Abstract
Given its propensity to metastasize, and lack of effective therapies for most patients with advanced disease, early detection of melanoma is a clinical imperative. Although there are no non-invasive techniques for definitive diagnosis of melanoma, and the “gold standard” remains biopsy with histologic examination, a variety of modalities may facilitate early melanoma diagnosis and the detection of new and changing nevi. This article reviews general clinical principles of early melanoma detection, and various modalities that are currently available or on the horizon, providing the clinician with an up-to-date understanding of management strategies for their patients with numerous or atypical nevi.
Learning objectives
At the conclusion of this learning activity, participants should: 1) understand the clinical importance of early melanoma detection; 2) appreciate the challenges of early melanoma diagnosis and which patients are at highest risk; 3) know general principles of early melanoma detection; 4) be familiar with current and emerging modalities that may facilitate early melanoma diagnosis and the detection of new and changing nevi; 5) know the advantages and limitations of each modality; and 6) be able to practice a combined approach to the patient with numerous or clinically atypical nevi.
INTRODUCTION
Melanoma has doubled in incidence in recent decades and is increasing more rapidly than any other cancer.1 There are an estimated 60,000 cases and over 8000 deaths associated with melanoma in the U.S. annually, with an average individual lifetime risk of melanoma approaching 1 in 75.2 Despite considerable efforts to develop new therapies for melanoma, patients with advanced disease continue to have a poor prognosis.3 Although many patients with melanoma localized to the skin are cured by surgical excision, increased time to diagnosis is associated with higher stage of disease, and those with regional lymphatic or metastatic disease respond poorly to conventional radiation and chemotherapy with 5-year survival rates ranging from 10 to 50%.3 The cost of treating melanoma rises significantly with disease stage, as less than 20% of patients with stage III–IV disease were responsible for 90% of the total annual cost for treating melanoma in 1997, which was estimated at $563 million.4 Thus earlier detection of melanoma is a key factor in improving patient survival and decreasing treatment costs.
There is currently no consensus in our specialty regarding the optimal modality or strategy for early melanoma detection. While isolated lesions can be biopsied, this becomes problematic in patients with numerous nevi or large clinically atypical nevi. In a recent survey of fellows of the American Academy of Dermatology regarding their management of patients with history of histologically-confirmed dysplastic nevi, Tripp et al5 reported that 99% recommended self-examinations, 75% performed total body skin exams on follow-up visits, 60% recommended ophthalmologic exams for some patients, 49% obtained baseline total body skin photography for most patients, and 23% used dermoscopy in their practice. Fifty-eight percent of practitioners recommended follow-up exams every 12 months, while 33% recommended 6-month follow-up visits in most patients.
Despite the considerable variability in practices in this area, it is likely that most dermatologists would agree on long-term goals for monitoring and melanoma detection (Table I). These include: identification and screening of high-risk patients, biopsy of melanomas early (rather than late), observation/monitoring of nevi (but not melanomas), and avoidance of unnecessary biopsies/excisions.
TABLE I.
Goals for monitoring and early melanoma detection
|
SCREENING
Who should be screened?
It is clearly impractical to screen everyone, and there are no consistent guidelines for melanoma screening.6 Screening efforts are most effective, however, when focused on patients known to be at increased risk. Classical risk factors for melanoma include: prior personal history of melanoma, positive family history of melanoma (≥2 members),7 numerous nevi, and presence (or history of) atypical/dysplastic nevi.8–10 Patients with history of a single melanoma have a 5–8% risk of developing a second melanoma.11,12 With respect to nevi, Holly et al13 reported relative risks of 1.6 for patients with 11–25 nevi, 4.4 for 26–50 nevi, 5.4 for 51–100 nevi and 9.8 for ≥100 nevi, and relative risks of 3.8 for 1–5 atypical nevi and 6.3 for ≥6 atypical nevi. Additional risks are associated with having fair skin (4-fold),14 red hair and blue eyes (2-fold),15 history of non-melanoma skin cancer (2–3 fold),16,17 and history of sunburns or excessive UV exposure (2-fold)18 including indoor tanning (2–3 fold).19 Other important aspects to consider are age and sex, as risk increases with age and men have higher melanoma incidence than women,20 and changing or new nevi are more likely to be melanoma in patients over age 50.21 All these risk factors can be assessed by history and physical examination. Annual screening of patients known to be at increased risk for melanoma is cost-effective,22 as is one-time screening of the general population over age 50 and bi-annual screening of siblings of melanoma patients.23 Several studies have shown that screening is associated with detection of thinner melanomas.24–26
Obstacles to screening
Although campaigns such as “Melanoma Monday” sponsored by the American Academy of Dermatology have increased public awareness,27 an insufficient number of dermatologists28,29 and lack of time for skin cancer screening by practicing dermatologists30 remain real obstacles to adequate melanoma screening and may account for delayed diagnosis. While primary care physicians could fill some of this gap, they are less likely to perform complete skin exams,31 and less likely than dermatologists to correctly diagnose melanoma according to several studies.29,32 In addition, melanomas biopsied by other physicians were associated with greater depth and increased mortality than those biopsied by dermatologists.33,34
Several studies have also shown that delays in melanoma diagnosis may be attributed to patient-related factors such as lack of concern.35,36 Males, the elderly, and those of lower educational status tend to have poorer rates of self-detection, longer delay before seeking medical attention, and greater melanoma tumor thickness.37,38
WHAT WE TELL PATIENTS
Recognizing melanoma
The ABCD acronym was devised in 1985 by Kopf and colleagues39 to help patients recognize several clinical features of melanoma: asymmetry, border irregularity, color variation, and diameter (>6 mm). While most melanomas tend to exhibit these features, amelanotic melanomas usually do not, and hence their diagnosis is often delayed.40,41 In addition, melanomas arising de novo (not within pre-existing nevi) will be smaller than 6 mm at an early stage. The acronym is also not very specific, as seborrheic keratoses which are very common in older patients often will exhibit “ABCD” features.
The history of change in a nevus is a red flag that may signal malignancy, and is noted at a significantly higher frequency in malignant than benign pigmented skin lesions.42 Indeed, short-term monitoring studies have revealed that most growing melanomas exhibit observable changes over a period of 3–6 months.43–45 Longer-term observational studies similarly found that melanomas tend to exhibit non-uniform growth patterns.46 Certain changes in nevi are associated with earlier melanoma detection, including changes in color, size, shape, elevation, and patient symptoms such as itching.47 Given these considerations, Polsky and colleagues recommended adding an “E” for evolving to the ABCD acronym to increase its sensitivity and specificity.48,49
New and changing nevi
Not all changes in nevi, however, are suspicious for melanoma. This is particularly true if the change is symmetric enlargement, which is expected as moles grow (particularly in younger patients). Other normal changes include uniform darkening of nevi, which may occur following sun exposure.50 Rates of change in nevi have been reported in the range of 4–6%,51–53 and we recently reported that only 96 of 5945 (1.6%) clinically atypical nevi exhibited interval changes over a 4-year monitoring period.54 Thus spontaneous changes in nevi, not attributed to other factors (see below), are uncommon in adults.
Although changes in nevi during pregnancy are thought to be common,55 there is little supporting evidence in the literature. Sanchez et al56 found that of 389 pregnant women examined, only 10% reported changes in their pigmented lesions and none of 28 lesions biopsied revealed significant histologic changes compared with similar pigmented lesions from age-matched women who were not pregnant. Pennoyer et al57 compared sizes of 129 nevi on the back in 22 pregnant women during their first and third trimesters, and did not find significant interval changes in nevus size. Akturk et al58 evaluated 97 nevi in 56 pregnant women in the first and third trimesters and did find significant increases in average nevus diameter, but predominantly in lesions on the front of the body. Approximately 6% of lesions developed dermoscopic changes, again predominantly on the front of the body.58 Zampino et al59 monitored 86 nevi located on the back in 47 pregnant women, and found significant progressive lightening of nevi without significant changes in size. Taken together, these studies suggest that changes in nevi during pregnancy are relatively uncommon and may largely result from skin expansion.
Nevi may also undergo reversible changes in color or texture that may be incited by chronic rubbing or other trauma. It is important to recognize that these types of changes are generally symmetric and uniform, while asymmetric changes in shape or color within a changing lesion, including ulceration or bleeding, would be suspicious for melanoma. It is suggested that morphologic changes in nevi subjected to mechanical irritation are due to increased numbers of suprabasal melanocytes and alterations in keratinocyte adhesion molecules.60 Selim et al61 examined 92 (non-surgically) traumatized nevi and in 20% noted pagetoid spread, almost always directly beneath zones of parakeratosis; cytologic atypia and mitoses in melanocytes were rarely seen.
Similarly, a new nevus may not be concerning, unless it appears different than the patient’s other existing nevi. New moles are expected in younger patients,62 and it is well-known that total nevus number generally peaks in the third decade before slowly declining in the seventh and eighth decades.63 Nevi may also regress in younger patients, as Siskind et al64 found in monitoring 230 facial nevi in 20 adolescents over a 4-year period. Although total nevus number increased by 56%, 61 (27%) nevi – predominantly small flat lesions – disappeared.64 In our practice, we have documented that it is not uncommon for “nevogenic” patients to develop new nevi into 40’s and 50’s. In addition, new nevi may arise in “eruptive” fashion in several clinical contexts. These include blistering skin diseases such as erythema multiforme,65 toxic epidermal necrolysis,66 and epidermolysis bullosa.67 Eruptive nevi have also been described in immunosuppressed patients following organ transplantation68 and HIV infection.69 Finally, it is important to note that only a small percentage of new or changing lesions will represent melanoma, although the likelihood increases significantly if the patient is over age 50.21
Role of self skin examination
Instructing patients to perform regular self skin examinations (SSE) is important for several reasons. First, melanomas are commonly detected by patients, although it is far more common for dermatologists to detect second primary tumors.33,70,71 Performing SSE has been associated with thinner melanomas25 and reduced mortality72 in some studies, although others found that patient-detected melanomas are more likely to be thicker than those detected by physicians.70,71 One study showed 58% sensitivity and 62% specificity for patient detection of artificial changes in mole size.73 However, diagnostic accuracy of SSE can be improved with the use of digital photography.74–76
Second, the SSE may be the only defense for patients who develop nodular melanomas which, due to their rapid growth, are more likely to arise between physician screening visits.36,77 Finally, SSE are important because they establish a role for the patient in sharing responsibility with the physician in early melanoma detection. Patients should initially be advised to perform SSE frequently so as to become familiar with the appearance of their nevi, and then transition to monthly SSE to detect new and changing nevi. After the initial period, monthly frequency for SSE seems optimal given the interval of clinical changes in developing melanomas (3–6 months, noted above). If SSE is performed too frequently, patients may not appreciate small or gradual changes over time.
