Abstract
We have developed and applied a method unifying fluorescence microscopy and mass spectrometry for studying spatial and temporal properties of proteins and protein complexes in yeast cells. To combine the techniques, first we produced a variety of DNA constructs that can be used for genomic tagging of proteins with modular fluorescent and affinity tags. The modular tag consists of one of the multiple versions of monomeric fluorescent proteins fused to a variety of small affinity epitopes. After this step we tested the constructs by tagging two yeast proteins, Pil1 and Lsp1, the core components of eisosomes, the large protein complexes involved in endocytosis in Saccharomyces cerevisiae, with a variety of fluorescent and affinity probes. Among the modular tags produced we found several combinations that were optimal for determining subcellular localization and for purifying the tagged proteins and protein complexes for the detailed analysis by mass spectrometry. And finally, we applied the designed method for finding the new protein components of eisosomes and for gaining new insights into molecular mechanisms regulating eisosome assembly and disassembly by reversible phosphorylation and dephosphorylation. Our results indicate that this approach combining fluorescence microscopy and mass spectrometry into a single method provides a unique perspective into molecular mechanisms regulating composition and dynamic properties of the protein complexes in living cells.
Fluorescent proteins have become invaluable probes for studying molecular processes in living cells with light microscopy techniques (1–3). Proteins, organelles, and entire cells can be selectively visualized using a variety of fluorescent proteins fused to the proteins of interest (1–6). Combined with genetics and molecular biology techniques fluorescence microscopy provides an efficient tool for observing molecular phenotypes useful for dissecting the pathways of cell cycle progression and cell response to internal and external signals (7). However, understanding the mechanism controlling the properties of proteins in cells can be a challenging task, frequently requiring a comprehensive characterization of the proteins at the molecular level.
The proteins tagged with green fluorescent protein (GFP)1 can be also purified using GFP antibodies. Cheeseman and Desai (8) and Cristea et al. (9) have enriched GFP-tagged proteins and protein complexes for further detailed analysis by MS. The MS-based methods for protein analysis are fast, sensitive, and able to identify both proteins in complex protein mixtures and residues bearing post-translational modifications (10, 11). Thus, the addition of affinity purification and mass spectrometry steps enabled the researchers to study protein interactions and the post-translational modifications in the context of the protein subcellular localization. Juxtaposition of the protein localization, composition of the protein complexes, and post-translational modifications frequently yield a unique perspective of the cellular processes and the molecular mechanisms of their regulation (12, 13).
Using fluorescent proteins also as affinity probes can be problematic in several instances. First of all, the good quality antibodies against the rapidly increasing number of fluorescent proteins (3, 6) are not yet readily available. Furthermore raising antibodies specifically recognizing fluorescent proteins originating from the same organism but fluorescing a different color can be difficult or even impossible because such proteins frequently differ by mutations of only a few amino acids (1–6). Thus, we seek an alternative approach to the design of tags suitable for subcellular localization and purification of proteins and protein complexes that is 1) independent of the availability of antibody to a specific form of a fluorescent protein, 2) suitable for multiplexing, i.e. simultaneous observation of subcellular localization of several proteins and affinity purification of the proteins and stably associated protein complexes, and 3) flexible and easy to modify to incorporate better versions of fluorescent proteins and affinity tags after they are discovered.
One possible solution that satisfies the stated requirements is to use a modular tag containing a version of a fluorescent protein fused to an affinity epitope. In this case we can decouple requirements for both modules and optimize the performance of each one independently for fluorescence microscopy and affinity purification experiments. To our knowledge, this possibility was first realized by Thorn and co-worker (14) who have fused 3HA (three repeats of YPYDVPDYA epitope from hemagglutinin protein) and 13MYC (13 repeats of EQKLISEEDL epitope, corresponding to a stretch of the C-terminal amino acids of the human c-MYC protein) tags to several variants of fluorescent proteins. The authors have argued that the fusion of the fluorescent proteins to the affinity epitopes may enable fluorescence and immunochemical analysis but did not test this idea. Cheeseman and Desai (8) fused the S-peptide and hexahistidine epitopes to the GFP protein to enable additional tandem purification steps. Su and co-workers (15) also fused a hexahistidine tag (His6) to GFP to purify recombinantly produced proteins. Although hexahistidine tag performs well for isolation of overexpressed recombinant proteins, it works poorly for affinity purification of low abundance, endogenously expressed proteins (16). A double affinity tag containing a single MYC epitope and hexahistidine was also used to purify recombinantly produced fluorescent proteins (6).
Here we describe the design and implementation of the modular fluorescent and affinity tags. These tags contain a variety of fluorescent proteins, which can be used exclusively for obtaining subcellular visualization, and several small epitope tags that can be utilized to perform two-step affinity purification. To test the performance of the constructs produced, we tagged two yeast proteins, Pil1 and Lsp1, the core components of eisosomes, with a variety of modular tags.
Eisosomes are large heterodimeric protein complexes recently discovered in Saccharomyces cerevisiae (17). There are ∼50–100 eisosomes in each mature yeast cell distributed uniformly in a characteristic dotted pattern at the cell surface periphery. Each eisosome contains ∼2000–5000 copies of Pil1 and Lsp1. It was shown that eisosomes serve as portals of endocytosis in yeast. The function of eisosomes is regulated by reversible phosphorylation (18, 19).
Among the constructs tested, we found several combinations of fluorescent protein and affinity tags that were optimal for determining subcellular localization and purification of the proteins and protein complexes. We applied these tags to further investigate eisosomes and found several new protein components of the complexes and obtained new insights into molecular mechanisms regulating eisosome integrity by reversible phosphorylation and dephosphorylation. Our results indicate that an approach combining fluorescence microscopy and mass spectrometry into a single method provides a unique perspective into molecular mechanisms regulating composition and dynamic properties of the protein complexes in living cells.
