Abstract
Social insects display task-related division of labour. In some species, division of labour is related to differences in body size, and worker caste members display morphological adaptations suited for particular tasks. Bumble-bee workers (Bombus spp.) can vary in mass by eight- to tenfold within a single colony, which previous work has linked to division of labour. However, little is known about the proximate mechanism behind the production of this wide range of size variation within the worker caste. Here, we quantify the larval feeding in Bombus impatiens in different nest zones of increasing distance from the centre. There was a significant difference in the number of feedings per larva across zones, with a significant decrease in feeding rates as one moved outwards from the centre of the nest. Likewise, the diameter of the pupae in the peripheral zones was significantly smaller than that of pupae in the centre. Therefore, we conclude that the differential feeding of larvae within a nest, which leads to the size variation within the worker caste, is based on the location of brood clumps. Our work is consistent with the hypothesis that some larvae are ‘forgotten’, providing a possible first mechanism for the creation of size polymorphism in B. impatiens.
Keywords: Bombus impatiens, division of labour, size polymorphism, alloethism, bumble-bees
1. Introduction
Division of labour as a key feature of social insect societies has been described for thousands of years (Aristotle 350 BC 2000; Beshers & Fewell 2001), and is considered a major reason for their ecological success (Wilson 1985, 1990; Robinson 1992). Division of labour implies that the worker caste is divided among the different jobs. Workers specialize, whether permanently or temporarily, on a subset of tasks. This is thought to increase the output efficiency when compared with a system where all workers perform all tasks indiscriminately (see reviews Oster & Wilson (1978), Robinson (1992), Beshers & Fewell (2001), although see Dornhaus 2008).
There are many ways in which labour might be divided (Beshers & Fewell 2001), including dominance (West-Eberhard 1969) or age (Rösch 1925; Lindauer 1953; Mirenda & Vinson 1981; Seeley 1982; Robinson 1992). Division of labour may also be based on morphology. Body shape and size are fixed in adults in the Hymenoptera, as in all other holometabolous insects. Morphological division of labour may be observed in several organisms, such as termites (Noirot & Pasteels 1987), aphids (Stern & Foster 1997) and ants (Hölldobler & Wilson 1990; Braendle et al. 2003). Ants provide some of the best cases of non-genetic polymorphisms (Huxley 1932; Wilson 1953, 1971), although see Hughes et al. (2003) and Rheindt et al. (2005), as approximately 15 per cent of ant genera display a form of worker polymorphism or size variation (Wheeler 1991). Sometimes this size variation is extreme, such as in Pheidologeton, where there is a 500-fold difference in mass between workers of a single colony (Wilson 2003). Leaf-cutting ants (Atta spp. and Acromyrmex spp.) are a well-known example of size polymorphisms in relation to a division of labour. Small workers inside the nest care for brood and tend the fungus garden, while large ants with larger mandibles forage and maintain the nest (Wilson 1980; Wetterer 1999).
Bumble-bee (Bombus spp.) workers display continuous size variation, symmetric around a single mean, where there may be an eight- to tenfold difference in mass between workers of the same nest (Cumber 1949; Plowright & Jay 1968; Michener 1974; Alford 1975; Garofalo 1978; Inouye & Kato 1992; Goulson 2003). Bumble-bee queens are typically singly mated (Schmid-Hempel & Schmid-Hempel 2000), and all workers in a nest are thus full ‘super sisters’ with a high degree of relatedness (r=0.75). This is interesting because size variation within a nest is therefore likely to be caused by trophic, not genetic, factors. As in leaf-cutting ants, this size polymorphism is linked with colony organization. Larger workers tend to serve as foragers (Richards 1946; Cumber 1949; Goulson et al. 2002), and are more likely to make the transition to foraging at a younger age (Brian 1952; Pouvreau 1989), while smaller workers carry out intranidal tasks, such as brood care and comb construction (Jandt & Dornhaus 2009), and are more likely never to initiate foraging (Free 1955; Yerushalmi et al. 2006), although task switching for all sizes is possible (Jandt & Dornhaus 2009).
Previous research has concentrated on the adaptive significance and fitness consequences of morphological division of labour (Wheeler 1928; Oster & Wilson 1978; Porter & Tschinkel 1985; Powell & Franks 2006). In bumble-bees, the larger foragers bring back more nectar per unit time (Goulson et al. 2002; Spaethe & Weidenmuller 2002), fly at cooler temperatures (Free & Butler 1959), possess more olfactory sensillae (Spaethe et al. 2007) and are probably less prone to predation (Goulson et al. 2002), all of which suggest that the size variation in the worker caste may be an adaptive feature in division of labour (Peat et al. 2005).
Many factors affect the ultimate size of an adult insect, such as temperature, humidity, food availability, hormones and larval competition (Wheeler 1991; Cnaani et al. 1997; Emlen & Nijhout 2000; Stern 2001; Davidowitz et al. 2003). In pollen-storing bumble-bee species such as Bombus impatiens, larvae develop in individual cells (Sladen 1912; Alford 1975; Goulson 2003). Their size as an adult is directly correlated with how much food they receive as larvae (Plowright & Jay 1968; Pendrel & Plowright 1981; Sutcliffe & Plowright 1988, 1990; Pereboom 2001; Pereboom et al. 2003). Larvae are fed by workers on a regurgitated mixture of pollen and nectar (Katayama 1973, 1975; Michener 1974; Alford 1975). As unequal food amounts during larval development cause size differences (Spaethe & Weidenmuller 2002), the mass of new workers is therefore controlled by the bees rearing them (Ribeiro 1994). The question then becomes, how do the workers rearing the new bees regulate which bee gets how much food? In other words, what is the mechanism by which size variation is created?
