Abstract
The environmental fungus Cryptococcus gattii emerged on Vancouver Island, British Columbia (BC), in 1999. By the end of 2006, it led to 176 cases and eight deaths – one of the highest burdens of C gattii disease worldwide. The present paper describes three cases, and the BC experience in the diagnosis and management of this infection. All three cases presented with pulmonary findings, including cryptococcomas and infiltrates. One also presented with brain cryptococcomas. Cases were diagnosed by chest and brain imaging, and laboratory evidence including serum or cerebrospinal fluid cryptococcal antigen detection and culture of respiratory or cerebrospinal fluid specimens. Genotyping of fungal isolates confirmed infection with C gattii VGIIa. Pulmonary cases were treated with fluconazole. One patient with central nervous system disease was treated with amphotericin B followed by fluconazole. Although this infection remains rare, clinicians should be aware of it in patients with a compatible clinical presentation who are either living in or returning from a trip to BC.
Keywords: Case series, Cryptococcus gattii, Diagnosis, Treatment
Abstract
Le champignon Cryptococcus gattii, qui est présent dans l’environnement, a fait son apparition sur l’île de Vancouver, en Colombie-Britannique (C.-B.), en 1999. À la fin de 2006, on dénombrait 176 cas d’infection et huit décès, l’un des fardeaux les plus lourds imputables à C. gattii dans le monde. Le présent article décrit trois cas et fait le point sur l’expérience de la Colombie-Britannique en matière de diagnostic et de traitement de cette infection. Dans les trois cas, on notait des symptômes pulmonaires, des cryptococcomes et des infiltrats, et l’un d’entre eux présentait aussi de cryptococcomes cérébraux. Les cas ont été diagnostiqués au moyen d’épreuves d’imagerie pulmonaires et cérébrales, et d’analyses de laboratoire, comme le dépistage de l’antigène du cryptocoque dans le sérum et le liquide céphalo-rachidien (LCR) et la culture de spécimens d’expectorations et de LCR. Le génotypage des isolats fongiques a confirmé l’infection à C. gattii VGIIa. Les infections pulmonaires ont été traitées par fluconazole. Un patient dont le système nerveux central était affecté a été traité par amphotéricine B, suivie de fluconazole. Bien que cette infection reste rare, les médecins doivent en soupçonner la présence si le tableau clinique concorde chez des gens qui vivent ou ont voyagé en Colombie-Britannique
Cryptococcus gattii, previously known as Cryptococcus neoformans var. gattii, was first recognized on Vancouver Island, British Columbia (BC), in 2001, and was subsequently found to have caused infections as early as 1999 (1,2). It is still unclear why this tropical fungus emerged in a temperate environment. There were 176 cases reported in BC between 1999 and 2006 (3). In the present report, three of these cases were selected to highlight the spectrum of illness, and diagnostic and management challenges caused by this pathogen.
CASE PRESENTATIONS
Case 1
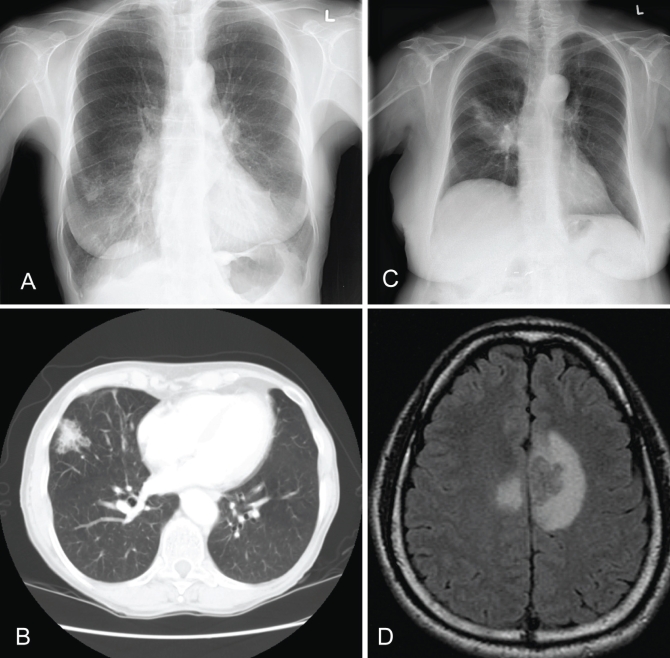
A 77-year-old female resident of Vancouver Island presented to her family physician for her annual checkup in 2002. She was a former 60 pack-year smoker. She suffered from chronic and recurrent sinusitis, and a long-standing dry, occasionally productive cough. She was not taking any medications on a regular basis and was HIV negative. In 1953, she had been treated for pulmonary tuberculosis. There were no new pulmonary or constitutional complaints. She denied fever, night sweats, weight loss, anorexia, fatigue, headache, shortness of breath, chest pain and hemoptysis. Her complete blood count, urinalysis, liver function tests, creatinine and electrolyte levels were all