EXPERIMENTAL PROCEDURES
Plasmid Vectors—
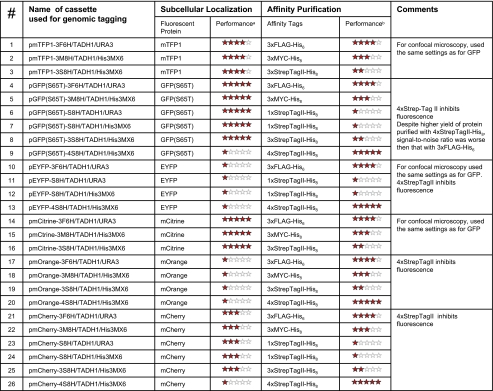
We developed multiple plasmid vectors (see supplemental Fig. 1 and Table I) that contain the DNA sequences of a variety of fluorescent proteins, i.e. GFP(S65T), mTFP1, mCitrine, mOrange, mCherry, and EYFP (2–6), fused to several versions of small double affinity tags, such as 3×FLAG-His6 (20), 3×MYC-His8 (6), n×StrepTagII-His8 (16) (where n = 1, 3, and 4 epitope repeats), terminators of transcription, bacterial and yeast selection markers, and some LoxP recombination sites (21). The vectors were produced by a standard cloning into a pEYFP-C1 vector (Clontech). The sequences of the vectors are shown in the supplemental data. The designed vectors can be used as templates for PCR-based genomic tagging (22, 23).
Table I.
Performance of the tested modular fluorescent and affinity tag constructs
The best results correspond to five stars, and the worst correspond to one star.
aAverage intensity and variation from cell to cell for Lsp1 protein tagged with modular tags. The data are based on at least three biological replicates.
bRelative protein purification yield.
Genomic Tagging, in Vivo Disruption, and in Vivo Mutagenesis—
The PCR primer pair, 5′-gene-specific primer (51 bases) + GAT CCG CTA GCG CTA CCG GTC-3′ (sense) and 5′-gene-specific primer (51 bases) + TAA TAC GAC TCA CTA TAG GGA GAC-3′(antisense), was selected to amplify the desired DNA construct coding for a specific modular tag. The amplified DNA was directly used for C-terminal tagging of the protein of interest by a homologous recombination technique (22, 23). In vivo disruption and mutagenesis of the genomic DNA was performed essentially as described previously (24). The typical molecular mass of a modular fluorescent and affinity tag is ∼30 kDa, which includes the molecular mass of a fluorescent protein, two affinity epitopes, and spacers between the tags and the protein (see DNA sequences in the supplemental data).
Yeast Strains—
All yeast strains were derived from MATa BY4741 bar1Δ::KanMX strain or MATα BY4742 strain. Transformed clones were selected based on the presence of a selectable marker and a fluorescence signal.
Cell Culturing—
Cells were cultured in 0.5–1 liter of YEPD medium (MP Biomedicals) at 30 °C to a density of 2–4 × 107 cells/ml. For the cell cycle arrest and release experiments, we cultured several liters of yeast cells. Each liter of a culture was incubated in YEPD medium containing 50 μg/liter α-factor (GenScript Corp.) for 3 h, spun down, washed three times with double deionized water, and then cultured in 1 liter of a regular YEPD medium containing 50 mg/liter Pronase enzyme (Sigma-Aldrich) added to the medium to digest the remaining α-factor (25).
Combined Fluorescence Microscopy and Mass Spectrometry Experiment—
A schematic diagram of the combined microscopy/mass spectrometry experiment is shown in Fig. 1. The cultured cells were quickly collected by centrifugation of the medium in two 500-ml buckets of a Hermle Z383 centrifuge (Denville Scientific) at ∼4000 × g for 5–10 min. A small portion of cells was immediately deposited on a glass slide for the fluorescence microscopy experiment performed on a Zeiss LSM510 confocal microscope. Images were later processed using Zeiss LSM Image Browser software.
Fig. 1.
The schematic diagram of the combined fluorescence microscopy and mass spectrometry experiment. After the cells are grown and quickly collected by centrifugation, a small portion of cells (∼10–100 μl) is sampled for fluorescence microscopy experiment. At this point, the experiment is performed by at least two investigators. One investigator obtains the images of sampled cells to establish subcellular localization of the studied protein, while the other one quickly collects the rest of the cells (∼1–4 g of cell pellet) and freezes them in liquid nitrogen. Frozen cells are later processed essentially as described under “Experimental Procedures” to determine the composition of the purified protein complexes and possible post-translational modifications on the purified proteins.
The rest of the sampled cells were frozen by dripping cells in a 50-ml Falcon tube filled with liquid nitrogen. The pellets of cells were stored at −80 °C until the proteins and protein complexes were affinity-purified according to the procedures described below.
Tandem Affinity Purification of the Protein Complexes—
Tandem affinity purification of proteins was performed essentially as described previously (20). The detailed protocol can be also found in the in the supplemental data. Briefly the fist purification step was performed by adding ∼5–10 mg of magnetic beads immobilized with antibody against a particular epitope to 5–10 ml of a crude cell extract. We used M-270 epoxy magnetic Dynabeads immobilized with anti-FLAG monoclonal antibody M2 (F3165, Sigma-Aldrich) when purifying proteins tagged with a fluorescent protein and the 3×FLAG-His6 affinity tag (DYKDHDGDYKDHDIDYKDDDDKHHHHHH) and a mouse monoclonal anti-c-MYC antibody (clone 9E10, Roche Applied Science) when purifying proteins tagged with a fluorescent protein and 3 × MYC-His8 epitope (three repeats of EQKLISEEDLG fused to octahistidine). Immobilization of the Dynabeads with the antibody was carried out essentially as described in the manufacturer's protocol, binding ∼10 μg of antibody/5 mg of beads. After 60–90 min of incubation, the beads were collected with a magnet and washed three times with 1 ml of IP buffer (20 mm Hepes, 2 mm MgCl2, 250 mm NaCl, 0.05% Tween 20, and protease inhibitor mixture (Sigma-Aldrich)). The enriched proteins were eluted with ∼300 μl of IP buffer containing a 3×FLAG peptide (Sigma-Aldrich) at a concentration of ∼400 μg/ml or with the c-MYC peptide (Sigma-Aldrich) at a concentration of ∼800 μg/ml for 30 min at 4 °C with constant rotation. The eluate was collected and diluted in 1 ml of IP buffer.
