Abstract
Objective: We investigated the association between inflammation, microvascular reactivity, and the development of peripheral diabetic neuropathy.
Research Design and Methods: We studied three groups: 55 healthy control subjects, 80 nonneuropathic patients, and 77 neuropathic diabetic patients. We also subdivided the neuropathic patients into a subgroup of 31 subjects with painless neuropathy and 46 with painful neuropathy. We measured the foot skin endothelium-dependent and -independent vasodilation, the nerve axon reflex-related vasodilation (NARV), and inflammatory cytokines and biochemical markers of endothelial function.
Results: The endothelium-dependent and -independent vasodilation and NARV were lower in the neuropathic group (P < 0.05). NARV was further reduced in the subgroup of painless neuropathy when compared to painful neuropathy (P < 0.05). Compared to the other two groups, the neuropathic group also had higher serum levels of PDGF AA/BB (P < 0.05), RANTES (P < 0.01), leptin (P < 0.0001), osteoprotegerin (P < 0.01), G-CSF (P < 0.05), sE-Selectin (P < 0.01), sICAM (P < 0.0001), sVCAM (P < 0.001), CRP (P < 0.0001), TNFα (P < 0.05), and fibrinogen (P < 0.05). Patients with painful neuropathy had higher sICAM-1 (P < 0.05) and CRP levels (P < 0.01) when compared to painless neuropathy. No major changes in the above results were observed in 78 diabetic patients who were seen for a second visit 21 months after the first visit.
Conclusions: Peripheral diabetic neuropathy is associated with increased biochemical markers of inflammation and endothelial dysfunction. Painful neuropathy is associated with further increase in inflammation and markers of endothelial dysfunction and preservation of the nerve axon reflex.
Peripheral diabetic neuropathy is associated with increased biochemical markers of inflammation and endothelial dysfunction, and these are more prominent in painful neuropathy.
Inflammation, which is currently considered to be a major factor in the development of type 2 diabetes, has also been proposed as a major factor in the development of diabetic neuropathy in animal models (1,2). In addition, numerous growth factors, such as vascular endothelial growth factor (VEGF), nerve growth factor, and TGFβ, have also been implicated in the pathogenesis of diabetic neuropathy and have been targeted as possible new therapeutic approaches (3,4). However, an additional problem is that all the above information is based on animal studies and very little information is available regarding human diabetes.
Furthermore, serious limitations exist in our current knowledge regarding the causes of neuropathic pain in diabetes. Previous studies in human diabetes have shown that there are no significant differences in peripheral small and large nerve fiber function and neurophysiological and pathological parameters between patients with painful and painless diabetic peripheral neuropathy (5,6,7). In addition, there are no data showing whether proinflammatory cytokines are associated with pain in human diabetic neuropathy.
The main aim of the present study was to assess possible associations between peripheral diabetic neuropathy and serum inflammatory cytokines and growth factors in human diabetes. An additional aim was to identify possible differences between painful and painless neuropathy. Finally, we opted to examine whether the observed cytokine and growth factor levels remain stable over a relatively long period of time. Our primary hypothesis was that inflammatory cytokines are associated with the development of peripheral neuropathy and neuropathic pain.
Subjects and Methods
Study subjects
We included 212 subjects, ages 21–80 yr, and divided them into three groups: 55 healthy, nondiabetic control subjects, 80 nonneuropathic diabetic patients, and 77 neuropathic diabetic patients. The neuropathic group was subsequently subdivided into patients with and without neuropathic pain according to the criteria described below. Patients with type 1 or 2 diabetes, according to American Diabetes Association criteria, were included (8). Exclusion criteria were: symptomatic peripheral arterial disease (ankle brachial index < 0.65 and/or symptoms of claudication), congestive heart failure, cardiac arrhythmias, stroke or transient ischemic attack, end stage renal failure, uncontrolled hypertension, severe dyslipidemia, chronic liver disease, or any other severe chronic medical condition requiring active treatment. The study was approved by the institutional review board of the Beth Israel Deaconess Medical Center. All participants gave informed consent for the original trials.
Clinical measurements
All clinical examinations and evaluations were conducted under fasting conditions. The cytokines and growth factors were measured using a Luminex 200 apparatus (Luminex Corporation, Austin, TX) and Millipore multiplex immunoassay panels (Millipore Corporation, Chicago, IL). Because there are very limited data available regarding the association of diabetic neuropathy to cytokines and growth factors, we opted to test a large array of these molecules. This approach was deemed the most suitable because it could provide us with a better view of possible associations and could lead to additional, specific, hypothesis-driven research.
Evaluation of peripheral diabetic neuropathy
Peripheral neuropathy was evaluated according to the guidelines of the San Antonio Consensus Statement and the American Diabetes Association statement on diabetic neuropathy (9,10,11). The neuropathic symptoms were evaluated by using a Neuropathy Symptom Score (NSS) and the clinical signs by using a Neuropathy Disability Score (NDS) as described elsewhere (5,11,12). More specifically, for the evaluation of the NSS, the participants were asked about the following symptoms in their feet or legs: 1) pins and needles; 2) abnormal cold or hot sensations; 3) lancinating pain; 4) deep aching pain; 5) burning pain (causalgia); and 6) irritation of the feet or legs by the bedclothes at night (hyperesthesia). Each symptom was scored with one point if it was present and two points if nocturnal exacerbation was also present. A score of four or more points was considered to be abnormal (4,11). For the evaluation of the clinical signs of peripheral diabetic neuropathy, the sensations of pain, touch, cold, and vibration were tested in both legs of all patients. A NDS more than five was considered to be abnormal (4,11).