within normal range. The chest radiograph demonstrated a new 2 cm nodule in the peripheral, lateral segment of the right middle lobe (RML) (Figure 1A). A computed tomography (CT) scan showed a 3 cm nodule in the RML with no surrounding consolidation (Figure 1B). In retrospect, a chest radiograph performed a few months earlier showed an ill-defined infiltrate in the RML, with possible right hilar adenopathy or prominence. A bronchoscopy was performed two weeks later. Encapsulated budding yeasts were seen on the direct smears. C gattii was isolated after two days of incubation. The strain was identified as a VGIIa molecular type by restriction fragment length polymorphism (RFLP). The serum cryptococcal antigen titre was 1:32. The patient received oral fluconazole 400 mg daily for three months. A follow-up chest radiograph demonstrated reduction in the nodule size and resolving RML infiltrates. The patient received oral fluconazole 200 mg daily for three additional months. Her repeat serum cryptococcal antigen titre was 1:64. No further treatment was provided, and the patient remained clinically well during the following year.
Figure 1).
Images from patients with Cryptococcus gattii infection. A Case 1: chest x-ray showing a 2 cm nodule in the peripheral, lateral segment of the right middle lobe. B Case 1: chest computed tomography scan showing a 3 cm nodule in the right middle lobe with no surrounding consolidation. C Case 2: chest x-ray showing right upper lobe consolidation and air bronchograms. D Case 3: magnetic resonance imaging scan of the head showing the presence of a ring-enhancing lesion
Case 2
A 75-year-old female resident of Vancouver Island presented to her family physician’s office in 2006 with a three-week history of productive cough with grey sputum that was blood streaked on one occasion. Complaints included fever, weight loss of approximately 2 kg, retrosternal chest pains, fatigue, headache, anorexia and weakness. She was a nonsmoker and had a history of fibromyalgia, hypertension, dyslipidemia, depression and gastroesophageal reflux disease. She was on various medications to treat these conditions but was not taking corticosteroids or other immunosuppressive medications. She was HIV negative. The patient was diagnosed with pneumonia based on physical examinations and chest radiographs, which demonstrated air space consolidation in the right and left upper lobes. She received a one-week course of clarithromycin without improvement. A sputum sample was collected one week later. Bacterial and mycobacterial cultures were negative. However, direct Gram stain demonstrated polymorphs and encapsulated yeast cells compatible with Cryptococcus species. C gattii was recovered from fungal sputum cultures after four days of incubation. RFLP revealed a VGIIa strain. The patient had ongoing respiratory symptoms and was admitted for management. A chest radiograph demonstrated worsening over time with right upper lobe consolidation and air bronchograms (Figure 1C). A CT scan of the lungs showed bilateral upper lobe involvement. The patient’s serum cryptococcal antigen titre was greater than 1:1024. A lumbar puncture was performed on day 4 after admission. The cerebrospinal fluid (CSF) findings were normal, and the CSF cryptococcal antigen and fungal cultures were negative. The patient was treated with oral fluconazole 400 mg daily. At her three-month follow-up, her sputum culture was negative, but her serum antigen titre remained greater than 1:1024. There was minimal improvement in the chest x-ray and CT scan, but the patient noted slow symptomatic improvement. The patient was continued on oral fluconazole 400 mg daily to complete one year of treatment, and she experienced complete resolution of symptoms.
