Abstract
Character evolution that affects ecological community interactions often occurs contemporaneously with temporal changes in population size, potentially altering the very nature of those dynamics. Such eco-evolutionary processes may be most readily explored in systems with short generations and simple genetics. Asexual and cyclically parthenogenetic organisms such as microalgae, cladocerans and rotifers, which frequently dominate freshwater plankton communities, meet these requirements. Multiple clonal lines can coexist within each species over extended periods, until either fixation occurs or a sexual phase reshuffles the genetic material. When clones differ in traits affecting interspecific interactions, within-species clonal dynamics can have major effects on the population dynamics. We first consider a simple predator–prey system with two prey genotypes, parametrized with data from a well-studied experimental system, and explore how the extent of differences in defence against predation within the prey population determine dynamic stability versus instability of the system. We then explore how increased potential for evolution affects the community dynamics in a more general community model with multiple predator and multiple prey genotypes. These examples illustrate how microevolutionary ‘details’ that enhance or limit the potential for heritable phenotypic change can have significant effects on contemporaneous community-level dynamics and the persistence and coexistence of species.
Keywords: predator–prey, eco-evolutionary, genetic diversity, rotifers, alga
1. Introduction
The last three decades have seen an accumulation of studies demonstrating that evolutionary change in ecologically important organismal traits often takes place at the same time and pace as ecological dynamics (‘eco-evolutionary dynamics’; Fussmann et al. 2007; Kinnison & Hairston 2007). Species as diverse as single-celled algae, annual plants, birds, fishes, crustaceans, insects and sheep are found to undergo rapid contemporary evolutionary changes in traits that adapt them within a few generations to changing or new environments (Reznick et al. 1997; Thompson 1998; Hairston et al. 1999; Sinervo et al. 2000; Cousyn et al. 2001; Reznick & Ghalambor 2001; Grant & Grant 2002; Heath et al. 2003; Yoshida et al. 2003; Olsen et al. 2004; Pelletier et al. 2007; Swain et al. 2007). Thus, the old assumption that evolutionary change is negligible on the time-scale of ecological interactions is now demonstrably incorrect.
Ultimately, evolution has the potential to transform how we think about and manage ecological systems, with implications for resource management (Heath et al. 2003; Swain et al. 2007; Strauss et al. 2008), conservation of biodiversity (Ferrière & Couvert 2004; Kinnison & Hairston 2007), invasive species management (Lavergne & Molofsky 2007) and ecosystem responses to environmental change (Hairston et al. 2005; Strauss et al. 2008). However, virtually all of the ecological theories developed to address these practical issues invoke, tacitly or explicitly, a separation of time-scales between ecological and evolutionary dynamics (see Ferrière & Couvert 2004). Frequently, that is valid, of course, but the accumulating evidence suggests that important questions may be answered incorrectly if we fail to account for the fact that we are studying and managing a ‘moving target’.
Here, we use the compound term ‘rapid contemporary evolution’ to refer to heritable changes within a population which occur at a rate fast enough to affect interspecific interactions while they are taking place. The descriptor ‘rapid’, by itself, leaves ambiguous the frame over which evolutionary rates are compared (rapid species diversification on a geological time-scale can be quite slow compared with the rate of heritable changes within a population). The adjective ‘contemporary’ (Hendry & Kinnison 1999) identifies the time-scale that interests us, but permits the degree of change to be either negligible or dramatic. Our intent is to explore the effect of evolution that both occurs within a few generations (the time-scale of ecological dynamics) and is substantial (ecological interaction strength is importantly altered), i.e. evolution that is both contemporary and rapid.
Post & Palkovacs (2009) note that a system of eco-evolutionary feedbacks requires both that the phenotypes must affect the environment in which they live and the environment must select on the distribution of genetically based phenotypes. A major component of ecological research has been the identification and measurement of interaction strengths among species, and between species and their chemical and physical environments (e.g. Cain et al. 2008; Molles 2008), and Post & Palkovacs (2009) provide a table of fine examples. At the same time, there is a growing literature showing that heritable phenotypic differences among individuals have substantial importance for how communities and ecosystems function (e.g. Johnson et al. 2006; Whitham et al. 2006; Urban et al. 2008; Palkovacs et al. 2009). Finally, extensive reviews of the literature (Hendry & Kinnison 1999; Kinnison & Hendry 2001; Palumbi 2001) leave no doubt that selection driven by strong ecological interactions results in marked adaptive evolutionary change that frequently takes place within a few generations.
A challenge, then, is to draw together each of these components within a single system to determine the importance of rapid contemporary evolution to ecological dynamics. Hairston et al. (2005) proposed an approach, first conceived by Monica Geber, in which the relative importance of temporal changes in a feature of the environment and heritable changes within a population are assessed in terms of the magnitudes and their contributions to ecological dynamics. They applied it to data on Galapagos finches (Grant & Grant 2002), freshwater copepods (Ellner et al. 1999) and laboratory microcosms (Yoshida et al. 2003). Pelletier et al. (2007) applied a similar method to Soay sheep, and Ezard et al. (2009) have used this approach on five populations of ungulates. In each case, evolutionary contributions to ecological dynamics were found to be substantial.
Some of the most striking examples of temporal ecological dynamics in nature are found in consumer–resource interactions. Theory indicates and empirical data confirm that population abundances often oscillate substantially as interactions vary in strength, and in some cases sign, over time. Many of the clearest examples of rapid contemporary evolution have been documented in these systems including predatory fish and their prey (Hairston & Walton 1986; Reznick et al. 1997; Ellner et al. 1999; Hairston & De Meester 2008), herbivorous insects and the plants they consume (Johnson et al. 2006, 2009) and parasites and pathogens and their hosts (Dube et al. 2002; Decaestecker et al. 2007; Duffy & Sivars-Becker 2007; Eldred et al. 2008). Owing to the propensity to oscillate, consumer–resource interactions are particularly good model systems for detailed laboratory study of eco-evolutionary dynamics over both long (Lenski & Travisano 1994; Boraas et al. 1998) and short term (Meyer & Kassen 2007). Investigations using simple laboratory microcosms of real consumer and resource species combined with mathematical models that incorporate observed interactions and predict resulting dynamics have proven to be particularly fruitful for uncovering some of the potential and diversity of eco-evolutionary dynamics (e.g. Yoshida et al. 2003, 2007; Meyer et al. 2006; Jones & Ellner 2007).
In this paper, we explore how evolutionary dynamics in traits that influence the strength and nature of interspecific interactions affect the temporal dynamics of species, and the reciprocal effects of population dynamics on maintenance of the heritable variation that allows evolution to continue. We begin by reviewing some of our relevant prior experimental and theoretical work on rapid contemporary evolution in predator–prey systems, and then use extensions of our previous models to address the following general questions:
How does evolution affect the dynamics of ecological communities, and their persistence, robustness and stability?
What are the dynamic possibilities when a wide range of heritable trait variation is present in a system, and what are the consequences of diminished genetic diversity and the correspondingly reduced potential for evolutionary change?