VISUAL DETECTION
In some cases, particularly with more advanced lesions, melanoma can be easily diagnosed by the naked eye. More commonly, however, we are faced with the difficult clinical differential diagnosis of atypical nevus vs. melanoma. In addition, benign-appearing amelanotic melanomas are easily missed.40,41,78 Multiple studies based on test photographs29,32 and retrospective analyses79 have estimated the success rate of dermatologists in correctly diagnosing melanoma at approximately 80%, although diagnostic accuracy in practice likely varies depending on years of experience and frequency of patients seen with pigmented lesions. Although physicians are able to detect melanoma at earlier stages than their patients,70,71 multiple studies have shown that dermatologists are better than other physicians at early detection.29,32 Initial melanomas found by dermatologists are more likely to be ≤0.75 mm in depth than those found by other physicians,33 and are thus associated with better survival rates and lower cancer-related mortality.34
Should we focus on nevi?
Melanomas are often described by patients as a “new mole”.80,81 As noted above, the presence of nevi is associated with increased (2–10 fold) melanoma risk.8–10,13 However, the annual risk of transformation to melanoma for a single nevus is estimated at only 1/200,000,82 raising the question of whether nevi are truly precursors of melanoma or simply a marker of melanoma risk. Given that 22–50% of melanomas show nevus origin histologically,9,80,83 it is likely that both scenarios are true. A recent study comparing nevus-derived and de novo melanomas did not find significant difference in tumor thickness when controlling for other prognostic factors.84 It is reasonable to conclude that at least half of melanomas arise de novo, from isolated melanocytes rather than from pre-existing nevi, and hence effective approaches to early melanoma detection ideally should identify and evaluate both new and changing nevi.
Signature nevi
Prior to close visual inspection of a particular nevus, it is useful to first look for other lesions on the patient displaying similar morphologic characteristics. Similar-appearing nevi, or recurring patterns within nevi, constitute a patient’s “signature” lesions. Common nevus signatures include uniformly brown nevi, and uniformly pink nevi in patients with fairer skin types. “Two-tone” nevi are represented by pink or light brown lesions with darker centers or darker lesions with more lightly pigmented centers. The “fried-egg” nevus contains a central portion that is elevated. These usually reveal benign histologic patterns, although mild dysplasia may be seen. Bolognia and colleagues first used the term “signature nevus” in the literature,85 and have described several of the less common varieties. “Eclipse” nevi display central tan or pink coloration with a darker peripheral rim, and are commonly found on the scalp in children.85 An inverse clinical pattern may also be seen, with darker central coloration and a lighter peripheral rim. Targetoid or “cockade” nevi 86,87 exhibit concentric patterns of pigmentation usually consisting of central and peripheral pigmented areas with an intervening area of hypopigmentation. “Halo” nevi are in various stages of regression due to inflammation,88 and most commonly present as hypopigmented skin surrounding a pigmented lesion that progressively lightens over time, although darkening of the central lesion has also been described.89
Nevi may display eccentric dark dots which can represent epidermal or dermal pigmentation90 or a clonal nevus.91 In their prospective analysis of 59 nevi with foci of hyperpigmentation, Bolognia et al90 found that most were benign but 3 lesions (5%) revealed melanoma. Nevi may also contain dark dots at the periphery, or more commonly scattered within the lesion (“shrapnel” or “ladybug” pattern). Perifollicular hypopigmentation may be associated with either terminal or vellus hairs within a nevus.92 The pattern of hypopigmentation will be circular if the hair is located within the nevus, and notched (making the border irregular) if the hair is at the edge of the nevus. Perifollicular hyperpigmentation within nevi has also been described.93 Although the presence of terminal hairs within a nevus is generally thought to be a benign sign, exceptions have been described.94 The presence of numerous small uniformly dark nevi constitutes the “cheetah” phenotype.95 Finally, the “ink-spot lentigo” is technically not a nevus, but refers to a reticulated black solar lentigo that appears to resemble a very dark spot of ink on the skin.96 These are often seen on a background of numerous or confluent lentigines. The various signature lesion types that have been described are summarized in Fig 1.
Figure 1.
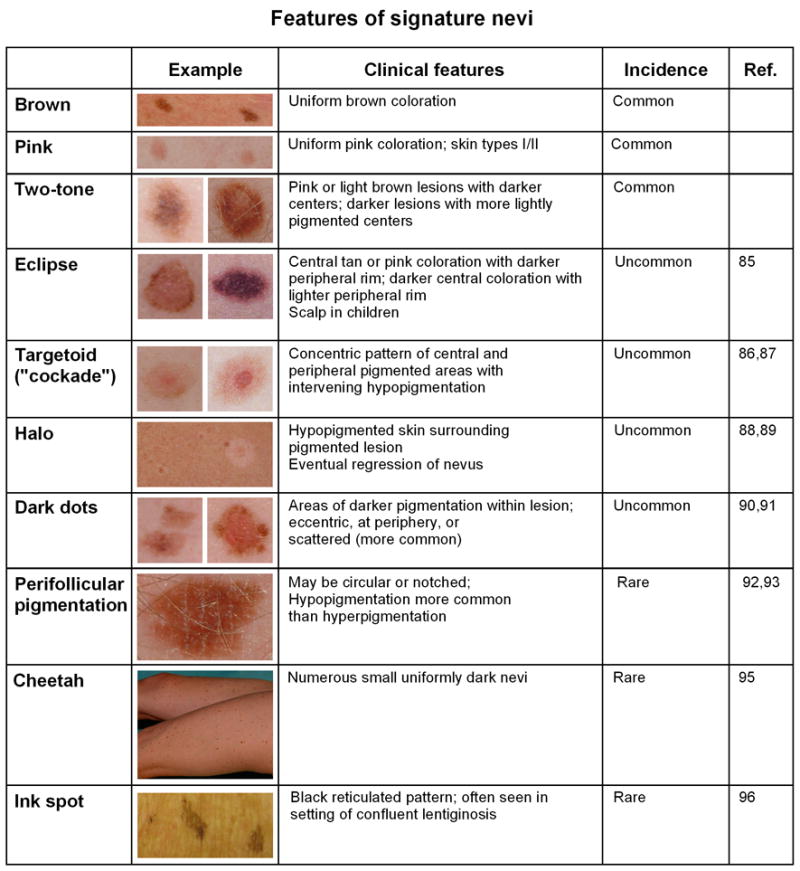
Summary of key features of signature nevi.
Importantly, these signature patterns generally do not reveal significant melanocytic atypia, but rather represent “biologically benign” explanations for irregularities in nevus pigmentation. Features of signature nevi can be appreciated without magnification. Thus when examining a patient with multiple clinically atypical nevi, recognition of signature lesions allows one to narrow down the number of lesions that may need further examination. Bolognia97 has compared taking this “low power” view to the dermatopathologist who examines a slide first at ×4 and ×20 magnification before focusing in on particular areas at higher magnification. Signature lesions may span a morphologic spectrum and their classification need not be confined to the specific types described above. In addition, patients may have more than one type of signature nevus. But once the signature pattern(s) is identified (i.e. which lesions go together), one can then determine which remaining lesions fit the pattern and which ones may require biopsy or further examination.
Ugly duckling sign
The “ugly duckling” sign is another useful clinical principle that represents the converse of the signature lesion – i.e. the lesion that does not go with all the rest. Once the signature nevus and its relatives have been identified, one may be able to appreciate other nevi that are not “part of the family”. Grob and Bonerandi98 coined the term in 1998 in describing two patients with melanoma: in one the melanoma was a brown-black lesion in a patient with predominantly red-brown nevi, while in the other case the melanoma was a uniformly dark lesion in a patient whose nevi predominantly displayed multiple colors and irregular borders. Thus the ugly duckling nevus can be an atypical nevus in a background of normal-appearing nevi, or a more normal-appearing lesion in a patient with multiple clinically atypical nevi. In our practice, we have also found it useful to appreciate an additional ugly duckling variant: the solitary clinically atypical lesion in a patient with few or no nevi. Gachon et al99 reported that for dermatologists, their immediate impression of a lesion frequently incorporates an unconscious reference to the ugly duckling sign. Scope et al100 found that the ugly duckling sign was highly sensitive for melanoma detection, even for non-dermatologists.
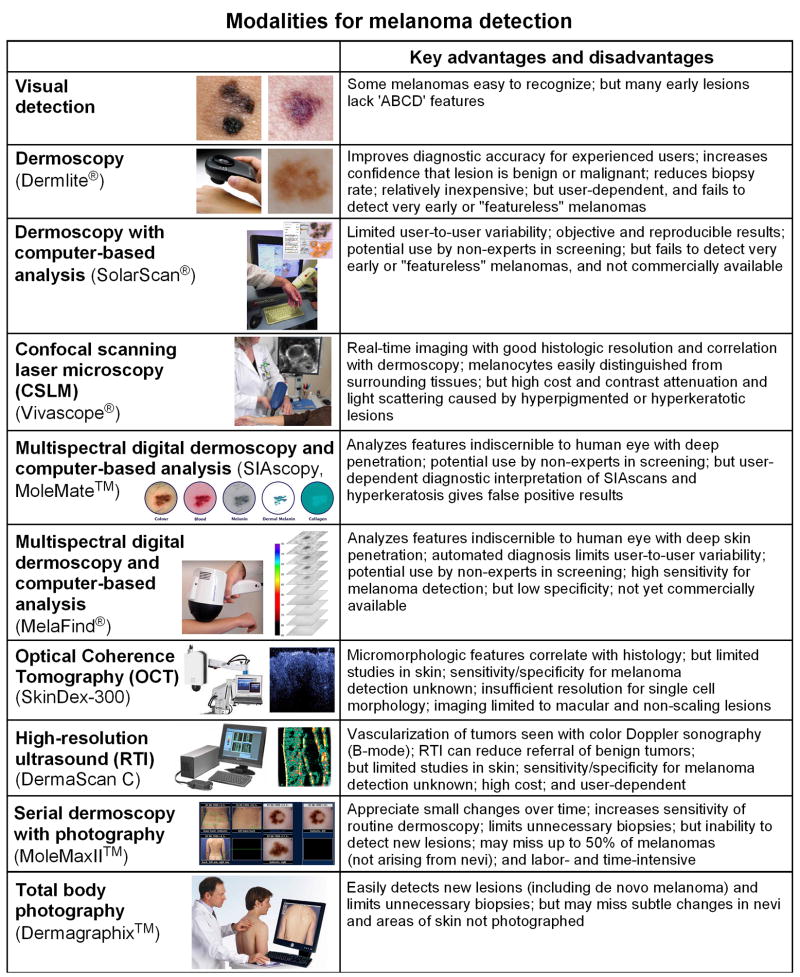
UNIDIMENSIONAL MODALITIES FOR MELANOMA DETECTION
A number of imaging modalities are currently available that may enhance the clinical examination of individual lesions, and decrease physician-to-physician variability (Fig 2). A comprehensive review of these modalities was published in the Journal in 2003.101 Here we focus on recent applications of these modalities to early melanoma detection.
Figure 2.
