Abstract
Dispersal and gene flow can have both positive and negative effects on population size, but little empirical support from nature exists for the negative effects. We test for such effects in a stream population of threespine stickleback (Gasterosteus aculeatus L.) that is subject to high gene flow from a lake and is thus maladapted to stream conditions. In this system, maladaptation increases with distance along the stream, and this increase is associated with decreasing population densities until stickleback are no longer present (2.5 km from the lake). We conducted field experiments to inform whether this association might reflect a negative role for gene flow in constraining population size and therefore causing a local range limit. We specifically tested predictions deriving from theory: peripheral populations should show partial local adaptation, be under strong selection and not simply be maintained by dispersal. First, a transplant experiment suggested a weak home-site advantage in the peripheral population. Second, a mark–recapture study showed directional selection for a stream-adapted phenotype in 1 of 2 years. Third, another mark–recapture experiment showed that dispersal is limited to the point that positive demographic effects of dispersal are probably minimal. We conclude that, although gene flow does constrain morphological maladaptation in the outlet stream population, the evidence for its contribution to population size and range limits is mixed. We discuss the implications of our work for the study of factors influencing the evolution of species' ranges.
Keywords: adaptational constraints, eco-evolutionary dynamics, migration load, migrational meltdown, species' range
1. Introduction
Evolutionary forces acting on ecological time scales are increasingly thought to influence population dynamics (Saccheri & Hanski 2006; Fussmann et al. 2007; Kinnison & Hairston 2007; Kokko & López-Sepulcre 2007; Zheng et al. 2009). Most of the work in this area has thus far focused on the role of one evolutionary force: natural selection. Natural selection, by favouring locally adapted genotypes, increases fitness and can thus increase population growth and long-term persistence (Burger & Lynch 1995; Gomulkiewicz & Holt 1995; Hairston et al. 2005; Hanski & Saccheri 2006). Other evolutionary forces can also influence population growth and persistence, but the effect of these forces is less certain. For example, gene flow can, in theory, have a variety of positive or negative consequences for adaptation (Holt & Gomulkiewicz 1997; Storfer 1999; Lenormand 2002; Tallmon et al. 2004; Garant et al. 2007). On the one hand, gene flow between selective environments can increase the frequency of locally maladapted genotypes, which might decrease fitness and thereby have negative consequences for population growth and persistence, i.e. migrational meltdown (Kirkpatrick & Barton 1997; Boulding & Hay 2001; Ronce & Kirkpatrick 2001). On the other hand, gene flow can supply small populations with new allelic variants, which might alleviate inbreeding depression (Tallmon et al. 2004) and increase adaptive potential (Holt & Gomulkiewicz 1997; Perron et al. 2007). At present, empirical support from natural systems exists for the positive effects of gene flow (reviewed in Tallmon et al. 2004), whereas support is generally lacking for the negative effects (Bridle & Vines 2007). This is unfortunate, especially given that the manipulation of gene flow between populations is a commonly advocated conservation measure (Storfer 1999; McLachlan et al. 2007). We here investigate the potentially negative effects of gene flow on population size in threespine stickleback (Gasterosteus aculeatus L.).
Our question and study system can be considered in the context of Kirkpatrick & Barton's (1997) model of how gene flow might limit species' ranges by hindering adaptation along a spatial gradient in selection (see also Case & Taper 2000; Filin et al. 2008). This model assumed that population density is high near the centre of a species' range and then decreases towards the range periphery. By contrast, the selective pressure (the optimal phenotype) changes linearly from one end of the range to the other, which is analogous to a latitudinal environmental gradient. The spatial variation of population density results in asymmetric gene flow from the centre of the range that hinders the adaptation of populations to extreme conditions at the periphery. Assuming that mean population growth rate (r) is a function of mean population fitness, Kirkpatrick & Barton (1997) showed that, if the selective gradient is sufficiently steep, gene flow into peripheral populations can depress population growth and ultimately limit range expansion (figure 1; but see Barton 2001). Despite some studies examining fitness variation across geographical ranges (e.g. Angert & Schemske 2005; Geber & Eckhart 2005), empirical tests in nature of the relevance of gene flow are lacking (Bridle & Vines 2007).
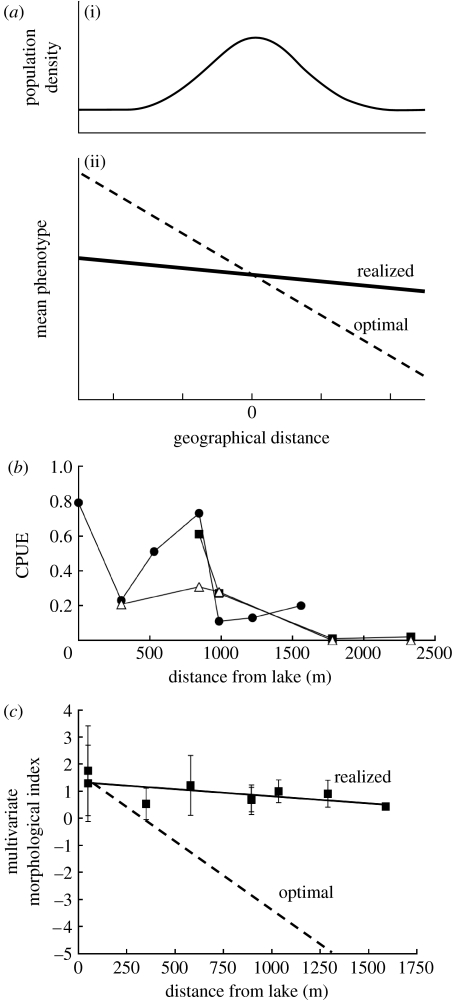
Figure 1.
Similarities between the Kirkpatrick & Barton (1997) model and the Misty Lake system. (a) Some major features of the Kirkpatrick & Barton (1997) model. Population densities are normally distributed around the centre of the range, which causes asymmetric gene flow towards the range periphery ((i) population density and (ii) mean phenotype vs. geographical distance). (b) The selective pressure changes linearly from one end of the range to the other, which leads asymmetric gene flow to cause an increasing mismatch between the realized (observed) and optimal mean phenotypes with increasing distance from the range centre. In the model, the population growth rate (r) is inversely related to this mismatch. (b) Population densities (catch-per-unit-effort (CPUE) as fish per trap hour) in the Misty outlet decrease with increasing distance from the lake. Data points correspond to the nine sites depicted in figure 2 (circles, 2003; squares, 2004; triangles, 2006). (c) Clinal variation in mean phenotype among the first seven sites in the Misty outlet (error bars are standard deviations; data collected in 2003–2004). The morphological index is the group centroid of the first discriminant function (grouping variable=site) calculated using four morphological traits thought to be under divergent selection between lakes and streams (Moore et al. 2007). The realized (observed) mean phenotype shows a much shallower cline than the optimal mean phenotype as predicted from habitat–phenotype associations (adapted from Moore et al. 2007).