Theory linking evolution and ecology has been published for decades, but theory unfettered by data can predict (and has predicted) any conceivable ecological dynamics, including stability, instability, chaos and criticality (the boundary between two different types of dynamics, e.g. stability versus cycles). To make specific predictions possible, our theory is closely tied to our experimental work on predator–prey chemostats that are laboratory analogues of freshwater planktonic food webs.
Natural freshwater plankton systems are often dominated by obligately asexual or facultatively sexual species, so our models assume that trait evolution occurs through changes in the frequency of different clonal lineages. However, models for quantitative trait dynamics in sexual species can be very similar to our models for clone-frequency dynamics, and can even be mathematically identical (depending on assumptions about the genetic variance for the quantitative trait; see Abrams & Matsuda 1997; Abrams 2005). As a result, our conclusions might also be relevant to sexually reproducing species. Models for clone-frequency change are also mathematically equivalent to models for multispecies interactions, but some predictions about clonal evolution result from constraints on the differences among clones within a species, which typically would not be realistic if we were modelling completely different species (Jones & Ellner 2007).
2. Background: predator and prey in the chemostat
In laboratory microcosms containing a rotifer, Brachionus calyciflorus, consuming an alga, Chlorella vulgaris, we observed predator–prey cycles with the classic quarter-period phase lag between the peaks in prey and predator densities when the algal prey were genetically homogeneous. By contrast, with a genetically variable prey population, cycle period increased three- to sixfold, and predator and prey cycles were exactly out of phase (Shertzer et al. 2002; Yoshida et al. 2003; figure 1), a phenomenon that cannot be explained by conventional predator–prey models. The algae in our microcosms vary genetically along a trade-off curve between defence against rotifer predation and ability to compete for limiting nutrients (Yoshida et al. 2004). As a result, they have an evolutionary response to fluctuations in rotifer density and nutrient availability that qualitatively changes the predator–prey dynamics.
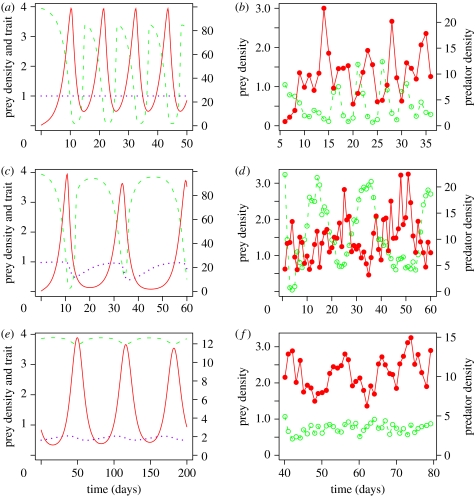
Figure 1.
Comparison of theoretical and experimental results for rotifer–algal chemostats with the rotifer B. calyciflorus as the predator (red circles) and the asexual green alga C. vulgaris as the prey (green circles). (a,b) Classical predator–prey cycles when algae are monoclonal (one genotype) and so cannot evolve (red solid curve, predator, green dashed curve, prey and purple dotted curve, average prey vulnerability to predation with 1=no defence and 0=complete invulnerability to attack). Data from Yoshida et al. (2003). (c,d) Evolutionary cycles when prey are genetically variable. Data from Fussmann et al. (2000). (e,f) Cryptic cycles when prey are genetically variable with low cost of defence. Data from Yoshida et al. (2007); spectral analysis confirms the presence of periodicity in the predator dynamics (P<0.01). Units: 106 cells ml−1 (algae), females ml−1 (rotifers) and mean palatability (prey trait). Additional experimental replicates for (b,d,f) can be found in the publications cited as the sources for the data.
To understand our experimental results, we formulated and analysed a general model for a predator–prey system with genetic variability in an asexually reproducing prey species (Yoshida et al. 2003, 2007; Jones & Ellner 2004, 2007). Our results show that the qualitative properties of evolution-driven cycles in our experimental system are a general consequence of rapid prey evolution in a predator–prey food chain, occurring in a specific but biologically relevant region of parameter space. Specifically, genotypes with better defence against predation become more common when predators are abundant, and less common (due to poorer competitive ability) when predators are rare. We then confirmed this theoretical prediction experimentally, with close qualitative and quantitative agreement between experimental results and a refined version of our evolutionary model (Yoshida et al. 2003; figure 1a–d). Finally, we verified the postulated trade-off between defence against predation and ability to compete for scarce nutrients (Yoshida et al. 2004).
A basic model for our experimental system is given by equation (2.1) below; this model will be the starting point for the new theoretical results that we present in this paper:
| (2.1) |
where x1, x2, y are the abundances of the two prey clones and the predator; S is the amount of a limiting substrate required for algal growth; and Q=p1x1+p2x2 is total prey ‘quality’ as perceived by the predator. Time has been rescaled so that the dilution rate (the fraction of medium replaced each day) is 1, and state variables have been rescaled so that the rate of substrate supply is 1 and a unit of prey consumption yields one net predator birth.
The prey types are assumed to be two genotypes within a single species, differing in two ways which we have documented in our experimental systems: their ‘palatability’ pi which determines their relative risk of being attacked and consumed by the predator, and their ability to compete for scarce resources, represented by ki. There is a trade-off between defence against predation and the ability to compete for nutrients when nutrient concentration is low (Yoshida et al. 2004), so the genotype with the lower value of p has the higher value of k. The models fitted to our experimental data also include predator age structure (Fussmann et al. 2000; Yoshida et al. 2003), mortality within the chemostat and a predator functional response that becomes linear (type-I) at low food density, but these features have no qualitative effect apart from stabilizing the system at very low dilution rates (Jones & Ellner 2007). The model's main qualitative predictions about potential effects of rapid contemporary prey evolution on predator–prey cycles are also robust to changes in the functional forms of the prey and predator functional responses (Jones & Ellner 2007). However, it is important for our results that the model does not allow the predator to preferentially attack and consume the less defended prey type. Prey genotypes are attacked in proportion to their abundance, and better defence means a higher chance of surviving an attack. These assumptions have been verified experimentally for our chemostat system (Meyer et al. 2006).
By mathematical and computational analysis of a general version of model (2.1), we have shown (Jones & Ellner 2007) that evolution-driven cycles occur when defence against predation is effective but cheap: p1 is much smaller than p2, but k1 is not much larger than k2. When the cost of defence is extremely low, a surprising phenomenon occurs, which we have called ‘cryptic dynamics’: the predator cycles in abundance, but the total prey population remains effectively constant through time (figure 1e). We know that the cost of defence is quite low for some genotypes in our algal study species (Meyer et al. 2006), and the cryptic dynamics predicted by the model have been observed in our experimental system (Yoshida et al. 2007; figure 1f). The constancy of total prey abundance occurs in the model due to near exact compensation between the defended and undefended genotypes. Each genotype is undergoing large changes in abundance, but as the cost of defence is made very low, the oscillations of the two genotypes become almost exactly out of phase with each other, leading to near constancy of their sum (Jones & Ellner 2007). In addition, the coefficient of variation in the trait becomes smaller and smaller relative to the coefficient of variation in the predators (compare the dotted curves in figure 1e versus figure 1c): only the predator continues to have cycles whose peaks are much higher than the troughs. Since defence is demonstrated to be cheap, or even free, in a number of natural and laboratory systems, especially those involving organisms with short generation times (Andersson & Levin 1999; Yoshida et al. 2004; Gagneux et al. 2006), cryptic dynamics may be more common than hitherto anticipated.
