Abstract
BACKGROUND AND OBJECTIVE:
Inhaled beta-agonist, anticholinergic and glucocorticoid medications are used to treat asthma and chronic obstructive pulmonary disease (COPD). The present study assessed the patterns of persistence with the above mentioned inhaled medications.
METHODS:
Prescription claims data from the Ontario Drug Benefit Program were analyzed to assess persistence (time to discontinuation) and compliance (percentage of days with doses available divided by days to last refill) of patients prescribed inhaled medications. Patients were grouped as naive (no inhaled medication in the previous year) or experienced (previous or current treatment), and by age (18 to 65 years of age and older than 65 years of age). Medications included ipratropium, ipratropium plus salbutamol, formoterol, formoterol plus budesonide, salmeterol, salmeterol plus fluticasone, and tiotropium.
RESULTS:
The database included 31,368 patients (4888 naive and 26,480 experienced) who were prescribed at least one of these medications. Fifteen per cent to 63% of patients continued on the index drug for more than six months, which decreased to 7% to 53% at 12 months, and 5% to 47% at 18 months. At 12 months, patients taking tiotropium had significantly longer persistence compared with other therapies (53% versus 7% to 30%; all P<0.0001), and fewer switches to alternative medications. Most naive patients had significantly shorter treatment persistence than experienced patients for all drugs (all P<0.0001), including tiotropium (27% versus 55%, P<0.0001). Compliance rates were similar for all drugs (ie, 76% to 94%) but were highest for tiotropium.
CONCLUSIONS:
These data demonstrated that persistence with inhaled treatment was low overall, but patients treated with tiotropium remained on therapy significantly longer than when treated with other medications, and patients naive to inhaled treatment had shorter treatment persistence than experienced patients.
Keywords: Anticholinergic, Asthma, Beta-agonist, Chronic obstructive pulmonary disease, Glucocorticoid, Medication compliance, Treatment persistence
Abstract
HISTORIQUE ET OBJECTIF :
Les bêta-agonistes, les anticholinergiques et les glucocorticoïdes en aérosol sont utilisés pour traiter l’asthme et la maladie pulmonaire obstructive chronique (MPOC). La présente étude visait à évaluer les profils de persistance à ces médicaments en aérosol.
MÉTHODOLOGIE :
Les données relatives aux réclamations d’ordonnances tirées du programme d’assurance-médicaments de l’Ontario ont été analysées pour évaluer la persistance (la période avant l’abandon) et l’observance (le pourcentage de jours avec des doses disponibles divisé par les jours depuis le dernier renouvellement) des patients à qui on a prescrit des médicaments en aérosol. Les patients ont été répartis entre les naïfs (aucun médicament en aérosol au cours de l’année précédente) et les expérimentés (traitement passé ou courant), selon l’âge (18 à 65 ans et plus de 65 ans). Les médicaments utilisés étaient l’ipratropium, l’ipratropium associé au salbutamol, le formotérol, le formotérol associé au budésonide, la salmétérol, le salmétérol associé au fluticasone et le tiotropium.
RÉSULTATS :
La base de données contenait 31 368 patients (4 888 naïfs et 26 480 expérimentés) à qui on avait prescrit au moins l’un de ces médicaments. De 15 % à 63 % des patients ont pris le médicament de référence pendant plus de six mois, mais ce pourcentage ne se situait plus qu’entre 7 % et 53 % à 12 mois et entre 5 % et 47 % à 18 mois. À 12 mois, les patients qui prenaient du tiotropium présentaient une persistance considérablement plus longue que ceux qui prenaient d’autres traitements (53 % par rapport à entre 7 % et 30 %; tous P<0,0001) et étaient moins nombreux à opter pour les médicaments parallèles. La plupart des patients naïfs étaient considérablement moins persistants au traitement que les patients expérimentés à l’égard de tous les médicaments (tous P<0,0001), y compris le tiotropium (27 % par rapport à 55 %, P<0,0001). Les taux de compliance étaient similaires pour tous les médicaments (c’est-à-dire de 76 % à 96 %), mais étaient les plus élevés avec le tiotropium.
