Abstract
Interactions among the component members of different symbioses are not well studied. For example, leaf-cutting ants maintain an obligate symbiosis with their fungal garden, while the leaf material they provide to their garden is usually filled with endophytic fungi. The ants and their cultivar may interact with hundreds of endophytic fungal species, yet little is known about these interactions. Experimental manipulations showed that (i) ants spend more time cutting leaves from a tropical vine, Merremia umbellata, with high versus low endophyte densities, (ii) ants reduce the amount of endophytic fungi in leaves before planting them in their gardens, (iii) the ants' fungal cultivar inhibits the growth of most endophytes tested. Moreover, the inhibition by the ants' cultivar was relatively greater for more rapidly growing endophyte strains that could potentially out-compete or overtake the garden. Our results suggest that endophytes are not welcome in the garden, and that the ants and their cultivar combine ant hygiene behaviour with fungal inhibition to reduce endophyte activity in the nest.
Keywords: Atta colombica, endophyte, Glomerella cingulata, inhibition, microbial interactions, microfungi
1. Introduction
Plants and insects host a wide diversity of symbiotic fungi. It is increasingly recognized that such symbioses affect host success and can ultimately regulate host populations, and thus community composition and diversity (Hajek & St Leger 1994; Gilbert & Strong 2007). For example, mycorrhizae associate with plant roots and enhance plant growth, which may indirectly modify the plant's success via interactions with competitors, foliar herbivores and pathogens (Bennett et al. 2006; Herre et al. 2007). While the reciprocal benefits of many host–fungal mutualisms have been studied, less understood are the interactions among the component members of different symbioses. Here, we address how leaf-cutting ants and their symbiotic fungal cultivar interact with foliar endophytic fungi, cryptic micro-organisms that form symbioses with plant hosts.
Foliar endophytic fungi (hereafter ‘endophytes’) live most of their life cycle within plant leaves and other above-ground plant tissues without causing any apparent signs of disease (Wilson 1995a). Endophyte-derived anti-herbivore defence has been described in some temperate grasses (Clay 1990) and trees (Wilson & Carroll 1994, 1997; Wilson 1995b; Preszler et al. 1996; Wilson & Faeth 2001). By contrast, tropical host–endophyte–herbivore interactions are only beginning to be studied (Van Bael et al. 2009). Endophytes can be extremely diverse in the leaves of tropical plants (Arnold et al. 2000), with endophyte communities that conservatively range from 10 to 20 species per host plant and generally exhibit low similarity among hosts (Van Bael et al. 2005; Arnold & Lutzoni 2007). Given this spectacular diversity, generalist herbivores, such as leaf-cutting ants, potentially interact with hundreds of foliar endophyte species.
Leaf-cutting ants (genera Atta and Acromyrmex, Myrmicinae) maintain an obligate symbiosis with their fungal cultivar (Leucocoprinus gongylophorus, Lepiotaceae, Basidiomycota; Weber 1972). The ants defoliate a wide diversity of plants in neotropical forests and often have an enormous effect on local flora and distribution of nutrients (Howard 1988; Farji-Brener & Silva 1996; Wirth et al. 2003; Sternberg et al. 2007). The worker ants cut leaves, carry them to the nest, clean them and use them to provision their fungal cultivar. In turn, the fungal cultivar partially degrades the leaf material and uses it to provide food for the ants. This ancient mutualism depends on the ants' hygienic behaviours: the fungal cultivar does not persist without its ant caretakers actively cleaning it (Currie & Stuart 2001; Mueller et al. 2005).
While the ants' cultivar symbiosis is well studied, the potential interactions among the ants' cultivar and endophytes are essentially unknown. In particular, few studies have been conducted in tropical areas where endophyte diversity is high and where most leaf-cutting ant species reside. Only one temperate study has involved experimental manipulations of endophyte densities: the study asked whether endophytes constitute a direct anti-herbivore defence to temperate grasses by reducing the overall likelihood of attack by Acromyrmex versicolor (Tibbets & Faeth 1999). While they did not find that foraging ants displayed a preference for leaves with or without endophytes, they did observe evidence of endophyte toxicity towards queens for some host plant–endophyte combinations (Tibbets & Faeth 1999). Importantly, Tibbets & Faeth (1999) explored interactions using Neotyphodium, a temperate grass endophyte species that is vertically transmitted (from mother to seed). By contrast, the predominant form of endophyte transmission in woody tropical plants is horizontal (from spore fall in the environment; Herre et al. 2007). Although selection for defensive mutualisms between endophytes and their plant hosts is likely to be more effective for vertically transmitted fungi (Herre et al. 1999; Faeth 2002), some examples of defensive mutualisms are known from horizontally transmitted fungi as well (Redman et al. 2001; Arnold et al. 2003; Mejia et al. 2008).
There has been no experimental work with horizontally transmitted endophytes and leaf-cutting ants. Previous descriptive work suggests that some fungal endophytes can enter and persist in the ants' gardens. Fisher et al. (1996) isolated apparent endophytes from the gardens of Atta cephalotes laboratory colonies and demonstrated that the endophyte composition changed when ants were offered a new food source. Rodrigues et al. (2008) also isolated species of known fungal endophytes from naturally occurring Acromyrmex sp. colonies in Brazil. Despite these observations, little is known about how ants and their fungal cultivar interact with endophytic fungi. Endophytes may act as pathogens to the ants or the garden, or may be beneficial to either or both. Control over which endophytes can remain in the garden may be a result of ant hygiene behaviour, the cultivar's behaviour or both. Alternatively, endophytes could be transient guests with neutral consequences for the ants and the cultivar (Poulsen & Currie 2006).