Summary of modalities for melanoma detection. See text for additional details. Images obtained from following websites: SolarScan, http://www.medgadget.com/archives; Vivascope-3000, http://www.lucid-tech.com/medical-imagers; MoleMate, http://www.astronclinica.com/products; Melafind, http://www.eosciences.com; SkinDex-300, http://www.isis-optronics.de/en/skindex and OCT image reprinted from Ref. 149 with permission from Elsevier; DermaScan C, http://www.cortex.dk; MoleMaxII, http://www.dermamedicalsystems.com; Dermagraphix, http://digitale-photographie.info/body-mapping_en.asp
Dermoscopy
Dermoscopy (also known as dermatoscopy or epiluminescence microscopy) is a well-established method in which skin lesions are viewed with a magnifier through an oil/gel interface (conventional immersion contact dermoscopy) or using cross-polarizing light filters (non-contact dermoscopy). These mediums limit the amount of reflected light, thus allowing improved and deeper visualization of pigmentary and vascular structures. Dermoscopy used to be more routinely practiced in Europe than in the U.S., however the advent of Dermlite® hand-held products (ranging in price from $300–$1000) has greatly expanded the use of non-contact dermoscopy. Benvenuto-Andrade et al102 compared the capabilities of various dermoscopic techniques and found moderate to excellent agreement for most colors and dermoscopic structures. However, they concluded that melanin (dots and streaks) appeared darker, blue nevi had more shades of blue, and vessels, red areas, and shiny-white streaks (fibrosis) were better visualized with non-contact polarized dermoscopy; on the other hand, milia-like cysts and comedo-like openings, peppering, lighter colors, and blue-white (regression) areas were better visualized with immersion-contact dermoscopy.102
Dermoscopy is not diagnostic, but can increase or decrease confidence that a melanocytic lesion is benign or malignant (see below),43,103–105 thus improving early detection of melanoma106,107 while reducing the need for unnecessary biopsies.108,109 The utility of this technique, not surprisingly, depends on the experience of its user.110–112 Piccolo et al113 reported that dermatologists with 5 years of experience using dermoscopy had 92% sensitivity and 99% specificity when diagnosing melanoma from dermoscopic images, compared to 69% sensitivity and 94% specificity for inexperienced physicians.
To increase objectivity, multiple dermoscopic scoring systems and algorithmic methods have been devised. These include the ABCD rule,114 Pattern analysis,115 Menzies method,116 7-point checklist,117 Modified ABC-point list,118 and CASH (color, architecture, symmetry, and homogeneity).119 There have been several studies comparing these methods, and while Pattern analysis had the highest sensitivity, specificity, and diagnostic accuracy for melanoma detection when used by dermatologists,120,121 the Menzies method proved better when used by non-dermatologist physicians.104 To overcome the expected variability in physician interpretation of dermoscopic images, several computer-based algorithms have been developed but none have proved superior. Blum et al122 developed an algorithm based on the evaluation of 64 different analytical parameters, which was used to evaluate 837 digital images of benign and malignant melanocytic lesions; diagnostic accuracy of 82–84% was comparable to that obtained using other established methods. Pellacani et al123 developed an algorithm based on asymmetry and border parameters that performed comparably to two experienced practitioners in evaluating a set of 331 dermoscopic images. SolarScan®, developed in Australia, combines acquisition of multiple dermoscopic images obtained using a remote-head color video camera with a computer algorithm.43 Using a data set of 2430 lesions, Menzies et al124 found that SolarScan® gave a sensitivity of 91% and specificity of 68% for melanoma, which was comparable to that of experts. SolarScan® is not currently available in the U.S.
The basic diagnostic strategy in dermoscopy typically involves a decision tree to guide whether particular lesions should be biopsied (Fig 3). First, a determination is made whether a lesion is melanocytic or not. The presence of pigmented networks, globules, dots, or streaks favors a melanocytic lesion. The second step for melanocytic lesions is to classify them as benign, suspicious, or malignant based on dermoscopic features using scoring systems or algorithms noted above. The principles of signature lesion and ugly duckling can also be applied, looking for consistent and variant dermoscopic features, respectively, among lesions in each patient. Suspicious lesions should be biopsied or closely monitored. Lesions determined to be non-melanocytic may then be screened for diagnostic features of seborrheic keratosis (milia-like cysts and comedo-like openings), dermatofibroma (central white scar-like patch), blue nevus (homogeneous blue color), angioma (red-blue-black lacunae), and basal cell carcinoma (arborizing vessels and blue-gray globules).125 Finally, lesions that do not display any of these characteristic features and do not appear to be melanocytic should be biopsied to rule out amelanotic melanoma. The major pitfall of dermoscopy is its failure to detect very early or “featureless” melanomas.41,126,127 Some practitioners may be discouraged from using dermoscopy because they feel it is too time-consuming, however Zalaudek et al128 found in a study of patients randomized to receive complete skin examination either with or without dermoscopy that use of dermoscopy increased the median examination time by 72 seconds.
Figure 3.
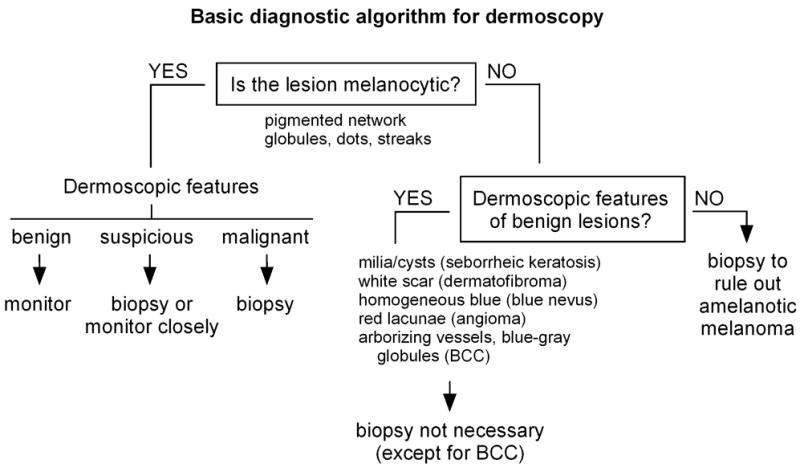
Basic diagnostic algorithm for dermoscopy. First, a determination is made whether the lesion is melanocytic. Based on dermoscopic features, melanocytic lesions can be classified as benign, suspicious, or malignant. Benign melanocytic lesions can be monitored, suspicious lesions should be biopsied or monitored closely, and malignant-appearing lesions should be biopsied. If non-melanocytic lesions have recognizable dermoscopic features of benign neoplasms, biopsy is not necessary. However, if non-melanocytic lesions cannot be otherwise identified, they should be biopsied to rule out amelanotic melanoma.
Confocal scanning laser microscopy
Confocal scanning laser microscopy (CSLM, also abbreviated CLSM or LSCM) is a non-invasive technique that allows for real-time in vivo imaging of skin lesions at variable depths in horizontal planes.129,130 In CSLM, a low-power laser beam in the visible or near infrared range is focused on the skin, then light reflected from this focal point is detected through a pinhole-sized spatial filter. Imaging depth increases with longer wavelengths, and in normal skin extends to the level of the dermis with a maximum depth of 350–400 μm.131 In a process termed “optical sectioning”, a series of sections through the thickness of the specimen are collected, assembled, and evaluated using specialized reconstruction software. CSLM can be used in either fluorescence or reflectance mode, the latter known as reflectance-mode confocal microscopy (RCM) which is more suitable for clinical applications.132 In RCM, melanocytes appear bright and can be easily visualized due to the capacity of melanin to backscatter the laser light; epidermal keratinocytes can also be seen.129 Melanocytic cells in nests can be appreciated in nevi, and intraepidermal melanocytes with disorganization of epidermal structure can be seen in melanoma.130,133 Several studies have shown that RCM provides good correlation with dermoscopy134–136 and with processed histologic sections.130,137,138 Clinically amelanotic melanoma may also be detected given the presence of melanosomes and rare melanin granules.133 Gerger et al139 generated RCM images of 117 melanocytic tumors prior to biopsy which were then evaluated by five trained observers. They found overall sensitivity of 88% and specificity of 98%, although using the presence or absence of monomorphic melanocytes as a single diagnostic criterion for melanoma increased sensitivity to 98%.139
Although RCM represents a rapid noninvasive technique that can aid in early diagnosis and management of melanocytic lesions, its high cost (approximately $75,000 for Vivascope®) currently limits its broader use. One limitation of this technique is strong contrast attenuation and light scattering caused by hyperpigmented or hyperkeratotic lesions.131 Additional potential uses of CSLM include imaging of skin lesions and their margins prior to biopsy and margin detection in freshly excised tumors, including non-melanoma skin cancers.131
Multispectral digital dermoscopy and computer-based analysis
In multispectral digital dermoscopy, sequences of images taken at different wavelengths (providing information from a range of depths in a lesion) are coupled with computer-based analysis. For each lesion, quantitative data are generated that can be used by the clinician to help decide whether the lesion should be biopsied. This technique offers the advantages of analyzing features indiscernible to the human eye, probing up to 2 mm below the surface, and limiting physician-to-physician variability. Two technologies have been described: SIAscopy™ and MelaFind®. The spectrophotometric intracutaneous analysis (SIA) scope is a chromophore imaging system that probes 1–2 cm areas of skin using wavelengths of 400–1000 nm. After eight narrow-band spectrally-filtered images are obtained, they are calibrated and entered into a series of algorithms to determine microarchitecture of the underlying skin. SIAscopy measures the amount of collagen, hemoglobin, melanin, and melanin distribution in the epidermis and dermis. This information is presented in the form of maps called SIAscans, which are then interpreted by the clinician. Moncrieff et al140 found that the presence of dermal melanin, collagen holes, and blood displacement with erythematous blush gave 83% sensitivity and 80% specificity for melanoma detection in 348 pigmented lesions referred for excisional biopsy. The SIAscope may be useful in the assessment of cosmetic interventions to reduce the appearance of aging by modification of skin color.141 MoleMate™ (approximately $8000) incorpoprates SIAscopy in a diagnostic algorithm specifically developed for use by primary care physicians.142 As with conventional dermoscopy, diagnostic accuracy of the SIAscope depends on the experience of the physician interpreting the SIAscans. In addition, hyperkeratosis in seborrheic keratoses can be interpreted as dermal melanin, giving false positive results – thus ideally, conventional dermoscopy can be coupled with SIAscopy to avoid diagnostic pitfalls.143 A newer version, (SIAscope V), provides higher-resolution images and is deemed superior to the older version (SIAscope II).143
MelaFind® acquires 10 images for eachlesion, encompassing the visible and near-infrared spectrum. Six scores are generated for each lesion based on constrained linear classifiers, with each classifier trained to differentiate melanoma from other pigmented lesions(low-grade dysplastic nevus, congenital nevus, common nevus, seborrheic keratosis, solar lentigo, and pigmented basal cellcarcinoma). A lesion is then recommended for biopsy if all 6 scores are above the threshold value. In the first published study by Elbaum et al,144 results from four clinical centers demonstrated 100% sensitivity and 85% specificity in diagnosing melanoma. More recently, Friedman et al145 used an imaging database of 990 small pigmented lesions to match a panel of 10 dermoscopists against MelaFind®. They found that while dermoscopists were able to correctly identify small melanomas with an average sensitivity of 39% and specificity of 82% and recommended small melanomas for biopsy with a sensitivity of 71% and specificity of 49%, MelaFind® achieved 98% sensitivity and 44% specificity.145 MelaFind® is not yet commercially available.