CONCLUSIONS :
Ces données démontrent que la persistance au traitement en aérosol est faible dans l’ensemble, mais que les patients traités au tiotropium maintiennent leur traitement considérablement plus longtemps que ceux qui sont traités par d’autres médicaments et que les patients naïfs au traitement en aérosol ont une persistance au traitement moins longue que les parents expérimentés.
Chronic obstructive pulmonary disease (COPD) encompasses a progressive group of disorders (ie, emphysema and chronic bronchitis) that cause airflow obstruction and breathing problems, as described by the World Health Organization and Canadian Thoracic Society (1). Guidelines set by the Global Initiative for Chronic Obstructive Lung Disease have defined COPD as a disease state characterized by airflow limitation that is not fully reversible (2,3). In contrast, airway limitations in asthma are usually more fully reversible (4,5). The classification of COPD severity is based on pulmonary function, which is calculated as forced expiratory volume in 1 s. In 2001, Health Canada reported that over three million Canadians cope with serious respiratory diseases such as asthma, COPD, lung cancer, influenza and pneumonia, bronchiolitis, tuberculosis, cystic fibrosis and respiratory distress syndrome (6). Approximately 3% of adults in Canada and 6% of adults in the United States have COPD (7,8), but the prevalence is higher because the disease is under-diagnosed.
Although COPD symptoms vary over time, inadequate treatment may be an important factor in the frequency and severity of exacerbations. Bronchodilators are the cornerstone of COPD treatment (3). Short-acting medications, such as beta-agonists (eg, salbutamol) and anticholinergics (eg, ipratropium), ameliorate acute dyspnea when used alone or in combinations. Long-acting bronchodilators, used for chronic treatment, include beta-agonists (formoterol and salmeterol) and tiotropium, an anticholinergic. Evidence to demonstrate that combining long-acting bronchodilators also confers benefits in lung function is emerging (9). Inhaled corticosteroids are the cornerstone of asthma treatment and their use in COPD is increasing alone as well as in combination with a bronchodilator (10). Taking less than the prescribed amount of medication, missing doses or stopping treatment for brief or extended periods will predispose the individual to increased symptoms.
Disease management necessitates treatment compliance and persistence. Based on definitions adopted by the International Society for Pharmacoeconomics and Outcomes Research (11), ‘treatment persistence’ is the duration of time from the initial prescription to the last refill, and ‘medication compliance’ is the proportion of doses taken as prescribed. Use of both terms provides a perspective on whether patients take medications daily or irregularly, and when they discontinue treatment altogether.
Various factors have been shown to influence compliance and persistence with oral medications, including patient age, the cost of the drug (copayment), the type of prescriber (specialist or primary care doctor), comorbidities, concomitant medications and dosing frequency (12,13). However, most studies (14–16) assessing the use of asthma and COPD treatments were performed in small populations as part of clinical trials. Blais et al (17) demonstrated that only 54% of COPD patients in Quebec continued using inhaled corticosteroids for one year, which decreased to 25% of patients over four years. With the increasing number of treatment options available, it is necessary to determine how consistently they are used by patients in community settings. Measures of medication persistence and compliance may help to explain why some patients do not respond to seemingly appropriate treatment or have exacerbations for no apparent reason.
The present study was designed as a retrospective, observational assessment of persistence and compliance among seven commonly used bronchodilators. The objective was to ascertain whether persistence differed among the bronchodilators, depending on whether patients were naive or had had previous experience with any of the medications.
METHODS
Data source
The present study was designed as a retrospective assessment of prescription claims data in Ontario. All patients were beneficiaries of the Ontario Drug Benefits Program, funded by the Ontario Ministry of Health and Long-Term Care. The plan offers coverage to Ontario residents 65 years of age and older, as well as to social assistance recipients of all ages. Patients are responsible for a copayment of $3 to $7 per prescription depending on their income level. Prescription claims included the drug name and the number of days that the medication was supplied (based on the prescribed dose and dose frequency [eg, one to four times daily]). To protect patient privacy, a unique patient identification number was externally generated to allow for longitudinal and multiproduct analysis at the individual patient level.