Here we experimentally manipulated the endophyte densities in leaves of a tropical vine, Merremia umbellata (Convolvulaceae), and used laboratory colonies of Atta colombica to explore the interactions among horizontally transmitted endophytes, leaf-cutting ants and their fungal cultivar. We asked (i) whether leaf-cutting ants preferentially choose to cut leaves with high or low endophyte densities, (ii) whether endophyte density affects handling time of leaves by the ants, (iii) whether leaf-cutting ants remove or reduce endophytic fungi in the process of preparing leaf material for the garden and (iv) whether the fungal cultivar inhibits growth of endophytic fungi. Our results suggest that horizontally transmitted endophytes are not welcome in the garden, and that the ants and their cultivar employ a sequential defensive strategy, combining ant hygiene behaviour with fungal inhibition to reduce microbial activity in the nest.
2. Material and methods
(a) Study species and natural history
This study was carried out at the Gamboa research station of the Smithsonian Tropical Research Institute (9°07′ N, 79°42′ W), Republic of Panama, where A. colombica is abundant. Colonies contain a single queen (mating with multiple males), as many as 2.5 million workers and up to 300 nest chambers. Foraging workers cut leaves from more than 100 species of trees, shrubs and vines, and bring them into their nests (Wirth et al. 2003), where medium- and small-sized workers start the substrate preparation. Within garden chambers, ants lick the surface of leaves to remove wax and surface micro-organisms (Quinlan & Cherrett 1977), and apply antimicrobial secretions from the exocrine metapleural glands (Fernández-Marín et al. 2003, 2006). Then they cut the leaves into little pieces (approx. 2 mm), chew them and add faecal drops to them before planting the leaf pieces in the garden (Quinlan & Cherrett 1977; Poulsen & Boomsma 2005). As a final step, ants then cut small pieces of mycelia from elsewhere in the garden and plant them over the fresh substrate. Within a few hours, the planted substrate is nearly covered by fungal growth, which is tended by ants that use a variety of behavioural and biochemical tactics to minimize growth of microbial contaminants (see Currie et al. 1999; Fernández-Marín et al. 2006). Merremia umbellata is a widespread Neotropical vine (Croat 1978) that grows in open areas, along the edges of forests, gaps and estuaries. It is commonly exploited by leaf-cutting ants in Gamboa (S. A. Van Bael 2004, personal observation). In Gamboa, foliar endophyte communities of M. umbellata are dominated by species of the genera Xylaria, Glomerella/Colletotrichum and Diaporthe, all of which appear to be horizontally transmitted from spore fall in the environment (S. A. Van Bael 2004, unpublished data). We screened the most common endophyte morphospecies of M. umbellata for their ability to sporulate in laboratory conditions, in order to select a species for leaf inoculation experiments and for manipulation of endophyte densities within plants. A strain of Glomerella cingulata (anamorph is Colletotrichum gloeosporioides) was used for leaf inoculation experiments. This fungus was present in approximately 50 per cent of 42 healthy leaves sampled. Thirty-two additional endophyte strains from five genera were used to test in vitro interactions against the garden cultivar. These additional strains were isolated from nine plant species and were collected within 20 km of Gamboa. Sequencing of the internal transcribed spacers and 5.8 s gene and translation elongation factor 1 α (tef1) were used to confirm the taxonomic affinity of the strains (see table S1 in electronic supplementary material 3).
(b) Experimental plants
Merremia umbellata plants were maintained in the greenhouse under a tent of clear plastic to prevent rain water from touching the leaves. Plants were watered at the soil level to minimize contact of water with leaf surfaces; foliar endophyte infections are reduced when water does not touch leaf material (Arnold et al. 2003). Half of the plants were designated as low-density (Elow) and the other half as high-density (Ehigh) endophyte plants. To vary the densities of endophytes in the plants, we created an inocula spray consisting of G. cingulata conidia (106–107 conidia ml−1) suspended in water and Tween 20 (a detergent that aids in dispersion and adhesion of conidia) for the Ehigh treatment. A similar spray that lacked conidia was used for the Elow treatment (appendix S1 in electronic supplementary material 1). During August–December 2006, we sprayed plants weekly with either the G. cingulata conidia (Ehigh) or the conidia-free control spray (Elow). Inoculations were followed by reisolations on eight different dates to confirm the efficacy of treatments (appendix S1 in electronic supplementary material 1). The mean (±s.e.) percentage of infection was 61±4 per cent in Ehigh leaves and 20±2 per cent in Elow leaves (t=8.0, d.f.=94, p<0.001).