Case 3
A 39-year-old male resident of Vancouver presented with headache, cough and low-grade fever for several weeks in 2005. He was a former smoker but was otherwise healthy, apart from digital clubbing of unknown cause; he was on no medications. He had travelled to Vancouver Island for a visit seven months before the onset of symptoms. There were no abnormal physical findings. A chest radiograph showed a 5 cm poorly defined opacity in the left upper lobe without cavitation or calcification. A magnetic resonance imaging (MRI) scan of the head showed the presence of three ring-enhancing lesions (Figure 1D). A lumbar puncture showed an opening pressure of 170 mm H2O. His CSF glucose level was 1.9 mmol/L (normal 2.2 mmol/L to 4.4 mmol/L), protein level was 1.23 g/L (normal lower than 0.45 g/L) and white blood cell count was 351×106 cells/L, of which 80% were lymphocytes, 15% were neutrophils and 4% were mononuclear cells. The CSF India ink smear was negative, but the CSF cryptococcal antigen titre was 1:16 and the serum cryptococcal antigen titre was 1:1024. C gattii was recovered from the CSF fungal culture, but not the routine CSF culture. RFLP revealed a VGIIa strain. C gattii was also recovered from a bronchial wash specimen. HIV serology was negative. During treatment with amphotericin B (AmB) deoxycholate (0.7 mg/kg/day), nephrotoxicity prompted a change to liposomal AmB (L-AmB) (3 mg/kg/day). The total duration of this treatment was 53 days. His symptoms gradually resolved, and he was stepped down to oral fluconazole 400 mg daily. During the following year, he remained clinically well. The follow-up MRI scan showed a reduction in size of the brain lesions. After 12 months of treatment, CSF cryptococcal antigen and culture were both negative. At that point, fluconazole was discontinued. The patient remained clinically well during the following year.
CLINICAL PRESENTATION
Based on self-reported data obtained during public health follow-up, three-quarters of C gattii patients in BC presented with pulmonary findings including single or multiple nodules, infiltrates, cavitary lesions and parenchymal consolidation, such as in cases 1 and 2 (3). The differential diagnosis in many cases includes lung cancer, as in case 1, and community-acquired pneumonia and tuberculosis, as in case 2. On chest radiograph, case 1 presented with a single nodule and no change in her chronic respiratory symptoms. Approximately 7% of BC cases with pulmonary disease present asymptomatically and are identified through routine chest radiographs (3). Among BC cases presenting with pulmonary findings, the most common presenting signs and symptoms include cough, dyspnea, chest pain and weight loss (3).
Close to one-fifth of BC cases present with central nervous system (CNS) disease with or without concomitant pulmonary disease. Case 3 presented with pneumonia, meningitis and brain cryptococcomas. The differential diagnosis included multiple bacterial abscesses and neoplasm. Common presenting signs and symptoms for CNS disease in BC patients include headache, fever, night sweats and neck stiffness (3). C gattii is less likely to cause disseminated or CNS disease than C neoformans, but more likely to form cryptococcomas in lungs and brain (4–7). These differences may be due to C gattii causing disease in patients who are not profoundly immunocompromised.
C gattii is thought to infect mostly immunocompetent persons (4,6,8). All three cases presented in the report were HIV negative. Among BC cases in 1999 to 2006, only four (2.9% of 138 with known HIV status) were HIV positive (3). However, a large proportion of patients infected with C gattii in BC smoked or had smoked (cases 1 and 3), had a history of lung disease (case 1) or cancer, or had recently been on systemic steroids (3). This has also been noted by others (4).
The mean age of patients infected with C gattii in BC between 1999 and 2006 was 59 years (range two to 92 years); 55% were male (3). The highest age-specific rate was observed in people 70 to 79 years of age, as illustrated by cases 1 and 2. Case 3 developed illness seven months after returning from an endemic area, which is consistent with incubation period estimates ranging from six weeks to 11 months, with a median of seven months (9,10).
DIAGNOSIS
In all three cases, imaging provided the first evidence of disease. Radiographs and CT scans of the chest and MRI scans of the brain are useful in identifying the focus of infection. The role of follow-up imaging in case management is less clear; cryptococcomas in the CNS may persist for years after successful therapy (11).
The most reliable specimens to diagnose pulmonary C gattii infection are bronchial washings (cases 1 and 3) and fine needle aspirates. However, when infiltrates are present, sputum (case 2) specimens may also be positive. When Cryptococcus infection is being considered, fine needle aspirates and biopsy specimens should be split for culture and histopathology.
Identification of encapsulated yeasts by microscopic examination of clinical specimens provides a preliminary diagnosis of Cryptococcus species. Calcofluor white – a fluorescent fungal stain – is the most sensitive (12), but Gram stain may also detect Cryptococcus species (Figure 2A). India ink stain can demonstrate negatively stained capsules in CSF specimens, although with limited sensitivity and specificity (Figure 2B). On fixed tissue sections, Cryptococcus species can also be identified using a mucicarmine stain. These direct and histological stains, however, do not differentiate between the species and varieties.