We suggest that some of the logistical difficulties associated with tests of the Kirkpatrick & Barton (1997) model might be circumvented by examining simpler range limits on smaller spatial scales, such as the distribution of fish along a single watercourse. We adopt this approach by considering the potential contribution of gene flow to the distribution of threespine stickleback along a single stream.
(a) The Misty system
The Misty Lake watershed (50°36′32″ N, 127°15′46″ W; Vancouver Island, British Columbia, Canada; figure 2) contains threespine stickleback that show genetically based adaptive differences between lake and inlet stream populations in a wide suite of morphological and behavioural traits (Lavin & McPhail 1993; Hendry et al. 2002; Delcourt et al. 2008; Sharpe et al. 2008). Most relevant here, lake fish have shallow (i.e. streamlined) bodies that are thought to be advantageous for sustained swimming in the pelagic habitat of the lake, whereas inlet fish have deeper bodies that are thought to confer increased manoeuvrability in structurally complex stream environments. Inlet fish also have a reduced number of gill rakers compared with lake fish, perhaps due to the lower abundance of planktonic prey in the stream (Berner et al. 2008). Misty lake and inlet stickleback also differ genetically in other aspects of body shape (Lavin & McPhail 1993; Hendry et al. 2002; Sharpe et al. 2008), behavioural traits such as aggressive and sexual display behaviour (Delcourt et al. 2008) and colour (Lavin & McPhail 1993). Lake and inlet fish thus show adaptive divergence and gene flow between the two populations is reduced (Hendry et al. 2002; Moore et al. 2007). This lower gene flow may be the result of several factors, including a partial barrier to dispersal at the lake–inlet transition (J.-S. Moore 2008, unpublished data). The present study focuses on Misty outlet stickleback, which resemble lake stickleback more closely than they resemble inlet stickleback (Hendry et al. 2002; Moore & Hendry 2005; Delcourt et al. 2008; Sharpe et al. 2008). Previous work has shown that this pattern is the result of gene flow from the lake that constrains adaptation to the outlet stream environment (Hendry et al. 2002; Moore & Hendry 2005; Moore et al. 2007; Berner et al. 2008).
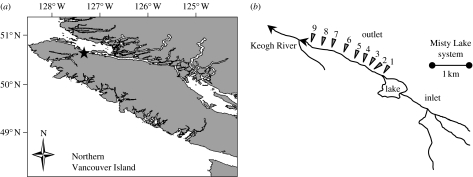
Figure 2.
The study sites. (a) Location of the Misty Lake watershed (star) on Vancouver Island, British Columbia, Canada. (b) Sampling locations and site numbers in the Misty outlet stream. The outlet is approximately 2.5 km long and flows into the Keogh River approximately 12 km upstream of the Pacific Ocean.
We here suggest that stickleback in the Misty outlet stream can be viewed as a small-scale, one-dimensional species range, which reveals key parallels to the Kirkpatrick & Barton (1997) model (figure 1). First, the selective environment in the outlet changes spatially in an almost linear fashion, with sites farther from the lake being more stream-like (i.e. faster/shallower water) than those nearer to the lake (Moore et al. 2007). Second, gene flow is very high from the lake population into the outlet population (mean pairwise FST=0.012, compared with 0.126 between lake and inlet; Moore et al. 2007). Third, population size (i.e. density) gradually decreases from the lake, reaching zero within a few kilometres (stickleback are absent from the much larger and faster Keogh River; figure 1b). Fourth, outlet stickleback show a gradual spatial cline in morphology, such that fish farther from the lake are more ‘stream-like’ (figure 1c; Moore & Hendry 2005). Fifth, the difference between observed and optimal morphologies (optimal morphology being estimated using the inlet as a benchmark and assuming it is not affected by gene flow; see Moore et al. (2007) for a discussion) in the outlet increases with increasing distance from the lake, suggesting greater migration load towards the ‘range’ periphery (Moore et al. 2007; figure 1c). This situation in the outlet is thus different from that in the inlet where linear trends in morphology, habitat and population densities are not apparent, and where gene flow from the lake is lower. What remains to be determined is whether the inability of stickleback to persist beyond the current range limit in the Misty system can, at least in part, be attributed to migration load.
Following Kirkpatrick & Barton (1997), several predictions should be verified if gene flow negatively influences population size along the outlet. First, peripheral populations should show partial local adaptation, i.e. peripheral populations will benefit from a partial home-site advantage in the peripheral habitats (figure 1a). The strongest test for such local adaptation is a reciprocal transplant experiment (Kawecki & Ebert 2004). However, low population densities in peripheral downstream sites far from the lake (the phenomenon we are trying to explain) prevented a fully reciprocal design. Instead, we tested for local adaptation at the peripheral site by comparing the survival of fish from different outlet sites when all were released at the peripheral site. Second, heavy migration load in peripheral populations should push phenotypes away from their local optimum, and thereby result in directional selection on key adaptive traits (Bolnick & Nosil 2007; Bolnick et al. 2008). We tested this prediction by comparing the mean body depth of marked fish before and after two different year-long intervals in the outlet.
We also evaluate an alternative ecological process that is potentially important in peripheral populations. That is, instead of gene flow restricting spatial distributions by hampering adaptation (as above), dispersal might increase spatial distributions through the demographic supplementation of a sink habitat (Dias 1996; Holt & Gomulkiewicz 2004). We consider this hypothesis by using mark–recapture experiments to estimate the number of dispersers into peripheral sites. Evidence against this positive effect of dispersal thus supplements our above tests for the negative effects.
2. Material and methods
All stickleback collections were made with unbaited minnow traps left overnight. Population densities were estimated for sites 1–9 based on catch-per-unit-effort (CPUE: fish/(trap×hour)) data collected over 3 years (figure 1b).
(a) Transplant experiment
Between 8 and 10 May 2006, 38 fish were collected from each of three sites: outlet 2; outlet 5; and outlet 8 (figure 2b). This sample size was the maximum possible (maintaining equal representation) because only 38 fish were captured at outlet site 8 despite high effort. This modest number of experimental individuals was inevitable and appropriate because we were concerned with performance at the range periphery, where densities are very low. Equal numbers of fish captured from the different sites were retained every day and held in the Marble River hatchery in origin-specific tanks at equal densities. The fish were measured (body length and body depth; see below for details), and marked with site-specific spine clips, a common marking method for stickleback (e.g. Hagen 1967; Hendry et al. 2002). Marked fish were held in tanks for a minimum of 24 hours (maximum of 72 hours), during which time none of the marked fish died. All marked fish were then released together at site 8 on 11 May 2006. The three groups differed significantly at the beginning of the experiment in body length (ANOVA: F=4.67, d.f.=109, p=0.011) but not body depth (F=1.55, d.f.=109, p=0.22), although trend for body depth was in the expected direction (site 8>site 5>site 2; see table 1.2 in appendix 1 of the electronic supplementary material). We focused our analyses of selection on relative body depth because (i) this trait was the easiest to quickly measure on larger numbers of fish in the field, (ii) it has a genetic basis (Sharpe et al. 2008), and (iii) it is clearly under divergent selection between lakes and streams (Hendry & Taylor 2004; Berner et al. 2008).