Study population
Patients 18 years of age or older who had new prescriptions for any of the study medications (index drug) between September 2003 and November 2003 were selected from the database. Patients were classified as ‘naive’ if they had no claims for any of the study medications in the previous year, ‘experienced’ if they had been taking one of the study medications in the previous year, ‘switchers’ if the index drug was discontinued and replaced by one of the other study medications, and ‘added’ if one of the other study medications were to be taken in addition to the index drug. Discontinuation was defined as no prescription refill for the index drug for more than 60 days. Only patients who were in the database for the entire period (2003 to 2005) were included in these analyses. Using data from 2000/2001, a parallel analysis of treatment persistence was performed with ipratropium and ipratropium plus salbutamol. These results were compared with data from 2004/2005.
Demographic data were available only for age and sex. Because medical claims data were not available, the diagnosis of pulmonary disease was based on a bronchodilator prescription. However, it was not possible to differentiate between asthma and COPD. Although these medications could be prescribed for both types of bronchial disease, tiotropium, ipratropium and ipratropium plus salbutamol are indicated only for COPD.
Definitions
Treatment persistence was defined as the proportion of patients refilling prescriptions within 60 days from the end of the previous prescription. A sensitivity analysis was performed using 30-day and 60-day gap periods. Medication compliance was defined as the proportion of days with doses taken as prescribed, or the percentage of days covered using a standard method (18). Compliance rates were calculated as the number of days of medication dispensed (excluding the final refill) divided by the number of days between the first and last dispensing. Patients who refilled a prescription early were considered to have leftover doses that were added to the amount received in the early refill.
Analyses
Descriptive statistics were used to characterize the sample. All analyses were based on a 12-month follow-up period after the index prescription except for treatment persistence analyses, which were based on an 18-month follow-up period. Treatment persistence, based on days to discontinuation, was analyzed using Kaplan-Meier survival analyses. Log-rank χ2 test was used to compare survival curves. Medication compliance rates were compared by paired comparisons between tiotropium (the only medication prescribed once daily) and the other drugs.
RESULTS
Patients
The database included 31,368 patients who were prescribed an inhaled bronchodilator or bronchodilator-corticosteroid fixed-dose combination. Within this population, 4888 (16%) patients were treatment-naive and 26,480 (84%) were experienced. The mean age for the naive and experienced patients was similar (69.9±13.7 and 70.1±13.2 years, respectively). When the cohort was divided by age, the mean age of the younger cohort (18 to 65 years) was 50.39±11.09 years, whereas the mean age of the older cohort (older than 65 years) was 75.90±6.58 years. Tables 1 and 2 describe the demographic data by drug.
TABLE 1.
Patient cohorts by drug
| Drug | Total n | Exp n | Naive n | ≤65 yrs n | >65 yrs n | Women n | Men n |
|---|---|---|---|---|---|---|---|
| Formoterol | 413 | 367 | 46 | 99 | 314 | 246 | 167 |
| Form-Budes | 3121 | 2668 | 453 | 735 | 2386 | 1860 | 1251 |
| Ipratropium | 2908 | 2052 | 856 | 654 | 2254 | 1561 | 1344 |
| Iprat-Salbut | 4333 | 2585 | 1748 | 923 | 3410 | 2389 | 1931 |
| Salmeterol | 1184 | 1099 | 85 | 308 | 876 | 688 | 495 |
| Salmet-Flut | 5155 | 4491 | 664 | 1274 | 3881 | 3021 | 2123 |
| Tiotropium | 14,254 | 13,218 | 1036 | 1650 | 12,604 | 6836 | 7406 |
| All | 31,368 | 26,480 | 4888 | 5643 | 25,725 | 16,601 | 14,717 |
Exp Experienced; Form-Budes Formoterol and budesonide; Iprat-Salbut Ipratropium and salbutamol; Salmet-Flut Salmeterol and fluticasone; yrs Years
TABLE 2.