(c) Choice trials
Do leaf-cutting ants preferentially choose to cut leaves with high or low densities of endophytes? Before choice trials, we first established that A. colombica accepted Ehigh or Elow M. umbellata leaves over a three-month feeding period without signs of damage to the colony or of delayed rejection (Herz et al. 2008) by the ants (S. A. Van Bael & H. Fernàndez 2006, unpublished data). To assess whether ants preferentially chose leaves with high or low endophyte densities, we provided 28 naive laboratory colonies (approx. 1 year old) with a single Ehigh and Elow M. umbellata leaf of approximately equal area and recorded behaviour hourly for 36 hours. All leaves were mature (at least 8 days old). The leaves were placed together side by side on an open sterile Petri dish (hereafter, the choice arena). We recorded the time until first contact with leaves, time until first cutting and total cutting time. The endpoint for total cutting time was when all pieces of the leaf had been cut and carried away from the choice arena. All times were converted to hours and then log transformed for statistical testing. We used a two-tailed t-test to test for differences in the time variables (using Systat v. 11, Chicago, IL). We report non-transformed values in all tables and figures.
(d) Ant substrate preparation trials
Do leaf-cutting ants remove or reduce endophytic fungi in the process of preparing leaf material for the garden? To address this question, we provided 16 naive colonies with a single Ehigh leaf and waited until we observed ants planting leaf pieces in the garden. Using sterile forceps, we removed leaf pieces from the garden and plated 20 ant-prepared leaf pieces per colony on a single 2 per cent malt extract agar (MEA) plate. We plated the ant-prepared pieces within 0–3 hours of planting by the ants. At the same time, we harvested a 2×1 cm piece of leaf from a part of the original leaf that was yet uncut by the ants. Using a sterile blade, we cut this leaf piece into 1×1 mm sections to mimic the average size of the pieces that ants planted in their garden. This resulted in approximately 200 leaf pieces. We used a haphazard selection of these leaf pieces for two purposes: (i) to plate and observe what grew out of non-ant-prepared pieces and compare with plates that had ant-prepared pieces, and (ii) to confirm that the fungus we applied was growing endophytically and not just from residue conidia on the surface of leaves. For purpose (i), we selected 20 pieces per colony and plated them on 2 per cent MEA without surface sterilization. For purpose (ii), we selected 40 more leaf pieces per colony to demonstrate that the applied fungi was both inside and outside of the leaf tissue. To test for viable fungal conidia on the surface of leaf pieces, we pressed 20 leaf pieces per colony onto a 2 per cent MEA plate and then removed the leaf pieces after approximately 10 min. To confirm that the fungus was indeed growing endophytically, we surface sterilized 20 leaf pieces per colony using sequential immersion in 70 per cent ethanol and 10 per cent commercial bleach. We then plated them on 2 per cent MEA and did not remove the leaf pieces (appendix S2 in electronic supplementary material 2). All the plates were incubated for 8 days and assessed for fungal growth on days 4 and 8. We calculated the proportion of leaf pieces from which G. cingulata grew for each plate as our response variable. Our focal comparison for statistical analysis was between paired plates that had ant-prepared and non-ant-prepared leaf pieces (paired by colony). The proportion values could not be normalized with transformations, so we used a Wilcoxon signed-rank test (using Systat v. 11).
(e) Garden fungi and endophytic fungi interaction trials
The purpose of interaction trials between garden fungi and endophytic fungi was to rapidly assess whether the fungal cultivar inhibited growth of a wide range of endophytic fungi in vitro. We were unable to perform the reverse experiment, to see whether endophytic fungi affect the ants' cultivar growth, because of the extremely slow relative growth rate of the ants' cultivar in vitro.
To address whether the cultivar inhibits growth of endophytic fungi in vitro, we conducted three experiments that compared the growth of endophytic fungi in the presence and absence of the garden cultivar. All in vitro interactions were assessed on plates with potato dextrose agar (PDA). In the first experiment, we tested for the effect of the ants' cultivar from 15 unique A. colombica colonies on the growth of our focal endophyte strain, G. cingulata. We did this by comparing the growth of G. cingulata on PDA plates with 4 mm diameter plugs from a pure culture of the ants' fungal cultivar versus plates with empty plugs of PDA. Fungal cultivar plugs from 15 different A. colombica colonies were placed in the centre of PDA plates with a point inoculation of G. cingulata 1 cm away. For a control, empty plugs of PDA (without the fungal cultivar) were placed in the centre of PDA plates with a point inoculation of G. cingulata 1 cm away. We also simultaneously grew plates with a point inoculation only of G. cingulata. All plates were duplicated for a second replicate of each interaction.
In a second experiment, we grew 32 endophyte strains (comprising seven species from five genera; table S1 in electronic supplementary material 3) in the presence and absence of the ants' fungal cultivar. Interactions were set up as described above, with each endophyte strain growing with a plug of the ants' cultivar, an empty plug or alone. A third experiment was designed to confirm the results of the second experiment. The third experiment reduced the contact between mycelium of the endophytes and the ants' cultivar in in vitro trials (detailed in appendix S2 in electronic supplementary material 2).
All plates were kept at room temperature, with approximately 12 hours light and dark each, and were photographed after 48 and 72 hours. One endophyte species that grew particularly slowly was photographed again after 192 hours. The diameter of fungal colonies was measured from the photos using the software ImageJ (http://rsb.info.nih.gov/ij). The diameters from replicate treatments were averaged. We compared the endophyte colony diameter on plates with cultivar plugs and empty plugs using a paired, two-tailed t-test. For the second experiment with multiple endophyte species, we calculated the percentage decrease x as x=((a−b)/b)×100, where x was the percentage decrease in endophyte colony diameter in the presence of the garden cultivar, a was the endophyte colony diameter growing with a plug of the ants' cultivar and b was the endophyte colony diameter growing with a plug that did not contain the ants' cultivar. We calculated the growth rate of individual endophyte strains by dividing the colony diameter (in cm) when the endophyte grew in a plate alone by time (in days). We used simple linear regression to compare the percentage decrease with each strain's growth rate.