Optical coherence tomography (OCT)
Optical coherence tomography (OCT) uses a fiber-optic Michelson interferometer with a low-coherence length broadband light source, reaching a penetration depth of about 1 mm (depending on scattering properties of tissue), while lateral resolution is determined by the numeric aperture of the objective.146 The reflectivity of different tissue components (such as melanin and cell membranes) provides contrast in the images,146 and micromorphologic features correlate with histopathologic findings.147 While the resolution is insufficient to reveal morphology of single cells, lesion architecture can be evaluated and correlated with surface dermoscopic parameters (pigment network and brown globules).148 Gambichler et al149 examined a panel of melanomas and benign nevi by OCT and demonstrated that melanomas showed increased architectural disarray, less defined dermal-epidermal borders, and vertically-oriented icicle-shaped structures not seen in nevi. While OCT has been used routinely to evaluate ocular lesions,150 its utility for skin lesions has not been fully established as sensitivity/specificity studies for melanoma detection have not been reported. OCT appears to be best suited for macular and non-scaling lesions, as histopathological structures may be less clearly visualized in hyperkeratotic or raised lesions.148
Reflex transmission imaging
High-resolution B-mode ultrasound has primarily been used in the past to assess depth/thickness of melanoma tumors.151,152 Reflex transmission imaging (RTI) is a form of high-resolution ultrasound that can be used in combination with white light digital photography for classification of pigmented lesions.153 Rallan et al154 used RTI to derive sonographic parameters for melanomas and benign pigmented lesions in a group of patients referred by primary care physicians. They found significant quantitative differences to allow discrimination between melanomas, seborrheic keratoses, and nevi to potentially reduce the referral of benign tumors by 65% without missing melanoma.154 The cost for DermaScan C (2-D mode) is approximately $50,000–$60,000. Given the limited reports using RTI in the literature and its high cost, its future utility as a primary imaging modality for melanoma detection is not clear.
MODALITIES FOR DETECTING NEW AND CHANGING LESIONS
Paradigms for side-by-side comparisons
The modalities described in the preceding sections are unidimensional, in that they represent imaging and evaluation of lesions at a single point in time. However, confirmation that a lesion is new or assessment of change in a nevus – both clearly important determinations for early melanoma detection – require observations at multiple points in time. There are currently two paradigms promulgated in the literature to facilitate “side-by-side” comparisons of nevi for the purpose of documenting changes over time. Both modalities involve comparing previously taken photographs with examinations in real time.
Serial dermoscopy and photography
The first paradigm involves monitoring suspicious nevi for changes over time using serial dermoscopy and photography. At the initial examination, lesions to be followed are subjected to digital epiluminescence (dermoscopic) microscopy (DELM). The digital photographs are linked to a body map, and DELM images are stored for future visits. At follow-up examinations, the same lesions are re-photographed and serial DELM images are reviewed to assess changes in particular lesions. The generation, archiving, and retrieval of DELM images are usually accomplished using an accessory camera and instrument such as Molemax™, SolarScan®, or VivaCam® (complete systems range from approximately $10,000–$30,000). The major advantage of this approach is the high resolution of DELM, allowing one to observe small changes in lesions over time. Several studies employing MoleMax II™ found that DELM monitoring was useful for patients with multiple atypical nevi in early melanoma diagnosis and decreased the number of biopsies.51,52,155 Haenssle et al53 monitored 7001 atypical nevi in 530 patients (median follow-up period of 32 months), and reported that chance of success for melanoma detection among lesions suspicious by dermoscopic criteria increased from 8.3% to 17% when additional DELM-documented changes were present. In addition, one third of the melanomas detected were exclusively identified by DELM-documented changes, indicating that DELM increased the sensitivity of the ELM analysis.53 Short-term DELM monitoring of 318 suspicious melanocytic lesions by Menzies et al43 found that 7 of 61 (11%) lesions showing morphologic changes proved to be early melanoma, none of which displayed classic surface microscopic features of melanoma. In addition, Robinson et al52 reported that DELM monitoring led to increased confidence and comfort with SSE in high-risk patients. Detection of a high fraction of in situ (compared to invasive) melanomas in these studies suggests improved sensitivity for early melanoma detection.43,51–53
Several pitfalls are intrinsic to this approach. First, only a subset of a patient’s lesions is being monitored in this way, so lesions not imaged (i.e. those not deemed clinically atypical at the initial visit) cannot be assessed for changes at follow-up visits. Three studies involving monitoring of high-risk patients found that the majority of nevus-derived melanomas did not develop from clinically-atypical nevi.9,46,54 Thus such a screening strategy will likely miss melanomas that ultimately arise from benign-appearing nevi. Second, this approach does not allow for detection of new lesions since only pre-existing lesions will have been photographed. Given that at least half of melanomas arise de novo and not from nevi,9,80,83 up to 50% of expected melanomas might be missed. Indeed, Kelly et al9 found that 13 of 20 (65%) melanomas in patients with clinically atypical nevi arose as new lesions. Third, while most short-term monitoring studies have revealed observable dermoscopic changes in growing melanomas,43–45 other longer-term studies found that most dermoscopic changes in clinically atypical nevi proved to be histologically inconsequential.54,112 Finally, acquiring, archiving, and retrieving DELM images at each visit is laborious and time consuming,54 and may adversely affect patient compliance for follow-up.112
Total body photography
The second paradigm for achieving side-by-side comparisons involves use of total body cutaneous photography. Clinical regional photographs capturing all existing nevi as well as “nevus-free” areas of skin are taken using standard poses156 at the initial visit, and these then serve as a baseline for comparison during follow-up examinations. At follow-up examinations, photographs can be used to appreciate new or changing lesions. The focus of this approach is on monitoring for the development of new lesions, with the capacity for detection of changing nevi dependent on the resolution of the photographs. The photographs may be printed and kept in the patient’s chart, or electronically archived. We have found MIRROR™ DermaGraphix software (approximately $4500 plus $500 annual renewal fee) to be useful for photo management and its built-in zoom function helpful for nevus comparisons. High-resolution photographs and zoom capability are also features of DigitalDerm™ MoleMapCD (approximately $300 cost to patient), in which patients are sent to designated photographers, primarily located in Arizona and the Southwestern U.S., and CD-ROMs containing the photographs and specialized software are then returned to the physicians.
The effectiveness of total body photography in early melanoma detection and reduction of biopsies in high-risk patients was first demonstrated by Kelly et al9 and subsequently in other studies.21,46,157,158 While this approach will easily detect new lesions, it is unlikely to observe small changes in nevi over time, and it is possible that some areas of skin may be covered by undergarments or hair and thus missed in baseline photographs.
Combined photographic approach
Selection of which photographic approach to use may be guided by the type of changes in nevi most likely to occur in a particular patient. For example, in younger patients it may be problematic to establish a baseline using total body photography if they are expected to develop many new nevi in the short term; dermascopic monitoring of particular nevi may be more appropriate if the focus of their surveillance is identifying changes in pre-existing lesions over time. On the other hand, total body photography may be more suitable for older patients with fewer nevi, in whom melanoma would be more likely to present as a new rather than changing nevus. It should be noted, however, that use of dermoscopic or total body photography need not be mutually exclusive. Practitioners may find it useful to incorporate aspects of both modalities. For example, regional photographs may be added for patients in whom DELM images are being monitored. Alternatively, DELM images can be generated for a subset of nevi for closer monitoring in patients undergoing total body photography. Such a combined photographic approach has been advocated in the literature.53,159
APPROACH TO THE PATIENT WITH NEVI
When physicians are confronted with patients having numerous or clinically atypical nevi, there may be a tendency to remove lesions in a prophylactic manner. Such practice of nevus removal may be sought by the patient to reduce their melanoma risk, or promulgated by the physician out of fear of missing a melanoma. It is clear that complete removal of a patient’s nevi will not prevent melanoma, which is just as (if not more) likely to arise from isolated epidermal melanocytes anywhere on the skin than from pre-existing nevi.9,80,83 However, it is unclear to what extent “molectomy” may reduce long-term melanoma risk in high-risk patients as (to our knowledge) this has not been formally studied. Nevertheless, application of general clinical principles combined with a patient-tailored approach is the best means for achieving the common goals for early melanoma detection (Table I).
An algorithm for initial evaluation of patients who may be at increased risk for melanoma is depicted in Fig 4. At the initial visit, the patient’s level of risk can be assessed by history and physical examination (see above for risk factors). It is useful to ask if the patient is concerned about any lesions, or aware of any that are new or changing. A complete skin examination may discover lesions suspicious for melanoma. Identification of the signature nevus and its relatives can narrow down the number of lesions which need further examination by dermoscopy or another imaging modality. Ugly duckling lesions, and other lesions about which either the physician or patient is concerned may represent melanoma, should be biopsied/removed. In our recent monitoring study of high-risk patients, we found that many melanomas were diagnosed at the first visit and represented the most clinically atypical lesion.54 Consequently it may be useful, particularly in patients who have not previously had many nevi removed, to biopsy such a lesion (if present) at the first visit. Doing so can provide a “histologic baseline” for the patient’s most clinically atypical lesion, and reassure both the patient and the physician that that lesion is not melanoma. For patients with nevi, one must decide which photographic paradigm or combinatorial approach will be employed for future assessment of changes. For patients receiving total body photography, lesions in odd locations may be separately photographed, and for isolated lesions a single digital or Polaroid-type photo may be sufficient. In addition, one may decide to remove some nevi that are poorly suited for photographic surveillance such as very dark lesions in which pigmentary changes would be difficult to appreciate over time. Patients should be counseled on sun protection and SSE. The recommended frequency for follow-up visits generally ranges from 6–12 months, but should be dictated by estimated level of patient risk and patient confidence/competence in performing SSE. For patients with numerous or clinically atypical nevi, where one might be concerned about missing a melanoma at the first visit, it can be reassuring to re-examine the patient in 3–4 months to confirm that no lesions have undergone photographic change. Once lesions are confirmed to be stable, longer intervals can be established for follow-up visits.
Figure 4.
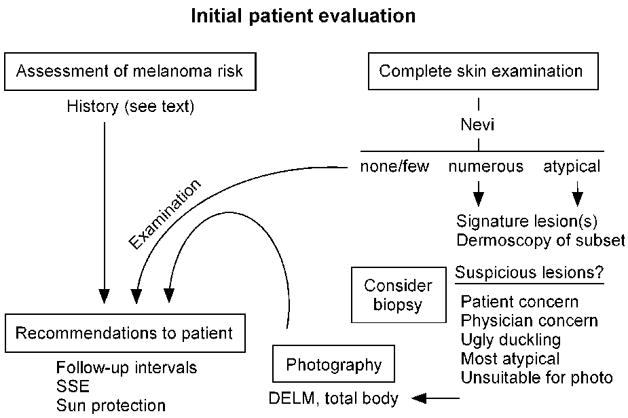
Algorithm for initial evaluation of patients who may be at increased risk for melanoma. See text for details.