We used mark–recapture methods to estimate survival probability for the three release groups. In 2006, the release site was sampled for marked individuals on four separate occasions: days 16, 17, 31 and 32 after release. On each of these days, we distributed 100 traps over a 150 m section of the stream centred on the release site. The traps were left overnight, after which time the captured fish were examined for spine clips. At this time, additional spine clips were added in a scheme that allowed reconstruction of the prior capture history of each individual. These capture histories were evaluated with Cormack–Jolly–Seber models (Pollock et al. 1990; Lebreton et al. 1992) in program Mark (White & Burnham 1999), thus allowing independent estimates of survival (ϕ) and recapture (p) probabilities. Alternative models were evaluated using model selection, and parameters were estimated using model averaging (i.e. multi-model inference; Burnham & Anderson 2002; for details see appendix 1 in the electronic supplementary material). Survival probabilities of the three release groups were compared based on overlap of the 95% confidence intervals generated by program Mark under model averaging (White & Burnham 1999; Burnham & Anderson 2002).
The above analysis evaluated survival over only one month in the summer. Longer term survival was evaluated by sampling again for potential survivors 1 year later (19–22 May 2007). To ensure that essentially all surviving fish were captured at this time, resampling involved intense effort (4905 traps×hours) at the release site, as well as upstream and downstream (approx. 200 m from the release site in both directions). Moreover, sampling was done without replacement, so as to obtain a depletion curve. We then used Fisher's exact probability test to determine whether the number of recaptured individuals differed among the original release groups.
(b) Selection on body depth
We next tested the hypothesis that migration load in the outlet leads to natural selection favouring deeper bodies. Selection analysis requires large sample sizes (Hersch & Phillips 2004), and so we performed this experiment at site 5, where stickleback densities are relatively high (figure 1b) but where maladaptation should still be substantial enough to optimize effect size (figure 1c). At this site, we captured large numbers of fish in 2005 (n=751) and 2006 (n=609). For each captured fish, we used (i) a ruler to measure body length (the tip of the upper jaw to the end of the caudal fin, mm), (ii) a calliper to measure body depth (contact of the first and second predorsal pterygiophores to the bottom of pelvic girdle, perpendicular to the lateral line, 0.1 mm) and (iii) spine clips to provide a year-specific mark. Processing was done in the field, all fish were measured by the same investigator (J.-S.M.), and all fish were released within a few minutes of capture. Surviving marked fish were then recaptured in May of 2006 (5541 trap×hours; 61 recaptures) and 2007 (980 trap×hours; 54 recaptures). Recaptured fish were identified based on their spine clips and were re-measured for body length and body depth. If selection favours deeper bodies, the average length-standardized (see below) body depth of marked survivors in year n should be greater than the average length-standardized body depth of all fish marked in year n−1. In turn, if gene flow from the lake occurs, the mean phenotype of new unmarked fish should again be maladaptively shallow-bodied.
Within a cohort, body depth might change over a year owing to both selection and growth. We removed effects of growth by allometrically standardizing body depth to a common body length (Reist 1986; see appendix 2 in the electronic supplementary material). This approach was justified because the relationship between log body length and log body depth was very strong and linear across all fish (R2=0.95; p<0.0005). We then used single-factor ANOVA, followed by a Tukey test, to determine which of the five samples (all fish marked in 2005, 2006 and 2007, and marked survivors in 2006 and 2007) differed significantly from each other in standardized body depth. These comparisons were then used to infer the action of selection and gene flow (see §4).
(c) Dispersal
We used mark–recapture methods to consider the potential role of dispersers on demography in the peripheral population. Our first step was to get an estimate of dispersal patterns in outlet stickleback. On 11 and 13 May 2006, we therefore captured, marked (site-specific spine clips) and released 252 fish at outlet site 5 and 206 fish at outlet site 2. These sites were chosen because the large numbers of local fish would allow good estimates and would be potentially relevant to source–sink dynamics in peripheral sites. Both sites were then resampled for marked fish after two and four weeks by using 99 minnow traps deployed overnight (three traps every 10 m along 320 m of stream centred on the release site). This particular design reduces the effects of distance weighting on dispersal distance distributions (DDDs; Porter & Dooley 1993; Albanese et al. 2003). Moreover, preliminary mark–recapture experiments performed a year earlier showed that dispersal beyond that distance range was rare (J.-S. Moore 2005, unpublished data).
Our examination of dispersal began by testing for a downstream bias in the number of moves (Χ2-test) and the median distance moved (Mann–Whitney U-test). These tests were performed for each site/sampling interval, except for outlet site 2 after four weeks, which had too few recaptured fish (n=2). We next fit alternative probability density functions (PDFs) to the empirical DDDs (again, except for outlet site 2 in week 4). The PDFs chosen for fitting (detailed in appendix 3 of the electronic supplementary material) are those commonly used for stream fish (Skalski & Gilliam 2000; Coombs & Rodríguez 2007; appendix 3 in the electronic supplementary material), and were fit to the data using the ‘optim’ routine in R (R Development Core Team 2006). The PDF that best fit the data was that with the lowest Akaike information criterion score (i.e. the lowest score represents the best fit; Burnham & Anderson 2002).
We next estimated the number of individuals from outlet sites 1 to 7 that might be expected to disperse at least as far as the peripheral outlet site 8. This number was estimated for each site (details in appendix 3 of the electronic supplementary material, table 3.2) as the product of the estimated population size (combining CPUE and mark–recapture) and the probability that an individual would migrate downstream at least as far as site 8 (based on the best-fit PDFs; details in appendix 3 of the electronic supplementary material, table 3.1). This procedure thus estimates the number of migrants over the two- and four-week capture intervals of the experiment. The number of migrants per year (roughly one generation) can be extrapolated by using the increase in variance of the DDD between the two sampling intervals (two and four weeks), assuming that the increase is constant throughout the year. This assumption may not hold true, however, since water levels fluctuate throughout the year. Fortunately, information on dispersal over the entire year could be gained by examining whether fish marked at sites 2 and 5 in 2005 and 2006 were captured at site 8 in 2006 and 2007.