3. Results
(a) Feeding trials
In ‘choice’ trials, A. colombica workers did not show a preference towards leaves with high (Ehigh) or low (Elow) densities of G. cingulata; they cut both leaves simultaneously (table 1). They spent significantly more time, however, in the process of cutting the Ehigh leaves and removing them from the choice arena (table 1).
Table 1.
Results from choice trials where 28 naive A. colombica colonies chose between one leaf each of high (Ehigh) and low (Elow) endophytic fungi densities. (Italic value represents a statistically significant difference at a value of p<0.05.)
| variable | Ehigh mean±s.e. | Elow mean±s.e. | t | d.f. | p-value |
|---|---|---|---|---|---|
| time until first contact (hours) | 1.1±0.4 | 1.1±0.3 | −1.1 | 17 | 0.303 |
| time until first cutting (hours) | 3.0±0.7 | 5.1±1.2 | −1.6 | 27 | 0.118 |
| total cutting time (hours) | 4.2±0.6 | 2.9±0.5 | 3.7 | 27 | 0.001 |
(b) Ant substrate preparation trials
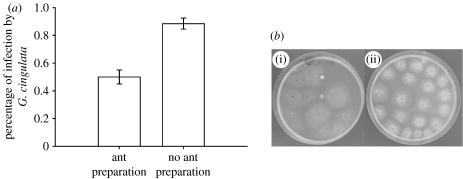
The Ehigh leaves that were presented to A. colombica workers had viable G. cingulata conidia on the outer surface of leaf pieces (mean±s.e.=41±8% of leaf pieces that were pressed onto plates), living endophytically within leaf tissues (70±6% of leaf pieces that were surface-sterilized). In the process of preparing Ehigh leaves for planting in their gardens, A. colombica workers reduced the mean incidence of G. cingulata by 44 per cent (ant-prepared leaf pieces versus unsterilized, non-ant-prepared leaf pieces; figure 1). This reduction included both G. cingulata conidia that were on the surface of leaves and the G. cingulata that had penetrated leaf tissues and was growing within leaf tissues. Thus, while ant preparation did not completely remove all G. cingulata, the infection rate of endophytes was significantly lower in ant-prepared leaf pieces (Wilcoxon signed-rank test, Z=3.6, p<0.001, n=18 colonies).
Figure 1.
Ants reduce G. cingulata associated with leaves before planting the leaves in their fungal gardens. (a) Mean (±s.e.) proportion of leaf pieces that were colonized by G. cingulata in treatments where leaf pieces were prepared by ants or not prepared by them (without surface sterilization). (b) A photo of plates from assays of (i) prepared and (ii) non-prepared leaf pieces. The large, white halos around leaf pieces are G. cingulata. In (i), two leaf pieces with small white growth show pieces that were colonized by the ants' slower-growing cultivar L. gongylophorus.
(c) Garden fungi and endophyte interaction trials
In all of the in vitro interactions between G. cingulata and the ants' cultivar, G. cingulata grew slower in the presence of cultivar (colony diameter mean±s.e.=1.86±0.03 cm) than without it (2.30±0.03 cm, paired t=10.0, d.f.=15, p<0.001, after 48 hours). The pattern of inhibition was consistent among trials, with a dark brown colour developing in the media between the cultivar and the endophyte. This discoloration did not appear in plates where the cultivar or endophytes grew alone. The endophyte eventually grew around but not over the cultivar.
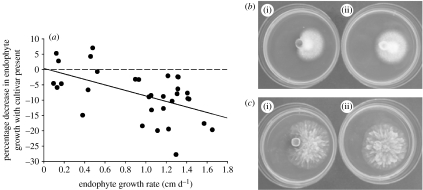
The ants' cultivar reduced the growth rate of most endophytic fungi tested (28 out of 32 interactions, paired t=−5.5, d.f.=31, p<0.001). The intensity of the inhibition, however, was relatively and absolutely greater for more rapidly growing endophyte strains (figure 2; table 2). Thus, inhibition by the ants' cultivar appears to be more effective against rapidly growing endophytes that could potentially out-compete or overtake the garden. The in vitro inhibition results were confirmed in a supporting experiment (appendix S2 in electronic supplementary material 2).
Figure 2.
Most endophyte species show reduced growth in the presence of the fungal cultivar, with faster-growing endophytic species showing a greater reduction than slower-growing endophytes. (a) Percentage decrease in colony diameter from 32 endophyte strains of 7 species correlates to endophyte growth rate; r2=0.29, p=0.002. (b) Comparison of Colletotrichum sp.1 in the (i) presence and (ii) absence of the fungal cultivar after 48 hours growth. (c) Comparison of Endomelanconiopsis endophytica (Rojas et al. 2008) in the (i) presence and (ii) absence of the fungal cultivar after 72 hours growth.
Table 2.