Quantitative sensory testing measurements included the assessment of vibration perception threshold (VPT) and cutaneous perception threshold. The vibration perception threshold was measured at the great toe on the dominant side of each patient using a Biothesiometer (Bio-medical Instruments, Newbury, OH). A VPT greater than 25 volts was considered to be abnormal (12). A set of six Semmes-Weinstein monofilaments was employed to evaluate the cutaneous perception threshold. Each nylon monofilament was applied to the plantar surface of the great toe of both feet, and the smallest monofilament that was felt by the patient with his or her eyes closed was recorded. Inability to feel the pressure applied by a 5.07 monofilament was considered to be abnormal (12).
The patient characterization was based on the clinical examination that was performed at the baseline visit. The diagnosis of peripheral neuropathy was made when at least two of the three tests (NSS, NDS, and quantitative sensory testing) were abnormal (9). The diagnosis of painful neuropathy was made when the NSS was at least four points.
Vascular reactivity measurements
Vascular reactivity measurements were performed according to previously described techniques (12). The blood flow response to the iontophoresis of 1% acetylcholine chloride solution and of 1% sodium nitroprusside was assessed at the dorsum of the foot. Acetylcholine induces endothelium-dependent vasodilation. It also stimulates c-nociceptive fibers, resulting in vasodilation in skin areas adjacent to the site of its administration, and this response, called the nerve axon reflex-related vasodilation (NARV), has been used to evaluate the c-nociceptive fiber action (13,14,15). The sodium nitroprusside induces endothelium-independent vasodilation and does not elicit a NARV response (15).
The skin blood flow before and after iontophoresis was evaluated by employing a Laser Doppler Perfusion Imager (LDPI Lisca 2.0; Lisca Development AB, Linkoping, Sweden), as previously described (12,16,17). The NARV was measured by employing a single point laser probe and a DRT4 Laser Doppler Blood Flow Monitor (Moor Instruments, Millwey, Devon, UK) (13,14). Previous studies in our unit have reported a satisfactory reproducibility for the above techniques, 13.1% for the endothelium-dependent vasodilation, 11.4% for the non-endothelium-dependent vasodilation, and 32.6% for the NARV (12).
Second visit
All diabetic patients were asked to return for a follow-up visit approximately 18–24 months after the first visit. The main reason for including this visit was to assess whether there were any major changes in the observed levels of cytokines and growth factors during the initial visit. The same tests that were performed during the baseline were also performed for the exit visit.
Statistical analysis
Statistical analysis was performed in collaboration with a biostatistician (C.G.). The analysis was undertaken by univariate techniques and modeling the data through multiple linear regression using the Minitab v.15 statistical package (Minitab, State College, PA). For normally distributed data, the ANOVA was used, followed by the Fisher’s multiple comparison test to identify differences between groups. For nonparametrically distributed data, the Kruskal-Wallis test was used. We also employed multiple linear regression analysis to assess the differences in cytokines and growth factors among the study groups after adjusting for age, gender, and medications. The analysis of correlation between normally distributed variables was done through Pearson correlation coefficient. For nonparametric data, the Spearman correlation coefficient was used. The comparison between baseline and exit visit measurements was performed by employing the paired t test for parametrically distributed data and the Wilcoxon matched pair signed rank test for nonparametrically distributed data. The contribution of cytokines and growth factors in the variation of neuropathy measurements was assessed by univariate and multivariate stepwise regression analysis.
Results
The comparisons of the demographics among the three groups are shown in Table 1. All groups were matched for age and gender. The two diabetic groups were also matched for the type of diabetes. The known diabetes duration was longer in the neuropathic group (P < 0.01). Body mass index was lower in the control group but similar in the two diabetic groups, whereas the systolic blood pressure and serum creatinine levels were higher in the neuropathic group (P < 0.01). As expected, all measurements of neuropathy were higher in the neuropathic group compared with the other two groups. In comparisons between the painless and painful neuropathic subgroups, the only observed differences were the known duration of diabetes, which was higher in the painless subgroup (P < 0.01), and the NSS, which was higher in the painful neuropathic group, as was expected from the selection criteria (P < 0.0001). Insulin treatment was more common in the neuropathic group (P < 0.0001), whereas metformin treatment was more common in the subgroup with painful neuropathy when compared with the group with painless neuropathy (P < 0.01).
Table 1.