The proteins tagged with a variety of fluorescent proteins and several repeats of StrepTagII epitope (WSHPQFEKG) and octahistidine (His8) were first purified using Strep-Tactin magnetic beads essentially as recommended by the manufacturer (Qiagen) and then eluted with biotin (Sigma-Aldrich). The second purification step was performed using polyhistidine tag and 20 μl of Cobalt TALON Dynabeads (Invitrogen). The enriched proteins were efficiently eluted with an SDS running buffer containing 250 mm imidazole and separated by SDS-PAGE. Alternatively the purified protein complexes were left on the beads for on-bead digestion.
Gel Electrophoresis—
The eluted proteins were separated by SDS-PAGE using 4–20% gradient gels (Pantera S, B-Bridge) or 4–20% microgels (Life-Gels, Life Therapeutics). The gels were stained with a colloidal Coomassie stain (GelCode, Pierce). The bands were exercised and digested with 5–7 μl of a 1 pmol/μl trypsin solution in ammonium bicarbonate. The tryptic peptides were extracted according to a standard in-gel digestion/peptide extract procedure (10). The details of the protocol can also be found in supplemental Protocol II.
Digestion of the Protein Complexes—
After the final wash of TALON beads with 2 × 1 ml of IP buffer and 2 × 1 ml of 50 mm ammonium bicarbonate buffer, the proteins were digested directly on the beads with 10 μl of trypsin solution (1 pmol/μl) in 10–50 mm ammonium bicarbonate buffer for 6–12 h at 37 °C.
Sample Preparation—
1–3 μl of a mixture of either synthetic peptides or tryptic peptides was deposited on the interchangeable MALDI target and allowed to dry. 2 μl of a saturated solution of α-cyano-4-hydroxycinnamic acid matrix was then added to the spot and again allowed to dry. The sample spots were then washed two times with 10% MeOH in 0.1% TFA by applying a 5–7-μl droplet on the top of the sample for 15–30 s and then quickly aspirating it.
Identification of Proteins by Mass Spectrometry—
We used two MALDI mass spectrometers, the orthogonal time-of-flight instrument prOTOF 2000 (PerkinElmer Life Sciences) and the MALDI-ion trap mass spectrometer vMALDI-IT (Thermo Finnigan) to identify proteins. The two instruments can be used separately or sequentially to interrogate the same samples deposited on the MALDI target plate, which can be loaded into either of the mass spectrometers (20).
Typical analysis started with acquisition of single stage MALDI-MS spectra of the samples using the prOTOF mass spectrometer. This time-of-flight instrument provides accurate measurements of the m/z values of the monoisotopic peaks with a typical accuracy 5–15 ppm in the m/z range 500–4000. The spectra then were analyzed with the “m/z” program (version 2002.10.01 by Ronald Beavis, Beavis Informatics Ltd.), which can automatically find and label the first isotopes of ion peaks in the spectra. We set the major settings of the program as follows: peak centroid, 6; signal-to-noise ratio, 1.5; and resolution, 10,000. This procedure usually results in detection of 50–300 ion peaks in the single spectrum measured in the range 500–4000 m/z with intensities above a signal-to-noise ratio of 1.5 (20). The detected values were saved as text files.
The monoisotopic values from the lists can be directly used to identify proteins in simple protein mixtures. We used the XProteo search engine (20) to identify proteins based on tryptic mapping. Searching for the S. cerevisiae proteins in the National Center for Biotechnology Information (NCBI) non-redundant database, version October 16, 2006 was performed with the following typical settings: protein mass, 0–300 kDa; protein pI, 1–14; mixture search, auto; enzyme, trypsin; maximum missed cleavage sites, auto; mass type, mono; charge state, MH+; mass tolerance, 0.025 Da; and allowed default modifications, methionine oxidation and phosphorylation. The XProteo search engine uses a signal detection theory to evaluate the search results and, for each protein candidate, outputs a quality index d′ and receiver operating characteristics curve. A “threshold of identification” is set at d′ equals 4. In this case, a protein candidate is identified with the probability ∼0.99 with false alarm rate 0.05.
We confirmed all species detected in the single stage MALDI by MS/MS analysis. A computer program, “AutoMSMS,” written in house using AutoIt Basic-like scripting language was used to create MS/MS data acquisition methods for the vMALDI-IT. The program automatically opens the text files containing lists of the detected m/z values and pastes them into the method files together with the defined isolation width (3 Da for ions with m/z <2000 and 4 Da for ions with 2000 < m/z < 4000), energy of activation (35%), and ion activation time (30 ms). These method files can be executed later by native Xcalibur software controlling the ion trap. The vMALDI-IT is capable of fast acquisition of high quality MS/MS spectra (∼3 s per spectrum) of peptides presented in the sample at a femtomole level (20).
The MS/MS spectra were converted to DTA format using the in-house written “DTA converter” program utilizing an original subroutine written by Yates and co-workers (32). The DTA data files containing accurate m/z values of parent peptides and their MS/MS spectra were supplied to the XProteo search engine set to operate in MS/MS mode. Searching was performed in the in NCBI non-redundant database (version October 16, 2006) of S. cerevisiae proteins with the following typical settings: protein mass, 0–300 kDa; protein pI, 1–14; mixture search, auto; enzyme, trypsin; maximum missed cleavage sites, auto; mass type, mono; charge state, MH+; precursor tolerance, 0.025 Da; fragment tolerance, 0.35 Da; and instrument, MALDI_I_TRAP. No possible modifications were considered in this mode. The XProteo search engine again produced a quality index d′ and receiver operating characteristics curve for each protein candidate based on the combined scores evaluated from accuracy of measurements of m/z values of parent ions and the accuracy and intensity of fragments detected in the MS/MS spectra using a proprietary, Bayesian formula-based algorithm.