An algorithm for management of patients with nevi is depicted in Fig 5. At each follow-up visit, the patient should be queried about any new or changing lesions they may have noted on SSE. Such lesions can then be assessed during clinical examination, using baseline photographs to confirm changes and to detect any new or changing lesions. Particular attention should be given to lesions on the back, buttocks and posterior legs, which are common sites for melanoma and difficult for patients to monitor by SSE. Indications for biopsy/removal of nevi in this setting are enumerated in Fig 5. These include patient concern (particularly if symptomatic) or physician concern about a lesion, ugly duckling, photographic confirmation of change (particularly asymmetric changes in previously symmetric or clinically atypical lesions), new lesion that is clinically atypical, and any new lesion in a patient over age 50. Gachon et al99 conducted a prospective study to investigate the major principles used by 135 dermatologists to aid in melanoma recognition (and whether a lesion should be excised), and found that most relied on assessment of overall pattern, ugly duckling sign, and knowledge of recent change.
Figure 5.
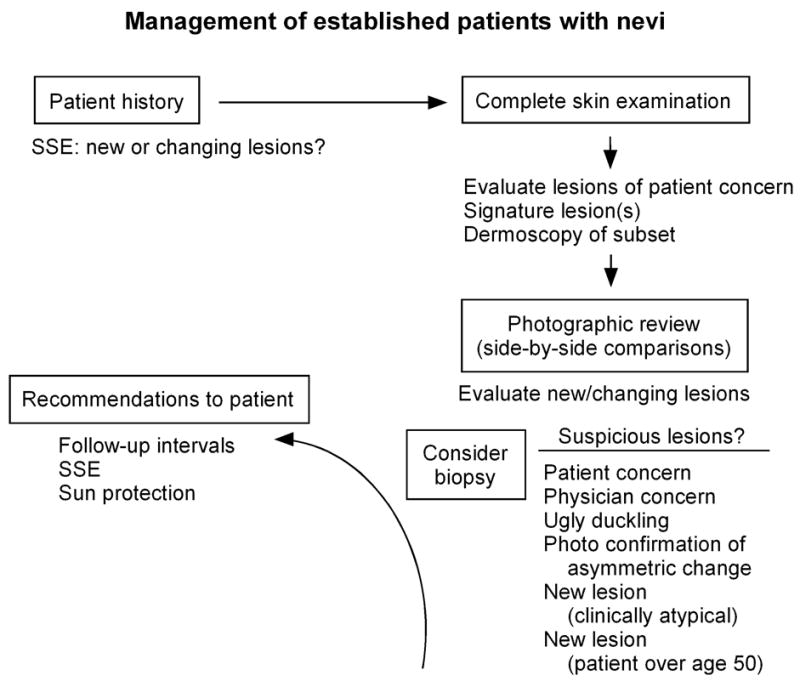
Algorithm for management of established patients with nevi. See text for details.
CONCLUSIONS
Although no definitive non-invasive technique is available for diagnosing melanoma, several modalities are now at our disposable to assist the physician in early melanoma detection. Dermoscopy can increase confidence that a melanocytic lesion is benign or malignant. It is likely that computer-based analysis of dermoscopic images and multispectral digital dermoscopy will be increasingly utilized to increase objectivity and reduce physician-to-physician variability. While there may be a role for CSLM/RCM, OCT and RTI in the future, more studies are needed and their availability is limited. Some type of photography is essential for determining whether individual nevi are new or have undergone changes. The limitations of any given modality necessitate a combined approach to maximize success in early melanoma detection. Implementation of a successful melanoma screening program, incorporating SSE, multiple detection modalities, and the general clinical principles outlined in this review should reduce melanoma mortality through earlier detection. A measure of successful implementation would be to have no patients diagnosed with invasive melanoma on a follow-up visit.
Acknowledgments
D.G. is supported by the Department of Dermatology, the Huntsman Cancer Foundation, and the NIH.
Abbreviations
- CSLM
confocal scanning laser microscopy
- DELM
digital epiluminescence microscopy
- OCT
optical coherence tomography
- RCM
reflectance-mode confocal microscopy
- RTI
reflex transmission imaging
- SIA
spectrophotometric intracutaneous analysis (i.e. SIAscope)
- SSE
self skin examination
Footnotes
Conflicts of interest: None declared.
References
- 1.Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275–80. doi: 10.1001/archderm.135.3.275. [DOI] [PubMed] [Google Scholar]
- 2.Geller AC, Swetter SM, Brooks K, Demierre MF, Yaroch AL. Screening, early detection, and trends for melanoma: current status (2000–2006) and future directions. J Am Acad Dermatol. 2007;57:555–72. doi: 10.1016/j.jaad.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 4.Tsao H, Rogers GS, Sober AJ. An estimate of the annual direct cost of treating cutaneous melanoma. J Am Acad Dermatol. 1998;38:669–80. doi: 10.1016/s0190-9622(98)70195-1. [DOI] [PubMed] [Google Scholar]
- 5.Tripp JM, Kopf AW, Marghoob AA, Bart RS. Management of dysplastic nevi: a survey of fellows of the American Academy of Dermatology. J Am Acad Dermatol. 2002;46:674–82. doi: 10.1067/mjd.2002.121029. [DOI] [PubMed] [Google Scholar]
- 6.Heymann WR. Screening for melanoma. J Am Acad Dermatol. 2007;56:144–5. doi: 10.1016/j.jaad.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Tucker MA, Fraser MC, Goldstein AM, Elder DE, Guerry Dt, Organic SM. Risk of melanoma and other cancers in melanoma-prone families. J Invest Dermatol. 1993;100:350S–5S. doi: 10.1111/1523-1747.ep12470264. [DOI] [PubMed] [Google Scholar]
- 8.Bataille V, Bishop JA, Sasieni P, Swerdlow AJ, Pinney E, Griffiths K, et al. Risk of cutaneous melanoma in relation to the numbers, types and sites of naevi: a case-control study. Br J Cancer. 1996;73:1605–11. doi: 10.1038/bjc.1996.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly JW, Yeatman JM, Regalia C, Mason G, Henham AP. A high incidence of melanoma found in patients with multiple dysplastic naevi by photographic surveillance. Med J Aust. 1997;167:191–4. doi: 10.5694/j.1326-5377.1997.tb138843.x. [DOI] [PubMed] [Google Scholar]
- 10.Tucker MA, Halpern A, Holly EA, Hartge P, Elder DE, Sagebiel RW, et al. Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma. Jama. 1997;277:1439–44. [PubMed] [Google Scholar]
- 11.Goggins WB, Tsao H. A population-based analysis of risk factors for a second primary cutaneous melanoma among melanoma survivors. Cancer. 2003;97:639–43. doi: 10.1002/cncr.11116. [DOI] [PubMed] [Google Scholar]
- 12.Dummer R, Panizzon R, Bloch PH, Burg G. Updated Swiss guidelines for the treatment and follow-up of cutaneous melanoma. Dermatology. 2005;210:39–44. doi: 10.1159/000081482. [DOI] [PubMed] [Google Scholar]
- 13.Holly EA, Kelly JW, Shpall SN, Chiu SH. Number of melanocytic nevi as a major risk factor for malignant melanoma. J Am Acad Dermatol. 1987;17:459–68. doi: 10.1016/s0190-9622(87)70230-8. [DOI] [PubMed] [Google Scholar]
- 14.Nikolaou VA, Sypsa V, Stefanaki I, Gogas H, Papadopoulos O, Polydorou D, et al. Risk associations of melanoma in a Southern European population: results of a case/control study. Cancer Causes Control. 2008;19:671–9. doi: 10.1007/s10552-008-9130-0. [DOI] [PubMed] [Google Scholar]
- 15.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040–59. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Bower CP, Lear JT, Bygrave S, Etherington D, Harvey I, Archer CB. Basal cell carcinoma and risk of subsequent malignancies: A cancer registry-based study in southwest England. J Am Acad Dermatol. 2000;42:988–91. [PubMed] [Google Scholar]
- 17.Hemminki K, Dong C. Subsequent cancers after in situ and invasive squamous cell carcinoma of the skin. Arch Dermatol. 2000;136:647–51. doi: 10.1001/archderm.136.5.647. [DOI] [PubMed] [Google Scholar]
- 18.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Ting W, Schultz K, Cac NN, Peterson M, Walling HW. Tanning bed exposure increases the risk of malignant melanoma. Int J Dermatol. 2007;46:1253–7. doi: 10.1111/j.1365-4632.2007.03408.x. [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Devesa SS, Fears TR, Hartge P. Cancer surveillance series: changing patterns of cutaneous malignant melanoma mortality rates among whites in the United States. J Natl Cancer Inst. 2000;92:811–8. doi: 10.1093/jnci/92.10.811. [DOI] [PubMed] [Google Scholar]
- 21.Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch Dermatol. 2005;141:998–1006. doi: 10.1001/archderm.141.8.998. [DOI] [PubMed] [Google Scholar]
- 22.Freedberg KA, Geller AC, Miller DR, Lew RA, Koh HK. Screening for malignant melanoma: A cost-effectiveness analysis. J Am Acad Dermatol. 1999;41:738–45. doi: 10.1016/s0190-9622(99)70010-1. [DOI] [PubMed] [Google Scholar]
- 23.Losina E, Walensky RP, Geller A, Beddingfield FC, 3rd, Wolf LL, Gilchrest BA, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21–8. doi: 10.1001/archderm.143.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masri GD, Clark WH, Jr, Guerry Dt, Halpern A, Thompson CJ, Elder DE. Screening and surveillance of patients at high risk for malignant melanoma result in detection of earlier disease. J Am Acad Dermatol. 1990;22:1042–8. doi: 10.1016/0190-9622(90)70149-c. [DOI] [PubMed] [Google Scholar]
- 25.Carli P, De Giorgi V, Palli D, Maurichi A, Mulas P, Orlandi C, et al. Dermatologist detection and skin self-examination are associated with thinner melanomas: results from a survey of the Italian Multidisciplinary Group on Melanoma. Arch Dermatol. 2003;139:607–12. doi: 10.1001/archderm.139.5.607. [DOI] [PubMed] [Google Scholar]
- 26.Hansson J, Bergenmar M, Hofer PA, Lundell G, Mansson-Brahme E, Ringborg U, et al. Monitoring of kindreds with hereditary predisposition for cutaneous melanoma and dysplastic nevus syndrome: results of a Swedish preventive program. J Clin Oncol. 2007;25:2819–24. doi: 10.1200/JCO.2007.11.4108. [DOI] [PubMed] [Google Scholar]
- 27.Chiarello SE. Every day is melanoma Monday. J Am Acad Dermatol. 1996;35:649. doi: 10.1016/s0190-9622(96)90705-7. [DOI] [PubMed] [Google Scholar]
- 28.Resneck J, Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50–4. doi: 10.1016/j.jaad.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Chen SC, Pennie ML, Kolm P, Warshaw EM, Weisberg EL, Brown KM, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678–82. doi: 10.1111/j.1525-1497.2006.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Federman DG, Kravetz JD, Kirsner RS. Skin cancer screening by dermatologists: prevalence and barriers. J Am Acad Dermatol. 2002;46:710–4. doi: 10.1067/mjd.2002.120531. [DOI] [PubMed] [Google Scholar]
- 31.Geller AC, O’Riordan DL, Oliveria SA, Valvo S, Teich M, Halpern AC. Overcoming obstacles to skin cancer examinations and prevention counseling for high-risk patients: results of a national survey of primary care physicians. J Am Board Fam Pract. 2004;17:416–23. doi: 10.3122/jabfm.17.6.416. [DOI] [PubMed] [Google Scholar]