Mean±s.e. growth rate of endophyte colony (diameter in cm d−1) in the presence and absence of the ants' fungal cultivar.
| endophyte taxa | growth rate of endophyte with garden plug (cm d−1) | growth rate of endophyte with empty plug (cm d−1) | % difference mean ± s.e. | n |
|---|---|---|---|---|
| Sordariomycete sp. | 0.13±0.01 | 0.13±0.01 | −1±3 | 5 |
| Xylaria sp. | 0.47±0.02 | 0.47±0.02 | 0±2 | 5 |
| Diaporthe sp. | 1.17±0.05 | 1.37±0.08 | −14±2 | 5 |
| Endomelanconiopsis endophytica | 1.12±0.04 | 1.22±0.04 | −8±2 | 5 |
| Colletotrichum sp.1 | 0.92±0.02 | 1.07±0.04 | −14±2 | 5 |
| Colletotrichum sp.2 | 1.09±0.07 | 1.19±0.09 | −8±4 | 4 |
| Colletotrichum sp.3 | 0.85±0.04 | 0.90±0.02 | −6±2 | 3 |
4. Discussion
Here, we have shown that leaf-cutting ants take more time cutting M. umbellata leaves with high levels of endophytes, and that their leaf preparation process reduces the incidence of endophyte infection before leaves are planted in the garden. Moreover, the fungal cultivar appears to inhibit growth of endophytic fungi in pure culture, particularly endophytes that show rapid growth in vitro. These findings show that a combination of ant behaviours and the fungal cultivar's inhibition reduces densities of endophytes that enter and persist in the nest.
(a) Endophytes and ants
We did not observe any worker preference for leaves with high or low endophyte densities. While this could be interpreted as a lack of conflict between ants and endophytes, past observations have shown that (i) foraging ants cut many things that never become incorporated into the nest and (ii) delayed rejection of leaf material can occur, via chemical signalling between ants and their cultivar (North et al. 1997; Herz et al. 2008). While workers appeared indiscriminate in whether they harvested endophyte-rich or endophyte-poor food first, the ants spent more time cutting and removing leaves with high endophyte densities from the choice arena. We do not know why, but we suggest that increased cutting time could reflect increased leaf toughness. Leaves from one tropical tree species, Theobroma cacao, have more lignin and cellulose in leaves with high endophyte densities (S. Maximova & E. A. Herre 2005, unpublished data). Whether M. umbellata leaves with high endophyte densities also show increased lignin and cellulose is unknown. Increased leaf toughness slows herbivore feeding by caterpillars (Choong 1996) and causes recruitment by larger workers in A. colombica foraging trails (Clark 2006). Further experiments are necessary to see whether the extra time spent cutting by A. colombica feeding on endophyte-rich leaves translates into a cost for ants and a net benefit for host plants. Moreover, further experiments are needed to measure the total preparation time of leaf material (after cutting) in the presence and absence of endophytes.
We observed that ants reduced G. cingulata from the surface and the interior of the leaf material before planting it in the garden. The observation that endophytes are reduced, but not removed completely, by the ants is consistent with earlier reports of apparent endophyte presence in leaf-cutting ant gardens (Fisher et al. 1996; Rodrigues et al. 2008). Quinlan & Cherrett (1977) similarly showed that ant behaviour reduced but did not completely remove surface fungi from leaves. Surface fungi are likely to be spores from random environmental spore fall, whereas interior endophytes generally are extending their mycelium within leaf material, and are in some cases forming defensive mutualisms with their hosts (Herre et al. 2007). Given that there is some signal of host specificity for at least some endophytes and their hosts (Arnold et al. 2000), the endophytes in material cut by ants may be predictable. Thus, we suggest that the mechanisms that ants and their cultivar have developed to defend against endophytes are likely to be under greater selection than the mechanisms against the random assemblage of fungi from environmental spore fall on leaf surfaces. Our current set of experiments, however, does not rule out that one type of anti-fungal mechanism exists for surface and interior fungi.
(b) Endophytes and the ants' cultivar
Keeping the ants' garden micro-organism-free has been thought to be primarily the responsibility of the ants, but our work joins other recent studies in suggesting that the fungal cultivar also takes an active role in shaping the community of microbes in the nest. For example, Bot et al. (2001) and Poulsen & Boomsma (2005) showed that the ants' fungal cultivar actively rejected mycelial fragments from gardens of neighbouring colonies, with greater rejection between more distantly related cultivars. Here, we have further extended the idea that the ants' cultivar is not merely a passive domesticated crop, but an active player in the mutualism. Moreover, the ants' fungal cultivar appears to play a role in reducing the growth rate of a diverse range of endophytic fungi. Because the growth rate reduction was greater for more rapidly growing endophyte strains, the inhibition by the ants' cultivar appears more effective against endophytes that could out-compete or overtake the garden via rapid growth.
The inhibition from the cultivar towards endophytes that we observed in vitro can be described in terms of combative interactions, as described in wood-decaying basidiomycetes (Boddy 2000). While evidence of mycoparasitism was not observed, antagonism via diffusible antibiotics was probable due to the discoloration of the media that were present in the interaction site between the fungal cultivar and the endophytes (figure 2), and due to reduced growth of endophytes even without hyphal contact (appendix S2 in electronic supplementary material 2). Discoloration was not observed when the fungal cultivar or endophyte grew alone in culture. While previous authors have isolated antibiotic compounds from ants' cultivar species related to L. gongylophorus (Wang et al. 1999), the role of such antibiotics has not been explored (Mueller 2002). Our current set of experiments did not determine whether the ants' cultivar, the endophyte or both fungi were actively involved in the antagonism.