The MS/MS data were also examined for the presence of the neutral loss of 98 Da in the fragmentation spectra that is a characteristic signature of phosphorylation. Detection of the neutral loss of 98 Da was facilitated by using the “Ion Map” function of the Qual Browser of the Xcalibur program controlling the trap (20). The interpretation and assignment of fragments in the fragmentation spectra of phosphopeptides was performed manually using PROWL computer tools available from Brian Chait's laboratory at the Rockefeller University.
Isotopic Differentiation of Interactions as Random or Targeted (I-DIRT) Technique—
The I-DIRT technique was implemented exactly as described previously (30) to distinguish between the specific and nonspecific interactors in the affinity-purified complexes (see also supplemental data).
RESULTS
Development of a Modular Fluorescent and Affinity Tags—
The DNA vectors produced were used as templates for PCR-based genomic tagging of proteins with a variety of modular fluorescent and affinity tags (see supplemental Fig. 1 and Table I). We tagged two yeast proteins, Pil1 and Lsp1, i.e. the core components of eisosomes (17, 18), by incorporating different DNA cassettes at the C termini of the protein genomic sequences. Then we experimented with cells expressing the tagged proteins as a model system to optimize the performance of the modular fluorescent and affinity tags. The schematic diagram of the combined fluorescence microscopy/mass spectrometry experiment is shown in Fig. 1.
Optimization of the Performance of Fluorescent and Affinity Tags—
The characteristic distribution of the eisosome particles at the cell periphery (17) was obtained in all cases of tested modular fluorescent and affinity tags (supplemental Fig. 2). However, not all of the fluorescent probes performed well. We found that GFP(S65T), mCitrine, and mTFP1 provide the brightest fluorescent signals. The mCherry protein produced a good signal when we used the 568-nm wavelength of an excitation laser. The fluorescence signals of EYFP and mOrange proteins were weak, and many cells expressing the proteins did not produce fluorescence. An erratic performance of these proteins may be due to several reasons, including long folding time compared with a typical cell cycle period in yeast (∼90 min) and high sensitivity to the pH in the microenvironment (3–5). Interference of the affinity and fluorescent probes could also be an additional factor, which we discuss below.
We also investigated the performance of the affinity probes for purification of proteins and protein complexes. To carefully compare purification yields that can be achieved using different epitopes, we incorporated several affinity tags, i.e. 3×FLAG, 4×StrepTagII (four repeats of WSHPQFEK peptide), and octahistidine (His8; HHHHHHHH) in tandem at the C terminus of Lsp1-GFP(S65T) fusion protein. The protein was purified from equal amounts of yeast cells but applying different tandem purification schemes. The results show that tandem purification using 4×StrepTagII-His8 yielded more protein after the two-step purification procedure (supplemental Fig. 3A). However, the two-step tandem purification using a StrepTagII-His8 tag usually resulted in noisier “pullouts” affected by endogenously biotinylated yeast proteins. Although the addition of increasing amounts of avidin to the cell lysates helped to compete out some of the biotinylated proteins from the Strep-Tactin resin (26), there was always a substantial amount of impurity proteins in the background. In general, the amount of impurity proteins was lesser when we used 3×FLAG-His6 and 3×MYC-His8 tags resulting in higher signal-to-noise ratios of the enrichment procedure.
We also compared the purification yield of Lsp1 fused to the different modular tags, mCherry-3×FLAG-His6, mCherry-3×MYC-His8, mCherry-3×StrepTagII-His8, and mCherry-4×StrepTag II-His8. The highest purification yield was again achieved using the 4×StrepTagII-His8 tandem purification scheme albeit with higher impurity content. The 3×MYC-His8 tandem purification produced less protein under conditions identical to 3×FLAG-His6 tandem purification but still provided enough protein for staining and MS analysis. The efficiency of 3×StrepTagII dropped drastically compared with 4×StrepTagII (supplemental Fig. 3B).
Compatibility of fluorescent and affinity probes is another important factor for designing the modular tagging system. For example, 4×StrepTagII affinity tag strongly compromised and, in some cases, entirely quenched fluorescence when it was fused with a short or long linker to the N or C terminus of GFP(S65T) or mCherry tag. The effect was reproducible in cases when other than Pil1 and Lsp1 proteins were tagged with the same tags. Interestingly decreasing the number of StrepTagII repeats from four to three or one reduced this problem but also drastically reduced the protein purification yield (supplemental Fig. 3B). Perhaps binding of one of the StrepTagII repeats to a fluorescent protein may unfavorably affect its structure and result in suppression of fluorescence (27).
Presently we selected a pair of modular tags, GFP-3×FLAG-His6 and mCherry-3×MYC-His8, among a variety of tested constructs (Table I). The two modular tags enabled us to simultaneously track two proteins in a cell and to co-purify the proteins and associated complexes without noticeable cross-interference of the probes (Fig. 2). Using these tags we performed the combined fluorescence microscopy/mass spectrometry experiments to investigate the compositions and the dynamic properties of eisosomes.
Fig. 2.
Shown are subcellular localization/co-localization (A) and purification/co-purification of the yeast proteins Pil1-GFP-3×FLAG-His6 and Lsp1-mCherry-3×(c-MYC)-His8 genomically tagged with different modular tags (B).The order of the tags used for affinity purification is indicated. The control and the principal experiments are indicated with − and +. The identities of proteins corresponding to the major bands are shown. The details on the identified proteins can be found in the supplemental material.
Localization-driven Exploration of the Composition of Protein Complexes—
Fig. 3 shows a schematic diagram of several strategies that can be readily implemented using the combined fluorescence microscopy/mass spectrometry experiments. In one strategy (Fig. 3A), we use the detected pattern of protein subcellular localization as a clue to whether the identified proteins could be associated in the protein complexes. We start by tagging the protein of interest and first explore the protein subcellular localization. In parallel, we purify the tagged protein and identify the co-purified proteins using mass spectrometry. The identified proteins are consequently tagged with a fluorescent and affinity tags, and we again start with establishing subcellular localization of the identified proteins. Thus, in this walking strategy, the localization step is followed by purification, separation, and identification of the new interacting proteins. The new identified proteins either exhibit or do not exhibit similar patterns of localization indicating the possibility that they could form either stable or transient complexes or even interact nonspecifically. This information is treated as a valuable clue for further experiments.