Clinical characteristics of studied subjects
| Controls (C) | DM–no neuropathy (No-PDN) | DM–neuropathy (PDN) | Painless neuropathy (Painless) | Painful neuropathy (Painful) | |
|---|---|---|---|---|---|
| n | 55 | 80 | 77 | 31 | 46 |
| Age (yr) | 55 ± 13 | 56 ± 14 | 58 ± 9 | 58 ± 11 | 58 ± 9 |
| Males | 30 (56) | 44 (55) | 51 (66) | 22 (71) | 29 (63) |
| Diabetes type (1/2) | 22/58 | 30/47 | 13/18 | 17/29 | |
| Diabetes duration (yr)a | 14 ± 11 | 20 ± 13 | 23 ± 16 | 17 ± 11 | |
| Body mass index (kg · m−2)b | 27.2 (22.6–30.1) | 31.0 (26.0–36.3) | 32.3 (27.0–37.68) | 31.9 (25.7–33.9) | 32.7 (28.1–40.1) |
| Systolic blood pressure (mm Hg)c | 122 ± 16 | 126 ± 16 | 146 ± 19 | 146 ± 18 | 145 ± 19 |
| Diastolic blood pressure (mm Hg) | 73 ± 9 | 73 ± 9 | 75 ± 9 | 74 ± 8 | 76 ± 9 |
| Heart rate (beats/min)d | 66 ± 8 | 71 ± 11 | 73 ± 11 | 73 ± 11 | 77 ± 11 |
| HbA1c (%)e | 5.6 ± 0.3 | 7.3 ± 1.3 | 8.1 ± 1.8 | 8.2 ± 1.9 | 8.0 ± 1.8 |
| Creatinine (mg/dl)f | 0.9 ± 0.2 | 1.0 ± 0.3 | 1.1 ± 0.5 | 1.1 ± 0.5 | 1.1 ± 0.6 |
| Total cholesterol (mg/dl)g | 200 ± 28 | 167 ± 46 | 175 ± 37 | 168 ± 28 | 180 ± 43 |
| HDL cholesterolh | 61 ± 20 | 58 ± 19 | 50 ± 13 | 53 ± 14 | 48 ± 11 |
| NSSi | 0 ± 0 | 1 ± 1 | 5 ± 4 | 1 ± 1 | 7 ± 3 |
| NDSj | 0 ± 1 | 2 ± 3 | 12 ± 7 | 13 ± 6 | 12 ± 7 |
| VPT (volts)k | 9 ± 5 | 14 ± 10 | 37 ± 14 | 38 ± 16 | 36 ± 13 |
| Semmes-Weinstein monofilamentsl | 3.86 ± 0.48 | 3.97 ± 0.56 | 5.90 ± 0.92 | 6.00 ± 0.80 | 5.82 ± 0.99 |
| Ankle brachial index | 1.1 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 |
| Insulinm | 32 (40) | 54 (70) | 22 (71) | 32 (70) | |
| Metforminn | 32 (40) | 23 (30) | 4 (13) | 19 (41) | |
| Sulfonylureas | 24 (30) | 16 (21) | 5 (16) | 11 (30) | |
| Thiazolidinediones | 17 (21) | 8 (10) | 3 (10) | 5 (11) | |
| Statins | 11 (20) | 39 (49) | 41 (53) | 18 (58) | 23 (50) |
| Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers | 6 (11) | 43 (54) | 46 (60) | 20 (65) | 26 (57) |
| Aspirin | 6 (11) | 26 (33) | 26 (34) | 13 (42) | 13 (28) |
| Antidepressantso | 5 (10) | 17 (23) | 25 (32) | 6 (20) | 19 (42) |
| Antiepileptics | 2 (4) | 5 (7) | 1 (1) | 1 (3) | 0 () |
Data are expressed as mean± sd or number (percentage). PDN, Peripheral diabetic neuropathy; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein.
No-PDN vs. PDN, P < 0.01, Painless vs. Painful, P < 0.01.
C vs. No-PDN, PDN, P < 0.01.
C, No-PDN vs. PDN, P < 0.0001.
C, No-PDN vs. PDN, P < 0.0001.
C vs. No-PDN vs. PDN, P < 0.0001.
C, No-PDN vs. PDN, P < 0.01.
C vs. No-PDN, PDN, P < 0.0001.
C, No-PDN vs. PDN, P < 0.001.
C vs. No-PDN vs. PDN, P < 0.0001, Painless vs. Painful, P < 0.0001.
C vs. No-PDN vs. PDN, P < 0.0001.
C vs. No-PDN vs. PDN, P < 0.0001.
C, No-PDN vs. PDN, P < 0.0001.
No-PDN vs. PDN, P < 0.0001.
Painless vs. Painful, P < 0.01.
Painless vs. Painful, P < 0.05.
The results from the vascular reactivity measurements are shown in Table 2. The resting blood flow was higher in the neuropathic group when compared with the other two groups (P < 0.0001). The response to the iontophoresis of acetylcholine (endothelium dependent) and sodium nitroprusside (endothelium independent) were lower in both diabetic groups when compared with the controls (P < 0.01), but no differences were found between the two diabetic groups. In contrast, the NARV that depends on the c-nociceptive fiber function was reduced in the neuropathic group when compared with the other two groups (P < 0.01). There were no differences in the response to acetylcholine and sodium nitroprusside between the painless and painful neuropathic subgroups. However, the NARV was higher in the painful subgroup when compared with the painless group (P < 0.05).
Table 2.