3. Results I: dynamic effects of prey genetic variability
The phenomenon of cryptic dynamics shows that the dynamics of a predator–prey interaction are not just determined by the presence or absence of contemporary evolution. The details matter, and the nature and extent of genetic variability for traits affecting the interaction are important details.
Our goal in this paper is to explore theoretically how changes in the suite of genotypes that are present in the prey and predator populations can affect dynamics at the population and community levels. We begin, in this section, by considering genetic variation in prey alone, with prey clones arrayed along a trade-off curve between competitive ability and defence against predation. Given a trade-off curve, how is the qualitative nature of the population-level dynamics affected by the presence or absence of specific genotypes along the curve, such as the presence or absence of well-defended types, or the number of alternative clones filling in the range of variation from low to high levels of defence? And how does the shape of the trade-off curve affect these predictions?
(a) Systems with two extreme prey types
A frequent outcome of competition between two prey clones in our model is selection for two extreme types: either the very least and most defended among the clones present in the population, or clones very near to those extremes. In such cases, the range of variation present (the values of p1 and p2) determines the outcome, and the shape of the trade-off curve is irrelevant.
Figure 2 summarizes how the population dynamics are affected by varying the range of palatability when only two extreme clones are present. When the better-defended clone has very effective defence (p1≤0.1), theory predicts the type of evolutionary cycles (EC, figure 2) observed by Yoshida et al. (2003), or possibly cryptic cycles (Yoshida et al. 2007), depending on the cost of defence. When defence is less effective, the system goes to a steady state (SS, figure 2). For a narrow range of p1-values (e.g. p1≈0.14 in figure 2), both prey types coexist at SS: the fraction of the defended prey clone is high but less than 100 per cent. This is followed by a substantial range of defence levels where the SS involves only the defended clone and the predator. Eventually, for moderate to low levels of defence (0.3≤p1<p2 in figure 2), there are classical consumer–resource cycles (figure 2) with the predator and only the defended clone. What happens when defence is nearly ineffective (p1≈p2) is very sensitive to the specifics of the trade-off curve. However, the qualitative pattern shown in figure 2 for high to moderately effective defence is robust to changes in parameter values, and also to some qualitative changes in the model (i.e. the form of the predator functional response, inclusion versus omission of predator age structure; Jones & Ellner 2004, 2007).
Figure 2.
Bifurcation diagrams for a model with two prey clones having palatabilities p1, and p2=1, with a linear trade-off between p and competitive ability. (a) The minimum and maximum of prey (green circles) and predator (red circles) populations, once the populations have settled onto their attractor (an equilibrium or limit cycle). EC, evolutionary cycles; SS, steady state; CRC, classical consumer–resource cycles. (b) The minimum and maximum of the fraction of the defended clone, p1, in the prey population.
The pattern predicted in figure 2 is somewhat counter-intuitive, because it says that improved defence by a defended clone is beneficial to the undefended clone. Specifically, an undefended clone can persist in the system only when the defended clone is nearly invulnerable to predation. What causes this pattern is indirect facilitation, a reversal of the familiar ‘apparent competition’ scenario. Increased defence by the defended prey (on its own) is bad for the predator, so it is good for undefended prey. As a prey lineage evolves better and better defence, eventually driving the predator population to low numbers, it opens the way for a superior competitor that is not paying a cost (however small) for anti-predator defence.
Some preliminary results (Becks et al. in preparation; figure 3) from chemostat experiments with Chlamydomonas reinhardii as the algal prey exhibit the pattern predicted by figure 2 with regard to EC versus SS coexistence. The Chlamydomonas system has the advantage that one prey defence trait, namely the formation of clumps that are less easily consumed by the predator, is visible and easily quantified, so we can track the dynamics of this trait along with the population dynamics. We have shown that prey clumping reduces the ability of predators to consume them, and that the tendency to clump is heritable (Becks et al. in preparation). However, clumping may not be the only defence trait that these prey can evolve, so the degree of clumping may not be a complete indication of the level of prey defence. A direct indicator of the effectiveness of prey defence is the relationship between prey abundance and predator population growth. The better defended the prey are, the lower the predator per capita rate of increase at any given prey abundance, and the higher the prey abundance required for the predator population to increase rather than decrease.
Figure 3.
Chemostat experiments (Becks et al. in preparation) with the rotifer B. calyciflorus as the predator (red solid curve and filled circles) and the asexual green alga Chlamydomonas reinhardii as the prey (green dashed curve and open circles) ((a) weaker defence: SS and (b) stronger defence: cycles). The circles and triangles are experimental measurements, and the curves are a smooth of the data by local polynomial regression. The purple curve (dotted curve and triangles) shows the mean clump size of the algal population. See the text for discussion of clumping as an anti-predator defence trait. Units are individuals ml−1 for predator population density, 104 cells ml−1 for prey population density and mean number of cells per clump.
In the experiment shown in figure 3a, the system settled to a SS with defended prey (as indicated by the occurrence of clumping). The level of prey defence in this experiment was such that the predator population had per capita rate of increase r≈0 at algal density of approximately 105 ml−1 (days 45–58). The experiment shown in figure 3b was run under the same conditions, but was initialized with prey drawn from a population that had been exposed to predation for several months. The prey in this latter experiment developed far more effective defence than in the former experiment, as seen by the fact that (for example) at days 25 and 60 the predator population was decreasing even though the algal density was approximately 2×105 ml−1. As predicted in figure 2, stronger prey defence was accompanied by a shift from SS to EC. A complete analysis of these experiments and additional replicates will be presented elsewhere (Becks et al. in preparation).
(b) Systems with more than two prey types
The details of the trade-off between defence and competitive ability become important when more than two prey types are initially present. Depending on the shape of the trade-off curve, it may be possible for more than two prey types to coexist with the predator. In that situation, the number of prey types present, the range of palatability between the least and most defended types and the shape of the trade-off curve together determine whether the system exhibits stable limit cycles, transient cycling followed by a coexistence equilibrium of one or more types, or exclusion of all but one prey genotype. The trade-off between defence against predation and competitive ability is modelled as follows:
| (3.1) |
where is the half-saturation constant for the completely undefended type; γ is the cost of defence; and b sets the shape of the trade-off curve. Increased defence results in an increase in the half-saturation constant ki(p), and thus a reduction in competitive ability relative to the undefended type in a predator-free environment. This curve (figure 4a) is only an example of many trade-off formulations that produce consistently similar results. For our purposes here, we set the minimum p-value as p1=0.01, and palatabilities pi take values between 0.01 and 1. Again, the mathematical model for the system is equation (2.1), but in this case with i=1, 2, …, N.
Figure 4.
