Abstract
Advanced glycation endproducts (AGEs) are present in the vasculature and are associated with vascular disease. We determined levels of AGEs in eight distinct adult vascular tissues using tissue microarray (TMA) technology and associated these levels with clinical characteristics. Medium-to-large caliber blood vessels were harvested from 100 adult autopsies to create 17 TMAs. AGE levels were evaluated by IHC using a polyclonal anti-AGE antibody on over 700 unique blood vessels. Slides were digitally scanned, and quantitative analysis was performed using a color deconvolution image analysis technique. Medial AGE staining was strongly correlated between all eight blood vessels. In the media, AGE staining levels were significantly higher at older ages (p=0.009), in white subjects (p<0.001) and with longer postmortem interval (PMI; p<0.0001). These associations remained significant after simultaneous adjustment for age, race/ethnicity, PMI, and diabetes status. Diabetes was associated with elevated AGE levels but only after adjustment for confounding by clinical variables including race/ethnicity, hypertension, and kidney function. This extensive vascular study shows that AGE accumulation in the macrovasculature is a global process affecting atherosclerosis-prone and -resistant vessels. It also suggests ethnicity has a previously undescribed role in vascular tissue AGE levels. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 57:559–566, 2009)
Keywords: advanced glycation endproducts, blood vessels, immunohistochemistry, diabetes
Advanced glycation endproducts (AGEs) play a major role in the development of diabetic vascular disease. AGEs are a collection of moieties formed by the Maillard reaction and are elevated with aging, diabetes, severe renal disease, dietary intake, and smoking (Vlassara and Palace 2002). AGEs induce vascular disease by increasing signaling along inflammatory pathways through the receptor for AGEs (RAGE) and the AGE receptor complex (Vlassara 2001; Ramasamy et al. 2008). AGEs also irreversibly cross-link extracellular matrix proteins such as collagen, resulting in vascular stiffening (Aronson 2003).
Small studies of AGEs in vascular tissues have established a link between AGEs and vascular disease (Nakamura et al. 1993; Kume et al. 1995). These studies found increased IHC staining with older subjects and within atherosclerotic plaques. One of the landmark studies in vascular AGEs correlated the presence of Nɛ-(carboxymethyl)lysine (CML) by semiquantitative IHC to diabetes and aging in a variety of tissues from 13 subjects (Schleicher et al. 1997).
Large-scale studies that compared robust phenotypic data and tissue AGE measures have used skin biopsies. Elevations in skin AGEs are associated with development of both micro- and macrovascular disease in persons with diabetes (Monnier et al. 1999,2005a; Genuth et al. 2005). Serum measures of AGEs are also used in large-scale studies (Monnier et al. 2005b). Unfortunately, these serum AGE studies have met with limited success in associating AGE levels with diabetic complications (Sharp et al. 2003; Thomas et al. 2004). This may be because of fluctuations in serum AGEs that occur as a result of dietary AGE intake (Uribarri et al. 2007).
Large-scale studies that combine robust phenotypic data and AGE levels in human vascular tissues do not exist. Procurement of human tissues and measurement of AGE levels in vascular tissue have proven difficult. High-performance liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR), all methods to accurately measure AGEs, are expensive, rigorous, and labor intensive, making large-scale studies cost prohibitive (Thornalley et al. 2003; Petrovic et al. 2005; Delatour et al. 2006; Mikulikova et al. 2007).
We overcame these problems by using tissue microarray (TMA) technology to measure AGEs in a high-throughput format (Kononen et al. 1998). TMA is a method of sampling multiple tissue blocks by punching small cores of donor tissue and arraying them in a single acceptor block, from which slides can be generated and assayed by IHC, immunofluorescence, ISH, or other methods (Takikita et al. 2007). We generated 17 99-core vascular TMAs from a prospective collection of blood vessels taken from adult subjects undergoing autopsy (Halushka et al. in press). These 17 TMA slides were stained with a polyclonal antibody to AGE. To analyze the IHC staining, we created novel tools and methods to quantitate staining intensity in a robust and efficient fashion (Cornish and Halushka in press; Halushka et al. in press).
This study was designed to determine whether AGE IHC staining intensity was correlated between atherosclerosis-prone and atherosclerosis-resistant vessels. We also sought to assess the association of AGEs with sociodemographic and clinical characteristics including age, race/ethnicity, sex, diabetes, and hypertension status. We hypothesized that AGE staining intensity would be higher at older ages, in smokers, and in persons with diabetes or renal disease. This is the first large-scale study to determine the distribution of AGEs in human vascular tissues using high-throughput technology.
