Abstract
The evolution of population dynamics in a stochastic environment is analysed under a general form of density-dependence with genetic variation in r and K, the intrinsic rate of increase and carrying capacity in the average environment, and in σe2, the environmental variance of population growth rate. The continuous-time model assumes a large population size and a stationary distribution of environments with no autocorrelation. For a given population density, N, and genotype frequency, p, the expected selection gradient is always towards an increased population growth rate, and the expected fitness of a genotype is its Malthusian fitness in the average environment minus the covariance of its growth rate with that of the population. Long-term evolution maximizes the expected value of the density-dependence function, averaged over the stationary distribution of N. In the θ-logistic model, where density dependence of population growth is a function of Nθ, long-term evolution maximizes E[Nθ]=[1−σe2/(2r)]Kθ. While σe2 is always selected to decrease, r and K are always selected to increase, implying a genetic trade-off among them. By contrast, given the other parameters, θ has an intermediate optimum between 1.781 and 2 corresponding to the limits of high or low stochasticity.
Keywords: density-dependent selection, environmental stochasticity, expected fitness, r and K selection, θ-logistic model, genetic trade-off
1. Introduction
Classical population genetics assumes a constant population size, such that the relative fitnesses of genotypes are constants unaffected by changes in population density or genotype frequencies. Alternatively, population growth is allowed such that the fitnesses of all genotypes are either independent of population density or identically dependent on population density, so that, respectively, their fitnesses or relative fitnesses remain constant. Under these assumptions, evolution in a constant environment maximizes the mean fitness or mean relative fitness in the population (Wright 1949, 1969; Fisher 1958; Crow & Kimura 1970).
Frequency-dependent selection occurs when genotypic fitnesses are functions of their frequencies. Even in a constant environment, frequency-dependent selection generally does not maximize the mean fitness or mean relative fitness in a population (Wright 1949, 1969; Crow & Kimura 1970). Common causes of frequency-dependent selection are asymmetrical competition for ecological resources among genotypes or phenotypes (Taper & Case 1985), and sexual selection involving asymmetrical competition for mates among male phenotypes (Lande 1980).
Density-dependent selection occurs when the fitnesses of genotypes within a population respond differently to changes in total population size or density. Density-regulation of a population in a constant environment implies that fitnesses decrease as population size increases. At equilibrium or carrying capacity in a constant environment the mean fitness per generation must equal one in a discrete-time model, or zero in a continuous-time model, with individuals on an average replacing themselves during their lifetime, preventing maximization of the mean fitness in long-term evolution.
In an early effort to introduce more ecological realism into classical population genetic models, MacArthur (1962) showed that in a constant environment, under density-dependent (but frequency-independent) selection, evolution maximizes the equilibrium size or carrying capacity of a population (reviewed by Roughgarden 1979; Boyce 1984). This important conclusion was later extended to models including age structure (Charlesworth 1994) and weak frequency-dependence of genotypic fitnesses (Nagylaki 1992), still assuming a constant environment.
A serious shortcoming of these models is the absence of environmental stochasticity, a ubiquitous feature of natural populations which causes both natural selection and population size to fluctuate (Gillespie 1991; Lande et al. 2003). Initial attempts to deal with this problem in an ecological context involved the qualitative concepts of r and K selection, asserting that under density-independent population growth, or at low population density, selection favours increased population growth rate, whereas at high population density selection favours increased carrying capacity or equilibrium population size (MacArthur & Wilson 1967). Regimes of r and K selection are thought to produce associated differences in life history, such that r-selected species in greatly variable environments have high reproductive rates and short lifespans, whereas K-selected species in more nearly constant environments have low reproductive rates and long lifespans (Pianka 1970, 1972; Desharnais & Constantino 1983; Boyce 1984). These concepts have been extended to life-history evolution by density-dependent selection on age-specific vital rates (Charlesworth 1994; Mueller 1997). Characteristics of a population which are simultaneously or sequentially selected to increase, such as r and K, or age-specific vital rates of survival and fecundity, are generally subject to a constraint or trade-off that inhibits their joint increase. Such trade offs often are manifested in negative genetic correlations between traits within a population (Lande 1982; Charlesworth 1994; Mueller 1997).