Anecdotally, it was suggested that the position of a larva during development, which is fixed, influences how much food she receives and, ultimately, her adult size. For example, the larvae at the periphery may receive less care than those in the middle. This was hypothesized not only for the making of reproductives, where it was observed that queens generally resulted from larvae in ‘favourable’ positions (Cumber 1949), but also for workers, where it was noted that larvae positioned at the border and bottom of the nest were visited less often by nurse bees (Sladen 1912) and grew ‘no larger than a housefly’. However, there has never been direct evidence that peripheral larvae are essentially forgotten. Indeed, the idea has been rejected as implausible (Goulson et al. 2002; Goulson 2003), probably because the thought of sloppy nurses neglecting larvae might at first appear at variance with the data supporting size variation as an adaptive feature in bumble-bees. However, whether size variation in bumble-bees is (or is not) today adaptive does not presuppose that it has (or has not) originally arisen out of the accidental neglect of some brood by nurse worker bees.
We argue that the mechanism by which workers achieve differential feeding of larvae resulting in worker polymorphism and the functional benefits of that polymorphism must be independently tested. Here, we investigate the relationship of position of larvae and their treatment by workers to elucidate the proximate mechanism by which worker size polymorphism is produced.
2. Material and methods
(a) Study organism
We obtained three queenright bumble-bee colonies (B. impatiens; colonies 1–3) from a commercial breeder (Koppert Biological Systems, Romulus, MI). At the start of the experiment, colonies typically had 15–30 workers with brood; each colony at its peak size had approximately 250 individual workers plus brood and honey stores. Colonies were housed in wooden boxes (22×22×11 cm) with screened ventilation holes and a Plexiglas cover to facilitate observations. The colonies were connected by plastic tubing to a foraging chamber (58×36×40 cm), where sugar solution was provided ad libitum. Pollen was delivered directly to the nest through a round opening in the Plexiglas cover daily before the start data collection (see below). Colonies were kept at room temperature. After a few days of habituation the bumble-bees behaved normally with the overhead lights that were on during the day.
(b) Data collection: number of feedings per larva
Data were collected approximately four times a week from 8 October to 14 November 2007, for colony 1 (23 observation days) and 22 February to 9 May 2008, for colonies 2 and 3 (39 observation days). We wished to know the feeding rates per individual larva from different nest zones over a daily 2 hours observation period. Brood are fed by workers for 5–9 days during larval development (Cnaani et al. 2002), so we were able to make observations on several brood cycles throughout the experiments. We drew eight concentric circles of increasing diameter on an acetate sheet that was taped to the Plexiglas cover, using the same sheets for all three colonies. Each circle was labelled with a letter, from A to H, with A being the zone directly over the centre of the colony. Each day, an observer would count the number of larvae within each zone. This allowed us to correct for the number of larvae per zone, which was essential as zones were of different size and varied in how many larvae were present (figure 1). The observer also photographed colonies 2 and 3 with the acetate sheet in place to provide a daily record of larval placement and number, and adult worker distribution.
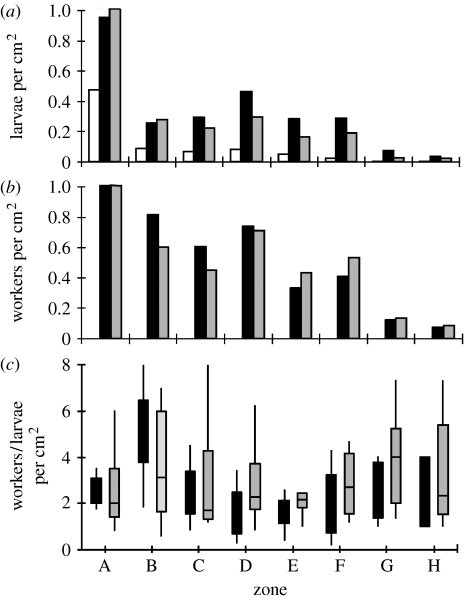
Figure 1.
Median density of larvae for (a) all three colonies (white bars, colony 1; black bars, colony 2; grey bars, colony 3), (b) workers for colonies 2 and 3 and (c) workers/larva per cm2 across days for each zone. Larval and worker density are highest in the centre of the colonies. However, the number of workers per larva did not increase or decrease in the periphery compared with the centre. Zones that share a letter are not significantly different from each other.
Structures such as honeypots and brood in bumble-bee nests are not ordered in a predictable pattern (Cameron 1989), and the queen will lay eggs throughout the nest during the entire life history of the colony. Therefore, at any one time, broods of different developmental stages may be located everywhere from the centre to the periphery of the colony.
Workers feed larvae by regurgitating a pollen/nectar mixture to larvae in a stereotyped manner that is easy to identify (Michener 1974). A worker will open a larval cell, insert her head and contract her abdomen to discharge the food (Katayama 1973, 1975; Ribeiro 1997). For each feeding event, the observer recorded the time and zone in which it took place. All zones were monitored simultaneously.
(c) Data collection: size of pupae
At the end of data collection for larval feeding, we froze the colonies and sectioned them according to zones. Pupae were measured with digital calipers (accurate to 100th mm) at their widest horizontal mid-section diameter. We measured pupae in zones at all depths, not taking into account that the nest has many levels in the vertical dimension, making our analysis a conservative estimate.
We measured two ‘types’ of pupae. First, we wished to measure all pupae at the end of their development cycle; these pupae were identified from earlier-stage pupae by a striped appearance on the pupal casing. Second, we wished to measure the pupal casings, which workers recycle as honeypots, of pupae that had enclosed earlier during the colony cycle. These are visually identifiable from pure-wax-constructed honeypots as the old pupa always had a vertically striped appearance on the casing. Therefore, whether we were measuring pupae that had not yet emerged or old pupal casings-turned honeypot, we measured only those with the striped appearance. In all, we measured 1259 pupae.