Materials and Methods
Study Population and Tissue Harvesting
One hundred adult autopsies were harvested at The Johns Hopkins Hospital or Bayview Medical Center. Tissues were taken from a variety of atherosclerosis-prone and -resistant large-to-medium caliber vessels. Tissues were fixed in 10% neutral buffered formalin (Cardinal Health; Dublin, OH) for a minimum of 24 hr, processed, and embedded in paraffin. Demographic and clinical information was collected from a review of patient medical records as described previously (Halushka et al. in press). The diagnosis of diabetes or hypertension was established only by documentation within the patient's medical record and corroborated with hemoglobin A1c (HbA1c) or medication use when available. There were insufficient premortem studies of vascular disease (cardiac catheterization, angiograms, carotid intimal-medial thickness, etc.) in this population to be used for analysis with the samples. Glomerular filtration rate (GFR) was estimated from premortem serum creatinine values using the Modification of Diet in Renal Disease (MDRD) equation (Prigent 2008). This study was approved by the institutional review board of The Johns Hopkins Hospital.
TMA Creation
Seventeen 99-core TMAs were created from 1683 vascular tissues. Generally, 15 vascular tissues from each individual, some in replicate, were included along with control tissues (placenta, smooth muscle cell line, and endothelial cell line). Each core (feature) was 1.5 mm in diameter. Explanation of the creation and validation of the vascular TMAs has been described previously in detail (Halushka et al. in press).
IHC
IHC was performed using standard protocols described previously (Maleszewski et al. 2007). Briefly, slides were cut from each TMA block and kept in an airtight container at −20C until use. After deparaffinization and rehydration, slides were washed in PBS and treated with 3% H2O2 for 10 min. High temperature antigen retrieval (HTAR) was not used (Miki Hayashi et al. 2002). Slides were blocked with Ultra V block (Thermo Scientific; Fremont, CA) and incubated with a polyclonal-AGE antibody (ab23722; Abcam, Cambridge, MA), at a 1:10,000 dilution for 1 hr at room temperature. Slides were incubated with anti-rabbit secondary antibody (R&D Systems; Minneapolis, MN), followed by incubation with high-sensitivity streptavidin-horseradish peroxidase conjugate, and finally incubated with DAB chromagen for exactly 10 min (R&D Systems).
The antigen for this polyclonal-AGE antibody was a proprietary mixture of AGE-human serum albumin and AGE-BSA (Abcam, unpublished data), which was not expected to cross-react with CML epitopes. A blocking experiment was performed in our laboratory with AGE-BSA derived from both glucose and glycolaldehyde protocols (Valencia et al. 2004). Both AGE-BSAs blocked antibody staining in a dose-dependent fashion, although the blocking was greater for glucose-derived AGE-BSA at comparable concentrations of antigen (data not shown).
Digital Slide Scanning and Staining Quantification
Stained slides were digitized using a ScanScope CS (Aperio; Vista, CA) at ×200 magnification. IHC staining for AGE was analyzed using color deconvolution techniques described in detail elsewhere (Cornish and Halushka in press). Briefly, the whole slide images were segmented into individual core images. The core images were annotated using ImageJ macros (Rasband 1997–2004), generating separate regions of interest (ROI) for the tunica intima (intima) and tunica media (media) of each vessel (Cornish and Halushka in press). Color deconvolution was performed on each core image, separating the RGB images into hematoxylin and DAB dye components (Ruifrok and Johnston 2001). A single DAB threshold value (90), separating background DAB from true staining, was selected empirically by identifying the cut-off value producing the strongest correlation between observer and digital scoring of brown staining percentage across multiple tissues and multiple thresholds. For each core image, the sum of the intensity values in the ROI pixels above the brown threshold was divided by the total area of the ROI, producing an average intensity value (AGE staining) for each core, which is unitless.
Separate AGE staining values were generated for eight blood vessels in up to 100 subjects (Table 1). An overall average AGE staining value was generated separately for the media and intima layers of each subject, which was designed to be a comprehensive measure of overall AGE accumulation in relative terms. This value was calculated by performing a Z-transformation of the log base 10 of each separate AGE staining score for a given vessel and averaging this across all eight vessels from a given subject. This final score was multiplied by 100 for regression analysis. Additional tissues present on the TMAs (skin, lung parenchyma, renal parenchyma, renal arcuate/interlobar, and retinal vessels) were not analyzed for this study.
Table 1.
