Abstract
Context: Transcription factor steroidogenic factor-1 (SF-1) plays a pivotal role in the control of adrenocortical cell steroidogenesis and proliferation. SF-1 amplification and overexpression are found in most cases of childhood adrenocortical tumors (ACTs).
Objective: Our objective was to investigate the effect of SF-1 inverse agonists of the alkyloxyphenol and isoquinolinone classes on the proliferation of human adrenocortical cell lines expressing SF-1 (H295R), in conditions of basal and increased SF-1 expression, or negative for SF-1 expression (SW-13).
Main Outcome Measures: Proliferation assays, immunoblots, flow cytometric analyses, steroid hormone assays, and reverse transcription quantitative PCR were used.
Results: SF-1 inhibitors of the alkyloxyphenol class displayed a dose-dependent inhibitory effect on both SF-1-positive and -negative ACT cells, whereas SF-1 inverse agonists of the isoquinolinone class selectively inhibited cell proliferation elicited by SF-1 overexpression. These drugs also inhibited stimulated steroid hormone secretion and CYP21 and CYP17 mRNA expression.
Conclusion: SF-1 inhibitors may represent a useful tool in the chemotherapy of ACTs.
Drugs targeting transcription factor SF-1 inhibit proliferation of adrenocortical carcinoma cells.
The nuclear receptor steroidogenic factor-1 (SF-1) (NR5A1) is an essential factor in adrenal and gonadal development in human and mice (1). SF-1-deficient mice exhibit male-to-female sex reversal, an impaired development of adrenals and gonads (2,3), defective pituitary gonadotrophs, and an agenesis of the ventromedial hypothalamic nucleus (4,5). SF-1 insufficiency has also been associated with metabolic disorders (6).
By using human adrenocortical tumor (ACT) cell cultures and transgenic mice analysis, we have recently defined a critical role for SF-1 dosage in regulating the proliferation of human adrenocortical cells and triggering tumor formation in mice (7,8). These findings are important to understand better the pathogenesis of childhood ACTs, in which SF-1 is amplified and overexpressed in most cases (9,10). Our previous studies indicated that SF-1 dosage is a critical factor for adrenal tumorigenesis and suggested that modulation of SF-1 activity may represent an important therapeutic target in childhood ACTs.
In various cell systems, heterologous expression of SF-1 leads to a constitutively active receptor, modulating the transcription of target genes in the absence of an exogenously added ligand (11). Whereas it is unclear whether SF-1 transcriptional activity is regulated by physiological ligands, one report suggested that SF-1 could be activated by various oxysterols, but it was not confirmed by subsequent studies (12,13). More recently, the crystal structure of the ligand binding domain of SF-1 was reported by several groups (14,15,16). These studies revealed a large binding pocket filled with phospholipids with the receptor adopting the canonical active conformation. Further characterization indicated that SF-1 preferentially binds phosphatidyl inositol bis- and trisphosphates, as well as different C12-C16 fatty acids, with high affinity. These phospholipids can be exchanged and modulate the interaction of SF-1 with coactivators. Nevertheless, these results failed to demonstrate a phospholipid-dependent regulation of SF-1 activity in vitro. On the other hand, studies by one group reported that sphingosine acts a negative regulator of SF-1 activity (17).
The identification of small molecules modulating SF-1 activity represents a potentially useful tool to better understand SF-1 involvement in both physiological and pathological situations. Recently, several studies were based on high-throughput screening (HTS) of the activity of chemical libraries on the constitutively active human SF-1. These studies led to the identification of two distinct classes of small molecules described as selective SF-1 inverse agonists (18,19,20).
To evaluate the effects of the SF-1 inverse agonists on the growth of ACT cells in the perspective of their potential therapeutic application, we have studied their action on the proliferation of adrenocortical cell lines that express, or not, SF-1. Here, we show that SF-1 inverse agonists of the isoquinolinone (IsoQ) class specifically decrease SF-1-driven proliferation of H295R cells. These compounds may represent a potential therapeutic tool in adrenocortical carcinoma.
Materials and Methods
Chemicals
AC-45594 [4-(heptyloxy)phenol] and octyloxyphenyl (OOP) were obtained from ACADIA Pharmaceuticals Inc. (San Diego, CA). SID7969543 and SID7970631 were purchased from Life Chemicals (Kiev, Ukraine). Compound nos. 31 and 32 were synthesized as described (20). All compounds were dissolved in dimethylsulfoxide (DMSO).