- 32.Brochez L, Verhaeghe E, Bleyen L, Naeyaert JM. Diagnostic ability of general practitioners and dermatologists in discriminating pigmented skin lesions. J Am Acad Dermatol. 2001;44:979–86. doi: 10.1067/mjd.2001.113442. [DOI] [PubMed] [Google Scholar]
- 33.Fisher NM, Schaffer JV, Berwick M, Bolognia JL. Breslow depth of cutaneous melanoma: impact of factors related to surveillance of the skin, including prior skin biopsies and family history of melanoma. J Am Acad Dermatol. 2005;53:393–406. doi: 10.1016/j.jaad.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Pennie ML, Soon SL, Risser JB, Veledar E, Culler SD, Chen SC. Melanoma outcomes for Medicare patients: association of stage and survival with detection by a dermatologist vs a nondermatologist. Arch Dermatol. 2007;143:488–94. doi: 10.1001/archderm.143.4.488. [DOI] [PubMed] [Google Scholar]
- 35.Krige JE, Isaacs S, Hudson DA, King HS, Strover RM, Johnson CA. Delay in the diagnosis of cutaneous malignant melanoma. A prospective study in 250 patients. Cancer. 1991;68:2064–8. doi: 10.1002/1097-0142(19911101)68:9<2064::aid-cncr2820680937>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Betti R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13:183–8. [PubMed] [Google Scholar]
- 37.Richard MA, Grob JJ, Avril MF, Delaunay M, Gouvernet J, Wolkenstein P, et al. Delays in diagnosis and melanoma prognosis (I): the role of patients. Int J Cancer. 2000;89:271–9. [PubMed] [Google Scholar]
- 38.Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12:389–94. doi: 10.1097/00008390-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Friedman RJ, Rigel DS, Kopf AW. Early detection of malignant melanoma: the role of physician examination and self-examination of the skin. CA Cancer J Clin. 1985;35:130–51. doi: 10.3322/canjclin.35.3.130. [DOI] [PubMed] [Google Scholar]
- 40.Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731–4. doi: 10.1067/mjd.2000.103981. [DOI] [PubMed] [Google Scholar]
- 41.Carli P, Massi D, de Giorgi V, Giannotti B. Clinically and dermoscopically featureless melanoma: when prevention fails. J Am Acad Dermatol. 2002;46:957–9. doi: 10.1067/mjd.2002.120569. [DOI] [PubMed] [Google Scholar]
- 42.Kittler H, Seltenheim M, Dawid M, Pehamberger H, Wolff K, Binder M. Morphologic changes of pigmented skin lesions: a useful extension of the ABCD rule for dermatoscopy. J Am Acad Dermatol. 1999;40:558–62. doi: 10.1016/s0190-9622(99)70437-8. [DOI] [PubMed] [Google Scholar]
- 43.Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Arch Dermatol. 2001;137:1583–9. doi: 10.1001/archderm.137.12.1583. [DOI] [PubMed] [Google Scholar]
- 44.Kittler H, Guitera P, Riedl E, Avramidis M, Teban L, Fiebiger M, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142:1113–9. doi: 10.1001/archderm.142.9.1113. [DOI] [PubMed] [Google Scholar]
- 45.Altamura D, Avramidis M, Menzies SW. Assessment of the optimal interval for and sensitivity of short-term sequential digital dermoscopy monitoring for the diagnosis of melanoma. Arch Dermatol. 2008;144:502–6. doi: 10.1001/archderm.144.4.502. [DOI] [PubMed] [Google Scholar]
- 46.Lucas CR, Sanders LL, Murray JC, Myers SA, Hall RP, Grichnik JM. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663–71. doi: 10.1067/mjd.2003.283. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz JL, Wang TS, Hamilton TA, Lowe L, Sondak VK, Johnson TM. Thin primary cutaneous melanomas: associated detection patterns, lesion characteristics, and patient characteristics. Cancer. 2002;95:1562–8. doi: 10.1002/cncr.10880. [DOI] [PubMed] [Google Scholar]
- 48.Abbasi NR, Shaw HM, Rigel DS, Friedman RJ, McCarthy WH, Osman I, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. Jama. 2004;292:2771–6. doi: 10.1001/jama.292.22.2771. [DOI] [PubMed] [Google Scholar]
- 49.Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE--an evolving concept in the early detection of melanoma. Arch Dermatol. 2005;141:1032–4. doi: 10.1001/archderm.141.8.1032. [DOI] [PubMed] [Google Scholar]
- 50.Stanganelli I, Rafanelli S, Bucchi L. Seasonal prevalence of digital epiluminescence microscopy patterns in acquired melanocytic nevi. J Am Acad Dermatol. 1996;34:460–4. doi: 10.1016/s0190-9622(96)90440-5. [DOI] [PubMed] [Google Scholar]
- 51.Kittler H, Pehamberger H, Wolff K, Binder M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. J Am Acad Dermatol. 2000;43:467–76. doi: 10.1067/mjd.2000.107504. [DOI] [PubMed] [Google Scholar]
- 52.Robinson JK, Nickoloff BJ. Digital epiluminescence microscopy monitoring of high-risk patients. Arch Dermatol. 2004;140:49–56. doi: 10.1001/archderm.140.1.49. [DOI] [PubMed] [Google Scholar]
- 53.Haenssle HA, Krueger U, Vente C, Thoms KM, Bertsch HP, Zutt M, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980–5. doi: 10.1038/sj.jid.5700119. [DOI] [PubMed] [Google Scholar]
- 54.Fuller SR, Bowen GM, Tanner B, Florell SR, Grossman D. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198–206. doi: 10.1111/j.1524-4725.2007.33254.x. discussion 205–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellis DL, Wheeland RG. Increased nevus estrogen and progesterone ligand binding related to oral contraceptives or pregnancy. J Am Acad Dermatol. 1986;14:25–31. doi: 10.1016/s0190-9622(86)70002-9. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez JL, Figueroa LD, Rodriguez E. Behavior of melanocytic nevi during pregnancy. Am J Dermatopathol. 1984;6 (Suppl):89–91. [PubMed] [Google Scholar]
- 57.Pennoyer JW, Grin CM, Driscoll MS, Dry SM, Walsh SJ, Gelineau JP, et al. Changes in size of melanocytic nevi during pregnancy. J Am Acad Dermatol. 1997;36:378–82. doi: 10.1016/s0190-9622(97)80212-5. [DOI] [PubMed] [Google Scholar]
- 58.Akturk AS, Bilen N, Bayramgurler D, Demirsoy EO, Erdogan S, Kiran R. Dermoscopy is a suitable method for the observation of the pregnancy-related changes in melanocytic nevi. J Eur Acad Dermatol Venereol. 2007;21:1086–90. doi: 10.1111/j.1468-3083.2007.02204.x. [DOI] [PubMed] [Google Scholar]
- 59.Zampino MR, Corazza M, Costantino D, Mollica G, Virgili A. Are melanocytic nevi influenced by pregnancy? A dermoscopic evaluation. Dermatol Surg. 2006;32:1497–504. doi: 10.1111/j.1524-4725.2006.32362.x. [DOI] [PubMed] [Google Scholar]
- 60.Tronnier M, Alexander M, Neitmann M, Brinckmann J, Wolff HH. [Morphological changes in melanocytic nevi induced by exogenous factors] Hautarzt. 2000;51:561–6. doi: 10.1007/s001050051172. [DOI] [PubMed] [Google Scholar]
- 61.Selim MA, Vollmer RT, Herman CM, Pham TT, Turner JW. Melanocytic nevi with nonsurgical trauma: a histopathologic study. Am J Dermatopathol. 2007;29:134–6. doi: 10.1097/01.dad.0000246176.81071.a6. [DOI] [PubMed] [Google Scholar]
- 62.Kittler H, Seltenheim M, Dawid M, Pehamberger H, Wolff K, Binder M. Frequency and characteristics of enlarging common melanocytic nevi. Arch Dermatol. 2000;136:316–20. doi: 10.1001/archderm.136.3.316. [DOI] [PubMed] [Google Scholar]
- 63.MacKie RM, English J, Aitchison TC, Fitzsimons CP, Wilson P. The number and distribution of benign pigmented moles (melanocytic nevi) in a healthy British population. Br J Dermatol. 1985;113:167–74. doi: 10.1111/j.1365-2133.1985.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 64.Siskind V, Darlington S, Green L, Green A. Evolution of melanocytic nevi on the faces and necks of adolescents: a 4 y longitudinal study. J Invest Dermatol. 2002;118:500–4. doi: 10.1046/j.0022-202x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- 65.Soltani K, Bernstein JE, Lorincz AL. Eruptive nevocytic nevi following erythema multiforme. J Am Acad Dermatol. 1979;1:503–5. doi: 10.1016/s0190-9622(79)80092-4. [DOI] [PubMed] [Google Scholar]
- 66.Goerz G, Tsambaos D. Eruptive nevocytic nevi after Lyell’s syndrome. Arch Dermatol. 1978;114:1400–1. doi: 10.1001/archderm.1978.01640210079034. [DOI] [PubMed] [Google Scholar]
- 67.Soltani K, Pepper MC, Simjee S, Apatoff BR. Large acquired nevocytic nevi induced by the Koebner phenomenon. J Cutan Pathol. 1984;11:296–9. doi: 10.1111/j.1600-0560.1984.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 68.Szepietowski J, Wasik F, Szepietowski T, Wlodarczyk M, Sobczak-Radwan K, Czyz W. Excess benign melanocytic naevi in renal transplant recipients. Dermatology. 1997;194:17–9. doi: 10.1159/000246050. [DOI] [PubMed] [Google Scholar]
- 69.Duvic M, Lowe L, Rapini RP, Rodriguez S, Levy ML. Eruptive dysplastic nevi associated with human immunodeficiency virus infection. Arch Dermatol. 1989;125:397–401. [PubMed] [Google Scholar]
- 70.Epstein DS, Lange JR, Gruber SB, Mofid M, Koch SE. Is physician detection associated with thinner melanomas? Jama. 1999;281:640–3. doi: 10.1001/jama.281.7.640. [DOI] [PubMed] [Google Scholar]
- 71.McPherson M, Elwood M, English DR, Baade PD, Youl PH, Aitken JF. Presentation and detection of invasive melanoma in a high-risk population. J Am Acad Dermatol. 2006;54:783–92. doi: 10.1016/j.jaad.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 72.Berwick M, Begg CB, Fine JA, Roush GC, Barnhill RL. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17–23. doi: 10.1093/jnci/88.1.17. [DOI] [PubMed] [Google Scholar]
- 73.Muhn CY, From L, Glied M. Detection of artificial changes in mole size by skin self-examination. J Am Acad Dermatol. 2000;42:754–9. doi: 10.1067/mjd.2000.104895. [DOI] [PubMed] [Google Scholar]
- 74.Oliveria SA, Chau D, Christos PJ, Charles CA, Mushlin AI, Halpern AC. Diagnostic accuracy of patients in performing skin self-examination and the impact of photography. Arch Dermatol. 2004;140:57–62. doi: 10.1001/archderm.140.1.57. [DOI] [PubMed] [Google Scholar]