Media and intima AGE staining values for each blood vessel
| Media
|
Intima
|
|||
|---|---|---|---|---|
| Blood vessel | AGE staining | N | AGE staining | N |
| Carotid | 136 ± 47 | 95 | 190 ± 20a | 92 |
| Coronary | 182 ± 30 | 95 | 191 ± 21a | 95 |
| Dorsalis pedis | 140 ± 38 | 86 | 149 ± 41a | 77 |
| Iliac | 160 ± 44 | 84 | 180 ± 28a | 81 |
| Internal mammary | 141 ± 44 | 97 | 159 ± 37a | 57 |
| Mesenteric | 171 ± 36 | 96 | 166 ± 36 | 76 |
| Pulmonary | 169 ± 36 | 92 | 201 ± 19a | 77 |
| Renal | 175 ± 37 | 90 | 173 ± 31 | 49 |
p≤0.001 between intima and media by t-test assuming equal variances.
Data are means ± SD. AGE, advanced glycation endproducts; n, number of blood vessels in the analysis.
Vascular Tissue Measurements
Intima and media thicknesses were measured using the calibrated (0.4982 μm/pixel) measuring tool in ImageJ (Rasband 1997–2004). The average distance in micrometers from the lumen to the internal elastic lamina was taken as the intimal thickness, and the distance from the internal elastic lamina to the adventitia was recorded as the medial thickness. Images that lacked a complete intima or media were not measured.
Statistical Analysis
Statistical analyses including t-tests assuming equal variances, χ2 tests, pairwise correlations, and multiple regression analysis were performed using Stata version 10.0 (Stata; College Station, TX).
Results
Study Population Characteristics
One hundred subjects, 76 white, 20 African-American, 2 Asian, and 2 Hispanic individuals, were included in this study. Patients ranged in age from 20 to 101 years of age (mean, 64 ± 16 years). The average age of diabetic subjects was 63 ± 14 years. The average postmortem interval (PMI) was 18 ± 6 hr (range, 4–28 hr). Thirty subjects had a previous diagnosis of diabetes (26 type 2 diabetes mellitus subjects, 3 steroid-induced diabetes subjects, and 1 type 1 diabetes mellitus subject), and 62 subjects were hypertensive. The average duration of diabetes was 9.3 ± 8.0 years. Non-white subjects were significantly more likely to have a history of diabetes compared with whites (50% vs 24%, p=0.01).
AGE IHC
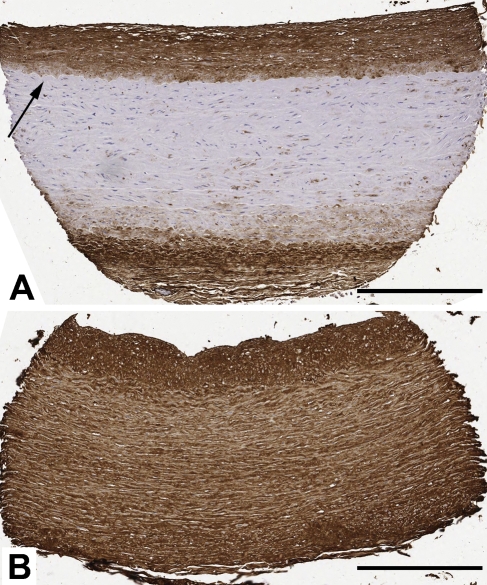
The polyclonal anti-AGE antibody stained cellular and extracellular matrix material within the vascular intima, media, and adventitia. Staining was generally weak within vascular smooth muscle cells and strong in the extracellular matrix material. The intima, which has more extracellular material, had more robust staining than the media (Table 1). Median AGE staining for the intima and media was variable and differed significantly between vessels. The staining intensity often changed abruptly at the internal elastic lamina (IEL) (Figure 1). In the media, staining ranged fully from absent to robust, whereas a narrower range of staining (moderate to robust) was appreciated in the intima of vascular tissues (Figure 1). Overall, the dynamic range of AGE staining (the range between minimum and maximum values) was 7.9-fold in the media and 3.2-fold in the intima. The overall average AGE staining value had a normal distribution (data not shown).
Figure 1.
Representative IHC staining for advanced glycation endproducts (AGEs) in two 1.5-mm features from a vascular tissue microarray (TMA). (A) Carotid artery from a 47-year-old male non-diabetic subject. The media and intima AGE staining scores are 17 and 200, respectively. Arrow denotes the internal elastic lamina. (B) Carotid artery from a 41-year-old female diabetic subject. The media and intima AGE staining scores are 207 and 225, respectively. Bar = 400 μm.
Measures of Completeness
We were able to generate AGE staining values for 92% (735/800) of media segments and 76% (605/800) of intima segments. Absent values resulted from missing tissue specimens or tissue that could not be evaluated. Frequently, in the absence of vascular disease, the intima was not of sufficient thickness to accurately select as a ROI. Thus, the vessels that are the least prone to vascular disease, the internal mammary artery and the renal artery (beyond the aortic bifurcation), had the fewest intimal ROIs (57 and 49, respectively). The media and intima thicknesses were determined for 92% and 99% of vessels, respectively.