Cell culture and proliferation assays
H295R cells overexpressing SF-1 in a doxycycline (Dox)-inducible fashion (H295R TR/SF-1) were cultured in DMEM/F-12 supplemented with 2% NuSerum (Becton Dickinson, Le Pont de Claix, France), 1% ITS Plus (Becton Dickinson), and antibiotics, as described (8). SW-13 cells were cultured in DMEM/F12 supplemented with 10% fetal calf serum and antibiotics. To measure proliferation, cells were seeded in duplicate in 24-well plates at the density of 3 × 104 cells per well, and cultured in medium without serum in the presence of either the indicated concentrations of the different compounds or DMSO vehicle with or without Dox (1 μg/ml; Sigma-Aldrich Corp., St. Louis, MO) added to the culture medium. Cells were counted after 4 d culture. Results are indicated as the average (± sem) of four independent experiments performed in duplicate.
Immunoblots
H295R/TR SF-1 cells were treated with the indicated concentrations of different compounds, or with DMSO vehicle in the absence or presence of Dox (1 μg/ml). Protein extracts were prepared by harvesting cells in Laemmli buffer [50 mm Tris-HCl (pH 6.8), 50% glycerol, 2% sodium dodecyl sulfate, and 0.02% bromophenol blue] containing 5% β-mercaptoethanol. Proteins were separated by SDS-PAGE and transferred to a polyvinylidene fluoride membrane. Immunoblot was performed using a chemiluminescence system for protein detection (ECL Plus; GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Primary antibodies used were: rabbit polyclonal anti-SF-1 (1:1000 dilution; Upstate Biotechnology Inc., Lake Placid, NY); and mouse monoclonal anti-β-tubulin (1:1000 dilution; Sigma- Aldrich).
Flow cytometry
H295R/TR SF-1 cells were fixed in 70% ethanol and then treated with ribonuclease A (50 μg/ml) for 30 min at 37C. DNA was stained with propidium iodide (50 μg/ml), and cells were analyzed for cell-cycle distribution with a FACScan instrument (BD).
Steroid assays
Aldosterone, cortisol, and dehydroepiandrosterone sulfate (DHEA-S) levels in culture supernatants were measured by specific immunoenzymatic assays (Diagnostics Biochem Canada Inc., London, Ontario, Canada).
Reverse transcription quantitative PCR (RT-qPCR)
A total of 500 ng total RNA was reverse transcribed using Superscript II reverse transcriptase (Invitrogen Corp., Carlsbad, CA). RT-qPCR was performed using the SYBRGreen I dye assay on a LightCycler 480 (Roche Applied Science, Indianapolis, IN) instrument using TATA-binding protein (TBP) as a reference transcript. Primer sequences used were: TBP, forward 5′-GAACATCATGGATCAGAACAACA-3′ and reverse 5′-ATTGGTGTTCTGAATAGGCTGTG-3′; CYP21, forward 5′-GTCATCATTCCGAACCTCCAA-3′ and reverse 5′-GAACTCATGTGGCCTCTCCC-3′; and CYP17, forward 5′-CTGAGCAAAGACAGCCTGGT-3′ and reverse 5′-GCTTGCATCAGTGTGTCCAG-3′.
Results were calculated using the ΔΔ threshold cycle method (21).
Results
Effects of alkyloxyphenol compounds on adrenocortical cell proliferation
We have recently shown that increased SF-1 dosage augments proliferation of human adrenocortical cells and induces ACTs in mice (8). By using a new HTS approach, Del Tredici et al. (18) recently described a class of alkyloxyphenol compounds defined by AC-45594 [4-(heptyloxy)phenol] and analogs that were described to represent selective SF-1 inverse agonists.