(a) Trade-off curves specified by equation (3.1). (b,c) Trajectories for a trade-off curve with b=2, γ=1, for (b) N=2, and (c) 32 initial prey types. Total prey are shown as dashed green curve and the predator is shown as solid red curve. A thin black line denotes the mean predator density, which remains approximately 0.05 for all three scenarios in (a–c). The dilution rate is d=0.7d−1, a setting for which we consistently observed cycles in our original experimental system (Jones & Ellner 2004). (d–f) Trajectories for a trade-off curve with b=0.5, γ=1, for (d) N=2, (e) 4 and (f) 32 initial prey types. Total prey are shown in green and the predator is shown in red. A thin black line denotes the mean predator density, which is approximately 0.05, 0.07 and 0.09, respectively, for the 2, 4 and 32 clone scenarios in (d–f).
For shape parameter b>1, the trade-off curve is convex (figure 4a) for most settings of the cost parameter γ and favours extreme types (figure 5a–c). In the limit (as time t →∞), the system behaves as a two-clone system: given p1 is small, and any initial number of types arrayed on the trade-off curve, there are stable EC for the dilution rate d=0.7 (figure 4a–c). As defence drops (p1 increases in value), there is first a narrow range of stable coexistence between the two extreme types, p1 and pN, together with the predator. As with the two-prey system, there is then a substantial range of defence levels where the SS involves only the most defended type (p1) and the predator. As the defence of the most defended type becomes moderate, similar to the two-prey system, there are then consumer–resource cycles. The small variation in cycle period observed in figure 4b,c for different numbers of initial prey types is a transient phenomenon, the duration of which increases as more prey types are added to the system and the trade-off curve gets ‘crowded’: each subsequent prey type becomes more similar to those immediately adjacent to it on the curve. Figure 5a–c shows surviving prey types for each of two initial scenarios: N=2, 4 or 32 prey types, when b=2. In each case, it is the extreme prey types (filled circles) that survive in the presence of the predators, with the most vulnerable prey type at very low densities and the best defended at high densities.
Figure 5.
Mean clonal frequencies (log scale, note differences in vertical scaling) for surviving prey types after 600 days at a dilution rate d=0.7d−1. (a–c) Assume that there were initially N=(a) 2, (b) 4 and (c) 32 prey types arrayed on a trade-off curve with shape parameter b=2 and cost parameter γ=1. In this case, the surviving clones are the extremes (filled circles), and after the transient phase—which increases in length as prey types are added to the trade-off curve—the system behaves approximately as a two-clone system: it exhibits stable limit cycles at this dilution rate. (d–f) Assume that there were initially N=(d) 2, (e) 4 and (f) 32 prey types arrayed on a trade-off curve with shape parameter b=0.5 and cost parameter γ=1. In this case, there is an internal equilibrium with the most defended type and one or more very well-defended types (filled circles). The number of coexisting types increases with the number of clones and the concavity of the trade-off curve. Clones are indexed in the order of increasing palatability.
For shape parameter b<1, the trade-off curve is concave (figure 4a), and favours intermediate types. Again assuming that the most defended type is nearly invulnerable to predation, p1≈0, as the number of prey clones increases above two types, there is an initial phase of transient EC followed by equilibrium during which there is a stable coexistence of two or more of the best-defended types. With an increase in prey types, the transient phase becomes shorter and shorter, and the dynamics are overall more stable with well-defended types dominating. In figure 4d–f, we illustrate what happens as prey clonal diversity is increased at the experimentally determined ‘cycling’ flow-through rate of d=0.7, and for p1≈0 and trade-off shape parameter b=0.5: given N=2 prey clones, the system exhibits stable EC with a period of approximately 30 days and stable cycle amplitudes. As the clones are increased to N=4, the cycles rapidly shorten in period and decline in amplitude with time as the system goes to equilibrium. By N=32, the transient phase comprises only one complete (evolutionary) cycle, and the system is otherwise in equilibrium. Predator densities very slowly increase with the number of initial clones, as the dominant clone shifts to an intermediate type (e.g. see figure 5, b=0.5, N=32). For further details and analysis of coexistence equilibria in our experimentally parametrized model, please see Jones & Ellner (2004).
4. Results II: dynamic effects of prey and predator genetic variability
In §3, we were able to give a rather detailed account of the effects of prey genetic diversity on community dynamics. Of course, there is no a priori reason why the predator in these systems should not also evolve. Indeed, we have shown that predator evolution does occur in our experimental rotifer–algal system and—just as prey evolution—can have strong effects on the stability of the observed predator–prey dynamics (Fussmann et al. 2003). We are only beginning to explore how contemporary coevolution of predator and prey affects the dynamics. Because both predator and prey in our rotifer–algal system are clonal populations, genetic diversity can easily be introduced at both trophic levels simultaneously and the effects on community dynamics can be studied. Given our experience with prey-evolution-only systems, we expect, however, that understanding the resulting eco-evolutionary dynamics will require a detailed mathematical analysis that accounts for the multitude of possible type-by-type interactions that occur within and across trophic levels.
We here present a first step in this direction by formulating a predator–prey model that contains up to six clones/genotypes within predator and prey levels and allows for the interaction of each predator with each prey type. So far, we are treating this model as an exploratory tool to understand the dynamical possibilities of this complex system, and intend to model concrete co-evolutionary predator–prey scenarios (i.e. rotifer and algal clones in the chemostat) in the future. The current model assumes logistic prey growth, a multispecies type-II functional response and is a system of up to 12 differential equations (M=6 prey and N=6 predator clones):
| (4.1) |
where xi and yi are the abundances of the six prey and predator clones, respectively, and are measures of effective total prey abundance as perceived by predator clone j. Parameters ri and dj are maximum prey clone growth rates and predator mortalities; parameters aji and bji determine the saturation value and steepness of the functional responses for pairwise predator–prey clone interactions. The total abundance of all prey clones is regulated by the carrying capacity K. If no clonal diversity is present (M=N=1), the system simplifies to the Rosenzweig–MacArthur model (Rosenzweig & MacArthur 1963). We parametrized in agreement with values encountered in plankton predator–prey systems (Fussmann & Heber 2002) that led to stable limit cycles for a system with a single prey and a single predator type (figure 6a). We introduced clonal diversity by generating aji and bji matrices with random uniform distribution (±5%) around the base values of a=7.5 and b=5.0. To see the effects of heritable trait variation, we contrasted the dynamics of a non-evolving (one-prey–one-predator) system using the base parameters, with the dynamics of evolving multiple-clone systems with randomly generated parameters.