Relationship Between Intima and Media
AGE staining was significantly higher in intima relative to media for the carotid, coronary, iliac, internal mammary, and pulmonary arteries (Table 1). Despite the absolute differences in AGE staining values, staining was proportional, with a strong correlation between intima and media AGE staining for each vessel (Table 2). There were significant pairwise correlations for media AGE staining across all eight blood vessels (Table 3). The intima staining did not correlate as frequently or as strongly across the eight vessels (data not shown).
Table 2.
Relationship between media and intima AGE staining for each blood vessel
| Blood vessel | Correlation coefficient (r) | p | N |
|---|---|---|---|
| Overall average | 0.68 | <0.0001 | 100 |
| Carotid | 0.40 | 0.0001 | 89 |
| Coronary | 0.49 | <0.0001 | 95 |
| Dorsalis pedis | 0.54 | <0.0001 | 77 |
| Iliac | 0.48 | 0.0001 | 77 |
| Internal mammary | 0.57 | <0.0001 | 57 |
| Mesenteric | 0.61 | <0.0001 | 75 |
| Pulmonary | 0.41 | 0.0002 | 77 |
| Renal | 0.32 | 0.03 | 49 |
Overall average represents an unweighted average of all eight vessels. The data were analyzed using a pairwise correlation test. AGE, advanced glycation endproducts; N, number of comparisons.
Table 3.
Relationship of AGE staining between blood vessels
| Carotid | Coronary | Dorsalis pedis | Iliac | Internal mammary | Mesenteric | Pulmonary | |
|---|---|---|---|---|---|---|---|
| Carotid | – | ||||||
| Coronary | 0.29a | – | |||||
| Dorsalis pedis | 0.44 | 0.38 | – | ||||
| Iliac | 0.41 | 0.57 | 0.45 | – | |||
| Internal mammary | 0.64 | 0.46 | 0.40 | 0.49 | – | ||
| Mesenteric | 0.43 | 0.44 | 0.44 | 0.59 | 0.42 | – | |
| Pulmonary | 0.45 | 0.28a | 0.27b | 0.53 | 0.57 | 0.37 | – |
| Renal | 0.45 | 0.63 | 0.37 | 0.70 | 0.46 | 0.52 | 0.41 |
p≤0.01.
p≤0.05.
Analysis performed by pairwise correlation test; values presented are r. For comparisons, N = 76–92.
All correlations are significant at p≤0.001 except where noted. AGE, advanced glycation endproducts.
Demographic and Clinical Characteristics and Vascular AGE Staining in Univariate and Multivariate Analyses
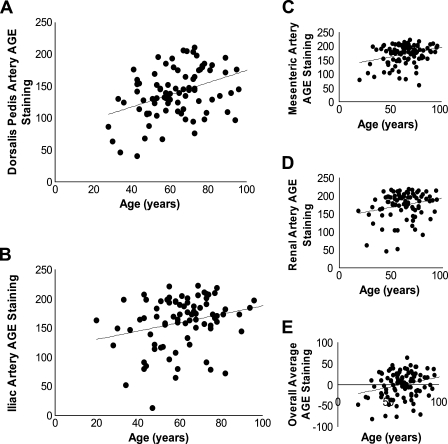
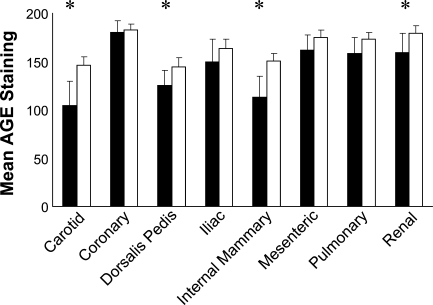
Patient age (in years) was positively and significantly associated with AGE staining for four of the eight vessels. The correlations were strongest for the dorsalis pedis and mesenteric arteries (Figure 2). The overall correlation (average across all eight vessels) between patient age and AGE staining was also significant (p=0.009). Across vessels, white subjects tended to have greater AGE staining compared with non-white subjects. Significant differences were observed in the carotid, dorsalis pedis, internal mammary, and renal arteries (Figure 3) and overall (p=0.002). There was no significant relationship between ethnicity/race and AGE staining in the intima (data not shown). We also did not observe differences by sex in AGE staining of either the intima or the media (data not shown). Diabetes, renal disease, and smoking are thought to increase AGE levels (Vlassara and Palace 2002). We did not observe significant differences in AGE staining between individuals with or without histories of these entities in our study population in univariate analyses (data not shown).
Figure 2.