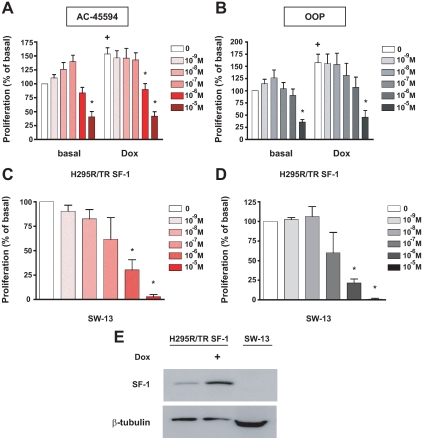
To study their biological function and define their therapeutic potential, we studied the effect of various doses of the AC-45594 compound and its analog OOP on human adrenocortical H295R/TR SF-1 cell proliferation, which overexpresses SF-1 in a Dox-inducible fashion. After 4 d treatment, both compounds inhibited proliferation of H295R/TR SF-1 cells cultured in basal conditions in a dose-dependent manner, with a maximal effect at 10 μm. In the presence of Dox, incubation with 1 μm of both drugs also markedly reduced cell proliferation induced by SF-1 overexpression (Fig. 1, A and B). We also evaluated the effect of treatment with AC-45594 and OOP on H295R/TR SF-1 cell cycle distribution after 4 d treatment. Both compounds at 10 μm induce a significant decrease in G0/G1 distribution and blocking of cells in early S phase. This phenomenon was particularly evident after treatment with OOP (supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Moreover, treatment with both drugs caused an increase of the percentage of sub-G1 (apoptotic) cells.
Figure 1.
Inhibition of human adrenocortical cell proliferation by alkyloxyphenols. A and B, H295R TR/SF-1 cells were treated with increasing doses of the AC-45594 (A) and OOP (B) compounds (at doses ranging from 10−9 to 10−5 m) in duplicate wells in the absence or presence of Dox (1 μg/ml). Data are expressed as the percentage of increment or reduction over the number of cells cultured in the presence of DMSO vehicle for 4 d (mean ± sem of four experiments). The difference in cell numbers between H295R TR/SF-1 cells cultured in the absence or presence of Dox is statistically significant (+; P < 0.01, t test). *, Values significantly different from DMSO control (P < 0.01, two way-ANOVA with Bonferroni posttest). C and D, Effect of AC-45594 (C) and OOP (D) compounds (doses ranging from 10−9 to 10−5 m) on proliferation of SW-13 cells. Data are expressed as the percentage of increment or reduction over the number of cells cultured in the presence of DMSO vehicle for 4 d (mean ± sem of four experiments). *, Values significantly different from DMSO control (P < 0.01, one way-ANOVA with Bonferroni posttest). E, Immunoblot showing increased expression of SF-1 after Dox treatment of H295R/TR SF-1 cells and lack of detectable SF-1 expression in SW-13 cells. With the purpose of increasing the sensitivity of the assay, SW-13 extracts were overloaded compared with H295R/TR SF-1 extracts, as shown by β-tubulin expression.
To assay for the specificity of this class of compounds on SF-1-dependent adrenocortical cell proliferation, we studied the effect of these drugs on the other human ACT cell line SW-13. Contrarily to H295R cells that retain the ability to secrete steroid hormones, SW-13, derived from a stage IV tumor, does not (22). The lack of the steroidogenic phenotype suggests that SW-13 cells are less differentiated than the H295R/TR SF-1 cell line. SF-1 expression is induced after a 4-d Dox treatment in H295R/TR SF-1 cells, whereas it cannot be detected in SW-13 cells (Fig. 1E). This cell line then represents a useful control to verify drug specificity on SF-1 activity. We observed that a 4-d treatment with AC-45594 and OOP also significantly inhibited proliferation of SW-13 cells in a dose-dependent fashion, with a high toxicity at 10 μm (Fig. 1, C and D). Together, these results suggest that the alkyloxyphenol compounds, even if they were characterized as bona fide SF-1 inverse agonists (18), do not specifically target SF-1 activity on cell proliferation in our system.