Usually, the reciprocal benefits of individual symbiotic relationships are studied in isolation from their positive and negative interactions with other organisms. Individual symbioses are compelling because (i) the organisms are involved in ecological interactions that are under strong selection and they evolve rapidly, and (ii) the direction of interactions can change and/or ‘cheating’ can occur (Thompson 1994). When interactions between component members of different symbioses occur, the result may be further shaped by competition or facilitation. The outcome of such ecological interactions between pairs of symbioses is likely to be influenced by the degree to which interests between each host and symbiont are aligned, which itself is shaped by mode of transmission (Herre et al. 1999). Evolutionary theory suggests that the interests of each symbiotic partner are more aligned when transmission is vertical rather than horizontal (Herre et al. 1999). The interaction of leaf-cutting ants and plant endophytes offers a model system to explore how transmission mode affects the outcome of interacting symbioses: nearly all transmissions of fungal symbionts in the leaf-cutting ants' cultivar system are vertical, while endophytes can be either vertically or horizontally transmitted to their host plants. Comparisons of how ants and their cultivar react to vertically versus horizontally transmitted endophytic fungi may highlight the importance of fungal symbiont transmission mode for effective defence against other fungi, and may provide more general predictions for how we can expect interactions among multiple symbioses to unfold.
In conclusion, we found that foliar endophytic fungi, which are neutral or beneficial to the host plant, are actively suppressed by the ants and their garden. This work opens many lines of future research with respect to the interacting symbioses and broader community-wide implications. First, further experiments are necessary to see longer-term effects of an endophyte-rich diet on the productivity of the ants'cultivar, and to check whether extra time spent cutting endophyte-rich leaves translates into a cost for the ants' cultivar and a net benefit for host plants. Alternatively, endophytes may present no net cost to ants and their cultivar yet may still play important roles in the leaf-cutting ant symbiosis. Next, we have focused on only a small group of endophytic fungi and host plants. Given the wide diversity of endophytes and plants cut by ants, further work in field and laboratory settings is necessary to establish the generality of the patterns observed here. Moreover, it is likely that different endophyte species will interact in different ways with ants and their cultivar. If endophytes prove costly to leaf-cutting ants and their cultivar, this may shape the usage of particular plants by ants as substrate or by endophytes as hosts. Broader implications could include the success or failure of certain host plants in areas with leaf-cutting ants, which in turn may shape plant community composition and diversity.
Acknowledgments
We thank G. Gilbert, P. Hebbar, M. Seid, L. Ramírez, Z. Maynard and L. Mejía for their advice, comments and help with endophyte collections; for help in the greenhouse and field, we thank E. Gomez, A. Portugal, B. Perez and L. Sosa. This research was funded by an NSF IRFP award (no. OISE-0401957) to S.A.V., an American Association of University Women post-doctoral fellowship to S.A.V., Smithsonian post-doctoral fellowships to S.A.V. and H.F.M. and a grant from the World Cacao Foundation to E.A.H. We thank the ANAM of Panama for permission to do research.
Supplementary Material
Details of conidia preparation, application, and reisolations
Supporting experiment for interactions among endophytes and the ants' cultivar
Description of endophytic fungal strains used for in vitro trials
References
- Arnold A.E., Lutzoni F. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology. 2007;88:541–549. doi: 10.1890/05-1459. doi:10.1890/05-1459 [DOI] [PubMed] [Google Scholar]
- Arnold A.E., Maynard Z., Gilbert G.S., Coley P.D., Kursar T.A. Are tropical fungal endophytes hyperdiverse? Ecol. Lett. 2000;3:267–274. doi:10.1046/j.1461-0248.2000.00159.x [Google Scholar]
- Arnold A.E., Mejia L.C., Kyllo D., Rojas E., Maynard Z., Robbins N., Herre E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl Acad. Sci. USA. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. doi:10.1073/pnas.2533483100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A.E., Alers-Garcia J., Bever J.D. Three-way interactions among mutualistic mycorrhizal fungi, plants, and plant enemies: hypotheses and synthesis. Am. Nat. 2006;167:141–152. doi: 10.1086/499379. doi:10.1086/499379 [DOI] [PubMed] [Google Scholar]