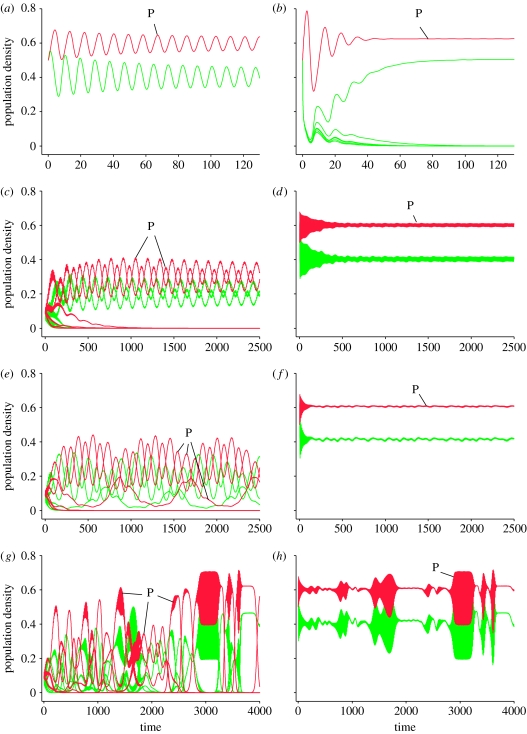
Figure 6.
Trajectories of predator–prey systems (equation (4.1)) with multiple prey (green) and predator (red; letter ‘P’) genotypes. (a) Benchmark system with one predator and one prey genotype. (b) Selection of a single prey type in a six-prey–one-predator system. (c) Selection of a two-prey–two-predator system in a six-prey–six-predator system. (d) Species dynamics of (c) (summation of predator and prey genotype abundances). (e) Selection of a three-prey–three-predator system in a six-prey–six-predator system. Note the two fast and one slowly cycling predator–prey pairs. (f) Species dynamics of (e). (g) Intermittent dynamics alternating between periods of dominance of single and multiple predator–prey pairs. (h) Species dynamics of (g). Parameters: ri=2.5, K=1, dj=1, aji=7.5±5%, bji=5.0±5%. High-frequency oscillations appear as continuously filled areas in (d,f–h).
What are the effects on predator–prey dynamics when diversity exists at both the predator and prey levels? Numerical simulations of equation (4.1) result in a wealth of dynamic scenarios but some interesting, repeatable patterns emerge.
(a) Selection processes among types are important and affect the dynamics at the species level
In all our simulations, only a subset of the six prey or predator types persisted in the long run. Selective survival of genotypes—due to natural selection—represents the evolutionary component of the eco-evolutionary dynamics we describe here. A frequently observed outcome in a six-prey–one-predator system is the selection of a single prey type that coexists with the predator at stable equilibrium (figure 6b), whereas a one-prey–one-predator system with the prey having the base parameter values displays limit cycle dynamics with the predator (figure 6a). Stabilization occurs because a prey type is selected that provides the highest growth rate in the presence of the predator, and at the same time shifts the predator–prey pair to the stable side of the Hopf bifurcation. Selection of genotypes at both predator and prey levels is the norm for the full six-prey–six-predator system, but very frequently systems of multiple prey and predator types are selected that coexist on compensatory cycles (i.e. predator and prey types alternate regularly in relative frequency and display oscillatory dynamics characterized by pairwise interactions of predator types with ‘their’ prey type; figure 6c,e). Figure 6e shows an interesting example of coexistence of three predator and three prey types. Each of the three distinguishable predator–prey pairs shows characteristic predator–prey dynamics with the typical shift between predator and prey peaks. One predator–prey pair cycles much more slowly than the other two, whose frequencies are approximately five times higher. These examples show that when prey and predator are evolving in tandem, the ‘cryptic cycles’ phenomenon can occur at both trophic levels, with rapid dynamics at the clone-frequency level resulting in total species abundances that remain nearly constant.
(b) High type diversity is rare
Most frequently, systems with two predator and two prey clones become selected. Complicated systems with many types appear to be unable to coexist in the homogeneous environment that this model represents. On the other hand, selection of just one clone of prey and predator each is not the most frequent outcome. It seems that our six-by-six interaction model is able to reproduce the essential dynamical patterns expected in homogeneous systems of much higher genetic diversity because they all reduce to systems of low to moderate diversity. Clonal genetic diversity in natural communities is often much higher (e.g. De Meester et al. 2006). This suggests that complex dynamics in complex community networks are not a likely mechanism that maintains high levels of genetic diversity in homogeneous natural systems.
(c) Genotype dynamics are wild, species and community dynamics are calm
The increased complexity of the multi-predator–prey system tends to lead to more complex and erratic dynamics at the genotype level (figure 6c,e,g), compared with the benchmark one-predator–one-prey system (figure 6a). However, species dynamics (the sums of the abundances of predator and prey types, respectively) become stabilized relative to the benchmark system, as also observed by Vos et al. (2001) for a similar model system. This is partly due to statistical averaging of time-series (Cottingham et al. 2001), but is primarily caused by the compensatory nature of the dynamics, i.e. the regular succession of different clones as opposed to their synchronous appearance and disappearance, and may lead to apparently steady-state dynamics at the species level (figure 6d,f). This is another instance of cryptic dynamics in which highly dynamic patterns of cyclical selection of genotypes are hidden under a calm surface of species dynamics. Because this type of cryptic dynamics can occur at very moderate levels of trait diversity (parameters a and b diverging only up to 5% from their mean value), it is likely to play an important role in real communities, even when within-species diversity is relatively low.
(d) Species dynamics may appear noisy or display intermittency
Owing to the complexity of the dynamics, genotype abundances never add up to smooth steady-state or cyclical species dynamics. Instead, equilibria appear ‘noisy’ (figure 6d,f) or the dynamics show intermittent patterns. Intermittency occurs because periods dominated by multiple genotype interactions alternate with periods of classical predator–prey oscillations (when only one prey and one predator genotype dominate; figure 6g,h). These simulations suggest that the noisy and intermittent patterns so frequently observed in natural communities are potentially due to intrinsic, cryptic genotype dynamics and are not necessarily the result of changing environmental factors.
The patterns described here emerged from a relatively simple mathematical model in which we—somewhat artificially—generated the potential for evolutionary dynamics by setting initial levels of genetic diversity of up to 12 genotypes. As such, we investigated the interaction between ecological dynamics and the dynamical process of natural selection, but neglected processes that are able to restore genetic diversity. In natural communities, mutation and immigration of genotypes are such processes, and future studies should consider their impact on eco-evolutionary dynamics. We suspect that the effects of pulsed mutation or immigration events will be understandable within the framework that we have presented here because they just reset genetic diversity to higher levels at fixed time intervals (as we did at the start of our simulations). However, continuous rates of mutation or immigration at the time-scale of the ecological dynamics may lead to dynamical outcomes not covered by our current analysis, and we see the inclusion of these processes as an important continuation of our work.
5. Discussion
Although the fields of ecology and evolutionary biology are both over a century old, we are only beginning to understand how tightly coupled the processes at the heart of these disciplines truly are. Becoming somebody else's dinner represents very strong natural selection, and it is hard to imagine the predator–prey interaction or any other strong interspecific interaction occurring without direct evolutionary consequences. But exactly that thinking is embedded in much of the ecological theory underpinning environmental and natural resource management. By studying some simple model food webs based directly on experiments, we hope to start developing ecological theory for a rapidly evolving biosphere.