Results of vascular reactivity
| Controls (C) | DM–no neuropathy (No-PDN) | DM–neuropathy (PDN) | Painless neuropathy (Painless) | Painful neuropathy (Painful) | |
|---|---|---|---|---|---|
| Resting skin blood flow (volts)a | 1.02 ± 0.37 | 1.04 ± 0.38 | 1.27 ± 0.41 | 1.23 ± 0.39 | 1.29 ± 0.43 |
| Acetylcholine response (%)b | 26 (12–41) | 17 (9–30) | 16 (8–24) | 14 (7–23) | 16 (8–25) |
| Sodium nitroprusside response (%)c | 33 (20–60) | 23 (12–33) | 17 (6–35) | 17 (6–33) | 17 (5–36) |
| NARV (%)d | 65 (19–149) | 40 (8–120) | 23 (6–51) | 10 (0–42) | 30 (13–55) |
Data are expressed as mean ± sd or median (first–third quartiles). PDN, Painful diabetic neuropathy.
C, No-PDN vs. PDN, P < 0.01.
C vs. No-PDN, PNN, P < 0.01.
C vs. No-PDN, PDN, P < 0.01.
C, No-PDN vs. PDN, P < 0.01, Painless vs. Painful, P < 0.05.
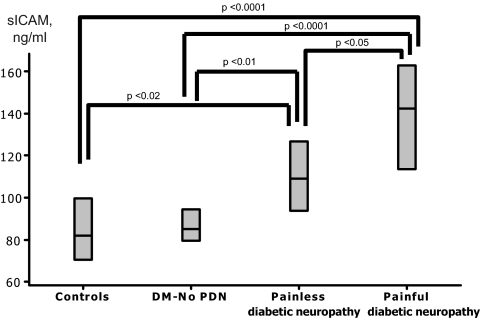
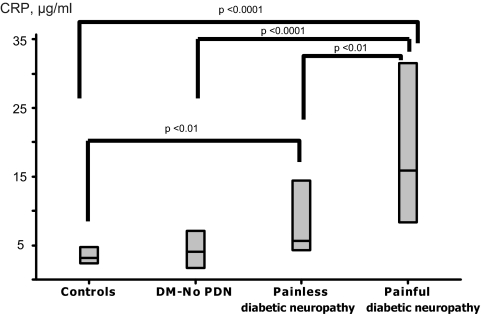
The results from growth factors and cytokine measurements are shown in Table 3. Platelet-derived growth factor (PDGF) AA was marginally lower in both diabetic groups when compared with the controls (P = 0.057) but PDGF AB/BB was higher (P < 0.05) and epidermal growth factor (EGF) (P < 0.01) was lower in the neuropathic group when compared with the other two groups. Apart from chemokine (C-X-C motif) ligand 10 (IP-10), no other differences were observed in the remaining measurements between the control and the nonneuropathic group. However, significant differences were found between these two groups and the neuropathic group. More specifically, the neuropathic group had higher levels of PDGF AA/BB (P < 0.05), regulated upon activation normal T cell expressed and secreted (RANTES) (P < 0.01), leptin (P < 0.0001), osteoprotegerin (OPG) (P < 0.01), granulocyte colony-stimulating factor (G-CSF) (P < 0.05), sE-Selectin (P < 0.01), soluble intercellular adhesion molecule (sICAM) (P < 0.0001), soluble vascular cell adhesion molecule (sVCAM) (P < 0.001), C-reactive protein (CRP) (P < 0.0001), TNFα (P < 0.05), and fibrinogen (P < 0.05). Patients with painful neuropathy had higher sICAM-1 (P < 0.05) (Fig. 1) and CRP levels (P < 0.01) (Fig. 2) when compared with the patients with painless neuropathy. Similar results were observed when analysis was adjusted for possible differences in the various treatments for diabetes, hypertension, and dyslipidemia that are presented in Table 1 (data not shown).
Table 3.