- Boddy L. Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol. Ecol. 2000;31:185–194. doi: 10.1111/j.1574-6941.2000.tb00683.x. doi:10.1111/j.1574-6941.2000.tb00683.x [DOI] [PubMed] [Google Scholar]
- Bot A.N.M., Rehner S.A., Boomsma J.J. Partial incompatibility between ants and symbiotic fungi in two sympatric species of Acromyrmex leaf-cutting ants. Evolution. 2001;55:1980–1991. doi: 10.1111/j.0014-3820.2001.tb01315.x. doi:10.1111/j.0014-3820.2001.tb01315.x [DOI] [PubMed] [Google Scholar]
- Choong M.F. What makes a leaf tough and how this affects the pattern of Castanopsis fissa leaf consumption by caterpillars. Funct. Ecol. 1996;10:668–674. doi:10.2307/2390178 [Google Scholar]
- Clark E. Dynamic matching of forager size to resources in the continuously polymorphic leaf-cutter ant, Atta colombica (Hymenoptera, Formicidae) Ecol. Entomol. 2006;31:629–635. doi:10.1111/j.1365-2311.2006.00826.x [Google Scholar]
- Clay K. Fungal endophytes of grasses. Annu. Rev. Ecol. Syst. 1990;21:275–297. doi:10.1146/annurev.es.21.110190.001423 [Google Scholar]
- Croat T.B. Stanford University Press; Stanford, CA: 1978. Flora of Barro Colorado Island. [Google Scholar]
- Currie C.R., Stuart A.E. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. Lond. B. 2001;268:1033–1039. doi: 10.1098/rspb.2001.1605. doi:10.1098/rspb.2001.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C.R., Scott J.A., Summerbell R.C., Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999;398:701–704. doi:10.1038/19519 [Google Scholar]
- Faeth S.H. Are endophytic fungi defensive plant mutualists? Oikos. 2002;98:25–36. doi:10.1034/j.1600-0706.2002.980103.x [Google Scholar]
- Farji-Brener A.G., Silva J.F. Leaf-cutter ants' (Atta laevigata) aid to the establishment success of Tapirira velutinifolia (Anacardiaceae) seedlings in a parkland savanna. J. Trop. Ecol. 1996;12:163–168. [Google Scholar]
- Fernández-Marín H., Zimmermann J.K., Wcislo W.T. Nest-founding in Acromyrmex octospinosus (Hymenoptera, Formicidae, Attini): demography and putative prophylactic behaviors. Insect. Soc. 2003;50:304–308. doi:10.1007/s00040-003-0687-z [Google Scholar]
- Fernández-Marín H., Zimmerman J.K., Rehner S.A., Wcislo W.T. Active use of the metapleural glands by ants in controlling fungal infection. Proc. R. Soc. B. 2006;273:1689–1695. doi: 10.1098/rspb.2006.3492. doi:10.1098/rspb.2006.3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.J., Stradling D.J., Sutton B.C., Petrini L.E. Microfungi in the fungus gardens of the leaf-cutting ant Atta cephalotes: a preliminary study. Mycol. Res. 1996;100:541–546. doi:10.1016/S0953-7562(96)80006-2 [Google Scholar]
- Gilbert G.S., Strong D.R. Fungal symbionts of tropical trees. Ecology. 2007;88:539–540. doi:10.1890/06-1671 [Google Scholar]
- Hajek A.E., St Leger R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994;39:293–322. doi:10.1146/annurev.en.39.010194.001453 [Google Scholar]
- Herre E.A., Knowlton N., Mueller U.G., Rehner S.A. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol. Evol. 1999;14:49–53. doi: 10.1016/s0169-5347(98)01529-8. doi:10.1016/S0169-5347(98)01529-8 [DOI] [PubMed] [Google Scholar]
- Herre E.A., Mejia L.C., Kyllo D.A., Rojas E., Maynard Z., Butler A., Van Bael S.A. Ecological implications of anti-pathogen effects of tropical fungal endophytes and mycorrhizae. Ecology. 2007;88:550–558. doi: 10.1890/05-1606. doi:10.1890/05-1606 [DOI] [PubMed] [Google Scholar]
- Herz H., Hölldobler B., Roces F. Delayed rejection in a leaf-cutting ant after foraging on plants unsuitable for the symbiotic fungus. Behav. Ecol. 2008;19:575–582. doi:10.1093/beheco/arn016 [Google Scholar]
- Howard J.J. Leafcutting ant diet selection—relative influence of leaf chemistry and physical features. Ecology. 1988;69:250–260. doi:10.2307/1943180 [Google Scholar]
- Mejia L.C., Rojas E., Maynard Z., Arnold A.E., Van Bael S.A., Samuels G.J., Robbins N., Herre E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control. 2008;46:4–14. doi:10.1016/j.biocontrol.2008.01.012 [Google Scholar]
- Mueller U.G. Ant versus fungus versus mutualism: ant–cultivar conflict and the deconstruction of the attine ant–fungus symbiosis. Am. Nat. 2002;160:S67–S98. doi: 10.1086/342084. doi:10.1086/342084 [DOI] [PubMed] [Google Scholar]
- Mueller U.G., Gerardo N.M., Aanen D.K., Six D.L., Schultz T.R. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 2005;36:563–595. doi:10.1146/annurev.ecolsys.36.102003.152626 [Google Scholar]
- North R.D., Jackson C.W., Howse P.E. Evolutionary aspects of ant–fungus interactions in leaf-cutting ants. Trends Ecol. Evol. 1997;12:386–389. doi: 10.1016/s0169-5347(97)87381-8. doi:10.1016/S0169-5347(97)87381-8 [DOI] [PubMed] [Google Scholar]