Standard ecological concepts of r and K selection fail to account for the fact that populations subject to substantial environmental fluctuations repeatedly experience periods of both high and low population densities, and therefore are subject to both r and K selection. It is widely understood, at least qualitatively, that the magnitude of environmental stochasticity and the pattern of population fluctuations govern the resolution of the trade-off between r and K selection. However, the few existing models of density-dependent (and frequency-independent) selection in a fluctuating environment failed to clarify this trade off or to produce any general evolutionary principle or a valid definition of expected genotypic fitness. In a logistic model of density-dependent selection with small environmental stochasticity, Heckel & Roughgarden (1980) showed that evolution can decrease r, sometimes also decreasing K. Using the more general θ-logistic model and allowing all the parameters to evolve, Turelli & Petry (1980) showed that density-dependent selection in a stochastic environment does not maximize the arithmetic, geometric or harmonic mean population size through time. They reiterated the conjecture of Slatkin & Maynard Smith (1979) that under fluctuating density-dependent selection no general definition of fitness exists, and concluded that no simple quantity is maximized by long-term evolution.
Here, we apply methods of diffusion theory to analyse a system of coupled ecological and evolutionary dynamics to derive an evolutionary maximum principle for density-dependent population dynamics in a fluctuating environment. We also obtain a general expression for the expected fitness of a genotype under fluctuating density-dependent (and frequency-independent) selection. The results reveal how the magnitude of environmental stochasticity and the form of density-dependence govern the trade-off between r and K selection. For simplicity, we analyse a continuous-time model with no age structure and single locus haploid (or asexual) inheritance, in which oscillations and chaotic dynamics are not possible; similar results would be found for a randomly mating population with diploid inheritance under weak selection.
2. Model and results
Let ni be the number or population density of the ith allele at a single locus in a large haploid population, or that of the ith genotype in a large asexual population, and denote the total population size or density by . The basic model is a stochastic differential equation for the change in numbers of each genotype, describing density-dependent growth in a random environment, assuming a large population size for each genotype so that demographic stochasticity and random genetic drift can be neglected,
| (2.1) |
The ith genotype has an intrinsic rate of increase in the average environment, ri. The per capita growth rate of each genotype responds to the total population density according to gi(N) which is an increasing function of N (except for possible Allee effects at very low population density) with gi(0)=0. Selection is density dependent, but frequency independent, when genotypic growth rates differ in their responses to population density, but do not depend on genotypic frequencies. The genotypic growth rates also are subject to environmental stochasticity. Assuming a stationary distribution of environmental states with no temporal autocorrelation, environmental fluctuations in genotypic growth rates can be modelled as white noise, the derivative of Brownian motion, dBi(t)/dt, with zero mean, E[dBi(t)]=0, and covariance cov[dBi(t), dBj(t)]=cij dt (Karlin & Taylor 1981).
The dynamics can then be analysed as a diffusion process characterized by the infinitesimal means and infinitesimal covariances of changes in ln ni and ln nj per unit time, given the vector of genotypic population densities n=(n1, n2, …) (Karlin & Taylor 1981; Gillespie 1991),
| (2.2a) |
| (2.2b) |
Consider two genotypes, or more generally one particular genotype versus all others. The diffusion process can be transformed to the natural log of total population density, ln N=ln(n1+n2), and the frequency of the first genotype, p=n1/(n1+n2), using the Ito transformation formulae (Karlin & Taylor 1981; Gillespie 1991). The infinitesimal moments of the diffusion process for population density and genotype frequency are
| (2.3a) |
| (2.3b) |
| (2.3c) |
| (2.3d) |
| (2.3e) |
Here is the density-independent ‘long-run growth rate’ of the population far below its carrying capacity (Cohen 1977, 1979; Tuljapurkar 1982), which could also be termed the stochastic intrinsic rate of increase. For given genotypic frequencies, the mean intrinsic rate of increase of the population in the average environment is r=pr1+(1−p)r2, and the environmental variance in population growth rate is . The mean density dependence is g(N)=pg1(N)+(1−p)g2(N). Identical formulae hold for multiple genotypes, provided that the partial derivatives with respect to p are interpreted as keeping constant the relative frequencies of the other genotypes (Wright 1969; Crow & Kimura 1970).
The infinitesimal mean and variance of ln N (equations (2.3a) and (2.3b)) take a standard form for density-dependent population dynamics in a random environment (Lande et al. 2003), but now explicitly incorporating genetic variation.
(a) Expected fitness of a genotype
The expected selection gradient in equation (2.3c) can be expressed in an alternative form that comes directly from the Ito transformation. This involves the differences between the expected fitnesses of the two genotypes, , as in classical models of deterministic density-independent selection (Fisher 1958; Wright 1969; Crow & Kimura 1970, chs 1, 5.9),
| (2.4a) |
| (2.4b) |
Note that in equation (2.4b) the subtracted term in brackets is (1/dt)cov[dBi(t), p dB1(t)+(1−p)dB2(t)]. Thus given N and p, the expected fitness of a genotype is its Malthusian fitness in the average environment (equation (2.1)) minus the covariance of its growth rate with that of the population.