Figure 2).
Photographs demonstrating diagnostic methods for identification of Cryptococcus neoformans and Cryptococcus gattii. A Cerebrospinal fluid Gram stain from a patient with cryptococcal meningitis, showing nonstaining capsule (bright field, original magnification × 1000); B India ink stain demonstrating nonstaining thick mucopolysaccharide capsule of a Cryptococcus cell (bright field, original magnification × 1000); C Potato-dextrose agar with colonies of Candida albicans (white) and C gattii (brown); D Canavanine-glycine-bromothymol plates with growth of C gattii (blue) and C neoformans (green); E Restriction fragment length polymorphism patterns of different Cryptococcus species
Cryptococcus antigen detection using latex-agglutination assays on CSF or serum specimens is useful in the initial diagnosis, as seen in all three cases above. The reported sensitivity for latex agglutination assays ranges from 54% to 100%, with higher sensitivity in patients with CNS infection or pneumonia (cases 2 and 3) (5,13,14). False-negative results may occur in cases with encapsulated nodules (case 1), or when patients have an overwhelming disease such that the amount of serum antigen in the sample is in excess of the amount of antibody in the assay (prozone effect) (15,16). False-positive results may occur with Trichosporon beigelii infection (17). Titres are occasionally used to monitor response to therapy, but the concordance between titre values and clinical progression is unclear; case 1 was clinically well after six months of treatment, although her titre remained elevated.
Only culture leads to Cryptococcus species and variety identification. Although Cryptococcus can be recovered on blood agar plates used routinely for bacterial culture, selective and enriched media may improve recovery of these yeasts, as demonstrated in case 3. Potato dextrose and birdseed agars are used to differentiate C neoformans and C gattii from other Cryptococcus species (Figure 2C). The canavanine-glycine-bromothymol agar allows for differentiation of C gattii from C neoformans based on the utilization of glycine and susceptibility to canavanine (18). Isolates producing a blue pigment on the canavanine-glycine-bromothymol agar can be presumptively identified as C gattii (Figure 2D). However, confirmation of C gattii requires subtyping by serotype or genotype methods.
There are currently no commercially available subtyping tests. Genotyping is, therefore, essential to confirm C gattii for patient management and further characterize isolates of Cryptococcus for molecular epidemiological surveillance. It identifies the molecular type of Cryptococcus, including VNI and VNII corresponding to C neoformans var. grubii, VNIV corresponding to C neoformans var. neoformans, VNIII, a C neoformans hybrid and the C gattii genotypes VGI, VGIIa, VGIIb, VGIII and VGIV. A number of typing methods have been described, including RFLP, random amplified polymorphic DNA, multilocus sequence typing, allele sequencing and electrophoretic karyotyping (19–23). RFLP is the most reliable (24) and has been implemented for the definitive identification of C gattii in BC (Figure 2E). All three cases were infected with C gattii VGIIa, which is the most common genotype in BC accounting for 83.7% (87 of 104) of culture-confirmed cases between 1999 and 2006. The rest of the BC human isolates are VGI and VGIIb types. This distribution is different from that observed in Australia where the majority of cases are due to VGI (8).
MANAGEMENT
The Infectious Diseases Society of America developed clinical practice guidelines for the management of cryptococcal disease in HIV-negative individuals (25). However, the management of cryptococcosis due to C gattii is not specifically addressed, likely because of the absence of randomized clinical trials for this infection and the few reported cases of C gattii infection in the United States before publication in 2000. Due to slower response to treatment, more frequent clinical relapses and, in CNS disease, more neurological sequelae (7), some clinicians treat C gattii infection more aggressively than C neoformans.
Although case 1 was treated, asymptomatic immunocompetent patients with solitary nodules may have favourable outcomes without antifungal therapy (26). Patients, such as case 2, presenting with pulmonary disease should be evaluated for the presence of asymptomatic CNS involvement, particularly if they are immunocompromised or have elevated serum cryptococcal antigen titres (25). An elevated titre suggests the presence of deep tissue infection and a high likelihood of disseminated disease, as in case 3.