3. Results
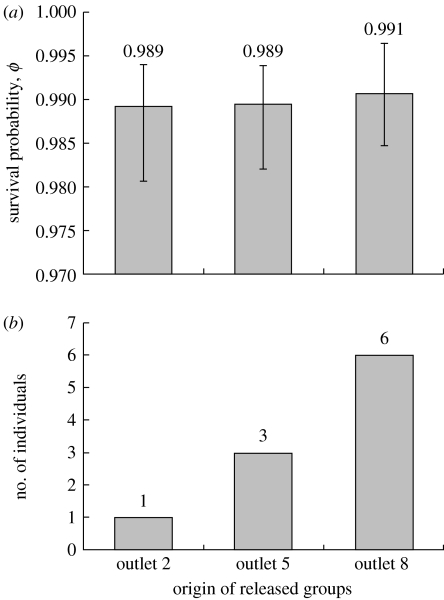
Of the 114 fish released at the range periphery (outlet site 8) at the start of the transplant experiment, 95 (83.3%) were recaptured at least once during the month-long assay. Survival estimates from program Mark were consequently very high (over 95%) for all three release groups, limiting our ability to detect differences among them. Although the trend was for local fish (site 8) to have the highest recapture rates, this pattern was weak and far from significant (appendix 1 in the electronic supplementary material; figure 3a). A year later, only 10 of the released fish were recaptured despite high effort. Here, the trend was again for the highest survival in the local (site 8) fish and the lowest survival in the most distant (site 2) fish. This pattern was the one expected under the hypothesis of partial local adaptation, but statistical significance was again lacking (Fisher's exact probability test; p=0.15; figure 3b). Moreover, note that the experiment used essentially all of the fish present at site 8 in 2006 and that we probably recaptured all surviving fish in 2007. This last result was confirmed by a depletion curve (not shown) that reached zero by the end of our sampling period in 2007. In short, our estimates may be closely representative of the actual population parameters at site 8.
Figure 3.
Results of the transplant experiment testing for local adaptation in fish transplanted from three different outlet sites (sites 2, 5 and 8) to outlet site 8. (a) Survival probabilities for the three groups one month after their release into site 8. Error bars are 95% confidence intervals calculated by program Mark. (b) The number of individuals from each release group captured 1 year after their release into site 8 (the number of released individuals was 38 for each of the three groups).
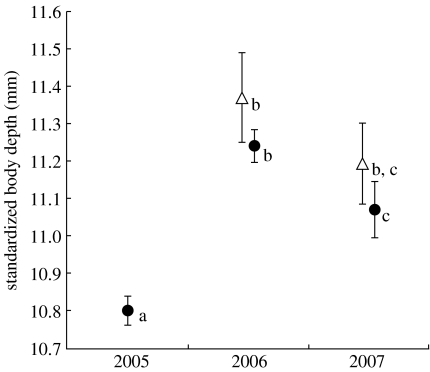
Average body depth differed among years and between marked and unmarked individuals in the two different year-long selection experiments (ANOVA: d.f.=1597, F=54.60, p<0.005). We found evidence for selection for deeper bodies at outlet site 5 in a year where gene flow effects appeared high but not in a year where they appeared low (figure 4). First, the starting body depth of all fish in 2005 was quite small, presumably reflecting high gene flow in the previous generations. Second, the mean size-standardized body depth of survivors from 2005 to 2006 was 5.3 per cent greater than the mean body depth of all fish in the starting 2005 sample, presumably reflecting viability selection over the year. Third, mean body depth did not differ between the survivors from 2005 to 2006 and the new fish marked in 2006, perhaps reflecting low maladaptive gene flow in that year. Fourth, mean body depth did not differ between the fish that survived from 2006 to 2007 and those present in the starting 2006 sample, presumably reflecting a lack of strong viability selection over the year.
Figure 4.
Results of year-long mark–recapture experiments testing for directional selection on body depth in the Misty outlet. Shown are the mean-standardized body depths of unmarked fish captured and marked in each year (circles) and of the marked individuals that survived from the previous year (triangles). Error bars show 95% confidence intervals. Homogeneous subsets from a Tukey test are indicated with lower-case letters.
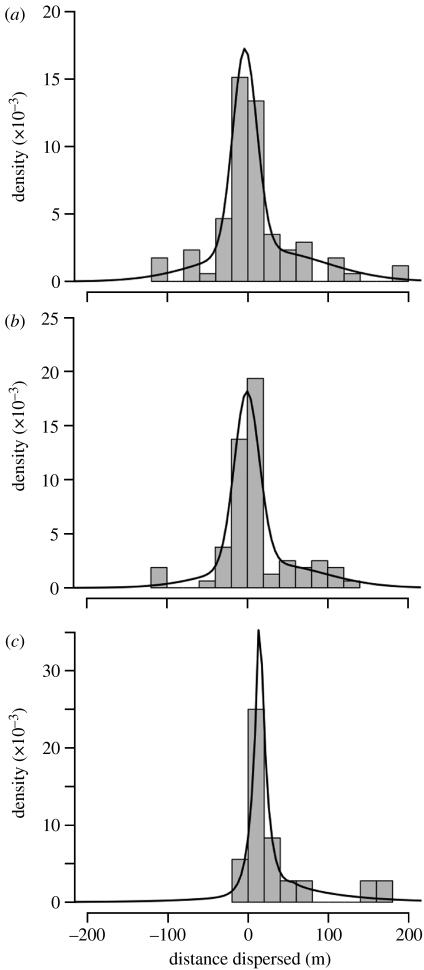
Dispersal does not appear high enough to maintain peripheral populations that would otherwise go extinct. First, all three dispersal distributions were leptokurtic (all γ>0), with very few individuals dispersing long distances (figure 5). Second, dispersal probabilities were not downstream-biased in any sample with respect to the number of moves (Χ2-tests; all p>0.05) or distance moved (Mann–Whitney U-tests; all p>0.05). Note, however, that it could still be true that migration rate (m) will be strongly asymmetric in the downstream direction because of decreasing population size in that direction. Third, variance of the empirical DDDs did not increase between weeks 2 and 4, suggesting that most of the movements observed were the result of normal home range movements rather than actual dispersal (Skalski & Gilliam 2000). This same observation suggested that the short-term dispersal rates could not be extrapolated to longer time scales. Overall, the estimated numbers of dispersers into site 8 were small: less than unity from sites 1–6 and either 98 (week 2 estimates) or 48 (week 3 estimates) from site 7 (see table 3.2 in appendix 3 of the electronic supplementary material). Even these estimates were probably much too high, given that we never captured a stickleback in outlet site 8 that had been marked at a different outlet site.
Figure 5.
(a–c) DDDs of marked individuals recorded two (outlet sites 2 and 5) and four (outlet site 5) weeks after release ((a) outlet 5, week 2; (b) outlet 5, week 4; (c) outlet 2, week 2). Positive distances represent upstream dispersal and negative distances represent downstream dispersal. The thick lines are the theoretical distributions that best fit the empirical distributions: a mixture of two normal distributions for both DDDs from outlet site 5 and a mixture of two Laplace distributions for outlet site 2 (see table 3.1 in appendix 3 of the electronic supplementary material).