Cytokine and growth factor results
| Controls (C) | DM–no neuropathy (No-PDN) | DM–neuropathy (PDN) | Painless neuropathy (Painless) | Painful neuropathy (Painful) | |
|---|---|---|---|---|---|
| PDGF AA (ng/ml)a | 23.2 (14.3–28.4) | 15.0 (8.5–26.1) | 14.8 (9.5–25.6) | 14.8 (10.0–31.3) | 15.7 (8.8–22.9) |
| PDGF AB/BB (ng/ml)b | 37.4 (20.9–65.3) | 36.4 (21.0–55.3) | 45.5 (31.3–81.7) | 51.0 (32.5–121.0) | 43.8 (27.2–72.1) |
| EGF (pg/ml)c | 61.4 (25.6–109.5) | 64.3 (30.8–122.5) | 40.7 (25.2–56.5) | 40.8 (26.5–54.1) | 39.7 (21.4–60.2) |
| Fibroblast growth factor (pg/ml) | 44.7 (32.3–66.0) | 36.1 (21.2–63.5) | 37.1 (25.3–50.5) | 36.8 (25.7–50.6) | 37.1 (24.7–50.7) |
| VEGF (pg/ml) | 112 (34–259) | 151 (43–459) | 169 (71–332) | 145 (63–260) | 185 (85–427) |
| RANTES (ng/ml)d | 46.8 (24.5–87.2) | 58.0 (25.4–93.2) | 71.3 (52.2–105.0) | 76.7 (51.9–114.2) | 69.9 (51.7–98.8) |
| Leptin(ng/ml)e | 5.7 (1.2–9.8) | 7.3 (2.2–23.0) | 16.3 (6.4–26.8) | 11.6 (4.4–26.8) | 19.2 (6.7–28.3) |
| OPG (pg/ml)f | 371 (219–496) | 301 (190–535) | 507 (306–625) | 428 (281–634) | 523 (326–634) |
| Receptor activator for nuclear factor κB ligand (pg/ml) | 17 (9–42) | 31 (13–71) | 23 (6–53) | 27 (7–60) | 17 (4–53) |
| G-CSF (pg/ml)g | 13 (7–22) | 14 (7–24) | 19 (12–26) | 21 (14–26) | 17 (12–27) |
| IP-10 (pg/ml)h | 171 (113–214) | 121 (81–156) | 130 (93–200) | 134 (101–223) | 128 (91–198) |
| Myeloperoxidase (ng/ml)i | 102 (43–229) | 105 (52–181) | 61 (33–147) | 64 (32–103) | 60 (33–166) |
| sE-Selectin (ng/ml)j | 18.1 (13.5–28.8) | 17.5 (14.5–23.6) | 31.6 (17.3–49.1) | 27.0 (17.2–38.2) | 32.5 (17.6–51.3) |
| sICAM-1 (ng/ml)k | 81 (59–112) | 85 (59–112) | 126 (92–176) | 109 (81–148) | 143 (95–192) |
| sVCAM-1 (ng/ml)l | 803 (602–1063) | 782 (560–1030) | 1000 (747–1180) | 890 (703–1165) | 1020 (749–1185) |
| CRP (μg/ml)m | 3.0 (1.8–6.7) | 4.1 (1.0–15.3) | 11.6 (4.4–46.7) | 5.6 (3.0–24.8) | 15.9 (5.7–52.0) |
| TNFα (pg/ml)n | 4.7 (3.4–7.6) | 5.3 (2.1–9.2) | 7.1 (5.0–9.3) | 7.0 (4.8–9.4) | 7.1 (5.1–9.2) |
| Fibrinogen (ng/ml)o | 360 (203–566) | 489 (259–864) | 750 (394–1180) | 612 (456–1320) | 759 (338–1170) |
Data are expressed as median (first–third quartiles). PDN, Peripheral diabetic neuropathy.
C vs. No-PDN and PDN, P = 0.057.
No-PDN vs. PDN, P < 0.05.
C, No-PDN vs. PDN, P < 0.01.
C, No-PDN vs. PDN, P < 0.01.
C, No-PDN vs. PDN, P < 0.0001.
C, No-PDN vs. PDN, P < 0.01.
C, No-PDN vs. PDN, P < 0.05.
C vs. No-PDN, P < 0.02.
C, No-PDN vs. PDN, P < 0.05.
C, No-PDN vs. PDN, P < 0.0001.
C, No-PDN vs. PDN, P < 0.0001, Painless vs. Painful, P < 0.05.
C, No-PDN vs. PDN, P < 0.001.
C, No-PDN vs. PDN, P < 0.0001, Painless vs. Painful, P < 0.01.
C, No-PDN vs. PDN, P < 0.05.
C, No-PDN vs. PDN, P < 0.0001.
Figure 1.
The sICAM serum levels were increased in patients with both painful and painless neuropathy when compared with the healthy controls and the nonneuropathic group [diabetes mellitus–no peripheral diabetic neuropathy (DM-No PDN)]. Furthermore, sICAM was increased in the patients with painful neuropathy when compared with patients with painless neuropathy.
Figure 2.
The CRP serum levels were increased in patients with painful neuropathy when compared with the healthy controls, the nonneuropathic group [diabetes mellitus–no peripheral diabetic neuropathy (DM-No PDN)], and the patients with painless neuropathy. Furthermore, CRP was increased in the patients with painless neuropathy when compared with the healthy controls.
The associations between measurements of neuropathy, growth factors, and other cytokines, considering all participating subjects as one group, are shown in Table 4. On stepwise multivariate analysis, sICAM, leptin, sVCAM, RANTES, PDGF-AA, and EGF contributed 12, 4, 2, 2, 2, and 1%, respectively, to the variation of the NSS. The variation of the NDS could be predicted by sICAM (13%), PDGF-AB-BB (2%), EGF (2%), and leptin (1%). The variation of the VPT could be predicted by sICAM (8%), EGF (2%), OPG (2%), and PDGF AB/BB (1%).
Table 4.
Relationship between neuropathy and cytokines
| NSS | NDS | VPT | |
|---|---|---|---|
| Acetylcholine response (%) | −0.14 (0.025) | −0.17 (0.006) | −0.18 (0.003) |
| Nerve axon reflex (%) | −0.19 (0.003) | −0.187 (0.002) | −0.20 (0.001) |
| PDGF AB/BB (ng/ml) | 0.15 (0.017) | ||
| RANTES (ng/ml) | 0.14 (0.027) | 0.18 (0.004) | 0.12 (0.046) |
| Leptin(ng/ml) | 0.28 (0.0001) | 0.19 (0.003) | |
| OPG (pg/ml) | 0.20 (0.001) | 0.18 (0.004) | 0.15 (0.014) |
| sE-Selectin (ng/ml) | 0.28 (0.0001) | 0.29 (0.0001) | 0.13 (0.31) |
| sICAM-1 (ng/ml) | 0.34 (0.0001) | 0.39 (0.0001) | 0.29 (0.0001) |
| sVCAM-1 (ng/ml) | 0.20 (0.001) | 0.19 (0.002) | 0.16 (0.011) |
| CRP (μg/ml) | 0.19 (0.003) | 0.12 (0.049) | |
| Fibrinogen (ng/ml) | 0.20 (0.001) | 0.16 (0.010) |
PDN, Peripheral diabetic neuropathy.