Haldane & Jayakar (1963) analysed a discrete-time diploid model of fluctuating density- and frequency-independent selection to derive the condition for maintenance of a genetic polymorphism: the geometric mean growth rate of the heterozygote must exceed that of either homozygote. Assuming weak selection, the long-run growth rate of a genotype in a continuous-time model (its Malthusian fitness in the average environment minus one-half of its environmental variance; equation (2.2a)) closely approximates the natural log of the geometric mean growth rate in a model with discrete non-overlapping generations.
The condition for maintenance of polymorphism in the Haldane–Jayakar model fostered the prevalent but erroneous definition of genotypic fitness in a fluctuating environment as the geometric mean or ‘long-run’ growth rate (e.g. Turelli 1981; Tuljapurkar 1982; Metz et al. 1992, 2008). This was corrected by Lande (2007, 2008) using a diffusion model to derive a result similar to equation (2.4b), omitting the density-dependent term for expected relative fitness because selection was assumed to be density independent. In a continuous-time model, identical additive terms in the density-dependent growth rates of all genotypes do not influence their relative fitnesses, or selection, which only depends on differences in Malthusian fitnesses among genotypes (Fisher 1958; Crow & Kimura 1970; Lande 2007, 2008). In the present model the density-dependent terms must be retained in the expected fitness of a genotype since the genotypic growth rates respond differently to changes in population density, causing selection to be density dependent.
(b) Maximization principle for long-term evolution
Using a model of density-dependent but frequency-independent selection in a constant environment, MacArthur (1962) showed that evolution maximizes the carrying capacity or equilibrium population size, and this conclusion was subsequently supported by other deterministic models (Roughgarden 1979; Charlesworth 1994). This principle does not apply to stochastic environments because fluctuations in population density produce a trade-off between selection for higher intrinsic rate of increase at low population density and selection for higher carrying capacity at high population density, summarized in the concepts of r and K selection (MacArthur & Wilson 1967; Pianka 1970, 1972; Desharnais & Constantino 1983; Boyce 1984; Mueller 1997).
The expected selection gradient in equation (2.3c) shows that, given N and p, the expected direction of evolution is always towards an increased per capita population growth rate. However, environmental stochasticity in selection (equation (2.3d)) often causes this expectation to be violated. Furthermore, density dependence of population dynamics dictates that the long-term average population growth rate must equal zero, apparently obviating long-term maximization of population growth rate. This raises the question of whether any general maximization principle exists for long-term evolution of density-dependent population dynamics in a fluctuating environment.
We analyse a general form of density dependence
| (2.5) |
allowing genetic variation in r, K and , with a constant functional form of density dependence, f(N), assumed to be a monotonically increasing function of N at high population density. For example, in the θ-logistic model f(N)=Nθ with positive θ (Gilpin & Ayala 1973), this implies that θ is constant and does not evolve. Note that in this model, if all genotypes have the same K but different r, then the selection is still density dependent because gi(N) differs among genotypes.
We perform an invasion analysis, deriving a condition for stochastic evolutionary stability of a population to discover a maximization principle for long-term evolution under fluctuating density-dependent (and frequency-independent) selection. This relies on the existence of a (quasi-) stationary distribution of population density in a genetically monomorphic population, obtained from the infinitesimal mean and variance of ln N (equations (2.3a) and (2.3b); Wright 1969; Karlin & Taylor 1981), assuming that r, K and are constants. The stationary distribution exists, and the population can persist, only if the long-run growth rate at low population density is positive, , neglecting the possibility of extinction due to demographic stochasticity and Allee effects (Lande et al. 2003).
Suppose that initially genotype 2 is fixed, p=0, and note that the expected per capita growth rate of the population (equations (2.2a) and (2.3a)), averaged over the stationary distribution of N, must equal 0. This implies when the population is monomorphic for genotype 2 that r2−c22/2=r2 E[f(N)]/f(K2) or
| (2.6a) |
Now introduce genotype 1 at a very low frequency p near 0 but in sufficiently large numbers that demographic stochasticity (as well as Allee effects) and random genetic drift can be neglected. Genotype 1 when rare will increase in frequency in the long run if its expected per capita growth rate (equations (2.2a) and (2.3a)), averaged over the stationary distribution of population density for genotype 2 when monomorphic, exceeds zero,
| (2.6b) |
This condition, proposed as an unproven conjecture by Turelli & Petry (1980), is exact in the present model because on the scale of ln N the infinitesimal variance, and the infinitesimal mean averaged over the stationary distribution of N, both are asymptotically constant (equations (2.3a) and (2.3b)). In the absence of age structure, autocorrelation of N caused by density dependence does not alter the long-run growth rate of the rare genotype in equation (2.6b) (Lande et al. 2003). The probability distribution of log population density for the rare type is therefore asymptotically normal with mean and variance both increasing linearly with time, implying that all sample paths for the population density of the rare type plotted against time eventually approach the same slope (Cohen 1977, 1979; Lande et al. 2003). Using equation (2.6a) to evaluate the expectation produces the condition for invasion by genotype 1,
| (2.6c) |
The reverse inequality gives the condition for invasion by genotype 2 into a population initially fixed for genotype 1, indicating that the genotype with the largest value of E[f(N)] eventually undergoes quasi-fixation, approaching arbitrarily close to fixation for an indefinitely long time. Thus, with a general form of density dependence, allowing evolution of r, K and , long-term evolution maximizes
| (2.7a) |
In a constant environment, , long-term evolution maximizes K because f(N) is assumed to be an increasing function of N at high population density, in agreement with classical findings for density-dependent selection (MacArthur 1962; Roughgarden 1979; Mueller 1997). With no genetic variance in either K or , evolution maximizes r as under density-independent selection in a constant environment (Fisher 1958; Wright 1969; Crow & Kimura 1970).