We now return to the questions we posed in §1: how rapid contemporary evolution can affect the dynamics of communities (question (i)), and then comparing the dynamic possibilities when genetic diversity is present versus absent (question (ii)). Even our simplest experimental systems (one predator and two prey genotypes) and the models developed to explain them generate a range of qualitatively different dynamics (§3). Changing the details of a trade-off between the cost of a defence and its effectiveness can dramatically alter the identity of the surviving prey types, as can environmental factors (such as microcosm flow-through rate). Adding realistic, if theoretical, levels of evolutionary complexity in the form of heritable variation in ecologically relevant traits of both predator and prey opens the door to even more possibilities (§4): selection of one prey type under some circumstances, and multiple types under others and dynamics varying from equilibrium to chaos. Conversely, evolutionary dynamics can sometimes make for greater simplicity at the species or community level, as complex dynamics at the genotype level may lead to constant or nearly constant population abundances, instead of cycles, in coexisting populations (§§3 and 4).
A challenge posed by these findings is to develop general theory for linked eco-evolutionary dynamics, so that we can understand which biological conditions give rise to different ecological outcomes, and why. One approach that we are currently exploring is the theory of slow–fast systems. This was used a decade ago (Khibnik & Kondrashov 1997) under the conventional assumption of slow evolutionary dynamics relative to fast ecological dynamics. To gain insights into possible effects of coupled and equally rapid evolutionary and ecological dynamics, we believe that it will be useful to consider the opposite limit where evolutionary change is fast relative to ecological dynamics (Cortez & Ellner in preparation). This is not biologically impossible—it could happen if many births, balanced by nearly as many deaths, result in slow changes in total population size but allow rapid change in genotype frequencies. Most importantly, it reduces model dimension and creates real possibilities for mapping out all dynamic possibilities and understanding when each of them can occur.
Because temporal dynamics provide information about underlying processes (e.g. Kendall et al. 2005; Ives et al. 2008), large-scale changes, such as consumer–resource cycles, are especially revealing about potential effects of rapid contemporary evolution. Several types of natural large-scale ecological dynamics have offered opportunities for studying rapid evolution in the wild, including invasions (e.g. Lavergne & Molofsky 2007; Kinnison et al. 2008), range expansion (Reznick et al. 2008), periodic outbreaks of infectious diseases (e.g. Eldred et al. 2008), annual population cycles driven by seasonality (Duffy & Sivars-Becker 2007) and trait cycles (Sinervo & Lively 1996; Sinervo et al. 2000). Systems at SS may also be shaped by rapid evolution, e.g. population stability may be an outcome of the interaction between potentially rapid ecological and evolutionary processes (e.g. Doebeli & Koella 1995; Zeineddine & Jansen 2005). But demonstrating what drives this outcome in natural settings may require experimental manipulations that are necessarily high cost and high effort (e.g. Fischer et al. 2001), and whose interpretation has often been problematic because of inadvertent confounding factors resulting from large-scale interventions (Turchin 2003). Natural dynamic processes may thus offer the best prospects for confronting theory with data on consequences of rapid evolution, which is critically important as the theory begins to develop.
Our most fundamental theoretical prediction is that the level of heritable variation in a trait affecting ecological interactions is a bifurcation parameter: small gradual quantitative changes in unseen evolutionary processes can cause large abrupt qualitative changes at the population and community levels. For the practical issues of ecological management and forecasting that we posed in §1, it is already widely recognized that we need to consider possible effects of evolutionary responses that are evoked by human impacts and interventions. Our results raise the converse issue: we may also need to consider the effects of evolutionary responses that are slowed or constrained when genetic variation is reduced by human impacts, such as habitat loss or fragmentation.
Acknowledgments
Our research has been supported primarily by the Andrew W. Mellon Foundation, the James S. McDonnell Foundation and the Natural Sciences and Engineering Research Council of Canada. We thank Andrew Hendry and two anonymous referees for their comments on the manuscript, and also the many Cornell undergraduates who assisted with data collection.
Footnotes
One contribution of 14 to a Theme Issue ‘Eco-evolutionary dynamics’.
References
- Abrams P.A. ‘Adaptive dynamics’ vs. ‘adaptive dynamics’. J. Evol. Biol. 2005;18:1162–1165. doi: 10.1111/j.1420-9101.2004.00843.x. doi:10.1111/j.1420-9101.2004.00843.x [DOI] [PubMed] [Google Scholar]
- Abrams P.A., Matsuda H. Prey adaptation as a cause of predator–prey cycles. Evolution. 1997;51:1742–1750. doi: 10.1111/j.1558-5646.1997.tb05098.x. doi:10.2307/2410997 [DOI] [PubMed] [Google Scholar]
- Andersson D.L., Levin B.R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 1999;2:489–493. doi: 10.1016/s1369-5274(99)00005-3. doi:10.1016/S1369-5274(99)00005-3 [DOI] [PubMed] [Google Scholar]
- Becks, L., Ellner, S. P., Jones, L. E. & Hairston Jr, N. G. In preparation. Changes in genetic variance can radically alter eco-evolutionary dynamics.
- Boraas M.E., Searle D.B., Boxhorn J.E. Phagotrophy by a flagellate selects for colonial prey: a possible origin of multicellularity. Evol. Ecol. 1998;12:153–164. doi:10.1023/A:1006527528063 [Google Scholar]
- Cain M.L., Bowman W.D., Hacker S.D. Sinauer Associates; Sunderland, MA: 2008. Ecology. p. 621. [Google Scholar]
- Cortez, M. & Ellner, S. P. In preparation. Understanding rapid evolution in predator–prey interactions using the theory of slow–fast dynamical systems. [DOI] [PubMed]
- Cottingham K.L., Brown B.L., Lennon J.T. Biodiversity may regulate the temporal variability of ecological systems. Ecol. Lett. 2001;4:72–85. doi:10.1046/j.1461-0248.2001.00189.x [Google Scholar]