Fig. 3.
Schematic diagram of the strategy for localization-driven exploration of the composition of the protein complexes (A) and investigation of the dynamic profiles of changes in protein subcellular localization, composition of protein complexes and abundances of post-translational modifications (B). a–g refer to the identified proteins.
Analysis of the gel bands corresponding to affinity-purified proteins of the eisosome complexes (see Fig. 2B, Fig. 4, and supplemental Fig. 3) consistently indicated the presence of three major proteins, Pil1, Lsp1, and Mrp8. The first two proteins, Pil1 and Lsp1, are the core eisosome proteins (17). The third protein frequently detected in the purified complexes is Mrp8, a putative mitochondrial ribosomal protein (see for example the Saccharomyces Genome Database). This protein has also been identified as a possible interacting partner of Pil1 and Lsp1 proteins in high throughput immunopurification (28) and two-hybrid experiments (29). The analysis of the localization of Mrp8 (see Fig. 4A) indicated that it does not co-localize with the eisosomes but rather resides in the cytoplasm of the cells. Nevertheless a small portion of Pil1 and Lsp1 proteins can be co-purified with the Mrp8-GFP-3×FLAG-His6 (Fig. 3A).
Fig. 4.
Subcellular localization of the tagged proteins and the composition of the protein complexes associated with them in yeast cells. The co-purified proteins were separated by SDS-PAGE and stained with colloidal Coomassie. Proteins were identified by a standard in-gel digestion/mass spectrometry procedure. A, subcellular localizations of Mrp8-GFP-3×FLAG-His6 and Lsp1-mCherry-3×MYC-His8 and the major components of the protein complexes purified with the tagged Mrp8. B, subcellular localization of Ygr130c-GFP-3×FLAG-His6 and the major components of the complexes co-purified with the tagged protein. C, subcellular localization of Ymr031c-GFP-3×FLAG-His6 and the composition of the major components of complexes co-purified with the tagged protein. D, subcellular localization of Ymr086-GFP-3×FLAG-His6 and the major components of the complexes purified with the tagged protein. The full list of the identified proteins, unique peptides found, and protein coverage can be found in the supplemental material.
To investigate whether Mrp8 protein interacts with Pil1 and Lsp1 protein in a specific or nonspecific manner we implemented the I-DIRT technique (30). We affinity-purified the Pil1 protein from a ∼1:1 mixture of ∼2 g of yeast cells expressing the tagged protein and grown in the presence of normal, undeuterated, lysine in the yeast medium and the wild type yeast cells (MATa BY4741) grown in the presence of deuterated dl-lysine-4,4,5,5-d4 in the yeast medium (see supplemental Fig. 5.) The two identified proteins, Pil1 and Lsp1, did not produce the deuterated versions of the tryptic peptides containing lysine residues, indicating that complexes of the two proteins were formed in the unlabeled cells. On the other hand, the other two identified proteins, Rsp17a and Mrp8, were represented by doublets of peaks. These doublets contain the “light,” undeuterated and “heavy,” deuterated versions of lysine residues, indicating that these two proteins are fast exchanging components of the Pil1 and Lsp1 complexes and thus, most probably, are nonspecific interactors.
On-bead digestion of the complexes co-purified with Pil1-GFP-3×FLAG-His6 followed by mass spectrometry (20) resulted in identification of several additional proteins, Tef1, Pma1, and Ygr130c (supplemental Fig. 6). Tef2 and Pma1 are impurities and are frequently found in control samples. However, Ygr130c was not identified in the control. The pictures of the protein subcellular localization from the yeast GFP fusion localization database (31) suggested a dotted pattern of localization at the cell periphery. Intrigued by this, we implemented the strategy for localization-driven exploration of the composition of protein complexes (Fig. 3A) and tagged Ygr130c protein with a modular GFP-3×FLAG-His6 tag and investigated the protein subcellular localization and protein interacting partners.
Fig. 4B confirms eisosome-like distribution of the protein tagged with GFP-3×FLAG-His6 at the cell periphery. On-bead digestion of the co-purified proteins followed by mass spectrometric analysis of the tryptic peptide mixture produced several top candidates: Ygr130c, Ymr031c, Pil1, Lsp1, and Ymr086w (supplemental Fig. 7). Fig. 4B also shows that these proteins were also identified by the standard SDS-PAGE separation/in-gel digestion/MS procedure (supplemental Protocol II).
Guided by detecting a characteristic pattern of localization, we again tagged Ymr086w and Ymr031c with one of our modular tags and determined first subcellular localization and then interacting partners of those proteins. Fig. 4, C and D, show that both proteins localize to the cell periphery and that Pil1 and Lsp1 are reciprocally co-purified with the query proteins. Although some other identified proteins may also exhibit the eisosome-like pattern of subcellular localization as for example Ycp4 (see the database of localization of yeast proteins (31)), at this particular point of our research we decided to focus our efforts on the most prominent candidates, Ygr130c, Ymr031c, and Ymr086w, and investigate whether these proteins are indeed the new integral components of eisosomes.
Fig. 5A shows that Ygr130c, Ymr031c, and Ymr086w tagged with GFP-3×FLAG-His6 co-localize with Lsp1 protein tagged with mCerry-3×MYC-His8 tag. To further investigate whether the proteins interact physically with eisosomes, we introduced modifications to the DNA sequence of Pil1 protein. During implementation of the “delitto perfetto” in vivo mutagenesis technique (24), we accidentally found that removal of 114 amino acids from the C terminus of Pil1 protein causes Lsp1 to relocalize into the cytoplasm and into several bright spots at the cell periphery (Fig. 5B). This effect is similar to the one observed after complete deletion of Pil1 protein from the cells (17). It confirms the importance of Pil1 protein, especially the C terminus, for proper localization of eisosome particles. We decided to use this phenomenon to deliberately change Lsp1 localization and to examine whether this, in turn, will change localization of the other proteins.
Fig. 5.