Effects of IsoQ compounds on adrenocortical cell proliferation
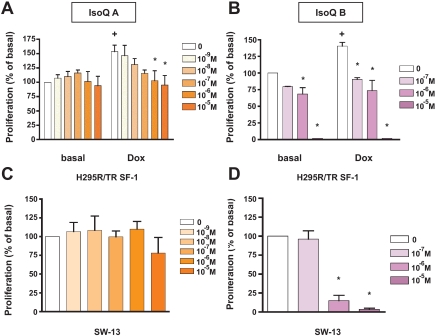
By using an ultra-HTS approach, Madoux et al. (19) identified and characterized two IsoQ compounds, SID7969543 and SID7970631, described as selective and efficacious inhibitors of SF-1 transcriptional activity. We tested the effects of these compounds on adrenocortical cell proliferation. SID7969543 (IsoQ A) had no significant effect on cell proliferation in basal conditions (Fig. 2A), as confirmed by cell cycle analysis (supplemental Fig. 2). However, in the presence of Dox, this compound dose dependently reduced proliferation induced by SF-1 overexpression to basal levels (Fig. 2A). Furthermore, IsoQ A did not affect SW-13 cell proliferation (Fig. 2C). Conversely, SID7970631 (IsoQ B) had a very strong effect on proliferation of H295R/TR SF-1 cells, both in the presence and absence of Dox, as well as of SW-13 cells (Fig. 2, B and D). These data are consistent with the higher cytotoxicity exhibited by IsoQ B compared with IsoQ A in a previous study (19).
Figure 2.
Inhibition of human adrenocortical cell proliferation by IsoQ compounds IsoQ A (SID7969543) and IsoQ B (SID7970631). A and B, H295R TR/SF-1 cells were treated with increasing doses of the IsoQ A (at doses ranging from 10−9 to 10−5 m; A) and IsoQ B compounds (at doses ranging from 10−7 to 10−5 m; B) in duplicate wells in the absence or presence of Dox (1 μg/ml). Data are expressed as the percentage of increment or reduction over the number of cells cultured in the presence of DMSO vehicle for 4 d (mean ± sem of four experiments). The difference in cell numbers between H295R TR/SF-1 cells cultured in the absence and presence of Dox is statistically significant (+; P < 0.01, t test). *, Values significantly different from DMSO control (P < 0.01, two way-ANOVA with Bonferroni posttest). C and D, Effect of IsoQ A (at doses ranging from 10−9 to 10−5 m; C) and IsoQ B (at doses ranging from 10−7 to 10−5 m; D) on proliferation of SW-13 cells. Data are expressed as the percentage of increment or reduction over the number of cells cultured in the presence of DMSO vehicle for 4 d (mean ± sem of four experiments). *, Values significantly different from DMSO control (P < 0.01, one way-ANOVA with Bonferroni posttest).
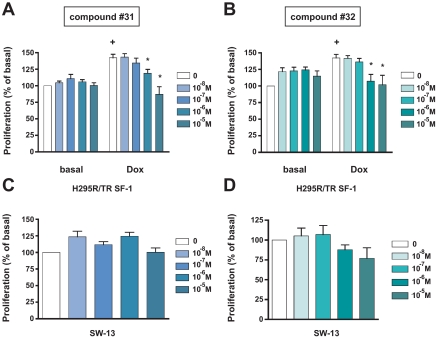
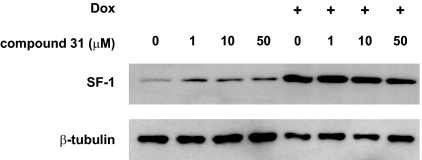
The discovery of the SF-1 modulating activities of the IsoQ A and B molecules led to the development of two derived compounds (named nos. 31 and 32 in Ref. 20) that were reported to have an improved selectivity and higher SF-1 inhibitory effects compared with IsoQ A (20). Treatment with the no. 31 and 32 compounds had no effect on basal H295R/TR SF-1 cell proliferation but significantly decreased the enhanced proliferation induced by SF-1 overexpression at doses starting from 1 μm (Fig. 3, A and B), whereas no effect was observed on SW-13 cell proliferation for both compounds (Fig. 3, C and D). Cell cycle analysis revealed an increase of the percentage of sub-G1 (apoptotic) cells after treatment with compound no. 31 (at 10 μm), with these effects being less evident with compound no. 32 (supplemental Fig. 3). These effects of the drugs might be due to interference with SF-1 expression. However, treatment with various doses of compound no. 31 had no effect on the expression of SF-1, both in basal conditions and after stimulation with Dox (Fig. 4). Equivalent results were obtained for compound no. 32 (data not shown).
Figure 3.