AGE staining correlates with patient age. Pairwise correlations between patient age in years and (A) dorsalis pedis artery (r = 0.40, p=0.0001), (B) iliac artery (r = 0.26, p=0.02), (C) mesenteric artery (r = 0.31, p=0.002), (D) renal artery (r = 0.23, p=0.03), and (E) Z-transformed average of all eight blood vessels (r = 0.26, p=0.009).
Figure 3.
Medial AGE staining by ethnicity for eight blood vessels. Solid bars are non-whites, and open bars are whites. Error bars are 95% confidence intervals. *p<0.05, t-test assuming equal variances.
Postmortem Interval and Vascular AGE Staining
There was a positive association between PMI and AGE staining across vessels in both the media (r = 0.40, p<0.0001, n=100) and intima (r = 0.38, p=0.0001, n=100) by pairwise correlation. PMI was positively associated with AGE staining of media in eight of nine vessels (all except the dorsalis pedis) and with AGE staining in the intima in five of nine blood vessels (carotid, dorsalis pedis, iliac, internal mammary, and mesenteric arteries). Length of PMI was not associated with patient age, race/ethnicity, diabetes, or hypertensive status (all p>0.05). Two other potentially confounding variables, length of formalin fixation before processing and slide to slide staining variation, did not affect AGE staining (data not shown).
Multivariable Analysis to Assess Independence of Associations of Clinical Variables With Media AGE Staining
We used linear regression analyses to assess whether the associations of the demographic and clinical variables we observed in univariate analysis persisted after simultaneous adjustment. Multivariable analyses are presented in Table 4. Model 1 included age, ethnicity, diabetes, and PMI. Model 2 included all variables in Model 1 plus hypertension, smoking (any history of), and kidney function as estimated by GFR. In Model 1, age, white ethnicity, and PMI were all independently associated with AGE staining. After further adjustment for hypertension, smoking, and kidney function in Model 2, diabetes was significantly associated with AGE staining (p=0.04), suggesting negative confounding by these variables. That is, adjustment for race/ethnicity and diabetes risk factors accounted for the higher proportion of diabetes cases and risk factors among the non-white subjects who had lower AGE levels. Thus, a significant association between AGE staining and diabetes status was observed after adjustment for these important confounding factors in our data (Table 4). Additional models exploring the possible effects of medication use did not appreciably alter our results.
Table 4.
Linear regression models of overall AGE staining (in arbitrary units) and clinical variables
| Model 1 (N = 100)
|
Model 2 (N = 83)
|
|||
|---|---|---|---|---|
| Variable | β | SE | β | SE |
| Age (per 1 year) | 0.91a | 0.37 | 0.60 | 0.58 |
| Ethnicity (white vs non-white) | 45.7a | 4.7 | 53.8a | 17.3 |
| Diabetes (yes vs no) | 17.97 | 13.37 | 34.3a | 16.6 |
| PMI (per 1 hr) | 4.03a | 0.93 | 4.2a | 1.1 |
| Hypertension (yes vs no) | – | – | −8.4 | 18.6 |
| Smoking (ever vs never) | – | – | 2.8 | 13.9 |
| Sex (male vs female) | – | – | −5.8 | 14.3 |
| Kidney function | ||||
| Estimated GFR (>60) | – | – | 1.0 (ref.) | 1.0 (ref.) |
| Estimated GFR (30–60) | – | – | −7.5 | 15.8 |
| Estimated GFR (<30) | – | – | −37.0 | 24.4 |
p<0.05.
AGE, advanced glycation endproducts; PMI, postmortem interval; GFR, glomerular filtration rate.
Artery Thickness and AGE Staining
Generally, reduced medial thickness significantly correlated with AGE staining (six of eight vessels, p<0.05 by pairwise correlation). Increasing intimal thickness was positively correlated with medial AGE staining for four of eight vessels (p<0.05 by pairwise correlation).
Discussion
To the best of our knowledge, this study represents the first large-scale survey of AGE staining in human vascular tissues using TMAs. This method of analysis enabled us to make several new observations and confirm and extend some known associations.
By studying eight blood vessels per subject, we showed that AGE accumulation is a pervasive process in human vasculature, irrespective of a given vessel's propensity for atherosclerosis. Thus, any clinical variable shown to increase vascular AGE levels does so in a global fashion. By evaluating the intima and media separately, we showed that the two layers are significantly correlated across all vessels despite differences in absolute AGE accumulation (Table 3). Often a clear demarcation between AGE staining of the intima and media was observed as if the IEL was a barrier to AGE accumulation (Figure 1A).