4. Discussion
(a) Drivers of population size and range limits in the Misty outlet
Many studies seek to explain the inability of species to persist beyond their range limits as resulting from spatial variation in environmental conditions (Hoffman & Blows 1994). In the Misty system, stickleback seem unable to persist in the downstream-most reaches of the outlet stream. These peripheral sites have relatively fast and shallow water (Moore et al. 2007) that stickleback probably find challenging given their mostly labriform mode of swimming (Walker 2004). For instance, resident stickleback are rarely caught in larger rivers with high water velocity, and are absent from the larger and faster Keogh River into which the Misty outlet stream flows (figure 2b; J.-S. Moore, unpublished data). Although these habitat characteristics might be the proximate cause of a population's inability to persist, they do not address the ultimate evolutionary question: why cannot populations adapt to conditions at the range periphery, and thereby persist in those conditions and expand their ranges?
One commonly invoked ultimate explanation is a lack of appropriate genetic variation (Antonovics 1976; Hoffman & Blows 1994; Bridle & Vines 2007). This explanation seems unlikely in the Misty system. First, stickleback have adapted to stream conditions in the Misty inlet (Hendry et al. 2002; Moore et al. 2007). Second, extreme stream-type morphology has evolved in parallel in many watersheds in the Northern Hemisphere since the last glaciation (Thompson et al. 1997; Hendry & Taylor 2004; Berner et al. 2008), suggesting sufficient levels of appropriate standing genetic variation in the marine ancestor. Alternatively, the outlet could have been colonized more recently than the inlet. Although we have no data to directly test this hypothesis, a variety of indirect evidence suggests that the populations are near a selection–gene flow equilibrium (see Hendry et al. (2002) for discussion). All of these observations suggest that some other factor must be limiting the spatial distribution of Misty outlet stickleback.
The implausibility of limited genetic variation explaining range limits in the outlet leads to the consideration of a main alternative: asymmetric gene flow from dense populations adapted to environmental conditions nearer the range centre. Misty outlet stickleback present several lines of evidence consistent with this hypothesis. First, previous work has shown that phenotypic adaptation is strongly limited by gene flow from the lake (figure 1; Hendry et al. 2002; Moore & Hendry 2005; Moore et al. 2007; Berner et al. 2008). Second, the magnitude of maladaptation increases along the outlet and this change is closely associated with declining population densities (figure 1). Third, our experimental results on adaptation and selection are at least partially consistent with the expectations from the Kirkpatrick & Barton (1997) model for how gene flow limits local population sizes and therefore constrains range limits. Fourth, dispersal data allow us to largely exclude an alternative hypothesis: peripheral populations might be maintained by the positive demographic influences of immigrants. We now discuss these last two points in further detail.
(b) Negative impacts of gene flow on population size and range limits?
Theory makes several predictions that should hold at peripheral sites in the Misty outlet if gene flow has a negative impact on population size and therefore contributes to range limits. One prediction is that peripheral populations should show partial local adaptation (Kirkpatrick & Barton 1997). In our study system, this would mean that local fish from outlet site 8, which is near the range limit, would show higher survival at site 8 than the fish from outlet sites 2 and 5, which have morphologies even further from the expected optimum in this part of the stream (Moore & Hendry 2005). A month-long assay during the summer was unable to directly inform this hypothesis because very few fish died, despite the fact that population densities were increased by the addition of fish from sites 2 and 5. Survival to the next year, however, was exactly as expected if some partial local adaptation was present: the highest for site 8 and the lowest for site 2 (figure 3b). This trend was not statistically significant owing to small sample sizes, but several counterpoints should be kept in mind. First, large differences were not expected given the only slight, and non-significant, morphological differences between fish from the different sites (figure 1 and §2). Second, we used all available fish from outlet site 8 and recaptured essentially all released fish, which means that our estimates were probably close to the actual population parameters. Note also that choice of a site with more abundant fish was not a better option, because our goal was to test for adaptation specifically in peripheral populations where few fish are present. We therefore conclude that the results of this experiment neither reject nor strongly support the model of Kirkpatrick & Barton (1997).
Another prediction is that migration load in peripheral populations should result in directional selection for a better-adapted phenotype (Kirkpatrick & Barton 1997; Bolnick & Nosil 2007; Bolnick et al. 2008). That is, recurrent immigration will repeatedly push mean phenotype away from the local optimum, leading to selection counteracting this effect. We found apparent signatures of this tug-of-war in the Misty outlet in only 1 year. In 2005, local fish at outlet site 5 had particularly shallow bodies, perhaps owing to high gene flow from farther upstream. The subset of these individuals that survived to 2006 had considerably deeper bodies, suggesting selection for stream-type morphology. This larger temporal change in an adaptively significant trait is very unlikely to be the result of genetic drift, particularly given the relative large population at this site (approx. 1400 individuals; see table 3.2 in appendix 3 of the electronic supplementary material). The change is also unlikely to be the result of phenotypic plasticity given the strong genetic basis for this trait in Misty stickleback (Lavin & McPhail 1993; Hendry et al. 2002; Sharpe et al. 2008). Additional fish captured in 2006 did not have shallower bodies, presumably owing to lower dispersal in that year. Accordingly, selection to 2007 was not detected. In short, selection for increased body depth was evident only in years when the outlet population had reduced body depth, a result consistent with theoretical expectations. It is also important to remember that we looked at only one of several traits influencing adaptation to lakes and streams (Hendry et al. 2002; Moore & Hendry 2005; Berner et al. 2008). Looking at these additional traits could reveal additional selection.
Maladaptation would also be expected if the peripheral site at the range margin was a demographic sink continually maintained by dispersal from further upstream. Two lines of evidence suggest that the demographic benefits of dispersal are limited in the peripheral stream habitats. First, stickleback exist in even more stream-like sites (e.g. faster/shallower) in other watersheds where gene flow is not a major constraint (Berner et al. 2008). (Range limits in these other systems, and the Misty inlet, seem determined by uninhabitable extreme conditions, rather than gene flow.) Second, our mark–recapture data suggest that dispersal is too low to maintain peripheral populations. That is, we documented very few long-distance movements (figure 5), and even these were probably home-range movements rather than dispersal (see §3). Moreover, we never captured any stickleback at the peripheral site (outlet site 8) that had been marked in upstream sites in more than 2 years of intensive sampling. We acknowledge, however, that dispersal may be episodic at certain times of year or in particular years that we did not sample.
In summary, we argue that dispersal (and therefore gene flow) must be high enough to constrain both neutral and adaptive genetic divergence (Hendry et al. 2002; Moore et al. 2007; Berner et al. 2008) but also low enough to minimize demographic benefits. We have several reasons for suggesting that this balance is possible. First, the peripheral population seems quite small (figure 1) and so even a few dispersers would represent a reasonable fraction (m) of the population. Second, adaptive divergence can be substantially constrained at levels of m (Hendry et al. 2001) that are so low as to have minimal demographic effects. Third, the peripheral population shows some morphological differences from sites further upstream (Moore & Hendry 2005; see appendix 1 in the electronic supplementary material), so it must contain a substantial fraction of residents (assuming no plasticity). We conclude that dispersal and gene flow in the Misty outlet are therefore more likely to have negative than positive effects on adaptation, population size and range limits.