There have been no randomized clinical trials for the treatment of pulmonary cryptococcosis. In one case series (27), a cure or clinical improvement was observed in 77 patients (84%) with pulmonary cryptococcosis due to C neoformans who received fluconazole (median dose 400 mg/day) for approximately three months or AmB plus or minus 5-flucytosine (5-FC). In BC, patients with mild to moderate pulmonary infection due to C gattii (cases 1 and 2) have usually been treated with fluconazole 400 mg daily for a minimum of three months. For immunocompetent patients with large or multiple pulmonary cryptococcomas due to C gattii, Australian experts favour a four-to six-week induction regimen with AmB and 5-FC, followed by fluconazole therapy for six to 12 months. Large cryptococcomas often require surgical resection (Australian Mycoses Interest Group, personal communication).
In CNS disease, induction therapy is recommended for at least two weeks. Case 3 was started on AmB. Studies (28–30) have demonstrated improved CSF sterilization and clinical outcomes for AmB at 0.7 mg/kg/day plus 5-FC compared with oral fluconazole 400 mg once daily in AIDS-related disease. AmB plus 5-FC was also associated with fewer failures and a shorter mean duration of CSF culture positivity (29). Due to AmB-associated nephrotoxicity, case 3 was switched to L-AmB. A similar clinical response has been shown with AmB 0.7 mg/kg/day and L-AmB 3 mg/kg/day or 6 mg/kg/day in AIDS-related cryptococcal meningitis (31). AmB lipid complex appears to be a less suitable alternative due to delayed CSF sterilization compared with AmB (32,33). Therefore, the lipid formulation of choice is L-AmB at a dosage of 3 mg/kg/day to 4 mg/kg/day. If the patient responds clinically, treatment can be changed to fluconazole 400 mg once daily for a minimum of 10 weeks (25). Case 3 had a good outcome after 12 months of treatment. It is not clear why he did not receive 5-FC. In Australia, at least a six-week induction regimen with AmB and 5-FC, followed by fluconazole consolidation therapy at 400 mg/day for at least six to 18 months is recommended for cerebral cryptococcomas. Patients with meningitis and no cryptococcomas are treated the same as patients with C neoformans meningitis (Australian Mycoses Interest Group, personal communication).
Cryptococcosis patients with opening pressures greater than or equal to 250 mm H20 more frequently have higher CSF cryptococcal antigen titres, positive India ink smears, headache, meningismus, papilledema, hearing loss, pathological reflexes and reduced survival (34). For patients who do not have contraindications to lumbar puncture, the recommended management of raised intracranial pressure (ICP) is repeated large-volume CSF removal by spinal tap. For those who do not respond or have recurring raised ICP, a ventriculoperitoneal shunt should be considered (35). Other adjunctive treatment should include consideration of corticosteroids in patients who develop clinical deterioration associated with inflammatory mass lesions during the course of mycologically successful antifungal therapy (36,37). Such patients may be experiencing immune reconstitution inflammatory syndrome (38).
PROGNOSIS
None of the patients in the present case series died of cryptococcosis. In BC, between 1999 and 2006, there were 14 deaths due to cryptococcosis (a case-fatality rate of 8.0%). In an Australian study (7), a 30% case-fatality rate was observed with C neoformans infection, but no deaths were observed in 20 patients with C gattii infection. However, the relapse rate was higher among patients with C gattii infection, and C gattii-infected patients more often required CSF shunt procedures, surgical resections or optic nerve decompression for raised ICP (7,39).
GEOGRAPHICAL RISK
In BC, the current areas at risk for C gattii exposure include the east coast of Vancouver Island and, to a lesser extent, the Lower Mainland (Sunshine Coast, Greater Vancouver Area and Fraser Valley) (3). There is also evidence of C gattii exposure in the Pacific Northwestern States (3,40,41).
CONCLUSION
C gattii infection should be suspected in patients who reside in or travel to affected areas in BC, presenting with pulmonary or CNS symptoms, particularly if they are HIV negative. Diagnosis is made through chest and/or brain imaging as well as culture of respiratory and/or CSF specimens. Genotyping of yeast recovered from culture is currently required to confirm C gattii infection. Patients with pulmonary cryptococcosis should be treated with fluconazole but may also require an induction with AmB and 5-FC. Patients with CNS disease require induction therapy with AmB and 5-FC followed by fluconazole consolidation therapy.
