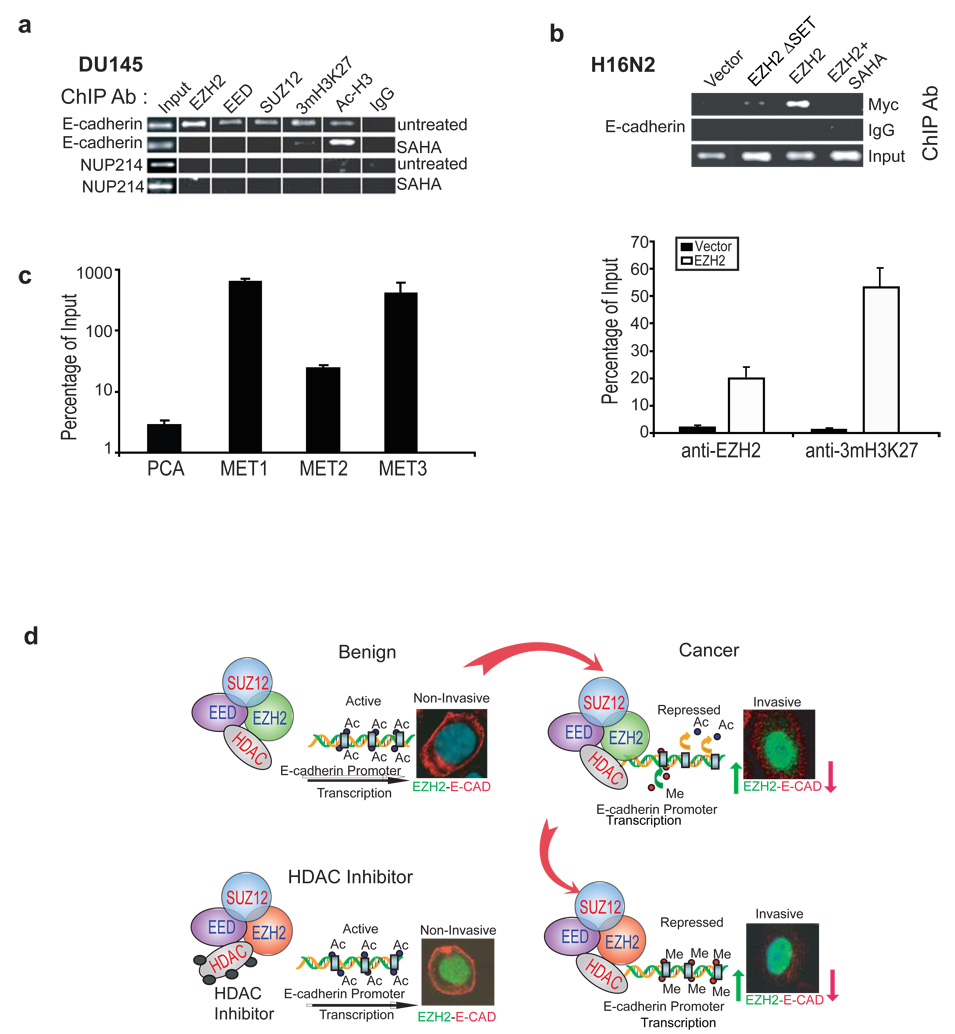
Figure 6. EZH2 and PRC2 members are localized at E-cadherin promoter.
(a) The endogenous PRC2 complex is recruited to the E-cadherin promoter. ChIP was carried out using antibodies against EZH2, EED, SUZ12, trimethyl-histone H3-Lys27 (3mH3K27), acetyl histone H3 (Ac-H3) and IgG control. Addition of 500nM SAHA curtails the recruitment of these complexes to the E-cadherin promoter, while acetylated histone levels increase at the E-cadherin promoter. Each ChIP experiment was repeated at least three times and a representative experiment is shown. (b) Ectopically expressed EZH2 binds the E-cadherin promoter and leads to H3K27 trimethylation. ChIP was carried out in H16N2 cells infected with EZH2 or control adenovirus and assayed by PCR analysis. The upper panel shows in the cells overexpressing myc tagged EZH2 constructs, EZH2 was found to bind the E-cadherin promoter. The lower panel showed by qPCR that EZH2 and 3mH3K27 co-occupy the E-cadherin promoter in the EZH2-overexpressing H16N2 cells. The enrichments in EZH2-overexpressing cells are significantly (p<0.001) higher than those in the vector-treated cells. Error bar: n = 3, mean ± SEM. (c) The E-cadherin promoter contains significantly (p<0.001) more H3K27 trimethylation in metastatic prostate cancer tissues (MET) relative to localized prostate cancer (PCa). ChIP experiments were performed using anti-3mH3K27 antibody in one localized prostate tumor and three independent metastatic prostate tumors. ChIP-enriched DNA and the input DNA were first amplified through ligation-mediated PCR. Equal amounts (50ng) of amplified ChIP DNA and the input DNA were then subjected to PCR, and enrichment by ChIP was assessed relative to the input DNA and normalized to the level of GAPDH. Error bar: n = 3, mean ± SEM. (d) A model depicting the mechanism of EZH2 mediated E-cadherin repression. In benign cells the E-cadherin promoter is not occupied by PRC2 complex. In cancer, the increased expression of PRC2 leads to tight binding to the promoter of E-cadherin, followed by deacetylation of histone H3 and subsequent trimethylation of lysine 27. This leads to repression of E-cadherin expression. Addition of HDAC inhibitors prevents the first step of histone deacetylation, and hence the EZH2 complex cannot methylate histone H3.