Second visit
Thirty-five nonneuropathic diabetic patients and 43 neuropathic patients (19 with painless and 24 with painful neuropathy) returned for a second visit. The time period between first and second visits was 21 ± 5 months (mean ± sd). There were no major changes in the prescribed medications between the first and second visits in all groups. In addition, there were no changes between first and second visits in NSS, NDS, VPT, Semmes-Weinstein monofilaments, and NARV. The response to acetylcholine improved in both the nonneuropathic [10%, −7:26 (median improvement, 25:75 percentile) (P < 0.05)] and neuropathic groups [13%, −6:26 (P < 0.05)], whereas the response to sodium nitroprusside improved only in the neuropathic group [12, 9:34 (P < 0.05)]. No significant changes were observed between the first and second visits in any of the measured growth factors and cytokines, glycemic control, and lipid levels.
Discussion
The main findings of the present study were that diabetic patients with peripheral neuropathy had increased serum levels of inflammatory cytokines and changes in the levels of various growth factors. Furthermore, patients with painful neuropathy had a further increase in inflammation, as indicated by higher levels of CRP and endothelial dysfunction, as indicated by the levels of sICAM. The NARV, a measurement of the c-nociceptive fiber action, was more impaired in patients with painless neuropathy than patients with painful neuropathy despite the fact that all other measurements of nerve function were similar in these two groups.
There are currently limited data regarding the association of peripheral diabetic neuropathy with vascular disease and inflammation. A large prospective study that followed patients with type 1 diabetes reported that the development of peripheral neuropathy was associated with known risk factors for the development of cardiovascular disease, including total and low-density lipoprotein cholesterol and triglycerides, body mass index, high levels of von Willebrand factor, hypertension, and smoking (18). The results of the present study further expand the association between vascular disease and neuropathy and reveal an association between peripheral diabetic neuropathy and biochemical markers of endothelial dysfunction, such as sE-Selectin, sICAM, sVCAM, and fibrinogen that are well-established risk factors for the development of cardiovascular disease (19,20,21).
Inflammation is a well-known risk factor for the development of macrovascular disease (22). Studies in our unit have shown that the vascular reactivity of the skin microcirculation (which includes both endothelium-dependent and -independent vasodilation) is impaired in patients with type 2 diabetes and in subjects at risk of developing type 2 diabetes, and this impairment is associated with inflammatory cytokines (23,24). The present study also indicates an association between a variety of inflammation markers and the development of peripheral diabetic neuropathy. To the best of our knowledge, there are no previous studies in human diabetes that demonstrated an association between diabetic neuropathy and systemic inflammation in human diabetes.
It should be emphasized that the above observed measurements are correlative and do not necessarily indicate causality. It can also be claimed that the observed measurements are secondary to diabetes and the inflammation that is associated with diabetes. Although the design of the current study, as is the case with the majority of human studies, does not allow definite conclusions about possible causality, we believe that there are some indications that the observed results may be related to the development of peripheral neuropathy. Thus, the lack of major differences between the control and nonneuropathic groups, although significant changes were observed between the nonneuropathic and the neuropathic diabetic patients, indicates that the observed changes are mostly associated to the presence of neuropathy rather than diabetes itself. Further support to this is also provided by the fact that the observed results were independent of the medications that were prescribed to treat hyperglycemia, hypertension, and dyslipidemia.
It is of interest that in addition to classic inflammatory cytokines that are known to be related to the diabetic state, such as TNFα and CRP, peripheral neuropathy was also associated with additional inflammatory cytokines that are products of a variety of cells that have not hitherto been involved in the development of this condition. Thus, RANTES is a cytokine produced by the platelets, the circulating lymphocytes, and the tissue cell monocytes and is involved in the initial stages of the development of atherosclerosis (25). Leptin is now considered to be a pleiotropic hormone with proinflammatory effects, and G-CSF is produced by monocytes, fibroblasts, and endothelial cells; is involved in the mobilization of granulocytes and progenitor cells from the bone marrow; and has been employed for the treatment of diabetic foot ulcers and acute myocardial infarction (26,27,28). It is also of interest that OPG was elevated in the neuropathic group, whereas no differences where observed in the receptor activator for nuclear factor κB ligand levels. These findings raise doubts regarding the hypothesized role of these two cytokines in the development of diabetic neuropathic osteoarthropathy (Charcot foot) (29). Surprisingly, the levels of myeloperoxidase were lower in the neuropathic group despite previous evidence regarding its role in increasing oxidative stress and contributing to cardiovascular disease (30). Finally, the levels of IP-10, a cytokine that has also been involved in the development of atheromatosis, were lower in the nonneuropathic group when compared with the controls, but no differences were observed between the two diabetic groups (31).