- 75.Weinstock MA, Nguyen FQ, Martin RA. Enhancing skin self-examination with imaging: evaluation of a mole-mapping program. J Cutan Med Surg. 2004;8:1–5. doi: 10.1007/s10227-003-0156-3. [DOI] [PubMed] [Google Scholar]
- 76.Chiu V, Won E, Malik M, Weinstock MA. The use of mole-mapping diagrams to increase skin self-examination accuracy. J Am Acad Dermatol. 2006;55:245–50. doi: 10.1016/j.jaad.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 77.Chamberlain AJ, Fritschi L, Kelly JW. Nodular melanoma: patients’ perceptions of presenting features and implications for earlier detection. J Am Acad Dermatol. 2003;48:694–701. doi: 10.1067/mjd.2003.216. [DOI] [PubMed] [Google Scholar]
- 78.Zand S, Lio PA, Mackool BT, Duncan LM. A slightly erythematous, firm papule on the upper arm--quiz case. Arch Dermatol. 2005;141:93–8. doi: 10.1001/archderm.141.1.93-e. [DOI] [PubMed] [Google Scholar]
- 79.Morton CA, Mackie RM. Clinical accuracy of the diagnosis of cutaneous malignant melanoma. Br J Dermatol. 1998;138:283–7. doi: 10.1046/j.1365-2133.1998.02075.x. [DOI] [PubMed] [Google Scholar]
- 80.Marks R, Dorevitch AP, Mason G. Do all melanomas come from “moles”? A study of the histological association between melanocytic naevi and melanoma. Australas J Dermatol. 1990;31:77–80. doi: 10.1111/j.1440-0960.1990.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 81.Thomas NE, Groben P. Invasive superficial spreading melanomas arising from clinically normal skin. J Am Acad Dermatol. 2004;51:466–70. doi: 10.1016/j.jaad.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 82.Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol. 2003;139:282–8. doi: 10.1001/archderm.139.3.282. [DOI] [PubMed] [Google Scholar]
- 83.Bevona C, Goggins W, Quinn T, Fullerton J, Tsao H. Cutaneous melanomas associated with nevi. Arch Dermatol. 2003;139:1620–4. doi: 10.1001/archderm.139.12.1620. discussion 4. [DOI] [PubMed] [Google Scholar]
- 84.Weatherhead SC, Haniffa M, Lawrence CM. Melanomas arising from naevi and de novo melanomas--does origin matter? Br J Dermatol. 2007;156:72–6. doi: 10.1111/j.1365-2133.2006.07570.x. [DOI] [PubMed] [Google Scholar]
- 85.Schaffer JV, Glusac EJ, Bolognia JL. The eclipse naevus: tan centre with stellate brown rim. Br J Dermatol. 2001;145:1023–6. doi: 10.1046/j.1365-2133.2001.04538.x. [DOI] [PubMed] [Google Scholar]
- 86.Mehregan AH, King JR. Multiple target-like pigmented nevi. Arch Dermatol. 1972;105:129–30. doi: 10.1001/archderm.1972.01620040089030. [DOI] [PubMed] [Google Scholar]
- 87.James MP, Wells RS. Cockade naevus: an unusual variant of the benign cellular naevus. Acta Derm Venereol. 1980;60:360–3. [PubMed] [Google Scholar]
- 88.Akasu R, From L, Kahn HJ. Characterization of the mononuclear infiltrate involved in regression of halo nevi. J Cutan Pathol. 1994;21:302–11. doi: 10.1111/j.1600-0560.1994.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 89.Huynh PM, Lazova R, Bolognia JL. Unusual halo nevi--darkening rather than lightening of the central nevus. Dermatology. 2001;202:324–7. doi: 10.1159/000051666. [DOI] [PubMed] [Google Scholar]
- 90.Bolognia JL, Lin A, Shapiro PE. The significance of eccentric foci of hyperpigmentation (‘small dark dots’) within melanocytic nevi. Analysis of 59 cases. Arch Dermatol. 1994;130:1013–7. [PubMed] [Google Scholar]
- 91.Huynh PM, Glusac EJ, Bolognia JL. The clinical appearance of clonal nevi (inverted type A nevi) Int J Dermatol. 2004;43:882–5. doi: 10.1111/j.1365-4632.2004.02133.x. [DOI] [PubMed] [Google Scholar]
- 92.Bolognia JL, Shapiro PE. Perifollicular hypopigmentation. A cause of variegate pigmentation and irregular border in melanocytic nevi. Arch Dermatol. 1992;128:514–7. doi: 10.1001/archderm.128.4.514. [DOI] [PubMed] [Google Scholar]
- 93.Morishima T, Endo M, Imagawa I, Morioka S. Clinical and histopathological studies on spotted grouped pigmented nevi with special reference to eccrine-centered nevus. Acta Derm Venereol. 1976;56:345–52. [PubMed] [Google Scholar]
- 94.Scope A, Tabanelli M, Busam KJ, Rabinovitz H, Braun RP, Marghoob AA. Dispelling the myth of the “benign hair sign” for melanoma. J Am Acad Dermatol. 2007;56:413–6. doi: 10.1016/j.jaad.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 95.Huynh PM, Glusac EJ, Alvarez-Franco M, Berwick M, Bolognia JL. Numerous, small, darkly pigmented melanocytic nevi: the cheetah phenotype. J Am Acad Dermatol. 2003;48:707–13. doi: 10.1067/mjd.2003.289. [DOI] [PubMed] [Google Scholar]
- 96.Bolognia JL. Reticulated black solar lentigo (‘ink spot’ lentigo) Arch Dermatol. 1992;128:934–40. [PubMed] [Google Scholar]
- 97.Bolognia JL. Too many moles. Arch Dermatol. 2006;142:508. doi: 10.1001/archderm.142.4.508. [DOI] [PubMed] [Google Scholar]
- 98.Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134:103–4. doi: 10.1001/archderm.134.1.103-a. [DOI] [PubMed] [Google Scholar]
- 99.Gachon J, Beaulieu P, Sei JF, Gouvernet J, Claudel JP, Lemaitre M, et al. First prospective study of the recognition process of melanoma in dermatological practice. Arch Dermatol. 2005;141:434–8. doi: 10.1001/archderm.141.4.434. [DOI] [PubMed] [Google Scholar]
- 100.Scope A, Dusza SW, Halpern AC, Rabinovitz H, Braun RP, Zalaudek I, et al. The “ugly duckling” sign: agreement between observers. Arch Dermatol. 2008;144:58–64. doi: 10.1001/archdermatol.2007.15. [DOI] [PubMed] [Google Scholar]
- 101.Marghoob AA, Swindle LD, Moricz CZ, Sanchez Negron FA, Slue B, Halpern AC, et al. Instruments and new technologies for the in vivo diagnosis of melanoma. J Am Acad Dermatol. 2003;49:777–97. doi: 10.1016/s0190-9622(03)02470-8. [DOI] [PubMed] [Google Scholar]
- 102.Benvenuto-Andrade C, Dusza SW, Agero AL, Scope A, Rajadhyaksha M, Halpern AC, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329–38. doi: 10.1001/archderm.143.3.329. [DOI] [PubMed] [Google Scholar]
- 103.Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343–50. doi: 10.1001/archderm.137.10.1343. [DOI] [PubMed] [Google Scholar]
- 104.Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol. 2005;141:1008–14. doi: 10.1001/archderm.141.8.1008. [DOI] [PubMed] [Google Scholar]
- 105.Argenziano G, Zalaudek I, Ferrara G, Johr R, Langford D, Puig S, et al. Dermoscopy features of melanoma incognito: indications for biopsy. J Am Acad Dermatol. 2007;56:508–13. doi: 10.1016/j.jaad.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 106.Pehamberger H, Binder M, Steiner A, Wolff K. In vivo epiluminescence microscopy: improvement of early diagnosis of melanoma. J Invest Dermatol. 1993;100:356S–62S. doi: 10.1111/1523-1747.ep12470285. [DOI] [PubMed] [Google Scholar]
- 107.de Troya-Martin M, Blazquez-Sanchez N, Fernandez-Canedo I, Frieyro-Elicegui M, Funez-Liebana R, Rivas-Ruiz F. [Dermoscopic study of cutaneous malignant melanoma: descriptive analysis of 45 cases] Actas Dermosifiliogr. 2008;99:44–53. [PubMed] [Google Scholar]
- 108.Carli P, de Giorgi V, Chiarugi A, Nardini P, Weinstock MA, Crocetti E, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683–9. doi: 10.1016/j.jaad.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 109.Carli P, De Giorgi V, Crocetti E, Mannone F, Massi D, Chiarugi A, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997–2001. Br J Dermatol. 2004;150:687–92. doi: 10.1111/j.0007-0963.2004.05860.x. [DOI] [PubMed] [Google Scholar]
- 110.Binder M, Schwarz M, Winkler A, Steiner A, Kaider A, Wolff K, et al. Epiluminescence microscopy. A useful tool for the diagnosis of pigmented skin lesions for formally trained dermatologists. Arch Dermatol. 1995;131:286–91. doi: 10.1001/archderm.131.3.286. [DOI] [PubMed] [Google Scholar]
- 111.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–65. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 112.Schiffner R, Schiffner-Rohe J, Landthaler M, Stolz W. Long-term dermoscopic follow-up of melanocytic naevi: clinical outcome and patient compliance. Br J Dermatol. 2003;149:79–86. doi: 10.1046/j.1365-2133.2003.05409.x. [DOI] [PubMed] [Google Scholar]
- 113.Piccolo D, Ferrari A, Peris K, Diadone R, Ruggeri B, Chimenti S. Dermoscopic diagnosis by a trained clinician vs. a clinician with minimal dermoscopy training vs. computer-aided diagnosis of 341 pigmented skin lesions: a comparative study. Br J Dermatol. 2002;147:481–6. doi: 10.1046/j.1365-2133.2002.04978.x. [DOI] [PubMed] [Google Scholar]
- 114.Nachbar F, Stolz W, Merkle T, Cognetta AB, Vogt T, Landthaler M, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30:551–9. doi: 10.1016/s0190-9622(94)70061-3. [DOI] [PubMed] [Google Scholar]
- 115.Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571–83. doi: 10.1016/s0190-9622(87)70239-4. [DOI] [PubMed] [Google Scholar]
- 116.Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55–62. doi: 10.1097/00008390-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 117.Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563–70. doi: 10.1001/archderm.134.12.1563. [DOI] [PubMed] [Google Scholar]
- 118.Blum A, Rassner G, Garbe C. Modified ABC-point list of dermoscopy: A simplified and highly accurate dermoscopic algorithm for the diagnosis of cutaneous melanocytic lesions. J Am Acad Dermatol. 2003;48:672–8. doi: 10.1067/mjd.2003.282. [DOI] [PubMed] [Google Scholar]
- 119.Henning JS, Dusza SW, Wang SQ, Marghoob AA, Rabinovitz HS, Polsky D, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45–52. doi: 10.1016/j.jaad.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 120.Annessi G, Bono R, Sampogna F, Faraggiana T, Abeni D. Sensitivity, specificity, and diagnostic accuracy of three dermoscopic algorithmic methods in the diagnosis of doubtful melanocytic lesions: the importance of light brown structureless areas in differentiating atypical melanocytic nevi from thin melanomas. J Am Acad Dermatol. 2007;56:759–67. doi: 10.1016/j.jaad.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 121.Carli P, Quercioli E, Sestini S, Stante M, Ricci L, Brunasso G, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148:981–4. doi: 10.1046/j.1365-2133.2003.05023.x. [DOI] [PubMed] [Google Scholar]