In a fluctuating environment with no genetic variation in K, long-term evolution maximizes (the ratio of the density-independent long-run growth rate to the deterministic growth rate), which may involve a trade-off between an increasing r and a decreasing . This differs somewhat from the result for density-independent selection, where evolution is a stochastic maximization of the long-run growth rate, (Lande 2007, 2008), because genetic variation in r produces density-dependent selection even when all genotypes have the same K, as explained after equation (2.5).
If a trade-off or negative genetic correlation exists between r and K, and all genotypes have the same environmental variance, then the magnitude of and the form of density dependence govern the long-term evolution of population dynamics. Low environmental variance favours an increased K, high environmental variance favours an increased r, and lower curvature (second derivative) of f(N) tips the balance towards an increased K.
For example under θ-logistic density dependence, f(N)=Nθ with θ a positive constant (Gilpin & Ayala 1973), larger θ produces lower curvature in f(N), with positive curvature for θ<1 and a negative curvature for θ>1. The logistic model is recovered when θ=1. The Gompertz form of density regulation with f(N)=ln N arises in the limit of θ→0, and the ceiling model of density-independent growth to a maximum population density K is approached when θ→∞ (Lande et al. 2003). The stationary distribution of N in a genetically monomorphic population subject to θ-logistic density dependence exists only when the density-independent long-run growth rate of the population is positive (see appendix A). Consistent with equation (2.7a) it yields
| (2.7b) |
which we have shown as the quantity maximized by long-term evolution. Armstrong & Gilpin (1977) derived a similar formula for the quantity maximized by fluctuating density-dependent selection in the logistic model, but their expression contains the mean density-independent mortality in place of and therefore constitutes essentially a reparametrization of MacArthur's deterministic model.
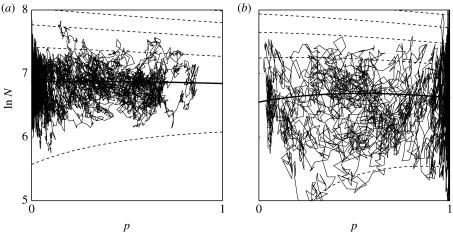
In the present model, sample paths of the two-dimensional process for N and p often display repeated rapid transitions between high and low gene frequencies, but the genotype with the largest E[f(N)] eventually undergoes quasi-fixation. The role of environmental stochasticity in governing the trade-off between r selection at low population density and K selection at high population density is illustrated in figure 1 by sample paths with logistic density dependence, θ=1.
Figure 1.
Simulated sample paths in a discrete-time version of the haploid model of fluctuating density-dependent selection with logistic regulation, f(N)=N. The frequency of genotype 1 is p and total population density is N. In the average environment, genotype 1 has a higher intrinsic rate of increase, r1=0.04, r2=0.03, and genotype 2 has a higher carrying capacity, K1=1000, K2=1050. Environmental variance in instantaneous fitness is the same for both genotypes, with correlation 0.5. (a) Low environmental stochasticity: c11=c22=0.005, c12=0.0025. (b) High environmental stochasticity: c11=c22=0.02, c12=0.01. Dotted lines are contours of the surface of per capita population growth rate, , as a function of p and ln N, the surface being negative at population densities above, and positive below, the zero-growth isocline (thick solid line). At any point on the surface the expected selection gradient is the slope with respect to p, holding N constant (equation (2.3c)). Contours indicate that for N well below the zero-growth isocline selection favours higher r (genotype 1), and for N well above the zero-growth isocline selection favours higher K (genotype 2). The sample path in each figure begins near the zero-growth isocline at p=1/2 and runs 10 000 time steps. The magnitude of environmental stochasticity determines how long-term evolution resolves the trade-off between r and K selection, with eventual quasi-fixation of genotype 2 under low stochasticity, and genotype 1 under high stochasticity.