We have also examined possible differences in the levels of various growth factors among the three groups. Our results showed no differences in fibroblast growth factor and VEGF, a marginal decrease in PDGF AA in both diabetic groups, an increase in the combined PDGF AB/BB levels in the neuropathic group, and a reduction in the EGF levels in the neuropathic group. Given the fact that the above growth factors have been mainly implicated in wound healing, their association with the development of peripheral neuropathy is intriguing, and further studies will be required to clarify their role in the development of peripheral neuropathy (32). However, it should also be emphasized that, as best seen by the results of the stepwise regression analysis, the contribution of the studied cytokines and growth factors to the observed nerve function measurement variation is rather limited, and other contributing factors should also be sought.
As mentioned in the introduction, the pathogenesis of pain in diabetic neuropathy is largely unknown. In the present study, we have found that the NARV, sICAM, and CRP were increased in patients with painful neuropathy when compared with patients with painless neuropathy. These results provide proof of concept for the first time that inflammation and endothelial dysfunction play a role in the development of painful neuropathy, and further studies will be required to explore these mechanisms.
A large number of nonneuropathic and neuropathic diabetic patients were seen for a second visit after a 21-month period, on average. In agreement with the majority of previous studies, no progression of the severity of the diabetic neuropathy was observed during this time period (33). The fact that there were also no changes in the cytokine and growth factor levels supports the hypothesis of an association between these parameters. However, longer studies that will allow the development of changes in the nerve function and evaluation of the role of cytokines and growth factors will be required to reach firm conclusions.
A mild improvement in the vascular reactivity of both the neuropathic and nonneuropathic diabetic patients was also observed, despite the lack of any changes in the severity of neuropathy, during the follow-up period. The reasons for this improvement are not known and cannot be explained by changes in factors that are known to influence vascular reactivity, such as changes in the glycemic control and lipid levels. Of interest, there were no changes in the NARV that evaluates the small fiber function. Therefore, the most possible explanation is that the observed improvement is nonspecific and is similar to nonspecific improvements that are seen in subjects that participate in the placebo arm of clinical trials.
The present study has its limitations. One limitation is that the nonneuropathic and neuropathic groups were not matched for the duration of diabetes and that the observed differences may be related to this mismatch. However, it should be pointed out that the duration of diabetes is rather long in both groups. Furthermore, inflammatory cytokines tended to be higher in the subgroup of painful neuropathy despite the fact that the duration of diabetes was shorter in this group when compared with the subgroup with painless neuropathy.
One additional limitation is that painful symptoms were evaluated employing only one technique, the NSS, and not using additional methods such as the visual analog scale. We chose the NSS because it captures symptoms that are specific for painful diabetic neuropathy, and we believe it is more appropriate in a study like this that is mainly interested in examining differences between painful and painless neuropathy and does not focus on possible therapeutic effects of new treatments where the use of a visual analog score scale would be more justified. Finally, another limitation could be the lack of electrophysiological and autonomic function measurements for the diagnosis of diabetic neuropathy. However, according to a recent statement by the American Diabetes Association, an accurate diagnosis of diabetic neuropathy can be made without the use of the above tests (10). Therefore, we believe that the employed methods are adequate to satisfactorily characterize the study population and that the addition of the above measurements would not add anything to the study and would not have any effect on the conclusions reached.
In conclusion, in the present study we have shown that human peripheral diabetic neuropathy is associated with increased biochemical markers of inflammation and endothelial dysfunction. Furthermore, painful neuropathy is associated with further increase in inflammation and markers of endothelial dysfunction and preservation of the nerve axon reflex. These results indicate that inflammation and endothelial dysfunction may be important contributors in the development of peripheral neuropathy in diabetes.
Footnotes
This work was partly supported by National Institutes of Health Grants R01-HL075678, R01-NS046710, and R01 DK076937 (to A.V.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 10, 2009
Abbreviations: CRP, C-reactive protein; EGF, epidermal growth factor; G-CSF, granulocyte colony-stimulating factor; IP-10, chemokine (C-X-C motif) ligand 10; NARV, nerve axon reflex-related vasodilation; NDS, Neuropathy Disability Score; NSS, Neuropathy Symptom Score; OPG, osteoprotegerin; PDGF, platelet-derived growth factor; RANTES, regulated upon activation normal T cell expressed and secreted; sICAM, soluble intercellular adhesion molecule; sVCAM, soluble vascular cell adhesion molecule; VEGF, vascular endothelial growth factor; VPT, vibration perception threshold.