Patient ages (years) by drug
| Drug | Mean ± SD All age groups | Mean ± SD ≤65 | Mean ± SD >65 |
|---|---|---|---|
| Formoterol | 68.60±14.00 | 48.19±12.17 | 75.04±6.18 |
| Form-Budes | 68.83±13.97 | 48.25±11.80 | 75.17±6.45 |
| Ipratropium | 70.91±13.31 | 51.09±10.87 | 76.66±6.87 |
| Iprat-Salbut | 71.23±13.42 | 50.57±10.76 | 76.82±7.12 |
| Salmeterol | 69.67±13.56 | 51.48±11.57 | 76.07±6.65 |
| Salmet-Flut | 68.66±14.69 | 47.48±12.25 | 75.62±6.48 |
| Tiotropium | 73.59±9.23 | 55.68±8.20 | 75.94±6.33 |
| All | 70.21±13.17 | 50.39±11.09 | 75.90±6.58 |
Formot-Budes Formoterol and budesonide; Iprat-Salbut Ipratropium and salbutamol; Salmet-Flut Salmeterol and fluticasone
Treatment persistence
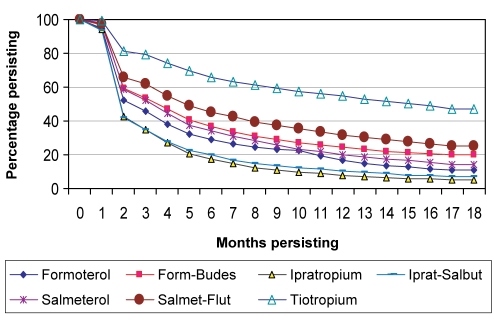
Only 15% to 63% of patients continued on the index drug for more than six months, which decreased to 7% to 53% at 12 months, and 5% to 47% at 18 months. Patients taking tiotropium had significantly longer persistence than patients taking all other drugs, with 63%, 53% and 47% of patients continuing for six, 12 and 18 months, respectively (Figure 1). Other than tiotropium, the combination of salmeterol plus fluticasone showed the highest persistence among the other drugs. Comparing the data from 2004/2005 with the data from 2000/2001 (ie, the period before tiotropium was available), there was little change in the treatment persistence rates for ipratropium and ipratropium plus salbutamol in both the experienced and naive patient groups.
Figure 1).
Treatment persistence. Differences between all drugs and tiotropium; P<0.0001. Form-Budes Formoterol and budesonide; Iprat-Salbut Ipratropium and salbutamol; Salmet-Flut Salmeterol and fluticasone
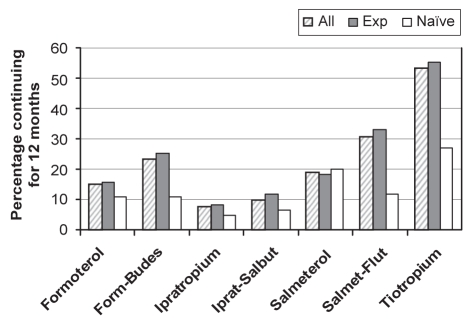
The most notable finding was that most naive patients had significantly shorter treatment persistence than experienced patients for all drugs (tiotropium: 27% versus 55% at 12 months, P<0.0001). Only 12% to 27% of naive patients taking drugs other than tiotropium persisted on treatment after six months, and 5% to 20% for more than 12 months (Figure 2). Although patients taking tiotropium had longer persistence than all other drugs (37% at six months, 27% at 12 months, and 24% at 18 months), this was significantly shorter than for experienced patients.
Figure 2).
Treatment persistence for experienced (Exp) and naive patients (12 months). Differences between all drugs and tiotropium for all patients as well as Exp and naive groups; P<0.0001. Form-Budes Formoterol and budesonide; Iprat-Salbut Ipratropium and salbutamol; Salmet-Flut Salmeterol and fluticasone
Days to discontinuation and refills
Mean days to discontinuation by 12 months ranged from 57 to 96 days overall, with significantly more days to discontinuation for the experienced group than the naive group (mean 97±79 versus 79±71, respectively, all P<0.001) (Table 3).
TABLE 3.