One important question that our study did not address is whether or not maladaptation will have a negative effect on demography only under hard selection (i.e. density independent; Saccheri & Hanski 2006; Kinnison & Hairston 2007). Scenarios involving both hard and soft selections can be envisioned for the Misty outlet. In the case of hard selection, fast-flowing water and complex habitats might favour a deeper body regardless of population densities. In this case, maladaptation might lower population growth rate (r) and persistence. In the case of soft selection, adaptation may be important in competition for limited prey resources or suitable nesting sites. In this case, population regulation might be mostly density dependent and some maladaptation at the population level would not have strong effects on population growth. The relative importance of hard versus soft selection (the term γ in Kirkpatrick & Barton 1997) will thus determine the strength of the effect of gene flow on population size. As such, enclosure experiments where population density is manipulated would be helpful in determining the strength of density-dependent effects in the Misty system.
(c) Implications for the evolution of species' ranges
Whether or not gene flow is an important force limiting population size and species ranges will be context dependent (Holt & Gomulkiewicz 2004; Bridle & Vines 2007; Garant et al. 2007). First, asymmetric gene flow will be stronger in situations where population densities are higher in the range centre (abundant centre hypothesis). Although this pattern is often assumed, it remains unclear whether or not it is characteristic of most species' ranges (Sagarin & Gaines 2002; Samis & Eckert 2007). Second, negative effects of gene flow probably will be the strongest across steep ecotones (relative to dispersal distance) rather than along shallow environmental gradients, such as on continental scales (Kirkpatrick & Barton 1997). Third, the negative effects of gene flow will be magnified compared with the positive effects only when adaptively significant genetic variation is readily available or where the demographic input of migrants is limited. Fourth, gene flow will have to be high if its effects are to predominate over other confounding factors, such as historical effects or interspecific competition at range margins (Case & Taper 2000).
Stickleback in the Misty outlet show close parallels to the theory that should make it a good candidate to detect negative effects of gene flow on aspects of demography: (i) population densities decrease away from the range centre (lake), (ii) the environmental gradient between lakes and streams is steep, (iii) dispersal in the outlet (as opposed to in the inlet) is high enough for gene flow to substantially constrain adaptive divergence in traits but probably not high enough to maintain a sink, and (iv) the Misty outlet population dramatically simplifies many of the factors that can confound large-scale tests. Our study therefore provided an opportunity to generate rare evidence for gene flow constraining species ranges, albeit on a small scale. Our results were generally consistent with this hypothesis. At the same time, however, none of our results was particularly strong, suggesting that the negative effects of gene flow are difficult to detect even under these apparently ideal conditions.
The ideal conditions for detecting gene flow's effects on range limits might be relatively rare in nature. These conditions should be carefully sought and examined to see if gene flow can have negative demographic effects under these most likely conditions. Our study provided one such test, and we found that the negative effects of gene flow were difficult to demonstrate. Although we acknowledge that gene flow can certainly have negative impacts on adaptation, we suspect that effects on range limits, particularly on large scales, may be relatively weak and therefore difficult to demonstrate. A particularly informative experiment would involve blocking migration to a population receiving gene flow and then tracking changes in population density. While it was impossible to perform such an experiment in the Misty system (it is enclosed in an ecological reserve where habitat alterations are prohibited), it could conceivably be done in other populations.
5. Conclusion
The capacity for evolutionary forces to drive ecological and demographic dynamics has long been stated (e.g. Chitty 1952), but there has been a recent surge of interest in the idea (Saccheri & Hanski 2006; Fussmann et al. 2007; Kokko & López-Sepulcre 2007; Zheng et al. 2009). This new enthusiasm for eco-evolutionary dynamics stems, at least in part, from the growing acceptance that evolution can occur over ecological time scales (Hendry & Kinnison 1999; Hairston et al. 2005; Carroll et al. 2007). Gene flow, being an important evolutionary force in many natural populations, has the potential to influence demographic processes. This potential has long been recognized in theory (for a review, see Holt & Gomulkiewicz 2004; Garant et al. 2007) but has only rarely been investigated in nature. Indeed, direct evidence for a negative impact of gene flow on population size, and species' ranges, is currently lacking (Bridle & Vines 2007). Part of this deficiency presumably stems from the difficulty of simultaneously estimating gene flow, adaptation and population dynamics in nature. Such integration is important, however, because dispersal and gene flow can have a variety of positive and negative effects (Garant et al. 2007). Our study simplified this problem by focusing on a natural system that circumvents many potentially confounding factors by focusing on a small-scale, one-dimensional range limit where phenotypes are known to be constrained by gene flow. The mixed evidence we find in support of gene flow effects on population size, and the implications for range limits, thus increase the need for future studies to help define the conditions under which gene flow will and will not be important.
Acknowledgments
Experiments were carried out in accordance with McGill University Animal Care Committee guidelines, and met the Canadian Council on Animal Care requirements.
V. Bahn, M. Mazerolle and X. Thibert-Plante helped with the design and analysis of the experiments. D. Berner, A.-C. Grandchamp, K. Hudson and N. Farrel helped with the field experiments. Western Forest Products, Inc., provided accommodation in the field. D. Anderson, G. Anderson and the Friends of the Marble River provided laboratory facilities. K. Samis and two anonymous reviewers provided their helpful comments on an earlier version of the manuscript. Finally, the Natural Sciences and Engineering Research Council of Canada provided funding through a Discovery grant (to A.P.H.) and a Canada Graduate Scholarship (to J-.S.M.).
Footnotes
One contribution of 14 to a Theme Issue ‘Eco-evolutionary dynamics’.