- Cousyn C., De Meester L., Colbourne J.K., Brendonck L., Verschuren D., Volckaert F. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc. Natl Acad. Sci. USA. 2001;98:6256–6260. doi: 10.1073/pnas.111606798. doi:10.1073/pnas.111606798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester L., Vanoverbeke J., De Gelas K., Ortells R., Spaak P. Genetic structure of cyclic parthenogenetic zooplankton populations—a conceptual framework. Arch. Hydrobiol. 2006;167:217–244. doi:10.1127/0003-9136/2006/0167-0217 [Google Scholar]
- Doebeli M., Koella J.C. Evolution of simple population dynamics. Proc. R. Soc. B. 1995;260:119–125. doi:10.1098/rspb.1995.0068 [Google Scholar]
- Dube D., Kim K., Alker A.P., Harvell C.D. Size structure and geographic variation in chemical resistance of sea fan corals Gorgonia ventalina to a fungal pathogen. Mar. Ecol. Prog. Ser. 2002;231:139–150. doi:10.3354/meps231139 [Google Scholar]
- Duffy M.A., Sivars-Becker L. Rapid evolution and ecological host–parasite dynamics. Ecol. Lett. 2007;10:44–53. doi: 10.1111/j.1461-0248.2006.00995.x. doi:10.1111/j.1461-0248.2006.00995.x [DOI] [PubMed] [Google Scholar]
- Decaestecker E., Gaba S., Raeymaekers J.A.M., Stoks R., Van Kerckhoven L., Ebert D., De Meester L. Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature. 2007;450:870–873. doi: 10.1038/nature06291. doi:10.1038/nature06291 [DOI] [PubMed] [Google Scholar]
- Eldred B.D., Dushoff J., Dwyer G. Host–pathogen interactions, insect outbreaks, and natural selection for disease resistance. Am. Nat. 2008;172:829–842. doi: 10.1086/592403. doi:10.1086/592403 [DOI] [PubMed] [Google Scholar]
- Ellner S., Hairston N.G., Jr, Kearns C.M., Babaï D. The roles of fluctuating selection and long-term diapause in microevolution of diapause timing in a freshwater copepod. Evolution. 1999;53:111–122. doi: 10.1111/j.1558-5646.1999.tb05337.x. doi:10.2307/2640924 [DOI] [PubMed] [Google Scholar]
- Ezard T.H.G., Côté S.D., Pelletier F. Eco-evolutionary dynamics: disentangling phenotypic, environmental and population fluctuations. Phil. Trans. R. Soc. B. 2009;364:1491–1498. doi: 10.1098/rstb.2009.0006. doi:10.1098/rstb.2009.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrière R., Couvert D. Cambridge Studies in Adaptive Dynamics. vol. 4. Cambridge University Press; Cambridge, UK: 2004. Evolutionary conservation biology. [Google Scholar]
- Fischer J.M., Klug J.L., Ives A.R., Frost T.M. Ecological history affects zooplankton community responses to acidification. Ecology. 2001;82:2984–3000. doi:10.2307/2679829 [Google Scholar]
- Fussmann G.F., Heber G. Food web complexity and chaotic population dynamics. Ecol. Lett. 2002;5:394–401. doi:10.1046/j.1461-0248.2002.00329.x [Google Scholar]
- Fussmann G.F., Ellner S.P., Shertzer K.W., Hairston N.G., Jr Crossing the Hopf bifurcation in a live predator–prey system. Science. 2000;290:1358–1360. doi: 10.1126/science.290.5495.1358. doi:10.1126/science.290.5495.1358 [DOI] [PubMed] [Google Scholar]
- Fussmann G.F., Ellner S.P., Hairston N.G., Jr Evolution as a critical component of plankton dynamics. Proc. R. Soc. B. 2003;270:1015–1022. doi: 10.1098/rspb.2003.2335. doi:10.1098/rspb.2003.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussmann G.F., Loreau M., Abrams P.A. Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 2007;21:465–477. doi:10.1111/j.1365-2435.2007.01275.x [Google Scholar]
- Gagneux S., Long C.D., Small P.M., Van T., Schoolnik G.K., Bohannan B.J.M. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–1946. doi: 10.1126/science.1124410. doi:10.1126/science.1124410 [DOI] [PubMed] [Google Scholar]
- Grant P.R., Grant B.R. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. doi:10.1126/science.1070315 [DOI] [PubMed] [Google Scholar]
- Hairston N.G., Jr, De Meester L. Daphnia paleogenetics and environmental change: deconstructing the evolution of plasticity. Int. Rev. Hydrobiol. 2008;93:578–592. doi:10.1002/iroh.200811057 [Google Scholar]
- Hairston N.G., Jr, Walton W.E. Rapid evolution of a life-history trait. Proc. Natl Acad. Sci. USA. 1986;83:4831–4833. doi: 10.1073/pnas.83.13.4831. doi:10.1073/pnas.83.13.4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston N.G., Jr, Lampert W., Caceres C.E., Holtmeier C.L., Weider L.J., Gaedke U., Fischer J.M., Fox J.A., Post D.M. Lake ecosystems: rapid evolution revealed by dormant eggs. Nature. 1999;401:446. doi:10.1038/46731 [Google Scholar]
- Hairston N.G., Jr, Ellner S.P., Geber M.A., Yoshida T., Fox J.A. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 2005;8:1114–1127. doi:10.1111/j.1461-0248.2005.00812.x [Google Scholar]
- Heath D.D., Heath J.W., Bryden C.A., Johnson R.M., Fox C.W. Rapid evolution of egg size in captive salmon. Science. 2003;299:1738–1740. doi: 10.1126/science.1079707. doi:10.1126/science.1079707 [DOI] [PubMed] [Google Scholar]
- Hendry A.P., Kinnison M.T. The pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. doi:10.2307/2640428 [DOI] [PubMed] [Google Scholar]
- Ives A.R., Einarsson Á., Jansen V.A.A., Gardarsson A. High-amplitude fluctuations and alternative dynamical states of midges in Lake Myvatn. Nature. 2008;452:84–87. doi: 10.1038/nature06610. doi:10.1038/nature06610 [DOI] [PubMed] [Google Scholar]
- Johnson M.T.J., Lajeunesse M.J., Agrawal A. Additive and interactive effects of plant genotypic diversity on arthropod communities and plant fitness. Ecol. Lett. 2006;9:24–34. doi: 10.1111/j.1461-0248.2005.00833.x. doi:10.1111/j.1461-0248.2005.00833.x [DOI] [PubMed] [Google Scholar]
- Johnson M.T.J., Vellend M., Stinchcombe J.R. Evolution in plant populations as a driver of ecological changes in arthropod communities. Phil. Trans. R. Soc. B. 2009;364:1593–1605. doi: 10.1098/rstb.2008.0334. doi:10.1098/rstb.2008.0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.E., Ellner S.P. Evolutionary tradeoff and equilibrium in an aquatic predator–prey system. Bull. Math. Biol. 2004;66:1547–1573. doi: 10.1016/j.bulm.2004.02.006. doi:10.1016/j.bulm.2004.02.006 [DOI] [PubMed] [Google Scholar]
- Jones L.E., Ellner S.P. Effects of rapid prey evolution on predator–prey cycles. J. Math. Biol. 2007;55:541–573. doi: 10.1007/s00285-007-0094-6. doi:10.1007/s00285-007-0094-6 [DOI] [PubMed] [Google Scholar]
- Kendall B.E., Ellner S.P., McCauley E., Wood S.N., Briggs C.J., Murdoch W.W., Turchin P. Population cycles in the pine looper moth Bupalus piniarius: dynamical tests of mechanistic hypotheses. Ecol. Monogr. 2005;75:259–276. doi:10.1890/03-4056 [Google Scholar]
- Khibnik A.I., Kondrashov A.S. Three mechanisms of Red Queen dynamics. Proc. R. Soc. B. 1997;264:1049–1056. doi:10.1098/rspb.1997.0145 [Google Scholar]
- Kinnison M.T., Hairston N.G., Jr Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 2007;21:444–454. doi:10.1111/j.1365-2435.2007.01278.x [Google Scholar]