Inhibition of human adrenocortical cell proliferation by IsoQ compound nos. 31 and 32. A and B, H295R TR/SF-1 cells were treated with increasing doses of the no. 31 (A) and 32 compounds (both at doses ranging from 10−8 to 10−5 m; B) in duplicate wells in the absence or presence of Dox (1 μg/ml). Data are expressed as the percentage of increment or reduction over the number of cells cultured in the presence of DMSO vehicle for 4 d (mean ± sem of four experiments). The difference in cell numbers between H295R TR/SF-1 cells cultured in the absence or presence of Dox is statistically significant (+; P < 0.01, t test). *, Values significantly different from DMSO control (P < 0.01, two way-ANOVA with Bonferroni posttest). C and D, Effect of the no. 31 (C) and 32 (D) compounds (both at doses ranging from 10−8 to 10−5 m) on proliferation of SW-13 cells. Data are expressed as the percentage of increment or reduction over the number of cells cultured in the presence of DMSO vehicle for 4 d (mean ± sem of four experiments).
Figure 4.
The no. 31 compound does not affect SF-1 expression in H295R/TR SF-1 cells. Treatment of cells cultured in the absence or presence of Dox (1 μg/ml) with increasing doses (ranging from 1–50 μm) of the no. 31 compound had no effect on SF-1 expression. β-Tubulin expression is shown as a loading control.
Effect of the IsoQ compounds on steroid production and steroidogenic gene expression
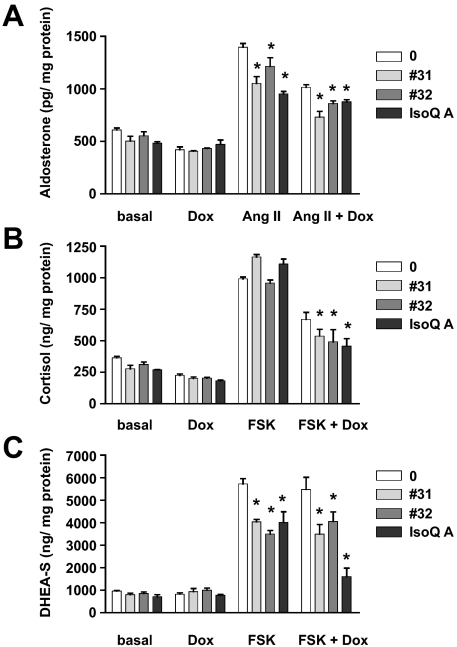
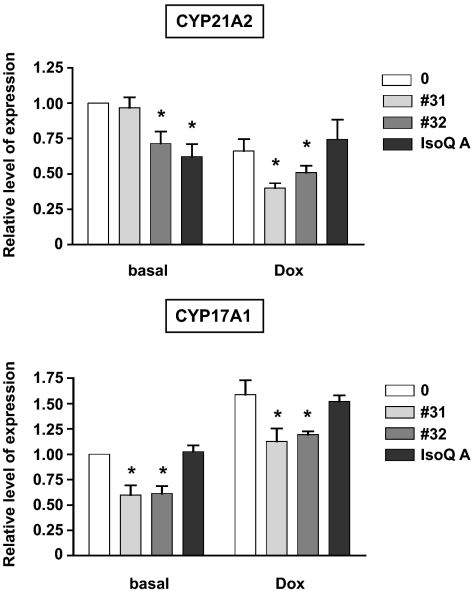
Because of the importance of SF-1 in the regulation of steroidogenesis, we investigated the effects of the IsoQ compounds that specifically affect adrenocortical cell proliferation on steroid production and steroidogenic enzyme gene expression. H295R cells have a mixed steroidogenic phenotype, secreting high doses of DHEA-S and lower doses of cortisol and aldosterone. We have previously shown that an increased SF-1 dosage decreases aldosterone and cortisol secretion in H295R/TR SF-1 cells (8,23). Consistent with their inverse agonist action on SF-1 activity, IsoQ A and compound nos. 31 and 32 significantly inhibited forskolin-stimulated DHEA-S secretion by H295R/TR SF-1 cells, in conditions of both basal and increased SF-1 dosage (Fig. 5C). These drugs also reduced angiotensin II (AngII)-stimulated aldosterone secretion and forskolin-stimulated cortisol secretion in the presence of increased SF-1 levels (Fig. 5, A and B). These results are consistent with the decreased levels of CYP21 and CYP17 transcripts induced by the no. 32 and IsoQ A compounds and no. 31 and 32 compounds, respectively (Fig. 6).
