, Kofod V, Munter J, Grimby G, Schnohr P, Jensen G 1984 Muscle strength and functional capacity in 78–81-year-old men and women. Eur J Appl Physiol 52:310–314 [DOI] [PubMed] [Google Scholar]
- Aniansson A, Hedberg M, Henning GB, Grimby G 1986 Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve 9:585–591 [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Bendall MJ, Pearson M 1988 Muscle strength in the triceps surae and objectively measured customary walking activity in men and women over 65 years of age. Clin Sci 74:85–89 [DOI] [PubMed] [Google Scholar]
- Reaven GM 1994 Syndrome X: 6 years later. J Intern Med Suppl 736:13–22 [PubMed] [Google Scholar]
- Abbasi AA, Drinka PJ, Mattson DE, Rudman D 1993 Low circulating levels of insulin-like growth factors and testosterone in chronically institutionalized elderly men. J Am Geriatr Soc 41:975–982 [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR 2001 Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D 1999 Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo study. J Clin Endocrinol Metab 84:573–577 [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ 1999 Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev 107:123–136 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A 1995 Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab 269:E820–E826 [DOI] [PubMed] [Google Scholar]
- Tenover JS 1992 Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab 75:1092–1098 [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Sih R, Hajjar R, Perry 3rd HM 1997 Testosterone and frailty. Clin Geriatr Med 13:685–695 [PubMed] [Google Scholar]
- Marin P, Krotkiewski M, Bjorntorp P 1992 Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissues. Eur J Med 1:329–336 [PubMed] [Google Scholar]
- Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, Holm G, Lindstedt G, Bjorntorp P 1992 The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 16:991–997 [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2005 Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90:1502–1510 [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Blackman MR 1993 Human growth hormone and human aging. Endocr Rev 14:20–39 [DOI] [PubMed] [Google Scholar]
- Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP 1981 Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest 67:1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranmanesh A, Lizarralde G, Veldhuis JD 1991 Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73:1081–1088 [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Pineyro MA, Roberson R, Blackman MR 1992 Growth hormone (GH)-releasing hormone-(1-29) twice daily reverses the decreased GH and insulin-like growth factor-I levels in old men. J Clin Endocrinol Metab 75:530–535 [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Kehayias JJ 1991 The meaning and measurement of lean body mass. Nutr Rev 49:163–175 [DOI] [PubMed] [Google Scholar]
- Watkins JC, Roubenoff R, Rosenberg IH 1992 Body composition: measure and screening of change with age. Boston: Foundation for Nutritional Advancement [Google Scholar]
- Munzer T, Harman SM, Hees P, Shapiro E, Christmas C, Bellantoni MF, Stevens TE, O’Connor KG, Pabst KM, St Clair C, Sorkin JD, Blackman MR 2001 Effects of GH and/or sex steroid administration on abdominal subcutaneous and visceral fat in healthy aged women and men. J Clin Endocrinol Metab 86:3604–3610 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjorntorp P, Sjostrom L, Bengtsson BA 1997 Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82:727–734 [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Verhovec R, Zizek B, Prezelj J, Poredos P, Clayton R 1999 Growth hormone (GH) treatment reverses early atherosclerotic changes in GH-deficient adults. J Clin Endocrinol Metab 84:453–457 [DOI] [PubMed] [Google Scholar]
- Colao A, Di Somma C, Rota F, Pivonello R, Savanelli MC, Spiezia S, Lombardi G 2005 Short-term effects of growth hormone (GH) treatment or deprivation on cardiovascular risk parameters and intima-media thickness at carotid arteries in patients with severe GH deficiency. J Clin Endocrinol Metab 90:2056–2062 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Lizarralde G, Urban RJ 1994 Combined deficits in the somatotropic and gonadotropic axes in healthy aging men: an appraisal of neuroendocrine mechanisms by deconvolution analysis. Neurobiol Aging 15:509–517 [DOI] [PubMed] [Google Scholar]
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St. Clair C, Pabst KM, Harman SM 2002 Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288:2282–2292 [DOI] [PubMed] [Google Scholar]
- Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, Urban RJ, Veldhuis JD 2002 Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab 87:5649–5657 [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N 2000 Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab 85:4500–4510 [DOI] [PubMed] [Google Scholar]
- Lucidi P, Lauteri M, Laureti S, Celleno R, Santoni S, Volpi E, Angeletti G, Santeusanio F, De Feo P 1998 A dose-response study of growth hormone (GH) replacement on whole body protein and lipid kinetics in GH-deficient adults. J Clin Endocrinol Metab 83:353–357 [DOI] [PubMed] [Google Scholar]
- Toogood AA, Shalet SM 1999 Growth hormone replacement therapy in the elderly with hypothalamic-pituitary disease: a dose-finding study. J Clin Endocrinol Metab 84:131–136 [DOI] [PubMed] [Google Scholar]
- Amato G, Mazziotti G, Di Somma C, Lalli E, De Felice G, Conte M, Rotondi M, Pietrosante M, Lombardi G, Bellastella A, Carella C, Colao A 2000 Recombinant growth hormone (GH) therapy in GH-deficient adults: a long-term controlled study on daily versus thrice weekly injections. J Clin Endocrinol Metab 85:3720–3725 [DOI] [PubMed] [Google Scholar]