Days to discontinuation for all, experienced (Exp) and naive patients
| Drug |
12 months |
18 months |
||||
|---|---|---|---|---|---|---|
| All | Exp | Naive | All | Exp | Naive | |
| Formoterol | 68±70 | 71±72 | 51±51 | 96±112 | 102±114 | 61±82 |
| Form-Budes | 74±70 | 78±72 | 54±54 | 96±105 | 102±108 | 64±81 |
| Ipratropium | 58±59 | 60±59 | 54±58 | 70±85 | 73±87 | 64±80 |
| Iprat-Salbut | 57±59 | 61±63 | 51±54 | 71±89 | 78±95 | 62±80 |
| Salmeterol | 76±70 | 76±71 | 66±66 | 103±112 | 104±111 | 97±122 |
| Salmet-Flut | 82±75 | 88±77 | 55±56 | 114±119 | 122±123 | 69±87 |
| Tiotropium | 96±78 | 97±79 | 79±71 | 145±133 | 150±135 | 103±108 |
Data presented as mean ± SD days. All Exp versus naive groups, P<0.00001. Form-Budes Formoterol and budesonide; Iprat-Salbut Ipratropium and salbu-tamol; Salmet-Flut Salmeterol and fluticasone
Treatment changes
The number of patients who switched to, or added, another study medication reflected a global dissatisfaction with the efficacy of the index drug. Most patients switched or added another drug during the first six months, with small additions thereafter. Comparison of the current data with that from the year 2000 (before tiotropium was available) revealed little differences in the proportion of naive patients persisting for 12 months. Switching from ipratropium plus salbutamol was lower in 2000 (54%) than the current year (62%), but not for salmeterol plus fluticasone (59% versus 58%, respectively). Days to discontinuation did not differ between 2000 (52 to 57 days, respectively) and the current year (51 to 55 days, respectively).
Compliance
Drug compliance rates ranged from 71% to 93%, with naive and experienced patients having similar rates. Tiotropium patients had the highest compliance rates (93% experienced, 88% naive), approximately 10% higher than any other drug.
DISCUSSION
The present study revealed two major concerns about the use of these medications. First, overall persistence with treatment was low across all medications, although differences were seen among different medications. However, patients stayed on the tiotropium treatment significantly longer than other daily medications. Second, among all medications, most naive patients had shorter treatment persistence than experienced patients. These findings were consistent for comparisons among all medications including those often used ‘as needed’.
Overall treatment persistence
The main finding was that patients did not persist long-term with treatment. Treatment persistence has been considered a global outcome encompassing efficacy, adverse effects, convenience and other attributes valued by patients (19–21). The pattern of early discontinuation has been demonstrated for other medication classes, but oral medications usually have a larger proportion of patients persisting for a year (21,22). The pattern of large numbers of patients discontinuing treatment during the first few months, with continually declining persistence as shown in the present study, indicates a lack of willingness to take the medication for more than a few months. Similar results were found in a study (20) of inhaled corticosteroid treatment persistence in the Netherlands where only 18% of all patients continued on the treatment for one year. In contrast, approximately one-half of Quebec patients in another study (17) persisted with inhaled corticosteroid for one year. Persistence with ipratropium formulations was probably affected by widespread use ‘as needed’ as well as a daily controller medication. These data suggest that there is a need for more research into how these formulations are being used by patients attempting self-management.
Although previous reports (17,20) have indicated that persistence with inhaled corticosteroid medications as a class was poor, the present analysis identified differences among individual medications. Significantly more patients persisted with tiotropium whether they were younger (65 years and younger), older (older than 65 years), newly treated (naive group) or had previously used bronchodilator medication (experienced group).
Differences between experienced and naive patients
Treatment-naive patients showed a different pattern of persistence compared with experienced patients, with significantly shorter treatment time (51 to 60 days) for all drugs except for tiotropium which was 79 days. These differences are reasonable, based on the assumption that previous use of these medications is a marker for severity. Data from the treatment-naive group also showed that the number of days to discontinuation was unchanged for the major medications between 2000/2001 and 2004/2005. Switching from ipratropium plus salbutamol was 8% lower in 2000 (before tiotropium was available). It is likely that the initial selection of the drug influenced treatment patterns, more than switching, when the drug had been available sometime in the year reviewed in the present analysis.