Figure 5.
Inhibition of steroid secretion by IsoQ compound nos. 31 and 32, and IsoQ A. Aldosterone (A), cortisol (B), and DHEA-S (C) levels (normalized by protein content) in the supernatant of H295R TR/SF-1 cells kept for 4 d in basal conditions or treated with Dox (1 μg/ml), stimulated with AngII (10 nm; A) or forskolin (FSK) (10 μg/ml; B and C) in the absence (AngII; forskolin) or presence (AngII plus Dox; forskolin plus Dox) of Dox. Where indicated, cells were treated with the no. 31 (pale gray) no. 32 (dark gray), or IsoQ A (black) compounds (10−5 m each) (mean ± sem of four experiments). *, Values significantly different from the corresponding untreated condition (P < 0.01, two way-ANOVA with Bonferroni posttest).
Figure 6.
Inhibition of steroidogenic enzyme mRNA levels by IsoQ compound nos. 31 and 32, and IsoQ A. CYP21A2 and CYP17A1 transcript levels were measured by RT-qPCR in H295R TR/SF-1 cells kept for 4 d in basal conditions or treated with Dox (1 μg/ml) in the absence or presence of the no. 31 (pale gray), no. 32 (dark gray), or IsoQ A (black) compounds (10−5 m each) (mean ± sem of four experiments). *, Values significantly different from the corresponding untreated condition (P < 0.01, two way-ANOVA with Bonferroni posttest).
Discussion
Although ACTs are a rare type of cancer, they are very aggressive and highly resistant to chemotherapy and radiotherapy (24). Therapeutic results are still unsatisfactory, with an overall survival rate at 5 yr of only about 50% (25). Current therapy for pediatric ACTs primarily consists of surgical resection of the tumor. Moreover, the use of the adrenolytic agent, mitotane (o,p-DDD), associated, or not, with DNA-damaging drugs, is the only medical therapy available up to date (24,25,26). Thus, a better knowledge of the molecular mechanisms underlying tumor growth and progression is necessary to develop more selective and specific treatments.
We have previously shown that the SF-1 gene is amplified and overexpressed in childhood ACTs (9,10), and that increased SF-1 dosage increases proliferation, decreases apoptosis of human adrenocortical cells, and induces ACTs in transgenic mice (7,8). These results indicate that SF-1 dosage is critical for adrenal tumorigenesis and suggest that modulation of SF-1 activity may represent an important therapeutic target in childhood ACTs. Our cellular model recapitulates several molecular features of childhood ACTs, and constitutes a useful tool to understand the molecular mechanism of ACT pathogenesis needed for the development and study of drugs targeting SF-1 transcriptional activity for ACT therapy. Indeed, we have shown previously that the SF-1 effect on adrenocortical cell proliferation requires this factor to be transcriptionally active through its activation function-2 domain (8).
Crystallographic studies have identified various types of phospholipids occupying the SF-1 ligand-binding pocket (14,15,16). Sphingosine was the first natural compound described to inhibit SF-1-dependent activation of the CYP17 gene (17). However, in our previous study, we observed no significant effect of sphingosine on dosage-dependent increase of H295R cell proliferation and the expression of the SF-1 dosage-regulated FATE1 gene. These data are consistent with the lack of effect of sphingosine observed in another study using a heterologous expression system (19).
The recent characterization of synthetic SF-1 inverse agonists (18,19,20) allowed us to investigate their biological effect in our cellular model of ACTs. Here, we have shown that the AC-45594 and OOP compounds of the alkyloxyphenol class inhibit proliferation of two different ACT cells lines, H295R and SW-13. However, their effects in our system do not seem to be selective for SF-1 because they are also active on SF-1-negative SW-13 cells. The absence of SF-1 expression in this cell line may explain their less differentiated phenotype, considering the fundamental role of SF-1 in the regulation of steroid biosynthesis.