(d) Statistical analyses
All statistics were performed using Minitab (Student v. 14). We used non-parametric statistics to analyse our data. We calculated a median-feeding rate for the eight zones (A–H) with data pooled from the three colonies, and then used the Kruskal–Wallis, as an analysis of variance between medians, to determine (i) whether there was a significant difference in larval feeding per individual larva among the eight zones and (ii) whether there was a significant difference in the pupal size among the eight zones. We justify our data pooling in two ways. First, we reanalysed both questions using Friedman's test that allows us to block for colonies. Second, we analysed each colony separately to demonstrate that this pattern is significant in each colony.
It was also important to compare specifically between zone pairs because we wished to know (i) whether feeding per larva decreased as one moved from A to H and (ii) whether pupae size decreased as one moved from A to H. If some larvae are ‘forgotten’, we would expect a significant decrease in feeding per larva and a significant decrease in pupae size from A to H. To determine this, we used a non-parametric multiple comparison procedure that is equivalent to the Tukey post hoc test (Zar 1984).
To determine whether timing had an effect on brood feeding, which has been previously reported as not being the case (Pereboom 1997), we performed regressions on each zone using date as the independent variable and feedings/larva as the response variable.
We wished to compare the spatial pattern of early and late feeding in the life cycle of the colony. We calculated a median-feeding rate for the eight zones (A–H) with data from all three colonies for before and after the appearance of reproductives, which is a significant point in colony life, after which workers may compete for reproductive opportunities. We used a Kruskal–Wallis test as above to separately analyse spatial pattern before and after this point.
Lastly, to determine whether there is a correlation between the median-feeding frequencies per larva in each zone with the median size of pupae in each zone, we used Spearman's rank correlation.
3. Results
(a) Workers and larvae are numerous in centre; ratio of workers/larva constant throughout nest
Colonies varied in their median number of larvae present on any given day (colony 1: 15.5; colony 2: 85; colony 3: 58; table 1). All three colonies had a similar distribution of number of larvae across zones: all three colonies had the highest density of larvae in the middle of the colony (figure 1a). Photograph analysis of colonies 2 and 3 (we did not have photographs of colony 1) demonstrated that both colonies also had the highest density of workers in the middle of the colony (figure 1b). However, the ratio of workers/larva did not follow a predictable spatial pattern in the nest. Median workers/larva ratios were significantly different among zones in colonies 2 and 3 (Kruskal–Wallis, H7=18.43, p=0.02; figure 1c). However, results of the post hoc test demonstrate that only zone B actually accounts for the significance, in that it was higher than zones D and E. Otherwise, the workers/larva ratio was not significantly different between zones.
Table 1.
Median number of feeding events per larva over 2 hours, median size of pupae (mm), median number of larvae across study days, median number of workers across study days (colony 1 data not available) and area of zones in zones A–H for all three colonies. Workers and larvae tend to be more concentrated in the middle. All colonies exhibited the trend of decreasing larval feeding and decreasing pupal size as one moved from A to H.
| A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|
| feeding/larva | ||||||||
| colony 1 | 4.03 | 4.58 | 4.14 | 3.52 | 3.19 | 2.56 | 1.50 | 1.50 |
| colony 2 | 4.59 | 4.36 | 4.06 | 3.15 | 3.10 | 2.11 | 1.99 | 0.73 |
| colony 3 | 2.57 | 4.33 | 2.79 | 1.95 | 1.27 | 1.89 | 1.06 | 1.05 |
| median | 4.03 | 4.36 | 4.06 | 3.15 | 3.10 | 2.11 | 1.50 | 1.05 |
| pupal size (mm) | ||||||||
| colony 1 | 5.15 | 4.80 | 4.88 | 3.94 | 3.94 | 3.68 | 3.30 | 3.08 |
| colony 2 | 7.78 | 7.72 | 7.51 | 7.41 | 7.37 | 7.40 | 7.02 | 6.90 |
| colony 3 | 7.48 | 7.61 | 7.59 | 7.49 | 7.35 | 7.07 | 7.16 | 6.52 |
| median | 7.48 | 7.61 | 7.51 | 7.41 | 7.35 | 7.07 | 7.02 | 6.52 |
| median number of larvae | ||||||||
| colony 1 | 1.5 | 2.0 | 3.0 | 3.0 | 3.0 | 1.0 | 1.0 | 1.0 |
| colony 2 | 3.0 | 6.0 | 13.0 | 17.0 | 17.0 | 12.0 | 11.0 | 6.0 |
| colony 3 | 4.0 | 6.5 | 10.0 | 11.0 | 10.0 | 8.0 | 4.5 | 4.0 |
| median | 3.0 | 6.0 | 10.0 | 11.0 | 10.0 | 8.0 | 4.5 | 4.0 |
| median number of workers | ||||||||
| colony 1 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| colony 2 | 7.0 | 19.0 | 27.0 | 27.0 | 20.0 | 17.0 | 19.0 | 12.0 |
| colony 3 | 7.0 | 14.0 | 20.0 | 26.0 | 26.0 | 22.0 | 21.0 | 14.0 |
| median | 7.0 | 16.5 | 23.5 | 26.5 | 23.0 | 19.5 | 20.0 | 13.0 |
| area of zones (cm2) | 3.14 | 23.27 | 44.44 | 36.61 | 59.87 | 41.24 | 154.30 | 159.66 |
(b) Larvae are fed more often closer to the centre of the nest
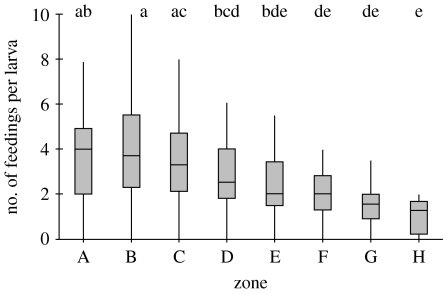
We found a significant difference among zones in feeding rates per larva when data are pooled across colonies (Kruskal–Wallis, H7=94.42, p<0.001; figure 2). This significance was maintained when colonies were analysed individually (colony 1: Kruskal–Wallis, H7=19.33, p=0.007; colony 2: Kruskal–Wallis, H7=52.75, p<0.001; colony 3: Kruskal–Wallis, H7=37.37, p<0.001) and when we reanalysed feeding frequencies per zone while blocking for colony (Friedman's test, S7=20.05, p=0.005). For all three colonies, the median number of feedings per larva decreases from the middle zones (A, B and C) to the periphery (table 1; figure 2). By comparing feeding between specific zone pairs, we found that feeding rates in zones A and B, the central zones, were significantly higher than those in zones F, G and H (figure 2). The feeding rate in zone H, which was the furthest from the centre, was significantly lower compared with zones A, B, C and D (figure 2).