Equation (2.7b) shows that long-term evolution always acts to increase r and K, and to decrease , in agreement with the expected selection gradient (equation (2.3c)). Joint evolution of parameters that are always under directional selection necessarily involves a trade-off among them (otherwise r and K would both evolve to infinity and to 0). By contrast, in the θ-logistic model, for given values of the other parameters θ has an intermediate optimum restricted to a narrow range depending only on the composite parameter (the density-independent long-run growth rate divided by half the environmental variance), which must be positive for the population to persist. Gilpin et al. (1976) incorrectly concluded that, given the values of the other parameters, θ is always selected to increase. Appendix A shows that in the limit of low stochasticity (a→∞) the optimal θ=2 and in the limit of high stochasticity (a→0) the optimal θ=eγ≈1.781, where Euler's γ≈0.577.
3. Discussion
A general formula for the expected fitness of a genotype under fluctuating density-dependent selection has not previously been discovered, and its existence has been doubted (Slatkin & Maynard Smith 1979; Turelli & Petry 1980). Even for density-independent selection, confusion about the definition of fitness in a fluctuating environment has persisted since Haldane & Jayakar (1963) derived a condition for the invasion of a rare allele and the maintenance of genetic polymorphism in a randomly mating population with discrete non-overlapping generations and single-locus diploid inheritance: the geometric mean growth rate for the heterozygote must exceed that of either homozygote. (To clarify this terminology for a diploid genetic model, the growth rate of a genotype refers to the rate of increase of a hypothetical pure population of that genotype, which is the same as its fitness per generation.) The Haldane–Jayakar result for long-term evolution led to the widespread but mistaken belief that the geometric mean growth rate, or its continuous time analogue, the long-run growth rate, constitutes fitness in a fluctuating environment (e.g. Turelli 1981; Tuljapurkar 1982; Metz et al. 1992). However, the expected fitness of a genotype should be defined not by the outcome of long-term evolution, but by the expected rate of change of gene frequency over a short time interval, such as a single generation.
This classical approach has been recently employed to define the expected relative fitness of a genotype under fluctuating density-independent selection (Lande 2007, 2008), which is here extended to density-dependent selection (equation (2.4b)). The expected fitness of a genotype, given N and p, is its Malthusian fitness in an average environment minus the covariance between its growth rate and that of the population. Thus the expected fitness of a genotype depends not only on the environmental variance in its own growth rate, but also on the correlation of growth rates between genotypes. In the Haldane–Jayakar model of fluctuating density-independent selection, correlation of growth rates between the two homozygous genotypes has a profound impact on the stationary distribution of gene frequency (Lande 2008).
It has also been unclear whether any simple quantity is maximized by a long-term evolution under density-dependent (and frequency-independent) selection in a fluctuating environment. Using the θ-logistic model with stochasticity and genetic variation in all the parameters, Turelli & Petry (1980) showed that evolution does not maximize either the arithmetic, geometric or harmonic mean population size. Under a general form of density-dependence, f(N) (equations (2.1) and (2.5)), allowing genetic variation in the intrinsic rate of increase and carrying capacity in the average environment, r and K, and the environmental variance in population growth rate, , we have established that long-term evolution maximizes E[f(N)] (equation (2.7a)). In the θ-logistic model, where f(N)=Nθ with θ a positive constant, long-term evolution maximizes (equation (2.7b)).
Our results (equations (2.7a) and (2.7b)) agree with MacArthur's finding that in a constant environment long-term evolution maximizes K, because f(N) is assumed to increase with N at a high population density (MacArthur 1962; Roughgarden 1979; Nagylaki 1992; Charlesworth 1994). Without genetic variation in K, long-term evolution maximizes ; this may involve a trade-off between an increasing r and a decreasing , which also occurs under density-independent selection in a fluctuating environment where evolution is a stochastic maximization of the long-run growth rate, (Gillespie 1991; Lande 2007, 2008). With no genetic variation in K, and identical environmental variance for all genotypes, evolution maximizes r as in classical population genetic models of density-independent selection in a constant environment (Fisher 1958; Wright 1969; Crow & Kimura 1970).