Acknowledgments
The authors thank Sultana Mithani and Min-Kuang Lee (both from the BC Centre for Disease Control) for their work in laboratory confirmation and molecular typing, physicians in British Columbia for their care of C gattii-infected patients, British Columbia Environmental Health Officers for follow-up of C gattii cases and the Australian Mycoses Interest Group for their interest and advice.
REFERENCES
- 1.Stephen C, Lester S, Black W, Fyfe M, Raverty S. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can Vet J. 2002;43:792–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Kidd SE, Hagen F, Tscharke RL, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc Natl Acad Sci USA. 2004;101:17258–63. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BC Centre for Disease Control BC Cryptococcus gattii surveillance summary, 1999–2006<http://www.bccdc.org/topic.php?item=109> (Version current at October 21, 2008).
- 4.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507–44. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Sorrell T, Nimmo G, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Clin Infect Dis. 2000;31:499–508. doi: 10.1086/313992. [DOI] [PubMed] [Google Scholar]
- 6.Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin N Am. 2002;16:837–74. doi: 10.1016/s0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 7.Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28–34. doi: 10.1093/clinids/21.1.28. [DOI] [PubMed] [Google Scholar]
- 8.Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39:155–68. [PubMed] [Google Scholar]
- 9.Lindberg J, Hagen F, Laursen A, Stenderup J, Boekhout T. Cryptococcus gattii risk for tourists visiting Vancouver Island, Canada. Emerg Infect Dis. 2007;13:178–9. doi: 10.3201/eid1301.060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDougall L, Fyfe M. Emergence of Cryptococcus gattii in a novel environment provides clues to its incubation period. J Clin Microbiol. 2006;44:1851–2. doi: 10.1128/JCM.44.5.1851-1852.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett JE, Hospenthal DR. Persistence of cryptococcomas on neuroimaging. Clin Infect Dis. 2000;31:1303–6. doi: 10.1086/317434. [DOI] [PubMed] [Google Scholar]
- 12.Koneman EW, Roberts GD, editors. Practical Laboratory Mycology. 3rd edn. Baltimore: Williams and Wilkins; 1985. p. 21. [Google Scholar]
- 13.Kaufman L, Blumer S. Proceedings of the Fourth International Conference on Mycoses, Brasilia, Brazil, June 1977. Washington, DC: Pan American Health Organization; 1977. Cryptococcosis: The awakening giant. (PAHO Scientific Publication no. 356). [Google Scholar]
- 14.Tanner DC, Weinstein MP, Fedorciw B, et al. Comparison of commercial kits for detection of cryptococcal antigen. J Clin Microbiol. 1994;32:1680–4. doi: 10.1128/jcm.32.7.1680-1684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamm AM, Polt SS. False negative cryptococcal antigen test. JAMA. 1980;244:1359. [PubMed] [Google Scholar]
- 16.Latex-cryptococcus antigen detection system REF CR1003, REF CR1004, REF CR1005, and Individual Reagents Norman: IMMY Immuno-Mycologics Inc; (Package insert.) [Google Scholar]
- 17.Mera WG, Roberts GD. Algorithms for detection and identification of fungi. In: Murray PR, Baron EJ, editors. Manual of Clinical Microbiology. 8th edn. ASM Press; 2003. pp. 1668–76. [Google Scholar]
- 18.Kwon-Chung KJK, Polacheck I, Bennett JE. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C) J Clin Microbiol. 1982;15:535–7. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt ME, Hutwagner LC, Kuykendall RJ, Pinner RW. Comparison of multilocus enzyme electrophoresis and random amplified polymorphic DNA analysis for molecular subtyping of Cryptococcus neoformans. The Cryptococcal Disease Active Surveillance Group. J Clin Microbiol. 1995;33:1890–5. doi: 10.1128/jcm.33.7.1890-1895.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casadevall A, Freundlich L F, Marsh L, Scharff MD. Extensive allelic variation in Cryptococcus neoformans. J Clin Microbiol. 1992;30:1080–4. doi: 10.1128/jcm.30.5.1080-1084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currie BP, Freundlich LF, Casadevall A. Restriction fragment length polymorphism analysis of Cryptococcus neoformans isolates from environmental (pigeon excreta) and clinical sources in New York City. J Clin Microbiol. 