Figure 2.
The number of feedings per larva is significantly different between different zones. Data are pooled for colonies. Letters above zones are results of non-parametric multiple comparison post hoc test. Zones that share a letter are not significantly different from each other.
(c) Pupae are larger closer to the centre of the nest
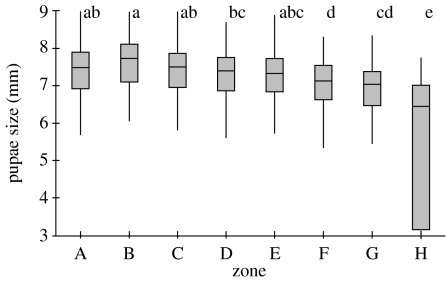
There was a significant difference in pupae size among zones when data are pooled across colonies (Kruskal–Wallis, H7=103.31, p<0.001; figure 3). This significance was maintained when colonies were analysed individually (colony 1: Kruskal–Wallis, H7=28.93, p<0.001; colony 2: Kruskal–Wallis, H7=58.23, p<0.001; colony 3: Kruskal–Wallis, H7=72.17, p<0.001) and when we reanalysed pupal size differences per zone while blocking for colony (Friedman's test, S7=18.89, p=0.009). For all three colonies, the median pupal size decreases from the middle zones (A, B and C) to the periphery (table 1; figure 3). Pupae in zones A and B, the central zones, were significantly larger than those in zones F, G and H (figure 3). Pupae size in zone H, which was furthest from the centre, was significantly smaller than all other zones (figure 3).
Figure 3.
Size of pupae is significantly different between different zones. Data are pooled for colonies. Letters above zones are results of non-parametric multiple comparison post hoc test. Zones that share a letter are not significantly different from each other.
(d) Date did not affect feedings/larva
For each of the eight zones, date was a non-significant variable in the regression analysis of feedings/larva (zone A: R2=0.001, F1,1=0.03, p=0.87; zone B: R2=0.001, F1,1=0.05, p=0.83; zone C: R2<0.001, F1,1=0.01, p=0.93; zone D: R2=0.01, F1,1=1.16, p=0.21; zone E: R2=0.009, F1,1=0.74, p=0.39; zone F: R2=0.006, F1,1=0.29, p=0.59; zone G: R2=0.004, F1,1=0.11, p=0.74; and zone H: R2<0.001, F1,1<0.01, p=0.99; data not shown). This confirms, as previously reported (Pereboom 1997), that overall feeding rates did not significantly change over the course of our experiment.
(e) Decrease in feedings/larva from centre to periphery is present before and after the appearance of reproductives
The overall spatial pattern that we report (figure 2) is also present when we separately analyse the results from the time before and after the appearance of reproductives. This confirms that the spatial pattern is robust across the colony cycle (see above). There was a significant difference among zones in feeding rates per larva when data are pooled across colonies both before (Kruskal–Wallis, H7=64.29, p<0.001) and after the appearance of reproductives (Kruskal–Wallis, H7=66.20, p<0.001; see figure 5 in the electronic supplementary material). For both time periods, the median number of feedings per larva decreases from the middle zones (A, B and C) to the periphery.
(f) Feeding rate per larva is correlated with pupae size
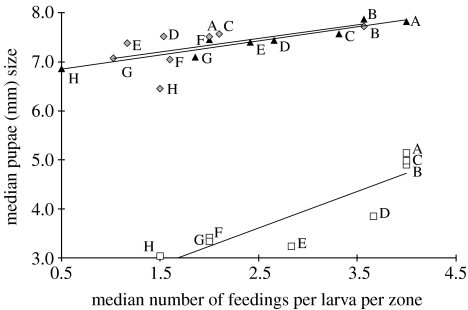
We found a significant positive correlation between feeding rate per larva and pupae size across zones A–H (Spearman's rank correlation, rs=0.976, n=8, p<0.001; figure 4), as both feeding rate and size of pupae increase from zones A to H. Colony 1 produced in general smaller pupae. However, all three colonies show the significant positive relationship between feeding and pupae size.
Figure 4.
Median-feeding frequency per larva positively correlates with median pupal size across zones A–H. Both increase across A–H. Data are shown for all three colonies. Squares, colony 1; triangles, colony 2; diamonds, colony 3.
4. Discussion
Our results clearly demonstrate that larvae at the periphery of the nest receive less feeding than those in the centre (figure 2). Further, pupae at the periphery of the nest are smaller than pupae in the centre of the nest (figure 3). These results were found consistently across all colonies (table 1) and support the hypothesis that peripheral brood receive less care. The differences in feeding rate based on larval location were present throughout the time course of the colony (see the electronic supplementary material, figure 5). They provide a possible mechanism for the production of size polymorphism in B. impatiens and, ultimately, morphological division of labour in bumble-bees.
This notion that larvae at the bottom and sides of the nest become small adults was first proposed almost a century ago (Sladen 1912; Cumber 1949); however, this is the first direct evidence, to our knowledge, that larval feeding and pupal size vary directly with distance from the nest centre.