Consistent with previous literature, we report the positive association of age with AGE staining and interpret this as validation of our quantitative staining method (Dyer et al. 1993; Schleicher et al. 1997). We observed this correlation between age and AGE staining in a population of largely middle-aged and older subjects (mean age, 64 years), without the benefit of a pediatric population for comparison, which had not been previously shown (Schleicher et al. 1997). We would predict that having vascular segments from healthy pediatric subjects on these TMAs would have further enhanced this association.
We identified a novel relationship between ethnicity and AGE staining of the media. The higher levels of AGE staining in white subjects persisted even after adjustment for age, sex, PMI, smoking status, and clinical variables (diabetes, hypertension, and kidney function). The non-white cohort (84% African American) had less AGE staining despite having a greater burden of diabetes. However, because the non-white cohort also had higher levels of detrimental risk factors (hypertension, poor kidney function), an association between AGE staining and diabetes status was observed only in our models that accounted for these race/ethnic differences. Our survey of the literature has found no previous reports of differences in tissue AGE levels by race/ethnicity, although this may be caused by the small sample size and race/ethnic homogeneity of previous studies. Possible causes include differences in genetics, diet, or environmental factors that we are unable to account for in this study (Krajcovicova-Kudlackova et al. 2002; Leslie et al. 2003; Kalousova et al. 2004).
There are some important limitations of this study. IHC has a relatively small dynamic range compared with other imaging methods such as immunofluorescence (IF) (Rimm 2006). However, IF is not amenable to the study of vascular tissues because of the presence of elastic fibers that result in unavoidable high background fluorescence. Despite this limitation, we were still able to recapitulate known associations of AGEs with clinical variables (aging and diabetes).
It is not entirely clear why a stronger association between diabetes status and AGE staining was not observed in these data, but heterogeneity in disease variables likely had an effect on our findings. To obtain a sufficient number of samples in a reasonable period of time (3 years), essentially every autopsy that conformed to our inclusion and exclusion criteria was used (Halushka et al. in press). As a result, there was great heterogeneity in diabetes duration (range, month to 36 years) and etiology of diabetes (type 1 diabetes mellitus, type 2 diabetes mellitus, steroid-induced, and diet controlled). Furthermore, the presence of individuals with undiagnosed diabetes may have resulted in misclassification of diabetes status in our data, further contributing to the lack of association (Wee et al. 2008). We found that diabetes was significantly associated with AGE staining after statistically correcting for the higher proportion of non-white with a poorer risk factor profile compared with the white subjects who were less likely to have diabetes and other risk factors but had significantly higher AGE levels.
Autolysis that occurs with extended postmortem interval has been thought to decrease cellular viability and lower antibody staining intensity (Martinez-Diaz et al. 2004). In this study, we found a consistent correlation between staining intensity and increasing PMI. Possible causes of this finding include the activity of the non-enzymatic Maillard reaction continuing after death or some other artifactual increase in AGEs, which may or may not be specific to the antibody used. The dorsalis pedis, the most peripheral artery studied and thus the most likely to rapidly cool to ambient temperature, was the only vessel that did not show a PMI-related medial AGE staining increase.
Intimal analysis did not provide as much information as medial analysis. A problem with analyzing the intima was the lack of sufficient intimal thickness to select as an ROI in many non-atherosclerotic vessels. Therefore, what could have been the weakest staining intimas were not evaluated. This partly explains the difference in dynamic range between medial and intimal staining (7.9 vs 3.2). The limited utility of the intima may also be methodological because the antibody was titrated to achieve maximal dynamic range in the media. The stronger intimal staining likely fully saturated the intensity measurement, creating an artificial ceiling to AGE staining scores.
It is important to point out the trade-offs between this new TMA method with quantitative IHC and older analytic methods. We learned in this study that large differences in AGE content of intima and media exist. In studies that may grind up vascular tissues for use in analytic devices such as HPLC, keeping an exact ratio of intima to media may be an underappreciated step to prevent confounding of results, particularly with variably sized atherosclerotic plaques (Vogt et al. 1982). However, the benefit of those methods is the determination of an exact amount of an AGE moiety/moieties vs our unitless value, which is a relative measure. By our method, staining was localized to a particular vascular layer and an AGE staining intensity was obtained for that ROI only. Also, by using TMA technology and autopsy material, we were able to analyze >700 separate blood vessel segments, which is 10 times more than any other study, in a robust and high-throughput manner (Nakamura et al. 1993; Schleicher et al. 1997; Nerlich and Schleicher 1999).
We specifically assessed the role of potential confounding variables such as days of formalin fixation before processing, slide to slide staining heterogeneity, and PMI. By identifying PMI as a confounding variable, we were able to account for it in our regression analysis unlike prior studies that used autopsy tissues but failed to perform this necessary analysis. The effect of these variables is unknown in prior IHC studies (Nakamura et al. 1993; Nerlich and Schleicher 1999; van Deutekom et al. 2008).