1994;32:1188–92. doi: 10.1128/jcm.32.5.1188-1192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfect JR, Ketabchi N, Cox GM, Ingram CW, Beiser CL. Karyotyping of Cryptococcus neoformans as an epidemiological tool. J Clin Microbiol. 1993;31:3305–9. doi: 10.1128/jcm.31.12.3305-3309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto Y, Kohno S, Koga H, et al. Random amplified polymorphic DNA analysis of clinically and environmentally isolated Cryptococcus neoformans in Nagasaki. J Clin Microbiol. 1995;33:3328–32. doi: 10.1128/jcm.33.12.3328-3332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latouche GN, Huynh M, Sorrell TC, Meyer W. PCR-restriction fragment length polymorphism analysis of the phospholipase B (PLB1) gene for subtyping of Cryptococcus neoformans isolates. Appl Envir Microbiol. 2003;69:2080–6. doi: 10.1128/AEM.69.4.2080-2086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Clin Infect Dis. 2000;30:710–8. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 26.Kerkering TM, Duma RJ, Shadomy S. The evolution of pulmonary cryptococcosis: Clinical implications from a study of 41 patients with and without compromising host factors. Ann Intern Med. 1981;94:611–6. doi: 10.7326/0003-4819-94-5-611. [DOI] [PubMed] [Google Scholar]
- 27.Pappas GP, Perfect JR, Cloud GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–9. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer AE, Rajanuwong A, Chierakul, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: A randomized trial. Lancet. 2004;363:1764–7. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 29.Larsen RA, Leal MAE, Chan SL. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS: A randomized trial. Ann Intern Med. 1990;113:183–7. doi: 10.7326/0003-4819-113-3-183. [DOI] [PubMed] [Google Scholar]
- 30.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 31.Hamill RJ, Sobel J, El-Sadr W, et al. Randomized double-blind trial of AmBisome (liposomal amphotericin B) and amphotericin B in acute cryptococcal meningitis in AIDS patients. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco. September 26 to 29; 1999. [Google Scholar]
- 32.Clemons KV, Stevens DA. Comparison of Fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob Agents Chemother. 1998;42:899–902. doi: 10.1128/aac.42.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharkey PK, Graybill JR, Johnson ES, et al. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22:315–21. [PubMed] [Google Scholar]
- 34.Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. Clin Infect Dis. 2000;30:47–54. doi: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 35.Liliang PC, Liang CL, Chang WN, et al. Shunt surgery for hydrocephalus complicating cryptococcal meningitis in human immunodeficiency virus-negative patients. Clin Infect Dis. 2003;37:673–8. doi: 10.1086/377208. [DOI] [PubMed] [Google Scholar]
- 36.Phillips P, Chapman K, Sharp M, et al. Dexamethasone in Cryptococcus gattii central nervous system infection. AMMI Canada-CACMID Conference; Vancouver. February 27 to March 2; 2008. (Abst K2). [DOI] [PubMed] [Google Scholar]
- 37.Lane M, McBride J, Archer J. Steroid responsive late deterioration in Cryptococcus neoformans variety gattii meningitis. Neurology. 2004;63:713–14. doi: 10.1212/01.wnl.0000134677.29120.62. [DOI] [PubMed] [Google Scholar]
- 38.Einsiedel L, Gordon DL, Dyer JR. Paradoxical inflammatory reaction during treatment of Cryptococcus neoformans var. gattii meningitis in an HIV-seronegative woman. Clin Infect Dis. 2004;39:e78–82. doi: 10.1086/424746. [DOI] [PubMed] [Google Scholar]
- 39.Garrity JA, Herman DC, Imes R, Fries P, Hughes CF, Campbell RJ. Optic nerve sheath decompression for visual loss in patients with acquired immunodeficiency syndrome and cryptococcal meningitis with papilledema. Am J Ophthalmol. 1993;116:472–8. doi: 10.1016/s0002-9394(14)71407-2. [DOI] [PubMed] [Google Scholar]
- 40.MacDougall L, Kidd S, Galanis E, et al. Spread of Cryptococcus gattii in British Columbia, Canada and its detection in the Pacific Northwest, USA. Emerg Infect Dis. 2007;13:42–50. doi: 10.3201/eid1301.060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upton A, Fraser JA, Kidd SE, et al. First contemporary case of human infection with Cryptococcus gattii in Puget Sound: Evidence for spread of the Vancouver Island outbreak. J Clin Microbiol. 2007;45:3086–8. doi: 10.1128/JCM.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]