Supplementary Material
3 appendices
References
- Albanese B., Angermeier P.L., Gowan C. Designing mark–recapture studies to reduce effects of distance weighting on movement distance distributions of stream fishes. Trans. Am. Fish. Soc. 2003;132:925–939. doi:10.1577/T03-019 [Google Scholar]
- Angert A.L., Schemske D.W. The evolution of species' distributions: reciprocal transplants across the elevation ranges of Mimulus cardinalis and M. lewisii. Evolution. 2005;59:1671–1684. doi:10.1554/05-107.1 [PubMed] [Google Scholar]
- Antonovics J. The nature of limits to natural selection. Ann. Mo. Bot. Gard. 1976;63:224–247. doi:10.2307/2395303 [Google Scholar]
- Barton N.H. Adaptation at the edge of a species' range. In: Silvertown J., Antonovics J., editors. Integrating ecology and evolution in a spatial context. Blackwell Science; Oxford, UK: 2001. pp. 365–392. [Google Scholar]
- Berner D., Adams D.C., Grandchamp A.-C., Hendry A.P. Natural selection drives patterns of lake–stream divergence in stickleback foraging morphology. J. Evol. Biol. 2008;21:1653–1665. doi: 10.1111/j.1420-9101.2008.01583.x. doi:10.1111/j.1420-9101.2008.01583.x [DOI] [PubMed] [Google Scholar]
- Bolnick D.I., Nosil P. Natural selection in populations subject to a migration load. Evolution. 2007;61:2229–2243. doi: 10.1111/j.1558-5646.2007.00179.x. doi:10.1111/j.1558-5646.2007.00179.x [DOI] [PubMed] [Google Scholar]
- Bolnick D.I., Caldera E.J., Matthews B. Evidence for asymmetric migration load in a pair of ecologically divergent stickleback populations. Biol. J. Linn. Soc. 2008;94:273–287. doi:10.1111/j.1095-8312.2008.00978.x [Google Scholar]
- Boulding E.G., Hay T. Genetic and demographic parameters determining population persistence after a discrete change in the environment. Heredity. 2001;86:313–324. doi: 10.1046/j.1365-2540.2001.00829.x. doi:10.1046/j.1365-2540.2001.00829.x [DOI] [PubMed] [Google Scholar]
- Bridle J.R., Vines T.H. Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol. Evol. 2007;22:140–147. doi: 10.1016/j.tree.2006.11.002. doi:10.1016/j.tree.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Burger R., Lynch M. Evolution and extinction in a changing environment: a quantitative-genetic analysis. Evolution. 1995;49:151–163. doi: 10.1111/j.1558-5646.1995.tb05967.x. doi:10.2307/2410301 [DOI] [PubMed] [Google Scholar]
- Burnham K.P., Anderson D.R. 2nd edn. Springer; New York, NY: 2002. Model selection and multimodel inference: a practical information–theoretic approach. [Google Scholar]
- Carroll S.P., Hendry A.P., Reznick D.N., Fox C.W. Evolution on ecological time-scales. Funct. Ecol. 2007;21:387–393. doi:10.1111/j.1365-2435.2007.01289.x [Google Scholar]
- Case T.J., Taper M.L. Interspecific competition, environmental gradients, gene flow, and the coevolution of species' borders. Am. Nat. 2000;155:583–605. doi: 10.1086/303351. doi:10.1086/303351 [DOI] [PubMed] [Google Scholar]
- Chitty D. Mortality among voles (Microtus agrestis) at Lake Vyrnwy, Montgomeryshire, in 1936–1939. Phil. Trans. R. Soc. Lond. B. 1952;236:505–552. doi:10.1098/rstb.1952.0009 [Google Scholar]
- Coombs M.F., Rodríguez M.A. A field test of simple dispersal models as predictors of movement in a cohort of lake-dwelling brook charr. J. Anim. Ecol. 2007;76:45–57. doi: 10.1111/j.1365-2656.2006.01188.x. doi:10.1111/j.1365-2656.2006.01188.x [DOI] [PubMed] [Google Scholar]
- Delcourt M., Räsänen K., Hendry A.P. Genetic and plastic components of divergent male intersexual behavior in Misty lake/stream stickleback. Behav. Ecol. 2008;19:1217–1224. doi:10.1093/beheco/arn084 [Google Scholar]
- Dias P.C. Sources and sinks in population biology. Trends Ecol. Evol. 1996;11:326–330. doi: 10.1016/0169-5347(96)10037-9. doi:10.1016/0169-5347(96)10037-9 [DOI] [PubMed] [Google Scholar]
- Filin I., Holt R.D., Barfield M. The relation of density regulation to habitat specialization, evolution of a species' range, and the dynamics of biological invasions. Am. Nat. 2008;172:233–247. doi: 10.1086/589459. doi:10.1086/589459 [DOI] [PubMed] [Google Scholar]
- Fussmann G.F., Loreau M., Abrams P.A. Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 2007;21:465–477. doi:10.1111/j.1365-2435.2007.01275.x [Google Scholar]
- Garant D., Forde S.E., Hendry A.P. The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct. Ecol. 2007;21:434–443. doi:10.1111/j.1365-2435.2006.01228.x [Google Scholar]
- Geber M.A., Eckhart V.M. Experimental studies of adaptation in Clarkia xantiana. II. Fitness variation across a subspecies border. Evolution. 2005;59:521–531. doi:10.1554/04-265 [PubMed] [Google Scholar]
- Gomulkiewicz R., Holt R.D. When does evolution by natural selection prevent extinction? Evolution. 1995;49:201–207. doi: 10.1111/j.1558-5646.1995.tb05971.x. doi:10.2307/2410305 [DOI] [PubMed] [Google Scholar]
- Hagen D.W. Isolating mechanisms in threespine stickleback (Gasterosteus) J. Fish. Res. Board Can. 1967;24:1637–1692. [Google Scholar]
- Hairston N.G., Jr, Ellner S.P., Geber M.A., Yoshida T., Fox J.A. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 2005;8:1114–1127. doi:10.1111/j.1461-0248.2005.00812.x [Google Scholar]
- Hanski I., Saccheri I. Molecular-level variation affects population growth in a butterfly metapopulation. PLoS Biol. 2006;4:e129. doi: 10.1371/journal.pbio.0040129. doi:10.1371/journal.pbio.0040129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A.P., Kinnison M.T. The pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi:10.2307/2640428 [PubMed] [Google Scholar]
- Hendry A.P., Taylor E.B. How much of the variation in adaptive divergence can be explained by gene flow: an evaluation using lake–stream stickleback pairs. Evolution. 2004;58:2319–2331. doi: 10.1111/j.0014-3820.2004.tb01606.x. doi:10.1554/04-376 [DOI] [PubMed] [Google Scholar]
- Hendry A.P., Day T., Taylor E.B. Population mixing and the adaptive divergence of quantitative traits in discrete populations: a theoretical framework for empirical tests. Evolution. 2001;55:459–466. doi: 10.1554/0014-3820(2001)055[0459:pmatad]2.0.co;2. doi:10.1554/0014-3820(2001)055[0459:PMATAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hendry A.P., Taylor E.B., McPhail J.D. Adaptive divergence and the balance between selection and gene flow: lake and stream stickleback in the Misty system. Evolution. 2002;56:1199–1216. doi: 10.1111/j.0014-3820.2002.tb01432.x. doi:10.1111/j.0014-3820.2002.tb01432.x [DOI] [PubMed] [Google Scholar]