The correlation across all vessels suggests that measurement of AGEs in the media of any medium-to-large caliber artery may be a marker of vascular tissue AGE levels throughout the body. What then is the ideal vessel to study? AGE staining in the dorsalis pedis artery was significantly and independently associated with aging and ethnicity, and it correlated with AGE levels in all other vessels studied. The dorsalis pedis is in an easily accessible location on the dorsal aspect of the foot, just beneath the skin. In the future, it may be a useful artery to study non-invasive imaging measures of vascular AGEs that would have global vascular applicability.
This study breaks ground in several areas. It is the first large-scale survey of AGE staining in human vascular tissues, encompassing eight unique vessels in 100 adult subjects. We found that, within a given individual, the AGE levels in these vessels are correlated, regardless of whether they were atherosclerosis-prone or atherosclerosis-resistant vessels. The overall medial AGE staining significantly correlated with increasing subject age, white ethnicity, and PMI in one model and with diabetes after further adjustment for comorbidites (hypertension and kidney function) in a second model. This TMA-based study represents a new method of large-scale vascular tissue analysis. We believe that future TMAs created from a single type of blood vessel from hundreds of individuals and derived from surgical (non-autopsy) materials will be of great use in evaluating specific AGEs such as CML, carboxyethyllysine, pentosidine, and others in relation to clinical variables and outcomes.
Acknowledgments
We thank Kristen Lecksell for excellent digital slide scanning and Dr. Frederick Brancati for helpful conversations.
This study was supported by a junior faculty award from the American Diabetes Association (1-05-JF-20 to MKH) and the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK076595 to ES) .
References
- Aronson D (2003) Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21:3–12 [DOI] [PubMed] [Google Scholar]
- Cornish TC, Halushka MK (In press) Color deconvolution for the analysis of tissue microarrays. Anal Quant Cytol Histol [PubMed]
- Delatour T, Fenaille F, Parisod V, Vera FA, Buetler T (2006) Synthesis, tandem MS- and NMR-based characterization, and quantification of the carbon 13-labeled advanced glycation endproduct, 6-N-carboxymethyllysine. Amino Acids 30:25–34 [DOI] [PubMed] [Google Scholar]
- Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW (1993) Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest 91:2463–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, et al. (2005) Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halushka MK, Cornish TC, Lu J, Selvin L, Selvin E (In press) Creation, validation, and quantitative analysis of protein expression in vascular tissue microarrays. Cardiovasc Pathol [DOI] [PMC free article] [PubMed]
- Kalousova M, Zima T, Popov P, Spacek P, Braun M, Soukupova J, Pelinkova K, et al. (2004) Advanced glycation end-products in patients with chronic alcohol misuse. Alcohol Alcohol 39:316–320 [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, et al. (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847 [DOI] [PubMed] [Google Scholar]
- Krajcovicova-Kudlackova M, Sebekova K, Schinzel R, Klvanova J (2002) Advanced glycation end products and nutrition. Physiol Res 51:313–316 [PubMed] [Google Scholar]
- Kume S, Takeya M, Mori T, Araki N, Suzuki H, Horiuchi S, Kodama T, et al. (1995) Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am J Pathol 147:654–667 [PMC free article] [PubMed] [Google Scholar]
- Leslie RD, Beyan H, Sawtell P, Boehm BO, Spector TD, Snieder H (2003) Level of an advanced glycated end product is genetically determined: a study of normal twins. Diabetes 52:2441–2444 [DOI] [PubMed] [Google Scholar]
- Maleszewski J, Lu J, Fox-Talbot K, Halushka MK (2007) Robust immunohistochemical staining of several classes of proteins in tissues subjected to autolysis. J Histochem Cytochem 55:597–606 [DOI] [PubMed] [Google Scholar]
- Martinez-Diaz F, Bernal-Gilar M, Gomez-Zapata M, Luna A (2004) Expression and significance of cell immunohistochemical markers (HHF-35, CD-31, Bcl-2, P-53 and apopDETEC) in hypertrophic cardiomyopathy. Histol Histopathol 19:9–14 [DOI] [PubMed] [Google Scholar]
- Miki Hayashi C, Nagai R, Miyazaki K, Hayase F, Araki T, Ono T, Horiuchi S (2002) Conversion of Amadori products of the Maillard reaction to N(epsilon)-(carboxymethyl)lysine by short-term heating: possible detection of artifacts by immunohistochemistry. Lab Invest 82:795–808 [DOI] [PubMed] [Google Scholar]