A, co-localization of Pil1, Ygr130, Ymro31c, and Ymr086w proteins tagged with a GFP-3×FLAG-His6 modular tag and Lsp1-mCherry-3×MYC-His8 protein. All proteins co-localize in eisosomes (17). B, proteins Ygr130, Ymr031, and Ymr086 physically interact with Lsp1 protein whose subcellular localization was changed by removing the last 114 amino acids at the C terminus of Pil1 protein. Truncation of Pil1 causes relocalization of Lsp1 and other physically interacting protein partners into several bright spots at the cell periphery. aa, amino acids.
Ygr130c, Ymr031c, and Ymr086w changed their localization together with Lsp1 in the cells in which the Pil1 protein was truncated at the C terminus (Fig. 5B). This result suggests that the newly discovered proteins physically interact at least with Lsp1 and thus are the integral components of eisosomes. The biological function of Ygr130c, Ymr031c, and Ymr086w and a possible involvement into endocytosis remain to be investigated.
Study of the Dynamic Properties of Protein Complexes: Application to the Studies of Protein Dephosphorylation Dynamics—
Fig. 3B is a schematic diagram of the experiments for studying the dynamic properties of the protein complexes. In this approach, we sample cells at the different stages of the cell cycle or cell response to a particular signal to obtain the dynamic profiles of changes in protein subcellular localization, composition of protein complexes, and abundances of post-translational modifications. Analysis of such profiles can frequently yield unique clues about the cellular processes and the molecular mechanisms of their regulation. Here we demonstrate the usefulness of the combined fluorescent microscopy/mass spectrometry experiments for the studies of the dynamics of eisosome dephosphorylation. We also show that from the measured changes in subcellular localization and abundances of phosphorylation sites we can deduce the existence of a phosphatase system involved in maintaining eisosome integrity during the cell cycle.
Eisosomes can exhibit dynamic behavior. Eisosome assembly and organization are controlled through reversible phosphorylation of its core proteins (18). It was shown that at least several kinases, including Pkh1 and Pkh2, are involved in eisosome phosphorylation. However, there is no information indicating the existence of a phosphatase system involved in eisosome dephosphorylation.
We measured the dynamics of eisosome dephosphorylation. First of all, we noticed that eisosomes of the MATa (BY4741) cells disassemble in response to the treatment with the α-mating pheromone (α-factor). At the same time, treatment of the control MATα (BY4742) cells with α-factor did not affect the distribution of eisosome particles (supplemental Fig. 13, A and B). Analysis of the mass spectra of Pil1 and Lsp1 purified from α-factor-treated or non-treated haploid MATa BY4741 revealed a number of striking differences (supplemental Fig. 13, C and D). The abundance of a Pil1 phosphorylated peptide detected in the mass spectra at m/z 2987.395 increased by more than a factor of 5 in the yeast cells incubated with α-factor. Phosphorylation of a homologous peptide from Lsp1 detected at m/z 2985.400 increased by more than a factor of 10 in the cells incubated with the α-factor. Fragmentation spectra of these peptides confirmed their identity and the position of the phosphorylated residues (supplemental Figs. 14 and 15). A drastic increase of the phosphorylation level after the treatment of cells with α-factor was noticed only at the reported phosphorylation sites. However, it was recently shown that eisosomes can be phosphorylated at a larger number of sites (18).
What is the dynamics of dephosphorylation of the detected sites? We sampled yeast cells at 0, 20, 40, 60, and 80 min after release from the α-factor for fluorescence microscopy and mass spectrometry experiments. Surprisingly a substantial portion of Pil1 and Lsp1 proteins remain in the cytoplasm of the cells for almost 1 h after removing the pheromone. After 1 h, the fluorescence of the cytoplasmic fraction of the protein quickly, within 10–15 min, dropped to the level observed in the cells with intact eisosomes (Fig. 6A). This process correlates well with dephosphorylation of Pil1 and Lsp1. Analysis of the mass spectra of the affinity-purified proteins indicates that the abundance of the peptides phosphorylated at Ser-230 and Thr-233 also dropped abruptly at ∼60–80 min after release from the α-factor block (Fig. 6, C and D). Noticeably the relative intensities of gel bands corresponding to the phosphorylated forms of the proteins also decreased at the same time.
Fig. 6.
A, subcellular localization of Pil1-GFP-3×FLAG-His6 after release from α-factor (αF). B, SDS-PAGE of Pil1 and Lsp1 proteins purified at the corresponding time points after release from α-factor. Note the doubling of bands as the result of post-translational modifications of both proteins. C, dynamics of the decrease of Pil1 phosphorylation. Note the decrease in abundance of phosphopeptide ALLELLDDp(SPVT)PGETRPAYDGYEASK (where p indicates phosphorylation) at m/z 2987.395 relative to the unphosphorylated form of the peptide at m/z 2907.423. D, dynamics of the decrease of Lsp1 phosphorylation. Note the decrease in abundance of phosphopeptide ALLELLDDp(SPVT)PGEAR at m/z 1875.885 relative to the unphosphorylated form of the peptide at m/z 1795.946. E, subcellular localization of the wild type (WT) and mutant forms of Pil1 and Lsp1. Simultaneous mutations of the phosphorylated residues Ser-230 and Thr-233 in both proteins (Pil1WT and Lsp1WT) to aspartic acid (Pil1D and Lsp1D) and alanine residues (Pil1A and Lsp1A) indicate the importance of phosphorylation/dephosphorylation events for the controlled assembly and disassembly of eisosomes.
The observed dynamics revealed a rather abrupt change in phosphorylation level of both proteins at around 60 min of cell cycle progression. We speculate that some unknown phosphatase is activated at that stage of the cell cycle that dephosphorylates Pil1 and Lsp1. Intriguingly this process coincides with the biogenesis of new eisosomes particles (33), the process in which new eisosomes are created in the new budding cells. It is tempting to hypothesize that both processes, biogenesis and protein accumulation in the cytoplasm of the cells, are related and controlled by reversible protein phosphorylation and dephosphorylation. Identification of a phosphatase system and the mechanisms of its activation could be the goal for future projects.