- Kinnison M.T., Hendry A.P. The pace of modern life II: from rates of contemporary microevolution to pattern and process. Genetica. 2001;112–113:45–164. [PubMed] [Google Scholar]
- Kinnison M.T., Unwin M.J., Quinn T.P. Eco-evolutionary vs. habitat contributions to invasion in salmon: experimental evaluation in the wild. Mol. Ecol. 2008;17:405–414. doi: 10.1111/j.1365-294X.2007.03495.x. doi:10.1111/j.1365-294X.2007.03495.x [DOI] [PubMed] [Google Scholar]
- Lavergne S., Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc. Natl Acad. Sci. USA. 2007;104:3883–3888. doi: 10.1073/pnas.0607324104. doi:10.1073/pnas.0607324104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski R.E., Travisano M. Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations. Proc. Natl Acad. Sci. USA. 1994;91:6808–6814. doi: 10.1073/pnas.91.15.6808. doi:10.1073/pnas.91.15.6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.R., Kassen R. The effects of competition and predation on diversification in the model adaptive radiation. Nature. 2007;446:432–435. doi: 10.1038/nature05599. doi:10.1038/nature05599 [DOI] [PubMed] [Google Scholar]
- Meyer J.R., Ellner S.P., Hairston N.G., Jr, Jones L.E., Yoshida T. Prey evolution on the time scale of predator–prey dynamics revealed by allele-specific quantitative PCR. Proc. Natl Acad. Sci. USA. 2006;103:10 690–10 695. doi: 10.1073/pnas.0600434103. doi:10.1073/pnas.0600434103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molles M.C., Jr . 4th edn. McGraw Hill; New York, NY: 2008. Ecology: concepts, applications. [Google Scholar]
- Olsen E.M., Heino M., Lilly G.R., Morgan M.J., Brattey J., Ernande B., Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. doi:10.1038/nature02430 [DOI] [PubMed] [Google Scholar]
- Palkovacs E.P., Marshall M.C., Lamphere B.A., Lynch B.R., Weese D.J., Fraser D.F., Reznick D.N., Pringle C.M., Kinnison M.T. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Phil. Trans. R. Soc. B. 2009;364:1617–1628. doi: 10.1098/rstb.2009.0016. doi:10.1098/rstb.2009.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi S.R. Norton; New York, NY: 2001. The evolution explosion: how humans cause rapid evolutionary change. p. 277. [Google Scholar]
- Pelletier F., Clutton-Brock T., Pemberton J., Tuljapurkar S., Coulson T. The evolutionary demography of ecological change: linking trait variation and population growth. Science. 2007;315:1571–1574. doi: 10.1126/science.1139024. doi:10.1126/science.1139024 [DOI] [PubMed] [Google Scholar]
- Post D.M., Palkovacs E.P. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B. 2009;364:1629–1640. doi: 10.1098/rstb.2009.0012. doi:10.1098/rstb.2009.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick D.N., Ghalambor C.K. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica. 2001;112:183–198. doi:10.1023/A:1013352109042 [PubMed] [Google Scholar]
- Reznick D.N., Shaw F.H., Rodd F.H., Shaw R.G. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. doi:10.1126/science.275.5308.1934 [DOI] [PubMed] [Google Scholar]
- Reznick D.N., Ghalambor C.K., Crooks K. Experimental studies of evolution in guppies: a model for understanding the evolutionary consequences of predator removal in natural communities. Mol. Ecol. 2008;17:97–107. doi: 10.1111/j.1365-294X.2007.03474.x. doi:10.1111/j.1365-294X.2007.03474.x [DOI] [PubMed] [Google Scholar]
- Rosenzweig M.L., MacArthur R.H. Graphical presentation and stability conditions of predator–prey interactions. Am. Nat. 1963;97:209–223. doi:10.1086/282272 [Google Scholar]
- Shertzer K.W., Ellner S.P., Fussmann G.F., Hairston N.G., Jr Predator–prey cycles in an aquatic microcosm: testing hypotheses of mechanism. J. Anim. Ecol. 2002;71:802–815. doi:10.1046/j.1365-2656.2002.00645.x [Google Scholar]
- Sinervo B., Lively C.M. The rock–paper–scissors game and the evolution of alternative male strategies. Nature. 1996;380:240–243. doi:10.1038/380240a0 [Google Scholar]
- Sinervo B., Svensson E., Comendant T. Density cycles and an offspring quantity and quality game driven by natural selection. Nature. 2000;406:985–988. doi: 10.1038/35023149. doi:10.1038/35023149 [DOI] [PubMed] [Google Scholar]
- Strauss S.Y., Lau J.A., Schoener T.W., Tiffin P. Evolution in ecological field experiments: implications for effect size. Ecol. Lett. 2008;11:199–207. doi: 10.1111/j.1461-0248.2007.01128.x. doi:10.1111/j.1461-0248.2007.01128.x [DOI] [PubMed] [Google Scholar]
- Swain D.P., Sinclair A.F., Mark Hanson J. Evolutionary response to size-selective mortality in an exploited fish population. Proc. R. Soc. B. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. doi:10.1098/rspb.2006.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.N. Rapid evolution as an ecological process. Trends Ecol. Evol. 1998;13:329–332. doi: 10.1016/s0169-5347(98)01378-0. doi:10.1016/S0169-5347(98)01378-0 [DOI] [PubMed] [Google Scholar]
- Turchin P. Princeton University Press; Princeton, NJ: 2003. Complex population dynamics. [Google Scholar]
- Urban M.C., et al. The evolutionary ecology of metacommunities. Trends Ecol. Evol. 2008;23:311–317. doi: 10.1016/j.tree.2008.02.007. doi:10.1016/j.tree.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Vos M., Moreno Berrocal S., Karamaouna F., Hemerick L., Vet L.E.M. Plant-mediated indirect effects and the persistence of parasitoid–herbivore communities. Ecol. Lett. 2001;4:38–45. doi:10.1046/j.1461-0248.2001.00191.x [Google Scholar]
- Whitham T.G., et al. A framework for community and ecosystem genetics: from genes to ecosystems. Nat. Rev. Genet. 2006;7:510–523. doi: 10.1038/nrg1877. doi:10.1038/nrg1877 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Jones L.E., Ellner S.P., Fussmann G.F., Hairston N.G., Jr Rapid evolution drives ecological dynamics in a predator–prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. doi:10.1038/nature01767 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Hairston N.G., Jr, Ellner S.P. Evolutionary trade-off between defence against grazing and competitive ability in a simple unicellular alga, Chlorella vulgaris. Proc. R. Soc. B. 2004;271:1947–1953. doi: 10.1098/rspb.2004.2818. doi:10.1098/rspb.2004.2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Ellner S.P., Jones L.E., Bohannan B.J.M., Lenski R.E., Hairston N.G., Jr Cryptic population dynamics: rapid evolution masks trophic interactions. PLoS Biol. 2007;5:1868–1879. doi: 10.1371/journal.pbio.0050235. doi:10.1371/journal.pbio.0050235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineddine M., Jansen V.A.A. The evolution of stability in a competitive system. J. Theor. Biol. 2005;236:208–215. doi: 10.1016/j.jtbi.2005.03.004. doi:10.1016/j.jtbi.2005.03.004 [DOI] [PubMed] [Google Scholar]