We also studied the effect of another class of recently described SF-1 inverse agonists of the IsoQ class, which were reported to display increased potency and selectivity compared with the alkyloxyphenols (19,20). Interestingly, we could show that SID7969543 (IsoQ A), and the no. 31 and 32 compounds selectively inhibit H295R cell proliferation in conditions of increased SF-1 expression. These drugs also decreased AngII-stimulated secretion of aldosterone and forskolin-stimulated secretion of cortisol and DHEA-S. This is consistent with the observed decrease of CYP21 and CYP17 mRNAs triggered by the IsoQ drugs. Overall, the effect of these SF-1 inverse agonists on hormonal secretion by H295R cells is probably accounted for by the sum of their actions on the expression of the different steroidogenic enzyme genes that are either activated or repressed by SF-1 (8,23).
The mechanism of action of the IsoQ and derived analogs remains to be elucidated. It will be interesting to determine whether these compounds bind inside the protein ligand binding pocket or act by a different mechanism, e.g. by interfering with the interaction of SF-1 with transcriptional cofactors. Their effects are SF-1-dosage dependent because we observed no effect of these compounds, either on basal H295R cell proliferation or on SF-1-negative SW-13 cells. We have previously shown that gene expression profiles and proliferation capacity are regulated by the intracellular levels of SF-1 in adrenocortical cells (7,8). Our new findings suggest that the IsoQ drugs preferentially target the activity of SF-1 on the expression of the set of genes that are most sensitive to its dosage and that are responsible for the increased cell proliferation in conditions of SF-1 overexpression. This class of drugs may then represent an interesting therapeutic tool to be associated with mitotane and newer drugs in ACT chemotherapy (26,27,28,29,30). In addition, suppression of steroid hormone secretion by ACT cells induced by those drugs may be beneficial to alleviate symptoms of virilization, and Cushing’s syndrome is nearly always associated with tumor burden. Our cellular model represents a useful model to characterize further the molecular mechanism of action of these compounds. Those analyses are crucial for the design of increasingly selective molecules targeting SF-1 in a cell-specific fashion with limited side effects.
Supplementary Material
Acknowledgments
We thank ACADIA Pharmaceuticals Inc., for the AC-45594 and octyloxyphenyl compounds, and M. Luconi for the gift of the SW-13 cells.
Footnotes
This study was supported by grants from Conseil Général 06 and Institut National du Cancer (to E.L.). The National Institutes of Health Grant 1U54MH084512 supported the effort of F.M. and P.H.
Disclosure Summary: The authors have nothing to declare.
First Published Online March 24, 2009
Abbreviations: ACT, Adrenocortical tumor; AngII, angiotensin II; DHEA-S, dehydroepiandrosterone sulfate; DMSO, dimethylsulfoxide; Dox, doxycycline; HTS, high-throughput screening; IsoQ, isoquinolinone; OOP, octyloxphenol; RT-qPCR, reverse transcription quantitative PCR; SF-1, steroidogenic factor-1; TBP, TATA-binding protein.
References
- Val P, Lefrançois-Martinez AM, Veyssière G, Martinez A 2003 A SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept 1:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J 1995 Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL 1995 The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol 9:478–486 [DOI] [PubMed] [Google Scholar]
- Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, Morohashi KI, Li E 1995 Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204:22–29 [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL 2002 Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology 143:607–614 [DOI] [PubMed] [Google Scholar]
- Doghman M, Arhatte M, Thibout H, Rodrigues G, De Moura J, Grosso S, West AN, Laurent M, Mas JC, Bongain A, Zambetti GP, Figueiredo BC, Auberger P, Martinerie C, Lalli E 2007 Nephroblastoma overexpressed/cysteine-rich protein 61/connective tissue growth factor/nephroblastoma overexpressed gene-3 (NOV/CCN3), a selective adrenocortical cell proapoptotic factor, is down-regulated in childhood adrenocortical tumors. J Clin Endocrinol Metab 92:3253–3260 [DOI] [PubMed] [Google Scholar]
- Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E 2007 Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol 21:2968–2987 [DOI] [PubMed] [Google Scholar]