Using an in vivo mutagenesis technique (24), we produced several yeast cell strains containing a combination of the different mutant versions of Pil1 and Lsp1 proteins tagged with fluorescent and affinity probes (see the complete, 3 × 3 mutation matrix in supplemental Fig. 16). Fig. 6E shows images of Pil1 and Lsp1 co-localization in the cells from the diagonal of the mutation matrix. Noticeably, simultaneous mutations of S230 and T233 in both proteins to either to aspartic acid or alanine residues produced enhanced phenotypes. Mutations S230D and T233D caused partial disassembly of eisosome particles or perhaps inability to assemble eisosome particles during eisosome biogenesis causing accumulation of the mutant proteins in the cytoplasm of the cells. Mutations S230A and T233A caused formation of fewer and brighter eisosomes as was described previously by Walther et al. (18). They showed that mutations of residues Ser-45, Ser-59, Ser-230, and Thr-233 to either aspartic acid or alanine residues in Pil1-GFP all have a profound effect on the integrity of eisosomes. Our experiments revealed the synergetic effect of simultaneous mutations in Pil1-GFP-3×FLAG-His6 and Lsp1-mCherry-3×MYC-His8 proteins, which had a stronger effect on eisosomes then the effects caused by mutation of the sites in a single protein (see Fig. 6E and supplemental Fig. 16).
DISCUSSION
We have combined fluorescence microscopy and mass spectrometry techniques for studying the composition and dynamic properties of protein complexes in the cells. To combine both techniques we designed and tested a variety of modular tags containing a fluorescent protein for visualization and two small epitope tags for two-step affinity purification. The modular construction of the tag allowed us to decouple requirements for the fluorescence and affinity probes and optimize the performance of each module independently for fluorescence microscopy and affinity purification experiments (see Table I). However, several modular tags exhibited strong coupling causing compromised performance of either the fluorescent protein or an affinity tag. It is one of our future goals to explore the cause and possible applications of this phenomenon.
Our results confirm the usefulness of the produced reagents and the combined fluorescence microscopy/mass spectrometry approach for studying composition and dynamic properties of eisosomes (17, 18, 33). The designed method enabled us to find new protein components of eisosomes and gain new insights into the molecular mechanisms regulating eisosome assembly and disassembly by reversible phosphorylation and dephosphorylation. In our future work, we will investigate the applicability of the method for studying proteins from different organisms, localized to different compartments, and present at low amounts in the cells. Our recent results with yeast anaphase-promoting complexes (20) (∼1000–5000 copies per cell (31)) and the securin-separase complexes (34) (∼10–100 copies per cell2) indicate a significant potential of the developed modular tags for studying proteins that exhibit a dynamic pattern of localization and that are present at exceedingly low abundance in the cell.
Acknowledgments
We are grateful to Peter Walter for discussions and suggestions and to Tobias Walther for sharing reagents and suggestions. We thank Roger Tsien's laboratory for sharing DNA coding for several fluorescence proteins. We also thank Chao Tang for continuing to develop the search engine XProteo we used to identify proteins.
Footnotes
Published, MCP Papers in Press, March 5, 2009, DOI 10.1074/mcp.M800397-MCP200
The abbreviations used are: GFP, green fluorescent protein; EYFP, enhanced yellow fluorescent protein; mTFP, monomeric teal fluorescent protein; MAT, mating type; YEPD, yeast extract peptone dextrose; I-DIRT, isotopic differentiation of interactions as random or targeted.
C. Deng, X. Xiong, and A. N. Krutchinsky, unpublished data.
This work was supported by the Sandler Family Fund.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
REFERENCES
- 1.Lippincott-Schwartz, J., and Patterson, G. H. ( 2003) Development and use of fluorescent protein markers in living cells. Science 300, 87–91 [DOI] [PubMed] [Google Scholar]
- 2.Zhang, J., Campbell, R. E., Ting, A. Y., and Tsien, R. Y. ( 2002) Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 3, 906–918 [DOI] [PubMed] [Google Scholar]
- 3.Chudakov, D. M., Lukyanov, S., and Lukyanov, K. A. ( 2005) Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 23, 605–613 [DOI] [PubMed] [Google Scholar]
- 4.Giepmans, B. N., Adams, S. R., Ellisman, M. H., and Tsien, R. Y. ( 2006) The fluorescent toolbox for assessing protein localization and function. Science 312, 217–224 [DOI] [PubMed] [Google Scholar]
- 5.Shaner, N. C., Steinbach, P. A., and Tsien, R. Y. ( 2005) A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 [DOI] [PubMed] [Google Scholar]
- 6.Shaner, N. C., Campbell, R. E., Steinbach, P. A., Giepmans, B. N., Palmer, A. E., and Tsien, R. Y. ( 2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 [DOI] [PubMed] [Google Scholar]
- 7.Goshima, G., Wollman, R., Goodwin, S. S., Zhang, N., Scholey, J. M., Vale, R. D., and Stuurman, N. ( 2007) Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316, 5823, 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheeseman, I. M., and Desai, A. ( 2005) A combined approach for the localization and tandem affinity purification of protein complexes from metazoans. Sci. STKE 2005, pl1. [DOI] [PubMed] [Google Scholar]
- 9.Cristea, I. M., Williams, R., Chait, B. T., and Rout, M. P. ( 2005) Fluorescent proteins as proteomic probes. Mol. Cell. Proteomics 4, 1933–1941 [DOI] [PubMed] [Google Scholar]