- 122.Blum A, Luedtke H, Ellwanger U, Schwabe R, Rassner G, Garbe C. Digital image analysis for diagnosis of cutaneous melanoma. Development of a highly effective computer algorithm based on analysis of 837 melanocytic lesions. Br J Dermatol. 2004;151:1029–38. doi: 10.1111/j.1365-2133.2004.06210.x. [DOI] [PubMed] [Google Scholar]
- 123.Pellacani G, Grana C, Seidenari S. Algorithmic reproduction of asymmetry and border cut-off parameters according to the ABCD rule for dermoscopy. J Eur Acad Dermatol Venereol. 2006;20:1214–9. doi: 10.1111/j.1468-3083.2006.01751.x. [DOI] [PubMed] [Google Scholar]
- 124.Menzies SW, Bischof L, Talbot H, Gutenev A, Avramidis M, Wong L, et al. The performance of SolarScan: an automated dermoscopy image analysis instrument for the diagnosis of primary melanoma. Arch Dermatol. 2005;141:1388–96. doi: 10.1001/archderm.141.11.1388. [DOI] [PubMed] [Google Scholar]
- 125.Kenet RO, Kang S, Kenet BJ, Fitzpatrick TB, Sober AJ, Barnhill RL. Clinical diagnosis of pigmented lesions using digital epiluminescence microscopy. Grading protocol and atlas. Arch Dermatol. 1993;129:157–74. [PubMed] [Google Scholar]
- 126.Braun RP, Gaide O, Skaria AM, Kopf AW, Saurat JH, Marghoob AA. Exclusively benign dermoscopic pattern in a patient with acral melanoma. Arch Dermatol. 2007;143:1213–5. doi: 10.1001/archderm.143.9.1213-b. author reply 5–6. [DOI] [PubMed] [Google Scholar]
- 127.Skvara H, Teban L, Fiebiger M, Binder M, Kittler H. Limitations of dermoscopy in the recognition of melanoma. Arch Dermatol. 2005;141:155–60. doi: 10.1001/archderm.141.2.155. [DOI] [PubMed] [Google Scholar]
- 128.Zalaudek I, Kittler H, Marghoob AA, Balato A, Blum A, Dalle S, et al. Time required for a complete skin examination with and without dermoscopy: a prospective, randomized multicenter study. Arch Dermatol. 2008;144:509–13. doi: 10.1001/archderm.144.4.509. [DOI] [PubMed] [Google Scholar]
- 129.Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946–52. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 130.Marghoob AA, Charles CA, Busam KJ, Rajadhyaksha M, Lee G, Clark-Loeser L, et al. In vivo confocal scanning laser microscopy of a series of congenital melanocytic nevi suggestive of having developed malignant melanoma. Arch Dermatol. 2005;141:1401–12. doi: 10.1001/archderm.141.11.1401. [DOI] [PubMed] [Google Scholar]
- 131.Gonzalez S, Gilaberte-Calzada Y. In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int J Cosmet Sci. 2008;30:1–17. doi: 10.1111/j.1468-2494.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 132.Meyer LE, Otberg N, Sterry W, Lademann J. In vivo confocal scanning laser microscopy: comparison of the reflectance and fluorescence mode by imaging human skin. J Biomed Opt. 2006;11:44012–6. doi: 10.1117/1.2337294. [DOI] [PubMed] [Google Scholar]
- 133.Busam KJ, Charles C, Lohmann CM, Marghoob A, Goldgeier M, Halpern AC. Detection of intraepidermal malignant melanoma in vivo by confocal scanning laser microscopy. Melanoma Res. 2002;12:349–55. doi: 10.1097/00008390-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 134.Scope A, Benvenuto-Andrade C, Agero AL, Halpern AC, Gonzalez S, Marghoob AA. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch Dermatol. 2007;143:176–85. doi: 10.1001/archderm.143.2.176. [DOI] [PubMed] [Google Scholar]
- 135.Scope A, Gill M, Benveuto-Andrade C, Halpern AC, Gonzalez S, Marghoob AA. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol. 2007;143:727–34. doi: 10.1001/archderm.143.6.727. [DOI] [PubMed] [Google Scholar]
- 136.Ahlgrimm-Siess V, Massone C, Koller S, Fink-Puches R, Richtig E, Wolf I, et al. In vivo confocal scanning laser microscopy of common naevi with globular, homogeneous and reticular pattern in dermoscopy. Br J Dermatol. 2008;158:1000–7. doi: 10.1111/j.1365-2133.2008.08460.x. [DOI] [PubMed] [Google Scholar]
- 137.Tannous ZS, Mihm MC, Flotte TJ, Gonzalez S. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260–3. doi: 10.1067/mjd.2002.118345. [DOI] [PubMed] [Google Scholar]
- 138.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125:532–7. doi: 10.1111/j.0022-202X.2005.23813.x. [DOI] [PubMed] [Google Scholar]
- 139.Gerger A, Koller S, Kern T, Massone C, Steiger K, Richtig E, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493–8. doi: 10.1111/j.0022-202X.2004.23569.x. [DOI] [PubMed] [Google Scholar]
- 140.Moncrieff M, Cotton S, Claridge E, Hall P. Spectrophotometric intracutaneous analysis: a new technique for imaging pigmented skin lesions. Br J Dermatol. 2002;146:448–57. doi: 10.1046/j.1365-2133.2002.04569.x. [DOI] [PubMed] [Google Scholar]
- 141.Matts PJ. New insights into skin appearance and measurement. J Investig Dermatol Symp Proc. 2008;13:6–9. doi: 10.1038/jidsymp.2008.6. [DOI] [PubMed] [Google Scholar]
- 142.Wood A, Morris H, Emery J, Hall PN, Cotton S, Prevost AT, et al. Evaluation of the MoleMate training program for assessment of suspicious pigmented lesions in primary care. Inform Prim Care. 2008;16:41–50. doi: 10.14236/jhi.v16i1.673. [DOI] [PubMed] [Google Scholar]
- 143.Hall PN, Hunter JE, Walter FM, Norris P. Use of a spectrophotometric intracutaneous analysis device in the real-time diagnosis of melanoma. Br J Dermatol. 2008;158:420–1. doi: 10.1111/j.1365-2133.2007.08324.x. [DOI] [PubMed] [Google Scholar]
- 144.Elbaum M, Kopf AW, Rabinovitz HS, Langley RG, Kamino H, Mihm MC, Jr, et al. Automatic differentiation of melanoma from melanocytic nevi with multispectral digital dermoscopy: a feasibility study. J Am Acad Dermatol. 2001;44:207–18. doi: 10.1067/mjd.2001.110395. [DOI] [PubMed] [Google Scholar]
- 145.Friedman RJ, Gutkowicz-Krusin D, Farber MJ, Warycha M, Schneider-Kels L, Papastathis N, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476–82. doi: 10.1001/archderm.144.4.476. [DOI] [PubMed] [Google Scholar]
- 146.Tadrous PJ. Methods for imaging the structure and function of living tissues and cells: 1. Optical coherence tomography. J Pathol. 2000;191:115–9. doi: 10.1002/(SICI)1096-9896(200006)191:2<115::AID-PATH589>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 147.Bechara FG, Gambichler T, Stucker M, Orlikov A, Rotterdam S, Altmeyer P, et al. Histomorphologic correlation with routine histology and optical coherence tomography. Skin Res Technol. 2004;10:169–73. doi: 10.1111/j.1600-0846.2004.00038.x. [DOI] [PubMed] [Google Scholar]
- 148.de Giorgi V, Stante M, Massi D, Mavilia L, Cappugi P, Carli P. Possible histopathologic correlates of dermoscopic features in pigmented melanocytic lesions identified by means of optical coherence tomography. Exp Dermatol. 2005;14:56–9. doi: 10.1111/j.0906-6705.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 149.Gambichler T, Regeniter P, Bechara FG, Orlikov A, Vasa R, Moussa G, et al. Characterization of benign and malignant melanocytic skin lesions using optical coherence tomography in vivo. J Am Acad Dermatol. 2007;57:629–37. doi: 10.1016/j.jaad.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 150.Shields CL, Materin MA, Shields JA. Review of optical coherence tomography for intraocular tumors. Curr Opin Ophthalmol. 2005;16:141–54. doi: 10.1097/01.icu.0000158258.01681.40. [DOI] [PubMed] [Google Scholar]
- 151.Hoffmann K, Jung J, el Gammal S, Altmeyer P. Malignant melanoma in 20-MHz B scan sonography. Dermatology. 1992;185:49–55. doi: 10.1159/000247403. [DOI] [PubMed] [Google Scholar]
- 152.Lassau N, Mercier S, Koscielny S, Avril MF, Margulis A, Mamelle G, et al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. AJR Am J Roentgenol. 1999;172:457–61. doi: 10.2214/ajr.172.2.9930803. [DOI] [PubMed] [Google Scholar]
- 153.Rallan D, Dickson M, Bush NL, Harland CC, Mortimer P, Bamber JC. High-resolution ultrasound reflex transmission imaging and digital photography: potential tools for the quantitative assessment of pigmented lesions. Skin Res Technol. 2006;12:50–9. doi: 10.1111/j.0909-725X.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 154.Rallan D, Bush NL, Bamber JC, Harland CC. Quantitative discrimination of pigmented lesions using three-dimensional high-resolution ultrasound reflex transmission imaging. J Invest Dermatol. 2007;127:189–95. doi: 10.1038/sj.jid.5700554. [DOI] [PubMed] [Google Scholar]
- 155.Kittler H, Binder M. Risks and benefits of sequential imaging of melanocytic skin lesions in patients with multiple atypical nevi. Arch Dermatol. 2001;137:1590–5. doi: 10.1001/archderm.137.12.1590. [DOI] [PubMed] [Google Scholar]
- 156.Halpern AC, Marghoob AA, Bialoglow TW, Witmer W, Slue W. Standardized positioning of patients (poses) for whole body cutaneous photography. J Am Acad Dermatol. 2003;49:593–8. doi: 10.1067/s0190-9622(03)02125-x. [DOI] [PubMed] [Google Scholar]
- 157.Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706–14. doi: 10.1111/j.0007-0963.2004.05892.x. [DOI] [PubMed] [Google Scholar]
- 158.Wang SQ, Kopf AW, Koenig K, Polsky D, Nudel K, Bart RS. Detection of melanomas in patients followed up with total cutaneous examinations, total cutaneous photography, and dermoscopy. J Am Acad Dermatol. 2004;50:15–20. doi: 10.1016/s0190-9622(03)02794-4. [DOI] [PubMed] [Google Scholar]
- 159.Kittler H. Commentary. Dermatol Surg. 2007;33:1205–6. [Google Scholar]