Previous theories of density-dependent selection failed to fully clarify the role of environmental stochasticity in mediating the trade-off between r and K selection. The concepts of r and K selection (MacArthur & Wilson 1967), and their extensions to age-structured populations (Charlesworth 1994; Mueller 1997), suggest that an increased r is favoured at low population density, while an increased K is favoured at high population density. Both quantities cannot simultaneously increase when a trade-off exists between them (MacArthur & Wilson 1967). Evolutionary constraints owing to such ecological trade-offs have been detected experimentally as a negative genetic correlation between r and K, or age-specific vital rates (Lande 1982; Charlesworth 1994; Mueller 1997). The basic concepts of r and K selection are conditional statements about the direction of selection and short-term evolution, given the current population density and gene frequency. These concepts do not account for repeated transitions between low and high population densities in a fluctuating environment, rendering ambiguous the fact on how the trade-off between r and K selection would be resolved.
If a trade-off (or negative genetic correlation) exists between r and K, and all genotypes have identical environmental variance, then the magnitude of and the form of density dependence govern the long-term evolution of population dynamics. Low stochasticity favours increased K and high stochasticity favours increased r, with lower curvature in the density dependence function (e.g. larger θ in the θ-logistic model) tipping the balance towards an increased K (equations (2.7a) and (2.7b)).
The importance of trade-offs between life-history characters including major components of fitness, and empirical approaches to their measurement are comprehensively reviewed by Roff (2002). Trade-offs should be measured either by estimating genetic correlations between life-history characters or by experimental manipulation to determine causal relationships, rather than by observation of phenotypic correlations within populations or among populations or species. Quantitative genetic methods for partitioning phenotypic variance and covariance into genetic and environmental components (Falconer & MacKay 1996; Lynch & Walsh 1998) control for unmeasured environmental variation among individuals, such as resource availability, which can produce positive environmental correlations between characters that mask negative genetic correlations (Dickerson 1955; Lande 1982; Bell & Koufopanou 1985; Reznick 1985). Experimental manipulations of life-history characters control for unmeasured (genetic and environmental) variation in resource acquisition among individuals, which can obscure the causal trade-offs (van Noordwijk & de Jong 1986; Houle 1991). Complex trade-offs among multiple life-history characters also can manifest as a mixture of positive and negative genetic correlations among the characters that constrain the evolution of net fitness (Dickerson 1955; Lande 1982; Pease & Bull 1988).
Even the present simple model with no age structure may have a complex genetic trade-off among the parameters. Because r and K are always selected to increase and is always selected to decrease, a trade off between them must occur to prevent these parameters from evolving towards infinity and zero respectively. By contrast, appendix A shows for the θ-logistic model that, given the values of the other parameters, θ has an intermediate optimum ranging from 1.781 to 2 corresponding respectively to the limits of high or low environmental stochasticity.
Under a constant functional form of density dependence, f(N), with haploid (or asexual) inheritance, a genetic polymorphism cannot be maintained and the genotype with the largest E[f(N)] eventually undergoes quasi-fixation. Figure 1 illustrates that sample paths of the coupled ecological and evolutionary process often perform repeated rapid excursions between high and low population size, involving frequent transitions between regimes of r and K selection, and between high and low gene frequencies, before eventual quasi-fixation of the genotype with the largest E[f(N)].
As for the coexistence of species competing for the same resources in a cyclical or stochastic environment (Chesson 2000), under density-dependent (and frequency-independent) fluctuating selection, the maintenance of genetic polymorphism with haploid (or asexual) inheritance would require genetic differences in the functional form of density dependence. This could occur in the θ-logistic model when genotypes differ substantially in θ and other parameter(s).
Results similar to those obtained here can be derived with diploid inheritance, as suggested by recent findings for fluctuating density-independent selection (Lande 2007, 2008). Invasion analysis for a diploid diallelic locus in a randomly mating population under weak selection, using the methods described above, would show that genetic polymorphism is maintained when E[f(N)] for (a hypothetical pure population of) heterozygotes exceeds that of either homozygote. Extension of the present theoretical results to more realistic modes of inheritance, including quantitative characters and age-structure, constitutes a formidable but worthwhile challenge for future research.
Acknowledgments
This work was supported by the Royal Society of London and by the Centre for Conservation Biology at the Norwegian University of Science and Technology.
Appendix A.
In a genetically monomorphic population subject to theta-logistic regulation, the diffusion process for the population size, N, has infinitesimal mean rN[1−(N/K)θ] and infinitesimal variance . Assuming that the population remains large, so that demographic stochasticity and Allee effects can be neglected, the stationary distribution of population size is
where (Diserud & Engen 2000; Sæther et al. 2008). This distribution exists only if a>0, and has moments given by
which when b=θ reduces to .