- 10.Aebersold, R., and Mann, M. ( 2003) Mass spectrometry-based proteomics. Nature 422, 198–207 [DOI] [PubMed] [Google Scholar]
- 11.Yates, J. R. ( 2004) Mass spectrometry as an emerging tool for systems biology. BioTechniques 36, 917–919 [DOI] [PubMed] [Google Scholar]
- 12.Cheeseman, I. M., Niessen, S., Anderson, S., Hyndman, F., Yates, J. R., III, Oegema, K., and Desai, A. ( 2004) A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 18, 2255–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristea, I. M., Carroll, J. W., Rout, M. P., Rice, C. M., Chait, B. T., and MacDonald, M. R. ( 2006) Tracking and elucidating alphavirus-host protein interactions. J. Biol. Chem. 281, 30269–30278 [DOI] [PubMed] [Google Scholar]
- 14.Sheff, M. A., and Thorn, K. S. ( 2004) Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 21, 661–670 [DOI] [PubMed] [Google Scholar]
- 15.Paramban, R. I., Bugos, R. C., and Su, W. W. ( 2004) Engineering green fluorescent protein as a dual functional tag. Biotechnol. Bioeng. 86, 6687–6697 [DOI] [PubMed] [Google Scholar]
- 16.Lichty, J. J., Malecki, J. L., Agnew, H. D., Michelson-Horowitz, D. J., and Tan, S. ( 2005) Comparison of affinity tags for protein purification. Protein Expr. Purif. 41, 98–105 [DOI] [PubMed] [Google Scholar]
- 17.Walther, T. C., Brickner, J. H., Aguilar, P. S., Bernales, S., Pantoja, C., and Walter, P. ( 2006) Eisosomes mark static sites of endocytosis. Nature 439, 998–1003 [DOI] [PubMed] [Google Scholar]
- 18.Walther, T. C., Aguilar, P. S., Fröhlich, F., Chu, F., Moreira, K., Burlingame, A. L., and Walter, P. ( 2007) Pkh-kinases control eisosome assembly and organization. EMBO J. 26, 4946–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, X., Lester, R. L., and Dickson, R. C. ( 2004) Pil1p and Lsp1p negatively regulate the 3-phosphoinositide-dependent protein kinase-like kinase Pkh1p and downstream signaling pathways Pkc1p and Ypk1p. J. Biol. Chem. 279, 22030–22038 [DOI] [PubMed] [Google Scholar]
- 20.Blethrow, J. D., Tang, C., Deng, C., and Krutchinsky, A. N. ( 2007) Modular mass spectrometric tool for analysis of composition and phosphorylation of protein complexes. PLoS ONE 4, e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauer, B. ( 1996) Multiplex Cre/lox recombination permits selective site-specific DNA targeting to both a natural and an engineered site in the yeast genome. Nucleic Acids Res. 24, 4608–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knop, M., Siegers, K., Pereira, G., Zachariae, W., Winsor, B., Nasmyth, K., and Schiebel, E. ( 1999) Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15, 963–972 [DOI] [PubMed] [Google Scholar]
- 23.Schneider, B. L., Seufert, W., Steiner, B., Yang, Q. H., and Futcher, A. B. ( 1995) Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast. 11, 13, 1265–1274 [DOI] [PubMed] [Google Scholar]
- 24.Storici, F., and Resnick, M. A. ( 2006) The delitto perfetto approach to in vivo site-directed mutagenesis and chromosome rearrangements with synthetic oligonucleotides in yeast. Methods Enzymol. 409, 329–345 [DOI] [PubMed] [Google Scholar]
- 25.Strathern, J. N., Amberg, D. C., and Burke, D. ( 2005) Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, pp. 165–167, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 26.Schmidt, T. G., Koepke, J., Frank, R., and Skerra, A. ( 1996) Molecular interaction between the Strep-tag affinity peptide and its cognate target, streptavidin. J. Mol. Biol. 255, 753–766 [DOI] [PubMed] [Google Scholar]
- 27.Sacchetti, A., and Alberti, S. ( 1999) Protein tags enhance GFP folding in eukaryotic cells. Nat. Biotechnol. 17, 1046. [DOI] [PubMed] [Google Scholar]
- 28.Krogan, N. J., Cagney, G., Yu, H., Zhong, G., Guo, X., Ignatchenko, A., Li, J., Pu, S., Datta, N., Tikuisis, A. P., Punna, T., Peregrín-Alvarez, J. M., Shales, M., Zhang, X., Davey, M., Robinson, M. D., Paccanaro, A., Bray, J. E., Sheung, A., Beattie, B., Richards, D. P., Canadien, V., Lalev, A., Mena, F., Wong, P., Starostine, A., Canete, M. M., Vlasblom, J., Wu, S., Orsi, C., Collins, S. R., Chandran, S., Haw, R., Rilstone, J. J., Gandi, K., Thompson, N. J., Musso, G., St Onge, P., Ghanny, S., Lam, M. H., Butland, G., Altaf-Ul, A. M., Kanaya, S., Shilatifard, A., O'Shea, E., Weissman, J. S., Ingles, C. J., Hughes, T. R., Parkinson, J., Gerstein, M., Wodak, S. J., Emili, A., and Greenblatt, J. F. ( 2006) Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 [DOI] [PubMed] [Google Scholar]
- 29.Ito, T., Chiba, T., Ozawa, R., Yoshida, M., Hattori, M., and Sakaki, Y. ( 2001) Comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. U. S. A. 98, 4569–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tackett, A. J., DeGrasse, J. A., Sekedat, M. D., Oeffinger, M., Rout, M. P., and Chait, B. T. ( 2005) I-DIRT, a general method for distinguishing between specific and nonspecific protein interactions. J. Proteome Res. 4, 1752–1756 [DOI] [PubMed] [Google Scholar]
- 31.Huh, W. K., Falvo, J. V., Gerke, L. C., Carroll, A. S., Howson, R. W., Weissman, J. S., and O'Shea, E. K. ( 2003) Global analysis of protein localization in budding yeast. Nature 425, 686–691 [DOI] [PubMed] [Google Scholar]
- 32.Sadygov, R. G., Cociorva, D., and Yates, J. R., III ( 2004) Large-scale database searching using tandem mass spectra: looking up the answer in the back of the book. Nat. Methods 1, 195–202 [DOI] [PubMed] [Google Scholar]
- 33.Moreira, K. E., Walther, T. C., Aguilar, P. S., and Walter, P. ( 2009) Pil1 controls eisosome biogenesis. Mol. Biol. Cell 20, 809–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt, L. J., Krutchinsky, A. N., and Morgan, D. O. ( 2008) Positive feedback sharpens the anaphase switch. Nature 454, 353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]