Although the feeding rate for individual larva is irregular and ‘stochastic’ (Pendrel & Plowright 1981; Cnaani & Hefetz 1994), previous work has shown that the average, population-level feeding rate at the colony level is constant (Pereboom 1997). This we confirm here. Our overall observed feeding rate was possibly elevated due to the pollen we provided immediately before the start of each observation period, which stimulated the workers' feeding of larvae (M. J. Couvillon 2008, personal observation). Sometimes feeding rates were slightly higher in zone B than zone A (table 1), which was immediately adjacent to the exact centre (zone A); however, this difference was never significant.
Previous work has demonstrated that spatial organization is relevant in social insect nests. Much of the work has been on adult workers, where their position within the nest is correlated with division of labour (in ants, Wilson 1976, Sendova-Franks & Franks 1995, Powell & Tschinkel 1999, Backen et al. 2000; wasps, Robson et al. 2000; honeybees, Rösch 1925, Lindauer 1953, Seeley 1982; bumble-bees, Jandt & Dornhaus 2009). There is also strong evidence to support that workers in some ant species spatially arrange brood to help workers organize brood care, such that the oldest larvae are moved to the nest periphery (Franks & Sendova-Franks 1992; Sendova-Franks et al. 2004). Bumble-bees are different, in that the position of any brood item is fixed throughout development.
Our data suggest that the density of larvae and workers is higher in the middle of the nest (figure 1a,b; table 1), although both workers and larvae are present throughout all zones. The ratio of workers/larva neither decreased nor increased as one moved to the periphery, as only zone B was significantly different from the other zones (figure 1c). Yet feedings/larva decreased towards the periphery. Perhaps some of the workers that we counted from photographs were not nurses actively engaged in tending brood, which agrees with previous data that nurse bees specifically were found in the nest centre (Jandt & Dornhaus 2009). Therefore, the absence of nurses (although not workers) at the periphery might provide an explanation of why feeding rates are lower there. Experimentally starved larvae are fed significantly earlier and more often, suggesting that the larvae somehow signal their status to workers (Smeets & Duchateau 2001; Pereboom et al. 2003). Larvae in the pathway of workers are fed more often than those in concealed areas (Stephen & Koontz 1973a,b), which suggests a contact chemoreception signal rather than a volatile one. Nurses may thus not actively ‘choose’ to starve larvae; rather, larvae at the periphery might be too far away from the main nurse traffic concentrated in the centre of the nest for a signal to be noted.
To our knowledge, honeybees, which are also fed by workers, do not neglect larvae at the periphery of the nest, although the regularity, organization and higher worker density of an Apis nest could account for their regular feeding and their relatively uniform-size worker distribution (Jay 1963). Similarly, stingless bee workers are also of uniform size (Waddington et al. 1986; Ramalho et al. 1998; Roulston & Cane 2000; Goulson et al. 2005); however, some stingless bee species have unpredictably patterned nests such as Bombus (e.g. Frieseomelitta varia). The uniform worker size may be achieved because, unlike B. impatiens, stingless bee larvae develop inside sealed, provisioned cells (Roubik 1989; Koedam et al. 1999).
There are some Bombus species that do provision their larvae as the stingless bees. In these pocket-making species, which we did not investigate here, larvae develop communally in ‘pockets’. Curiously, worker size variation is even greater in pocket makers than the unprovisioned pollen-storing species, such as B. impatiens (Pouvreau 1989; Goulson 2003). The pocket larvae might compete with each other for the provisioned food (Sladen 1912; Cumber 1949). If this is the case, the position of a larva within the pocket in relation to the other larvae might be significant. By contrast, we have shown here that the position of the individual larva within the nest for the pollen-storing B. impatiens is likely to be an important predictor of adult worker size.
We do not believe these data exclude the idea that size variation is an adaptive feature in bumble-bee colonies. Our data could support three possible scenarios. In one, accidental neglect of some larvae led to size variation that was then co-opted for division of labour. Size variation would therefore be an exaptation (Gould & Lewontin 1979; Gould & Vrba 1982), conferring adaptive benefits for fitness via division of labour, even if it did not initially evolve for that specific role. In a second scenario, size variation evolved as an adaptation for division of labour (Goulson et al. 2002; Goulson 2003), and we show here that the mechanism of creating that variation is differential feeding depending on position in the nest. The third scenario would be that size variation is not adaptive for division of labour. We did not test these scenarios, although an interesting implication of our work is that it raises the possibility of scenarios one and three.
However, there is supportive evidence for the second scenario: large bees are more effective as foragers (Goulson et al. 2002; Spaethe & Weidenmuller 2002), and they are, in fact, more likely to work as foragers (Goulson et al. 2002). However, it is important to note that specific pieces are missing from the puzzle. Less understood is the adaptive benefit of small workers or polymorphism per se. Pervading wisdom has suggested that small bees might better manoeuvre in the cramped confines of a nest (Free & Butler 1959); however, there is little direct support for small bees as superior nurses. In fact, when the worker size distribution in small groups of bees is experimentally manipulated such that the average worker is larger, these groups rear more bees (Cnaani & Hefetz 1994). If small bees are not, in fact, better nurses, what precisely is their adaptive function within a bumble-bee nest? Alternatively, perhaps the mechanism reported here, whereby position within the nest results in accidental neglect of peripheral larvae, is a constraint, and worker size variation is a result of this constraint, with selection neither acting for nor against it. Clearly, more work is needed in this area.
Acknowledgments
Many thanks to Shane Rusing, Jennifer Bonds and Jason Turner for their work with data collection and Jennifer Jandt for her helpful suggestions with the manuscript. This work was funded by a grant from the NIH (Postdoctoral Excellence in Research and Teaching (PERT) fellowship) to M.J.C.