- Figueiredo BC, Cavalli LR, Pianovski MA, Lalli E, Sandrini R, Ribeiro RC, Zambetti G, DeLacerda L, Rodrigues GA, Haddad BR 2005 Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab 90:615–619 [DOI] [PubMed] [Google Scholar]
- Pianovski MA, Cavalli LR, Figueiredo BC, Santos SC, Doghman M, Ribeiro RC, Oliveira AG, Michalkiewicz E, Rodrigues GA, Zambetti G, Haddad BR, Lalli E 2006 SF-1 overexpression in childhood adrenocortical tumours. Eur J Cancer 42:1040–1043 [DOI] [PubMed] [Google Scholar]
- Ito M, Yu RN, Jameson JL 1998 Steroidogenic factor-1 contains a carboxy-terminal transcriptional activation domain that interacts with steroid receptor coactivator-1. Mol Endocrinol 12:290–301 [DOI] [PubMed] [Google Scholar]
- Lala DS, Syka PM, Lazarchik SB, Mangelsdorf DJ, Parker KL, Heyman RA 1997 Activation of the orphan nuclear receptor steroidogenic factor 1 by oxysterols. Proc Natl Acad Sci USA 94:4895–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Bair SR 1998 25-Hydroxycholesterol is not a ligand for the orphan nuclear receptor steroidogenic factor-1 (SF-1). Endocrinology 139:3026–3029 [DOI] [PubMed] [Google Scholar]
- Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA 2005 Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–355 [DOI] [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE 2005 Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell 17:491–502 [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, Shelloe R, Mehra U, Eng K, Nguyen H, Settachatgul C, Powell B, Milburn MV, West BL 2005 The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci USA 102:7505–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs AN, Dammer E, Sewer MB 2006 Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology 147:5249–5258 [DOI] [PubMed] [Google Scholar]
- Del Tredici AL, Andersen CB, Currier EA, Ohrmund SR, Fairbain LC, Lund BW, Nash N, Olsson R, Piu F 2008 Identification of the first synthetic steroidogenic factor 1 inverse agonists: pharmacological modulation of steroidogenic enzymes. Mol Pharmacol 73:900–908 [DOI] [PubMed] [Google Scholar]
- Madoux F, Li X, Chase P, Zastrow G, Cameron MD, Conkright JJ, Griffin PR, Thacher S, Hodder P 2008 Potent, selective and cell penetrant inhibitors of SF-1 by functional ultra-high-throughput screening. Mol Pharmacol 73:1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J, Madoux F, Hodder P, Roush WR 2008 Synthesis of small molecule inhibitors of the orphan nuclear receptor steroidogenic factor-1 (NR5A1) based on isoquinolinone scaffolds. Bioorg Med Chem Lett 18:2628–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Leibovitz A, McCombs 3rd WM, Johnston D, McCoy CE, Stinson JC 1973 New human cancer cell culture lines. I. SW-13, small-cell carcinoma of the adrenal cortex. J Natl Cancer Inst 51:691–697 [PubMed] [Google Scholar]
- Ye P, Nakamura Y, Lalli E, Rainey WE 2009 Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology 150:1303–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berruti A, Ferrero A, Sperone P, Daffara F, Reimondo G, Papotti M, Dogliotti L, Angeli A, Terzolo M 2008 Emerging drugs for adrenocortical carcinoma. Expert Opin Emerg Drugs 13:497–509 [DOI] [PubMed] [Google Scholar]
- Michalkiewicz E, Sandrini R, Figueiredo B, Miranda EC, Caran E, Oliveira-Filho AG, Marques R, Pianovski MA, Lacerda L, Cristofani LM, Jenkins J, Rodriguez-Galindo C, Ribeiro RC 2004 Clinical and outcome characteristics of children with adrenocortical tumors. An analysis of 254 cases from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol 22:838–845 [DOI] [PubMed] [Google Scholar]
- Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A 2007 Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 356:2372–2380 [DOI] [PubMed] [Google Scholar]
- Betz MJ, Shapiro I, Fassnacht M, Hahner S, Reincke M, Beuschlein F; German and Austrian Adrenal Network 2005 Peroxisome proliferator-activated receptor-γ agonists suppress adrenocortical tumor cell proliferation and induce differentiation. J Clin Endocrinol Metab 90:3886–3896 [DOI] [PubMed] [Google Scholar]
- Doghman M, Cazareth J, Lalli E 2008 The T cell factor/β-catenin antagonist PKF115–584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab 93:3222–3225 [DOI] [PubMed] [Google Scholar]
- Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, Lerario AM, Maciel CC, Mattos GE, Jorge AA, Mendonca BB, Latronico AC 2008 Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab 93:3524–3531 [DOI] [PubMed] [Google Scholar]
- Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD 2009 Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab 94:204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.