Experienced patients, by definition, have a longer duration of medication use and had been taking medications before the start of the analysis period. This eligibility criterion indicates a more severe disease status than naive patients. In contrast, naive patients may have initiated a bronchodilator as the first step in treatment, later advancing to other medications when their disease was considered severe or they had advanced problems. In parallel with our findings, the analysis of only experienced patients taking an inhaled corticosteroid medication in the Netherlands study (20) showed persistence rates twice as high as in the overall population. They described an association to disease severity, with higher persistence among patients who had been hospitalized the year before the study. Blais et al (17) found that after hospitalization, only one-half of newly treated COPD patients persisted with inhaled corticosteroid treatment started for one year.
Compliance
An important characteristic that differentiates medications in terms of convenience is the daily dosing regimen (as defined in the prescription database). Compliance rates have shown to decrease with larger numbers of daily doses (23). Most of the medications were prescribed for two or more doses daily, whereas tiotropium was prescribed for once-daily dosing. Patients in the present study used approximately 74% to 99% of bronchodilator doses, indicating a very high rate of bronchodilator use as prescribed, until discontinuation. In comparison to the present study, Stoloff et al (24) showed that adult asthma patients had compliance rates of 68% for the salmeterol-fluticasone combination treatment. A population of older men who were followed in veterans’ hospitals reported small differences in compliance among COPD, asthma and mixed diagnostic groups (66%, 56% and 61% compliance, respectively) (25). Compliance rates may have been higher in the present study in comparison with others because a large proportion of patients were taking once-daily tiotropium.
Limitations
An important limitation of these analyses was the lack of an accurate diagnosis of COPD versus asthma. However, tiotropium and ipratropium (ipratropium plus salbutamol) are only indicated for use in COPD patients whereas all other investigated medications are indicated for both asthma and COPD. This may have introduced a bias because asthma patients may have not needed to continue on the prescribed medication once symptoms improved. COPD patients with regular symptoms are assumed to use medications more regularly than asthma patients.
As an observational study, there were no data on efficacy parameters, such as the number or severity of episodes. Thus, this type of database analysis cannot suggest that better effectiveness or fewer adverse events with tiotropium can lead to significantly higher usage rates compared with other medications. The focus of the study was to observe differences in medication use across a variety of bronchodilators between naive and experienced patients. The most conservative interpretation is to attribute the significantly better compliance and persistence with once-daily dosing regimen. A broader interpretation may be that patients continue to take tiotropium because it improves their symptoms with fewer adverse effects than some other medications (26,27). Further research is needed to explore this hypothesis. Additional analyses are also needed to determine what drugs are added or substituted when the first medication is not effective.
SUMMARY
Airway disorders may vary in severity. However, patients appeared to have had similar behaviour in continuing treatment after initiation among all drugs in the class except tiotropium. Tiotropium was significantly better accepted by patients in terms of their willingness to continue on treatment, lesser need to switch or add other medications, and better compliance with the once-daily dose regimen. Based on significantly lower persistence rates for naive patients, clinicians should address the importance of long-term treatment more carefully and consistently with their patients when initiating treatment with a bronchodilator. Future research should pursue reasons why treatment-naive patients do not continue on treatment, as well as why tiotropium was more favourably accepted by patients.
Acknowledgments
The authors would like to thank Michael Brogan of Brogan Inc (Ottawa, Ontario) for his assistance on the statistical analyses. The present study was supported by Boehringer Ingelheim (Canada) Ltd and Pfizer Canada Inc.
REFERENCES
- 1.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2003. Can Respir J. 2003;10(Suppl A):11A–65A. doi: 10.1155/2003/567598. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): Executive summary. Respir Care. 2001;46:798–825. [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma Global strategy for asthma management and prevention. <www.ginasthma.com/download.asp?intId=13> (Version current at October 13, 2006).
- 5.National Heart Lung and Blood Institute Guidelines for the diagnosis and management of asthma: Expert panel report 2. <http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf> (Version current at October 13, 2006).
- 6.Public Health agency of Canada. Respiratory disease in Canada <http://www.phac-aspc.gc.ca/publicat/rdc-mrc01/index.html> (Version current at October 13, 2006).
- 7.National Center for Health Statistics National Health Interview Survey (NHIS). <http://www.cdc.gov/nchs/nhis.htm> (Version current at October 13, 2006).