- Hersch E.I., Phillips P.C. Power and potential bias in field studies of natural selection. Evolution. 2004;58:479–485. doi:10.1554/03-383 [PubMed] [Google Scholar]
- Hoffman A.A., Blows M.W. Species borders: ecological and evolutionary perspectives. Trends Ecol. Evol. 1994;9:223–227. doi: 10.1016/0169-5347(94)90248-8. doi:10.1016/0169-5347(94)90248-8 [DOI] [PubMed] [Google Scholar]
- Holt R.D., Gomulkiewicz R. How does immigration influence local adaptation? A reexamination of a familiar paradigm. Am. Nat. 1997;149:563–572. doi:10.1086/286005 [Google Scholar]
- Holt R.D., Gomulkiewicz R. Conservation implications of niche conservatism and evolution in heterogeneous environments. In: Ferrière R., Dieckmann U., Couvet D., editors. Evolutionary conservation biology. Cambridge University Press; Cambridge, UK: 2004. pp. 244–264. [Google Scholar]
- Kawecki T.J., Ebert D. Conceptual issues in local adaptation. Ecol. Lett. 2004;7:1225–1241. doi:10.1111/j.1461-0248.2004.00684.x [Google Scholar]
- Kinnison M.T., Hairston N.G., Jr Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 2007;21:444–454. doi:10.1111/j.1365-2435.2007.01278.x [Google Scholar]
- Kirkpatrick M., Barton N.H. Evolution of a species' range. Am. Nat. 1997;150:1–23. doi: 10.1086/286054. doi:10.1086/286054 [DOI] [PubMed] [Google Scholar]
- Kokko H., López-Sepulcre A. The ecogenetic link between demography and evolution: can we bridge the gap between theory and data? Ecol. Lett. 2007;10:773–782. doi: 10.1111/j.1461-0248.2007.01086.x. doi:10.1111/j.1461-0248.2007.01086.x [DOI] [PubMed] [Google Scholar]
- Lavin P.A., McPhail J.D. Parapatric lake and stream sticklebacks on northern Vancouver Island: disjunct distribution or parallel evolution? Can. J. Zool. 1993;71:11–17. doi:10.1139/z93-003 [Google Scholar]
- Lebreton J.-D., Burnham K.P., Clobert J., Anderson D.R. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case-studies. Ecol. Monogr. 1992;62:67–118. doi:10.2307/2937171 [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends Ecol. Evol. 2002;17:183–189. doi:10.1016/S0169-5347(02)02497-7 [Google Scholar]
- McLachlan J.S., Hellmann J.J., Schwartz M.W. A framework for debate of assisted migration in an era of climate change. Conserv. Biol. 2007;21:297–302. doi: 10.1111/j.1523-1739.2007.00676.x. doi:10.1111/j.1523-1739.2007.00676.x [DOI] [PubMed] [Google Scholar]
- Moore J.-S., Hendry A.P. Both selection and gene flow are necessary to explain adaptive divergence: evidence from clinal variation in stream stickleback. Evol. Ecol. Res. 2005;7:871–886. [Google Scholar]
- Moore J.-S., Gow J.L., Taylor E.B., Hendry A.P. Quantifying the constraining influence of gene flow on adaptive divergence in the lake–stream threespine stickleback system. Evolution. 2007;61:2015–2026. doi: 10.1111/j.1558-5646.2007.00168.x. doi:10.1111/j.1558-5646.2007.00168.x [DOI] [PubMed] [Google Scholar]
- Perron G.G., Gonzalez A., Buckling A. Source–sink dynamics shape the evolution of antibiotic resistance and its pleiotropic fitness cost. Proc. R. Soc. B. 2007;274:2351–2356. doi: 10.1098/rspb.2007.0640. doi:10.1098/rspb.2007.0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock K.H., Nichols J.D., Brownie C., Hines J.E. Statistical inference for capture–recapture experiments. Wildl. Monogr. 1990;107:1–97. [Google Scholar]
- Porter J.H., Dooley J.L. Animal dispersal patterns: a reassessment of simple mathematical models. Ecology. 1993;74:2436–2443. doi:10.2307/1939594 [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment for statistical computing. [Google Scholar]
- Reist J.D. An empirical evaluation of coefficients used in residual and allometric adjustment of size covariation. Can. J. Zool. 1986;64:1363–1368. doi:10.1139/z86-203 [Google Scholar]
- Ronce O., Kirkpatrick M. When sources become sinks: migrational meltdown in heterogeneous habitats. Evolution. 2001;55:1520–1531. doi: 10.1111/j.0014-3820.2001.tb00672.x. doi:10.1111/j.0014-3820.2001.tb00672.x [DOI] [PubMed] [Google Scholar]
- Saccheri I., Hanski I. Natural selection and population dynamics. Trends Ecol. Evol. 2006;21:341–347. doi: 10.1016/j.tree.2006.03.018. doi:10.1016/j.tree.2006.03.018 [DOI] [PubMed] [Google Scholar]
- Sagarin R.D., Gaines S.D. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecol. Lett. 2002;5:137–147. doi:10.1046/j.1461-0248.2002.00297.x [Google Scholar]
- Samis K.E., Eckert C.G. Testing the abundant center model using range-wide demographic surveys of two coastal dune plants. Ecology. 2007;88:1747–1758. doi: 10.1890/06-1153.1. doi:10.1890/06-1153.1 [DOI] [PubMed] [Google Scholar]
- Sharpe D.M.T., Räsänen K., Berner D., Hendry A.P. Genetic and environmental contributions to the morphology of lake and stream stickleback: implications for gene flow and reproductive isolation. Evol. Ecol. Res. 2008;10:849–866. [Google Scholar]
- Skalski G.T., Gilliam J.F. Modeling diffusive spread in a heterogeneous population: a movement study with stream fish. Ecology. 2000;81:1685–1700. doi:10.2307/177317 [Google Scholar]
- Storfer A. Gene flow and endangered species translocations: a topic revisited. Biol. Conserv. 1999;87:173–180. doi:10.1016/S0006-3207(98)00066-4 [Google Scholar]
- Tallmon D.A., Luikart G., Waples R.S. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004;19:489–496. doi: 10.1016/j.tree.2004.07.003. doi:10.1016/j.tree.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Thompson C.E., Taylor E.B., McPhail J.D. Parallel evolution of lake–stream pairs of threespine sticklebacks (Gasterosteus) inferred from mitochondrial DNA variation. Evolution. 1997;51:1955–1965. doi: 10.1111/j.1558-5646.1997.tb05117.x. doi:10.2307/2411016 [DOI] [PubMed] [Google Scholar]
- Walker J.A. Dynamics of pectoral fin rowing in a fish with an extreme rowing stroke: the threespine stickleback (Gasterosteus aculeatus) J. Exp. Biol. 2004;207:1925–1939. doi: 10.1242/jeb.00994. doi:10.1242/jeb.00994 [DOI] [PubMed] [Google Scholar]
- White G.C., Burnham K.P. Program MARK: survival estimation from populations of marked animals. Bird Study. 1999;46:120–139. [Google Scholar]
- Zheng C., Ovaskainen O., Hanski I. Modelling single nucleotide effects in phosphoglucose isomerase on dispersal in the Glanville fritillary butterfly: coupling of ecological and evolutionary dynamics. Phil. Trans. R. Soc. B. 2009;364:1519–1532. doi: 10.1098/rstb.2009.0005. doi:10.1098/rstb.2009.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3 appendices