- Mikulikova K, Eckhardt A, Pataridis S, Miksik I (2007) Study of posttranslational non-enzymatic modifications of collagen using capillary electrophoresis/mass spectrometry and high performance liquid chromatography/mass spectrometry. J Chromatogr A 1155:125–133 [DOI] [PubMed] [Google Scholar]
- Monnier VM, Bautista O, Cleary P, Sell DR, Genuth S, Group at DER (2005a) Skin collagen-linked fluorescence predicts atherosclerosis progression in the epidemiology of diabetes interventions and complications (EDIC) study. Ann NY Acad Sci 1043:917 [Google Scholar]
- Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, Cleary PA, et al. (1999) Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 48:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, Sell DR, Genuth S (2005b) Glycation products as markers and predictors of the progression of diabetic complications. Ann NY Acad Sci 1043:567–581 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Horii Y, Nishino T, Shiiki H, Sakaguchi Y, Kagoshima T, Dohi K, et al. (1993) Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am J Pathol 143:1649–1656 [PMC free article] [PubMed] [Google Scholar]
- Nerlich AG, Schleicher ED (1999) N(epsilon)-(carboxymethyl)lysine in atherosclerotic vascular lesions as a marker for local oxidative stress. Atherosclerosis 144:41–47 [DOI] [PubMed] [Google Scholar]
- Petrovic R, Futas J, Chandoga J, Jakus V (2005) Rapid and simple method for determination of Nepsilon-(carboxymethyl)lysine and Nepsilon-(carboxyethyl)lysine in urine using gas chromatography/mass spectrometry. Biomed Chromatogr 19:649–654 [DOI] [PubMed] [Google Scholar]
- Prigent A (2008) Monitoring renal function and limitations of renal function tests. Semin Nucl Med 38:32–46 [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Yan SF, Herold K, Clynes R, Schmidt AM (2008) Receptor for advanced glycation end products: fundamental roles in the inflammatory response: winding the way to the pathogenesis of endothelial dysfunction and atherosclerosis. Ann NY Acad Sci 1126:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS (1997–2004) ImageJ. Bethesda, MD, National Institutes of Health
- Rimm DL (2006) What brown cannot do for you. Nat Biotechnol 24:914–916 [DOI] [PubMed] [Google Scholar]
- Ruifrok AC, Johnston DA (2001) Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23:291–299 [PubMed] [Google Scholar]
- Schleicher ED, Wagner E, Nerlich AG (1997) Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest 99:457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PS, Rainbow S, Mukherjee S (2003) Serum levels of low molecular weight advanced glycation end products in diabetic subjects. Diabet Med 20:575–579 [DOI] [PubMed] [Google Scholar]
- Takikita M, Chung JY, Hewitt SM (2007) Tissue microarrays enabling high-throughput molecular pathology. Curr Opin Biotechnol 18:318–325 [DOI] [PubMed] [Google Scholar]
- Thomas MC, Tsalamandris C, MacIsaac R, Medley T, Kingwell B, Cooper ME, Jerums G (2004) Low-molecular-weight AGEs are associated with GFR and anemia in patients with type 2 diabetes. Kidney Int 66:1167–1172 [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Battah S, Ahmed N, Karachalias N, Agalou S, Babaei-Jadidi R, Dawnay A (2003) Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J 375:581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Stirban A, Sander D, Cai W, Negrean M, Buenting CE, Koschinsky T, et al. (2007) Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and nondiabetic subjects. Diabetes Care 30:2579–2582 [DOI] [PubMed] [Google Scholar]
- Valencia JV, Weldon SC, Quinn D, Kiers GH, DeGroot J, TeKoppele JM, Hughes TE (2004) Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem 324:68–78 [DOI] [PubMed] [Google Scholar]
- van Deutekom AW, Niessen HW, Schalkwijk CG, Heine RJ, Simsek S (2008) Increased Nepsilon-(carboxymethyl)-lysine levels in cerebral blood vessels of diabetic patients and in a (streptozotocin-treated) rat model of diabetes mellitus. Eur J Endocrinol 158:655–660 [DOI] [PubMed] [Google Scholar]
- Vlassara H (2001) The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev 17:436–443 [DOI] [PubMed] [Google Scholar]
- Vlassara H, Palace MR (2002) Diabetes and advanced glycation endproducts. J Intern Med 251:87–101 [DOI] [PubMed] [Google Scholar]
- Vogt BW, Schleicher ED, Wieland OH (1982) epsilon-Amino-lysine-bound glucose in human tissues obtained at autopsy. Increase in diabetes mellitus. Diabetes 31:1123–1127 [DOI] [PubMed] [Google Scholar]
- Wee CC, Hamel MB, Huang A, Davis RB, Mittleman MA, McCarthy EP (2008) Obesity and undiagnosed diabetes in the U.S. Diabetes Care 31:1813–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]