- Flack S, Kraemer WJ, eds. 1997 Designing resistance training programs. In: Human kinetics. 2nd ed. Champaign, IL: Human Kinetics; 4, 98–100 [Google Scholar]
- Schroeder ET, Wang Y, Castaneda-Sceppa C, Cloutier G, Vallejo AF, Kawakubo M, Jensky NE, Coomber S, Azen SP, Sattler FR 2007 Reliability of maximal voluntary muscle strength and power testing in older men. J Gerontol A Biol Sci Med Sci 62:543–549 [DOI] [PubMed] [Google Scholar]
- Singh AB, Lee ML, Sinha-Hikim I, Kushnir M, Meikle W, Rockwood A, Afework S, Bhasin S 2006 Pharmacokinetics of a testosterone gel in healthy postmenopausal women. J Clin Endocrinol Metab 91:136–144 [DOI] [PubMed] [Google Scholar]
- Lansang MC, Williams GH, Carroll JS 2001 Correlation between the glucose clamp technique and the homeostasis model assessment in hypertension. Am J Hypertens 14:51–53 [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron, AD, Follmann DA, Sullivan G, Quon MJ 2000 Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410 [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Singh A, Bhasin S, Storer TW, Azen C, Davidson T, Martinez C, Sinha-Hikim I, Jaque SV, Terk M, Sattler FR 2003 Effects of an oral androgen on muscle and metabolism in older, community-dwelling men. Am J Physiol Endocrinol Metab 284:E120–E128 [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Zheng L, Yarasheski KE, Qian D, Stewart Y, Flores C, Martinez C, Terk M, Sattler FR 2004 Treatment with oxandrolone and the durability of effects in older men. J Appl Physiol 96:1055–1062 [DOI] [PubMed] [Google Scholar]
- Lewis MI, Fournier M, Storer TW, Bhasin S, Porszasz J, Ren SG, Da X, Casaburi R 2007 Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol 103:1299–1310 [DOI] [PubMed] [Google Scholar]
- Deley G, Kervio G, Van Hoecke J, Verges B, Grassi B, Casillas JM 2007 Effects of a one-year exercise training program in adults over 70 years old: a study with a control group. Aging Clin Exp Res 19:310–315 [DOI] [PubMed] [Google Scholar]
- Hobbs CJ, Plymate SR, Rosen CJ, Adler RA 1993 Testosterone administration increases insulin-like growth factor-I levels in normal men. J Clin Endocrinol Metab 77:776–779 [DOI] [PubMed] [Google Scholar]
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R 2003 AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 88:2673–2681 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL 1999 Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG 2001 Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 56:M266–M272 [DOI] [PubMed] [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry 3rd HM, Patrick P, Ross C 1997 Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 82:1661–1667 [DOI] [PubMed] [Google Scholar]
- Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE 2003 Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 58:618–625 [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT 2008 Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 299:39–52 [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton 3rd LJ, Smith GE, Khosla S, Jensen MD 2006 DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE 1990 Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6 [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, McHenry B 1996 Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab 81:3239–3243 [DOI] [PubMed] [Google Scholar]
- Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C 1996 Growth hormone replacement in healthy older men improves body composition but not functional ability [see comments]. Ann Intern Med 124:708–716 [DOI] [PubMed] [Google Scholar]
- Lange KH, Larsson B, Flyvbjerg A, Dall R, Bennekou M, Rasmussen MH, Orskov H, Kjaer M 2002 Acute growth hormone administration causes exaggerated increases in plasma lactate and glycerol during moderate to high intensity bicycling in trained young men. J Clin Endocrinol Metab 87:4966–4975 [DOI] [PubMed] [Google Scholar]
- Hennessey JV, Chromiak JA, DellaVentura S, Reinert SE, Puhl J, Kiel DP, Rosen CJ, Vandenburgh H, MacLean DB 2001 Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people. J Am Geriatr Soc 49:852–858 [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Pruitt L, Reim J, Hintz RL, Butterfield G, Hoffman AR, Marcus R 1994 Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab 79:1361–1366 [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM 1995 Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol 268:E268–E276 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM 2006 Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 91:1995–2010 [DOI] [PubMed] [Google Scholar]
- Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML, Stephens P 2006 Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 91:1621–1634 [DOI] [PubMed] [Google Scholar]
- Thuesen L, Jorgensen JO, Muller JR, Kristensen BO, Skakkebaek NE, Vahl N, Christiansen JS 1994 Short and long-term cardiovascular effects of growth hormone therapy in growth hormone deficient adults. Clin Endocrinol (Oxf) 41:615–620 [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Nieschlag E 2007 Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab 92:3844–3853 [DOI] [PubMed] [Google Scholar]
- Blazer DG, Barrett-Connor E, Brody BA, Califf RM, Costantino JP, Federman DD, Fried LP, Grady DG, Hazzard WR, Heymsfield SB, Lagakos SW, Litwin MS, Lombardo PA, Nelsen PS, Orowoll ES, Schover LR, Vaughan Jr ED 2003 Testosterone and Aging: Clinical Research Directions. Washington, DC: National Academies Press [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.