- 8.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 9.van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium nd ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000;55:289–94. doi: 10.1136/thorax.55.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackevicius CA, Chapman KR. Prevalence of inhaled corticosteroid use among patients with chronic obstructive pulmonary disease. Ann Pharmacother. 1997;31:160–4. doi: 10.1177/106002809703100204. [DOI] [PubMed] [Google Scholar]
- 11.International Society for Pharmacoeconomics and Outcomes Research ISPOR medication compliance and persistence special interest group. <http://www.ispor.org/sigs/medication.asp> (Version current at October 13, 2006).
- 12.Cramer JA. Partial medication compliance: The enigma in poor medical outcomes. Am J Manag Care. 1995;1:45–52. [Google Scholar]
- 13.Cramer JA, Spilker B, editors. Patient Compliance in Medical Practice and Clinical Trials. New York: Raven Press; 1991. [Google Scholar]
- 14.Tashkin DP, Rand C, Nides M, et al. A nebulizer chronolog to monitor compliance with inhaler use. Am J Med. 1991;91:33S–6S. doi: 10.1016/0002-9343(91)90260-5. [DOI] [PubMed] [Google Scholar]
- 15.Rand CS, Nides M, Cowles MK, Wise RA, Connett J. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. Am J Resp Crit Care Med. 1995;152:580–8. doi: 10.1164/ajrccm.152.2.7633711. [DOI] [PubMed] [Google Scholar]
- 16.Simmons MS, Nides MA, Rand CS, Wise RA, Tashkin DP. Unpredictability of deception in compliance with physician-prescribed bronchodilator inhaler use in a clinical trial. Chest. 2000;118:290–5. doi: 10.1378/chest.118.2.290. [DOI] [PubMed] [Google Scholar]
- 17.Blais L, Bourbeau J, Sheehy O, LeLorier J. Inhaled corticosteroids in COPD: Determinants of use and trends in patient persistence with treatment. Can Respir J. 2004;11:27–32. doi: 10.1155/2004/289420. [DOI] [PubMed] [Google Scholar]
- 18.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: Methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Sernyak M. Results of a naturalistic study of treatment options: Switching atypical antipsychotic drugs or augmenting with valproate. Clin Ther. 2004;26:905–14. doi: 10.1016/s0149-2918(04)90134-8. [DOI] [PubMed] [Google Scholar]
- 20.Breekveldt-Postma NS, Gerrits CM, Lammers JW, Raaijmakers JA, Herings RM. Persistence with inhaled corticosteroid therapy in daily practice. Respir Med. 2004;98:752–9. doi: 10.1016/j.rmed.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: A cross-national study. JAMA. 1998;279:1458–62. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 22.Catalan VS, Couture JA, LeLorier J. Predictors of persistence of use of the novel antidiabetic agent acarbose. Arch Intern Med. 2001;161:1106–12. doi: 10.1001/archinte.161.8.1106. [DOI] [PubMed] [Google Scholar]
- 23.Claxton AJ, Cramer JA, Pierce C. Medication compliance: The importance of the dosing regimen. Clin Ther. 2001;23:1296–310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 24.Stoloff SW, Stempel DA, Meyer J, Stanford RH, Carranza Rosenzweig JR. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol. 2004;113:245–51. doi: 10.1016/j.jaci.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Lee TA, Bartle B, McLaughlin T, Dirani R.Adherence to respiratory medications in VA patients with chronic lung disease Value Health 20058327(Abst) [Google Scholar]
- 26.Shukla VK, Chen S, Boucher M, Mensinkai S, Dales R.Long-acting β2-agonists for the maintenance treatment of chronic obstructive pulmonary disease in patients with reversible and non-reversible airflow obstruction: A systematic review of clinical effectiveness. <http://www.cadth.ca/index.php/en/hta/reports-publications/search/publication/613> (Version current at October 13, 2006).
- 27.Olin JL. Tiotropium: An inhaled anticholinergic for chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2005;62:1263–9. doi: 10.1093/ajhp/62.12.1263. [DOI] [PubMed] [Google Scholar]