References
- Kellogg AP, Wiggin TD, Larkin DD, Hayes JM, Stevens MJ, Pop-Busui R 2007 Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes 56:2997–3005 [DOI] [PubMed] [Google Scholar]
- Wang Y, Schmeichel AM, Iida H, Schmelzer JD, Low PA 2006 Enhanced inflammatory response via activation of NF-κB in acute experimental diabetic neuropathy subjected to ischemia-reperfusion injury. J Neurol Sci 247:47–52 [DOI] [PubMed] [Google Scholar]
- Veves A, King GL 2001 Can VEGF reverse diabetic neuropathy in human subjects? J Clin Invest 107:1215–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjaneyulu M, Berent-Spillson A, Inoue T, Choi J, Cherian K, Russell JW 2008 Transforming growth factor-β induces cellular injury in experimental diabetic neuropathy. Exp Neurol 211:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veves A, Young MJ, Manes C, Boulton AJ 1994 Differences in peripheral and autonomic nerve function measurements in painful and painless neuropathy. A clinical study. Diabetes Care 17:1200–1202 [DOI] [PubMed] [Google Scholar]
- Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, Boulton AJ 2001 Sural nerve fibre pathology in diabetic patients with mild neuropathy: relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology. Acta Neuropathol 101:367–374 [DOI] [PubMed] [Google Scholar]
- Sorensen L, Molyneaux L, Yue DK 2006 The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care 29:883–887 [DOI] [PubMed] [Google Scholar]
- 2007 Diagnosis and classification of diabetes mellitus. Diabetes Care 30:S42–S47 [DOI] [PubMed] [Google Scholar]
- 1988 Consensus statement: report and recommendations of the San Antonio Conference on Diabetic Neuropathy. Diabetes 37:1000–1004 [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D; American Diabetes Association 2005 Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28:956–962 [DOI] [PubMed] [Google Scholar]
- Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A 2000 Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 23:606–611 [DOI] [PubMed] [Google Scholar]
- Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, DeGirolami U, LoGerfo FW, Freeman R 1998 Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 47:457–463 [DOI] [PubMed] [Google Scholar]
- Kilo S, Berghoff M, Hilz M, Freeman R 2000 Neural and endothelial control of the microcirculation in diabetic peripheral neuropathy. Neurology 54:1246–1252 [DOI] [PubMed] [Google Scholar]
- Caselli A, Rich J, Hanane T, Uccioli L, Veves A 2003 Role of C-nociceptive fibers in the nerve axon reflex-related vasodilation in diabetes. Neurology 60:297–300 [DOI] [PubMed] [Google Scholar]
- Hamdy O, Abou-Elenin K, LoGerfo FW, Horton ES, Veves A 2001 Contribution of nerve-axon reflex-related vasodilation to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care 24:344–349 [DOI] [PubMed] [Google Scholar]
- Kubli S, Waeber B, Dalle-Ave A, Feihl F 2000 Reproducibility of laser Doppler imaging of skin blood flow as a tool to assess endothelial function. J Cardiovasc Pharmacol 36:640–648 [DOI] [PubMed] [Google Scholar]
- Morris SJ, Shore AC 1996 Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol 496:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH; EURODIAB Prospective Complications Study Group 2005 Vascular risk factors and diabetic neuropathy. N Engl J Med 352:341–350 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Roitman-Johnson B, Stamofer MJ, Allen J 1998 Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 351:88–92 [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Rifai N, Ridker PM 2002 Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation 106:820–825 [DOI] [PubMed] [Google Scholar]
- Meade TW, Mellows S, Brozovic M, Miller GJ, Chakrabarti RR, North WR, Haines AP, Stirling Y, Imeson JD, Thompson SG 1986 Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet 2:533–537 [DOI] [PubMed] [Google Scholar]
- Libby P 2002 Inflammation in atherosclerosis. Nature 420:868–874 [DOI] [PubMed] [Google Scholar]
- Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A 1999 Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 48:1856–1862 [DOI] [PubMed] [Google Scholar]
- Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A 2004 Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care 27:2450–2457 [DOI] [PubMed] [Google Scholar]
- Cavusoglu E, Eng C, Chopra V, Clark LT, Pinsky DJ, Marmur JD 2007 Low plasma RANTES levels are an independent predictor of cardiac mortality in patients referred for coronary angiography. Arterioscler Thromb Vasc Biol 27:929–935 [DOI] [PubMed] [Google Scholar]
- Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gómez-Reino JJ, Gualillo O 2005 Leptin, from fat to inflammation: old questions and new insights. FEBS Lett 579:295–301 [DOI] [PubMed] [Google Scholar]
- Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC 2005 Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care 28:2155–2160 [DOI] [PubMed] [Google Scholar]
- Ripa RS, Kastrup J 2008 G-CSF therapy with mobilization of bone marrow stem cells for myocardial recovery after acute myocardial infarction—a relevant treatment? Exp Hematol 36:681–686 [DOI] [PubMed] [Google Scholar]
- Jaffcoate WJ, Game F, Cavanaugh PR 2005 The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet 366:2058–2061 [DOI] [PubMed] [Google Scholar]
- Cuccurullo C, Iezzi A, Fazia ML, De Cesare D, Di Francesco A, Muraro R, Bei R, Ucchino S, Spigonardo F, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A, Cipollone F 2006 Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol 26:2716–2723 [DOI] [PubMed] [Google Scholar]
- Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD 1999 Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest 104:1041–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V 2005 Wound healing and its impairment in the diabetic foot. Lancet 366:1736–1743 [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Norell JE, Tritschler H, Schuette K, Samigullin R, Ziegler D, Bastyr 3rd EJ, Litchy WJ, O’Brien PC 2007 Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care 30:2619–2625 [DOI] [PubMed] [Google Scholar]