A rare genotype with parameters can invade a population initially fixed for genotype 0 if its long-run growth rate is positive (equation (2.6b)),
where E0 denotes the expectation with respect to the stationary distribution of the common resident genotype 0. Now assuming that r, K and are constants (identical for both genotypes), the long-run growth rate of the rare genotype is
which has the property G(θ0, θ0)=0. Now if an intermediate optimum value of θ exists then G(θ0, θ0)<0 for all θ≠θ0, and the optimum θ is the solution of . This produces the equation for the optimum θ
Here, Ψ(x)=d ln Γ(x)/dx is the digamma function (Abramowitz & Stegun 1972) the properties of which can be used to show that in the limit of low stochasticity (a→∞) the optimal θ=2, and in the limit of high stochasticity (a→0) the optimal θ=eγ≈1.781, where Euler's γ≈0.577. Numerical analysis shows that the solution to the above equation indeed represents a maximum rather than a minimum of G(θ, θ0).
Footnotes
One contribution of 14 to a Theme Issue ‘Eco-evolutionary dynamics’.
References
- Abramowitz M., Stegun I.A., editors. Handbook of mathematical functions. Dover; New York, NY: 1972. [Google Scholar]
- Armstrong R.A., Gilpin M.E. Evolution in a time-varying environment. Science. 1977;195:591–592. doi: 10.1126/science.835017. doi:10.1126/science.835017 [DOI] [PubMed] [Google Scholar]
- Bell G., Koufopanou V. The cost of reproduction. Oxf. Surv. Evol. Biol. 1985;3:83–131. [Google Scholar]
- Boyce M.S. Restitution of r- and K-selection as a model of density-dependent natural selection. Annu. Rev. Ecol. Syst. 1984;15:427–447. doi:10.1146/annurev.ecolsys.15.1.427 [Google Scholar]
- Charlesworth B. 2nd edn. Cambridge University Press; Cambridge, UK: 1994. Evolution in age-structured populations. [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. doi:10.1146/annurev.ecolsys.31.1.343 [Google Scholar]
- Cohen J.E. Ergodicity of age structure in populations with Markovian vital rates. III. Finite-state moments and growth rate; an illustration. Adv. Appl. Probab. 1977;9:462–475. doi:10.2307/1426109 [Google Scholar]
- Cohen J.E. Ergodic theorems in demography. Bull. Am. Math. Soc. 1979;1:275–295. doi:10.1090/S0273-0979-1979-14594-4 [Google Scholar]
- Crow J.F., Kimura M. Harper & Row; New York, NY: 1970. An introduction to population genetics theory. [Google Scholar]
- Desharnais R.A., Constantino R.F. Natural selection and density-dependent population growth. Genetics. 1983;105:1029–1040. doi: 10.1093/genetics/105.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson G.E. Genetic slippage in response to selection for multiple objectives. Cold Spring Harb. Symp. Quant. Biol. 1955;20:213–224. doi: 10.1101/sqb.1955.020.01.020. [DOI] [PubMed] [Google Scholar]
- Diserud O.H., Engen S. A general and dynamic species abundance model, embracing the lognormal and the gamma models. Am. Nat. 2000;155:497–511. doi: 10.1086/303339. doi:10.1086/303339 [DOI] [PubMed] [Google Scholar]
- Falconer D.S., MacKay T.F.C. 4th edn. Longman; London, UK: 1996. Introduction to quantitative genetics. [Google Scholar]
- Fisher R.A. 2nd edn. Dover; New York, NY: 1958. The genetical theory of natural selection. [Google Scholar]
- Gillespie J.H. Oxford University Press; Oxford, UK: 1991. The causes of molecular evolution. [Google Scholar]
- Gilpin M.E., Ayala F.J. Global models of growth and competition. Proc. Natl Acad. Sci. USA. 1973;70:3590–3593. doi: 10.1073/pnas.70.12.3590. doi:10.1073/pnas.70.12.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin M.E., Case T.J., Ayala F.J. θ-Selection. Math. Biosci. 1976;32:131–139. [Google Scholar]
- Haldane J.B.S., Jayakar S.D. Polymorphism due to selection of varying direction. J. Genet. 1963;58:237–242. doi:10.1007/BF02986143 [Google Scholar]
- Heckel D.G., Roughgarden J. A species near its equilibrium size in a fluctuating environment can evolve a lower intrinsic rate of increase. Proc. Natl Acad. Sci. USA. 1980;77:7497–7500. doi: 10.1073/pnas.77.12.7497. doi:10.1073/pnas.77.12.7497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle D. Genetic covariance of fitness correlates: what genetic correlations are made of and why it matters. Evolution. 1991;45:630–648. doi: 10.1111/j.1558-5646.1991.tb04334.x. doi:10.2307/2409916 [DOI] [PubMed] [Google Scholar]