Supplementary Material
The number of feedings per larva is significantly different between different zones both before and after the appearance of reproductives. Data pooled for colonies
References
- Alford D.V. Davis-Poynter; London, UK: 1975. Bumblebees. [Google Scholar]
- Aristotle 350 BC. Adamant Media Corporation; Boston, MA:: 2000. The history of animals. [Google Scholar]
- Backen S.J., Sendova-Franks A.B., Franks N.R. Testing the limits of social resilience in ant colonies. Behav. Ecol. Sociobiol. 2000;48:125–131. doi:10.1007/s002650000219 [Google Scholar]
- Beshers S.N., Fewell J.H. Models of division of labor in social insects. Annu. Rev. Entomol. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. doi:10.1146/annurev.ento.46.1.413 [DOI] [PubMed] [Google Scholar]
- Braendle C., Hockley N., Brevig T., Shingleton A.W., Keller L. Size-correlated division of labour and spatial distribution of workers in the driver ant, Dorylus molestus. Naturwissenschaften. 2003;90:277–281. doi: 10.1007/s00114-003-0426-3. doi:10.1007/s00114-003-0426-3 [DOI] [PubMed] [Google Scholar]
- Brian A.D. Division of labour and foraging in Bombus agrorum fabricius. J. Anim. Ecol. 1952;21:223–240. doi:10.2307/1959 [Google Scholar]
- Cameron S.A. Temporal patterns of division of labor among workers in the primitively eusocial bumble bee, Bombus griseocollis (Hymenoptera: Apidae) Ethology. 1989;80:137–151. [Google Scholar]
- Cnaani J., Hefetz A. The effect of workers size frequency-distribution on colony development in Bombus terrestris. Insectes Soc. 1994;41:301–307. doi:10.1007/BF01242301 [Google Scholar]
- Cnaani J., Borst D.W., Huang Z.Y., Robinson G.E., Hefetz A. Caste determination in Bombus terrestris: differences in development and rates of JH biosynthesis between queen and worker larvae. J. Insect Physiol. 1997;43:373–381. doi: 10.1016/s0022-1910(96)00106-0. doi:10.1016/S0022-1910(96)00106-0 [DOI] [PubMed] [Google Scholar]
- Cnaani J., Schmid-Hempel R., Schmidt J.O. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insectes Soc. 2002;49:164–170. doi:10.1007/s00040-002-8297-8 [Google Scholar]
- Cumber R.A. The biology of bumble-bees, with special reference to the production of the worker caste. Trans. R. Entomol. Soc. Lond. 1949;100:1–45. [Google Scholar]
- Davidowitz G., D'Amico L.J., Nijhout H.F. Critical weight in the development of insect body size. Evol. Dev. 2003;5:188–197. doi: 10.1046/j.1525-142x.2003.03026.x. doi:10.1046/j.1525-142X.2003.03026.x [DOI] [PubMed] [Google Scholar]
- Dornhaus A. Specialization does not predict individual efficiency in an ant. PLoS Biol. 2008;6:e285. doi: 10.1371/journal.pbio.0060285. doi:10.1371/journal.pbio.0060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen D.J., Nijhout H.F. The development and evolution of exaggerated morphologies in insects. Annu. Rev. Entomol. 2000;45:661–708. doi: 10.1146/annurev.ento.45.1.661. doi:10.1146/annurev.ento.45.1.661 [DOI] [PubMed] [Google Scholar]
- Franks N.R., Sendova-Franks A.B. Brood sorting by ants: distributing the workload over the work-surface. Behav. Ecol. Sociobiol. 1992;30:109–123. doi:10.1007/BF00173947 [Google Scholar]
- Free J.B. The division of labour within bumblebee colonies. Insectes Soc. 1955;2:195–212. doi:10.1007/BF02224381 [Google Scholar]
- Free J.B., Butler C.G. Collins; London, UK: 1959. Bumblebees. [Google Scholar]
- Garofalo C.A. Bionomics of Bombus (Fervidobombus) morio. 2. Body size and length of life of workers. J. Apic. Res. 1978;17:130–136. [Google Scholar]
- Gould S.J., Lewontin R.C. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. doi:10.1098/rspb.1979.0086 [DOI] [PubMed] [Google Scholar]
- Gould S.J., Vrba E.S. Exaptation: a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- Goulson D. Oxford University Press; Oxford, MA: 2003. Bumblebees: behaviour and ecology. [Google Scholar]
- Goulson D., Peat J., Stout J.C., Tucker J., Darvill B., Derwent L.C., Hughes W.O.H. Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim. Behav. 2002;64:123–130. doi:10.1006/anbe.2002.3041 [Google Scholar]
- Goulson D., Derwent L.C., Peat J. Evidence for alloethism in stingless bees (Meliponinae) Apidologie. 2005;36:411–412. doi:10.1051/apido:2005029 [Google Scholar]
- Hölldobler B., Wilson E.O. Belknap Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Hughes W.O.H., Sumner S., Van Borm S., Boomsma J.J. Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc. Natl Acad. Sci. USA. 2003;100:9394–9397. doi: 10.1073/pnas.1633701100. doi:10.1073/pnas.1633701100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley J.S. Dover; New York, NY: 1932. Problems of relative growth. [Google Scholar]