- Poulsen M., Boomsma J.J. Mutualistic fungi control crop diversity in fungus-growing ants. Science. 2005;307:741–744. doi: 10.1126/science.1106688. doi:10.1126/science.1106688 [DOI] [PubMed] [Google Scholar]
- Poulsen M., Currie C.R. Complexity of insect–fungal interactions: exploring the influence of microorganisms on attine ant–fungus associations. In: Bourtzis K., Miller T.A., editors. Insect symbiosis. vol. 2. CRC Press; Boca Raton, FL: 2006. pp. 57–77. [Google Scholar]
- Preszler R.W., Gaylord E.S., Boecklen W.J. Reduced parasitism of a leaf-mining moth on trees with high infection frequencies of an endophytic fungus. Oecologia. 1996;108:159–166. doi: 10.1007/BF00333227. doi:10.1007/BF00333227 [DOI] [PubMed] [Google Scholar]
- Quinlan R.J., Cherrett J.M. The role of substrate preparation in the symbiosis between the leafcutting ant Acromyrmex octospinosis (Reich) and its food fungus. Ecol. Entomol. 1977;2:161–170. doi:10.1111/j.1365-2311.1977.tb00877.x [Google Scholar]
- Redman R.S., Dunigan D.D., Rodriguez R.J. Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? New Phytol. 2001;151:705–716. doi: 10.1046/j.0028-646x.2001.00210.x. doi:10.1046/j.0028-646x.2001.00210.x [DOI] [PubMed] [Google Scholar]
- Rodrigues A., Bacci M., Mueller U.G., Ortiz A., Pagnocca F.C. Microfungal ‘weeds’ in the leafcutter ant symbiosis. Microb. Ecol. 2008;56:604–614. doi: 10.1007/s00248-008-9380-0. doi:10.1007/s00248-008-9380-0 (online only) [DOI] [PubMed] [Google Scholar]
- Rojas E., Herre E.A., Mejia L., Chaverri P., Samuels G.J. Endomelanconiopsis, a new anamorph genus in the Botryosphaeriaceae. Mycologia. 2008;100:760–775. doi: 10.3852/07-207. doi:10.3852/07-207 [DOI] [PubMed] [Google Scholar]
- Sternberg L.D., Pinzon M.C., Moreira M.Z., Moutinho P., Rojas E.I., Herre E.A. Plants use macronutrients accumulated in leaf-cutting ant nests. Proc. R. Soc. B. 2007;274:315–321. doi: 10.1098/rspb.2006.3746. doi:10.1098/rspb.2006.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.N. University of Chicago Press; Chicago, IL: 1994. The coevolutionary process. [Google Scholar]
- Tibbets T.M., Faeth S.H. Neotyphodium endophytes in grasses: deterrents or promoters of herbivory by leaf-cutting ants? Oecologia. 1999;118:297–305. doi: 10.1007/s004420050730. doi:10.1007/s004420050730 [DOI] [PubMed] [Google Scholar]
- Van Bael S.A., Maynard Z., Rojas E., Mejia L., Kyllo D.A., Herre E.A., Robbins N., Bischoff J.F., Arnold A.E. Emerging perspectives on the ecological roles of endophytic fungi in tropical plants. In: Dighton J., White J.F., Oudemans P., editors. The fungal community: its organization and role in the ecosystem. Taylor & Francis; London, UK: 2005. pp. 181–192. [Google Scholar]
- Van Bael S.A., Valencia M., Rojas E.I., Gómez N., Windsor D.M., Herre E.A. Effects of foliar endophytic fungi on the preference and performance of a leaf beetle, Chelymorpha alternans Boheman (Chrysomelidae: Cassidinae) Biotropica. 2009;41:221–225. doi:10.1111/j.1744-7429.2008.00476.x [Google Scholar]
- Wang Y., Mueller U.G., Clardy J. Antifungal diketopiperazines from symbiotic fungus of fungus-growing ant Cyphomyrmex minutus. J. Chem. Ecol. 1999;25:935–941. doi:10.1023/A:1020861221126 [Google Scholar]
- Weber N.A. Gardening ants: the attines. Mem. Am. Philos. Soc. 1972;92:1–146. [Google Scholar]
- Wilson D. Endophyte—the evolution of a term, and clarification of its use and definition. Oikos. 1995a;73:274–276. doi:10.2307/3545919 [Google Scholar]
- Wilson D. Fungal endophytes which invade insect galls—insect pathogens, benign saprophytes, or fungal inquilines. Oecologia. 1995b;103:255–260. doi: 10.1007/BF00329088. doi:10.1007/BF00329088 [DOI] [PubMed] [Google Scholar]
- Wilson D., Carroll G.C. Infection studies of Discula quercina, an endophyte of Quercus garryana. Mycologia. 1994;86:635–647. doi:10.2307/3760534 [Google Scholar]
- Wilson D., Carroll G.C. Avoidance of high-endophyte space by gall-forming insects. Ecology. 1997;78:2153–2163. doi:10.2307/2265952 [Google Scholar]
- Wilson D., Faeth S.H. Do fungal endophytes result in selection for leafminer ovipositional preference? Ecology. 2001;82:1097–1111. doi:10.2307/2679906 [Google Scholar]
- Wirth R., Herz H., Ryel R.J., Beyschlag W., Hölldobler B. Ecological studies. Springer; Berlin/Heidelberg, Germany; New York, NY: 2003. Herbivory of leaf-cutting ants: a case study on Atta colombica in the Tropical Rainforest of Panama. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of conidia preparation, application, and reisolations
Supporting experiment for interactions among endophytes and the ants' cultivar
Description of endophytic fungal strains used for in vitro trials