- Karlin S., Taylor H.M. Academic Press; New York, NY: 1981. A second course in stochastic processes. [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. doi:10.2307/2407393 [DOI] [PubMed] [Google Scholar]
- Lande R. A quantitative genetic theory of life-history evolution. Ecology. 1982;63:607–615. doi:10.2307/1936778 [Google Scholar]
- Lande R. Expected relative fitness and the adaptive topography of fluctuating selection. Evolution. 2007;61:1835–1846. doi: 10.1111/j.1558-5646.2007.00170.x. doi:10.1111/j.1558-5646.2007.00170.x [DOI] [PubMed] [Google Scholar]
- Lande R. The adaptive topography of fluctuating selection in a Mendelian population. J. Evol. Biol. 2008;21:1096–1105. doi: 10.1111/j.1420-9101.2008.01533.x. doi:10.1111/j.1420-9101.2008.01533.x [DOI] [PubMed] [Google Scholar]
- Lande R., Engen S., Sæther B.-E. Oxford University Press; Oxford, UK: 2003. Stochastic population dynamics in ecology and conservation. [Google Scholar]
- Lynch M., Walsh B. Sinauer; Sunderland, MA: 1998. Genetics and analysis of quantitative traits. [Google Scholar]
- MacArthur R.H. Some generalized theorems of natural selection. Proc. Natl Acad. Sci. USA. 1962;48:1893–1897. doi: 10.1073/pnas.48.11.1893. doi:10.1073/pnas.48.11.1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R.H., Wilson E.O. Princeton University Press; Princeton, NJ: 1967. The theory of island biogeography. [Google Scholar]
- Metz J.A.J., Nisbet R.M., Geritz S.A.H. How should we define ‘fitness’ for general ecological scenarios? Trends Ecol. Evol. 1992;7:198–202. doi: 10.1016/0169-5347(92)90073-K. doi:10.1016/0169-5347(92)90073-K [DOI] [PubMed] [Google Scholar]
- Metz J.A.J., Mylius S.D., Diekmann O. When does evolution optimize? Evol. Ecol. Res. 2008;10:629–654. [Google Scholar]
- Mueller L.D. Theoretical and empirical examination of density-dependent selection. Annu. Rev. Ecol. Syst. 1997;28:269–288. doi:10.1146/annurev.ecolsys.28.1.269 [Google Scholar]
- Nagylaki T. Springer; Berlin, Germany: 1992. Introduction to theoretical population genetics. [Google Scholar]
- Pease C.M., Bull J.J. A critique of methods for measuring life history trade-offs. J. Evol. Biol. 1988;1:293–303. doi:10.1046/j.1420-9101.1988.1040293.x [Google Scholar]
- Pianka E.R. On r- and K-selection. Am. Nat. 1970;104:592–597. doi:10.1086/282697 [Google Scholar]
- Pianka E.R. r and K selection or b and d selection? Am. Nat. 1972;106:581–588. doi:10.1086/282798 [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. doi:10.2307/3544698 [Google Scholar]
- Roff D. Sinauer; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Roughgarden J. Macmillan; New York, NY: 1979. Theory of population genetics and evolutionary ecology: an introduction. [Google Scholar]
- Sæther B.-E., et al. Forms of density regulation and (quasi-) stationary distributions of population sizes in birds. Oikos. 2008;117:1197–1208. doi:10.1111/j.0030-1299.2008.16420.x [Google Scholar]
- Slatkin M., Maynard Smith J. Models of coevolution. Q. Rev. Biol. 1979;54:233–263. doi:10.1086/411294 [Google Scholar]
- Taper M.L., Case T.J. Quantitative genetic models for the coevolution of character displacement. Ecology. 1985;66:355–371. doi:10.2307/1940385 [Google Scholar]
- Tuljapurkar S.D. Population dynamics in variable environments. III. Evolutionary dynamics of r-selection. Theor. Popul. Biol. 1982;21:141–165. doi:10.1016/0040-5809(82)90010-7 [Google Scholar]
- Turelli M. Temporally varying selection on multiple alleles. J. Math. Biol. 1981;13:115–129. doi:10.1007/BF00276870 [Google Scholar]
- Turelli M., Petry D. Density-dependent selection in a random environment: an evolutionary process that can maintain stable population dynamics. Proc. Natl Acad. Sci. USA. 1980;77:7501–7505. doi: 10.1073/pnas.77.12.7501. doi:10.1073/pnas.77.12.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noordwijk A.J., de Jong G. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 1986;128:137–142. doi:10.1086/284547 [Google Scholar]
- Wright S. Adaptation and selection. In: Jepson G.L., Simpson G.G., Mayr E., editors. Genetics, paleontology and evolution. Princeton University Press; Princeton, NJ: 1949. pp. 365–389. [Google Scholar]
- Wright, S. 1969 Evolution and the genetics of populations The theory of gene frequencies, vol. 2. Chicago, IL: University of Chicago Press.