- Inouye T., Kato M. Inter and intra specific morphological variation in bumblebee species and competition in flower utilization. In: Hunter M.D., Ohgushi T., Price P.W., editors. Effects of resource distribution on animal–plant interactions. Academic Press; San Diego, CA: 1992. pp. 393–427. [Google Scholar]
- Jandt J., Dornhaus A. Spatial organization and division of labor in the bumble bee, Bombus impatiens. Anim. Behav. 2009;77:641–651. doi:10.1016/j.anbehav.2008.11.019 [Google Scholar]
- Jay S.C. The development of honeybees in their cells. J. Apic. Res. 1963;2:117–134. [Google Scholar]
- Katayama E. Observations on the brood development in Bombus ignitus (Hymenoptera, Apidae). II. Brood development and feeding habits. Kontyû. 1973;41:203–216. [Google Scholar]
- Katayama E. Egg-laying habits and brood development in Bombus hypocrita (Hymenoptera, Apidae). II. Brood development and feeding habits. Kontyû. 1975;43:478–496. [Google Scholar]
- Koedam D., Contrera F.A.L., Imperatriz-Fonesca V.L. Clustered male production by workers in the stingless bee Melipona subnitida Ducke (Apidae, Meliponinae) Insectes Soc. 1999;46:387–391. doi:10.1007/s000400050161 [Google Scholar]
- Lindauer M. Division of labour in the honeybee colony. Bee World. 1953;34:63–90. [Google Scholar]
- Michener C.D. Harvard University Press; Cambridge, MA: 1974. The social behaviour of the bees. [Google Scholar]
- Mirenda J.T., Vinson S.B. Division of labor and specification of castes in the red imported fire ant Solenopsis invicta Buren. Anim. Behav. 1981;29:410–420. doi:10.1016/S0003-3472(81)80100-5 [Google Scholar]
- Noirot C., Pasteels J.M. Ontogenetic development and evolution of the worker caste in termites. Cell. Mol. Life Sci. 1987;43:851–952. doi:10.1007/BF01951642 [Google Scholar]
- Oster G.F., Wilson E.O. Princeton University Press; Princeton, NJ: 1978. Caste and ecology in the social insects. [PubMed] [Google Scholar]
- Peat J., Tucker J., Goulson D. Does intraspecific size variation in bumblebees allow colonies to efficiently exploit different flowers? Ecol. Entomol. 2005;30:176–181. doi:10.1111/j.0307-6946.2005.00676.x [Google Scholar]
- Pendrel B.A., Plowright R.C. Larval feeding by adult bumble bee workers (Hymenoptera, Apidae) Behav. Ecol. Sociobiol. 1981;8:71–76. doi:10.1007/BF00300817 [Google Scholar]
- Pereboom, J. J. M. 1997 …while they banquet splendidly the future mother… PhD thesis, Utrecht University, The Netherlands.
- Pereboom J.J.M. Size dimorphism in bumblebees: a result of caste specific differences in fat body metabolism? Neth. J. Zool. 2001;51:323–333. doi:10.1163/156854201753247578 [Google Scholar]
- Pereboom J.J.M., Velthuis H.H.W., Duchateau M.J. The organisation of larval feeding in bumblebees (Hymenoptera, Apidae) and its significance to caste differentiation. Insectes Soc. 2003;50:127–133. doi:10.1007/s00040-003-0639-7 [Google Scholar]
- Plowright R.C., Jay S.C. Caste differentiation in bumblebees (Bombus Latr—Hym). 1. Determination of female size. Insectes Soc. 1968;15:171–192. doi:10.1007/BF02223465 [Google Scholar]
- Porter S.D., Tschinkel W.R. Fire ant polymorphism: the ergonomics of brood production. Behav. Ecol. Sociobiol. 1985;16:323–336. doi:10.1007/BF00295545 [Google Scholar]
- Pouvreau A. Contribution to the study of polyethism in bumblebees, Bombus Latr (Hymenoptera, Apidae) Apidologie. 1989;20:229–244. doi:10.1051/apido:19890305 [Google Scholar]
- Powell S., Franks N. Ecology and the evolution of worker morphological diversity: a comparative analysis with Eciton army ants. Funct. Ecol. 2006;20:1105–1114. doi:10.1111/j.1365-2435.2006.01184.x [Google Scholar]
- Powell S., Tschinkel W.R. Ritualized conflict in Odontomachus brunneus and the generation of interaction-based task allocation: a new organizational mechanism in ants. Anim. Behav. 1999;58:965–972. doi: 10.1006/anbe.1999.1238. doi:10.1006/anbe.1999.1238 [DOI] [PubMed] [Google Scholar]
- Ramalho M., Imperatriz-Fonesca V.L., Giannini T.C. Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidiodes (Apidae, Hymenoptera) Apidologie. 1998;29:221–228. doi:10.1051/apido:19980302 [Google Scholar]
- Rheindt F.E., Strehl C.P., Gadau J. A genetic component in the determination of worker polymorphism in the Florida harvester ant Pogonomyrmex badius. Insectes Soc. 2005;52:163–168. doi:10.1007/s00040-004-0787-4 [Google Scholar]
- Ribeiro M.F. Growth in bumble bee larvae: relation between development time, mass, and amount of pollen ingested. Can. J. Zool. 1994;72:1978–1985. doi:10.1139/z94-270 [Google Scholar]
- Ribeiro, M. F. 1997 Larval nutrition in the bumblebee Bombus terrestris and its influence in caste differentiation. PhD thesis, Utrecht University, The Netherlands, p. 129.
- Richards O.W. Observations on Bombus agrorum (Fabricius) (Hymen, Bombindae) Proc. R. Entomol. Soc. Lond. A. 1946;21:66–71. [Google Scholar]
- Robinson G.E. Regulation of division-of-labor in insect societies. Annu. Rev. Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. doi:10.1146/annurev.en.37.010192.003225 [DOI] [PubMed] [Google Scholar]
- Robson S.K.A., Bean K., Hansen J., Norling K., Rowe R.J., White D. Social and spatial organization in colonies of a primitively eusocial wasp, Ropalidia revolutionalis (de Saussure) (Hymenoptera: Vespidae) Aust. J. Entomol. 2000;39:20–24. doi:10.1046/j.1440-6055.2000.00135.x [Google Scholar]
- Rösch G.A. Untersuchungen über die Arbeitsteilung im Bienenstaat. 1. Teil: Die Tätigkeiten im normalen Bienenstaate und ihre Beziehungen zum Alter der Arbeitsbienen. Z. vergl. Physiol. 1925;2:571–631. [Google Scholar]
- Roubik D.W. Cambridge University Press; Cambridge, UK: 1989. Ecology and natural history of tropical bees. [DOI] [PubMed] [Google Scholar]
- Roulston T.H., Cane J.H. The effect of diet breadth and nesting ecology on body size variation in bees (Apiformes) J. Kans. Entomol. Soc. 2000;73:129–142. [Google Scholar]
- Schmid-Hempel R., Schmid-Hempel P. Female mating frequencies in Bombus spp. from central Europe. Insect. Soc. 2000;47:36–41. doi:10.1007/s000400050006 [Google Scholar]
- Seeley T.D. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav. Ecol. Sociobiol. 1982;11:287–293. doi:10.1007/BF00299306 [Google Scholar]
- Sendova-Franks A.B., Franks N.R. Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Anim. Behav. 1995;50:121–136. doi:10.1006/anbe.1995.0226 [Google Scholar]
- Sendova-Franks A.B., Scholes S.R., Franks N.R., Melhuish C. Brood sorting by ants: two phases and differential diffusion. Anim. Behav. 2004;68:1095–1106. doi:10.1016/j.anbehav.2004.02.013 [Google Scholar]
- Sladen F.W.L. Macmillan and Co; London, UK: 1912. The bumble-bee. [Google Scholar]
- Smeets P., Duchateau M.J. Feeding behaviour in the bumblebee Bombus terrestris. Belg. J. Zool. 2001;131:11–18. [Google Scholar]
- Spaethe J., Weidenmuller A. Size variation and foraging rate in bumblebees (Bombus terrestris) Insectes Soc. 2002;49:142–146. doi:10.1007/s00040-002-8293-z [Google Scholar]
- Spaethe J., Brockmann A., Halbig C., Tautz J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften. 2007;94:733–739. doi: 10.1007/s00114-007-0251-1. doi:10.1007/s00114-007-0251-1 [DOI] [PubMed] [Google Scholar]
- Stephen W.P., Koontz T. The larvae of the Bombini. I. Interspecific variation in larval head characteristics. Melanderia. 1973a;13:1–12. [Google Scholar]
- Stephen W.P., Koontz T. The larvae of the Bombini. II. Developmental changes in the preadult stages of Bombus griseocollis (Degeer) Melanderia. 1973b;13:14–29. [Google Scholar]
- Stern D. Body-size evolution: how to evolve a mammoth moth. Curr. Biol. 2001;11:R917–R919. doi: 10.1016/s0960-9822(01)00554-1. doi:10.1016/S0960-9822(01)00554-1 [DOI] [PubMed] [Google Scholar]
- Stern D.L., Foster W.A. The evolution of sociality in aphids: a clone's-eye view. In: Choe J.C., Crespi B.J., editors. Social behavior in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 150–165. [Google Scholar]
- Sutcliffe G.H., Plowright R.C. The effects of food-supply on adult size in the bumble bee Bombus terricola Kirby (Hymenoptera, Apidae) Can. Entomol. 1988;120:1051–1058. [Google Scholar]
- Sutcliffe G.H., Plowright R.C. The effects of pollen availability on development time in the bumble bee Bombus terricola K (Hymenoptera, Apidae) Can. J. Zool. Rev. Can. Zool. 1990;68:1120–1123. doi:10.1139/z90-166 [Google Scholar]
- Waddington K.D., Herbst L.H., Roubik D.W. Relationship between recruitment systems of stingless bees and within-nest worker size variation. J. Kans. Entomol. Soc. 1986;59:95–102. [Google Scholar]
- West-Eberhard M.J. The social biology of polistine wasps. Misc. Publ. Mus. Zool. Univ. Mich. 1969;140:1–101. [Google Scholar]
- Wetterer J.K. Ecology and evolution of worker size-distribution in leaf-cutting ants (Atta spp. and Acromyrmex spp.) Sociobiology. 1999;34:119–144. [Google Scholar]
- Wheeler D.E. The developmental basis of worker caste polymorphism in ants. Am. Nat. 1991;138:1218–1238. doi:10.1086/285279 [Google Scholar]
- Wheeler W.M. Kegan Paul, Trench, Trubner and Co; London, UK: 1928. The social insects: their origin and evolution. [Google Scholar]
- Wilson E.O. The origin and evolution of polymorphism in ants. Q. Rev. Biol. 1953;28:136–156. doi: 10.1086/399512. doi:10.1086/399512 [DOI] [PubMed] [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]
- Wilson E.O. Behavioral discretization and the number of casts in an ant species. Behav. Ecol. Sociobiol. 1976;1:141–154. doi:10.1007/BF00299195 [Google Scholar]
- Wilson E.O. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). I. The overall pattern in A. sexdens. Behav. Ecol. Sociobiol. 1980;7:143–156. doi:10.1007/BF00299520 [Google Scholar]
- Wilson E.O. The sociogenesis of insect colonies. Science. 1985;228:1489–1495. doi: 10.1126/science.228.4707.1489. doi:10.1126/science.228.4707.1489 [DOI] [PubMed] [Google Scholar]
- Wilson E.O. Ecology Institute; Oldendorf/Luke, Germany: 1990. Success and dominance in ecosystems: the case of the social insects. [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge, MA: 2003. Pheidole in the new world. [Google Scholar]
- Yerushalmi S., Bodenhaimer S., Bloch G. Developmentally determined attenuation in circadian rhythms links chronobiology to social organization in bees. J. Exp. Biol. 2006;209:1044–1051. doi: 10.1242/jeb.02125. doi:10.1242/jeb.02125 [DOI] [PubMed] [Google Scholar]
- Zar J.H. Prentice Hall; Englewood Cliffs, NJ: 1984. Biostatistical analysis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of feedings per larva is significantly different between different zones both before and after the appearance of reproductives. Data pooled for colonies