Abstract
Three cases of severe rash associated with the use of atazanavir are described. In all cases, the rash was maculopapular and pruritic. Rash onset occurred eight to 11 days after initiation of therapy, and resolved with atazanavir discontinuation. Clinicians prescribing atazanavir should be aware of this potential adverse effect.
Keywords: Atazanavir, Rash
Abstract
Les auteurs décrivent ici trois cas d’érythème grave associé à l’emploi de l’atazanavir. Dans les trois cas, l’érythème était de nature maculopapulaire et prurigineuse. L’érythème est apparu entre les jours 8 et 11 suivant le début du traitement par atazanavir et s’est résorbé à l’arrêt du traitement. Les médecins qui prescrivent l’atazanavir doivent être au courant de cette réaction indésirable potentielle.
Atazanavir is an azapeptide protease inhibitor licensed for the treatment of HIV (1,2). Ritonavir-boosted atazanavir, in combination with two nucleoside (or nucleotide) reverse transcriptase inhibitors, is currently one of the recommended options for first-line HIV therapy (1). Atazanavir has a pharmacokinetic profile that permits once daily administration (2). Additionally, it is reported to cause fewer abnormalities in the plasma lipid profile than other protease inhibitors (2,3). These features make atazanavir an attractive option for patients. In clinical trials, atazanavir has been generally well tolerated. However, rash has been reported in 1% to 6% of study participants (4–6). To date, there are few publications describing atazanavir-associated dermatological adverse events in any detail (7,8). The current report presents three cases of severe rash that occurred shortly after the initiation of therapy with atazanavir.
CASE PRESENTATIONS
Case 1
A 33-year-old antiretroviral-naive Aboriginal Canadian woman, who was known to be HIV-positive for over 10 years, was started on antiretroviral therapy on August 30, 2007. Her CD4 count two months previously was 247 cells/μL, and her corresponding viral load was 8230 copies/mL. She had previously tested negative for human leukocyte antigen (HLA)-B*5701, suggesting that she would be at a very low risk of developing a hypersensitivity reaction to abacavir. A combination of Kivexa (GlaxoSmithKline USA; abacavir 600 mg and lamivudine 300 mg) one tablet orally daily, ritonavir 100 mg orally daily and atazanavir 300 mg orally daily was chosen based on ease of administration and adverse effect profile.
Eight days after starting her antiretroviral medications, she developed a new-onset rash. She presented to the emergency department (Health Sciences Centre, Winnipeg, Manitoba) on September 9, 2007, with a progressive rash over two days, pruritis, subjective fever and chills, and mild numbness to her lips. She did not report any dyspnea. Apart from HIV, her medical history was significant for mild asthma, hepatitis C, migraines, depression and previous Graves’ disease. Her medications, in addition to the antiretrovirals, included lorazepam and trimethoprim-sulfamethoxazole. She had been receiving both of these medications for over four months without any adverse effects.
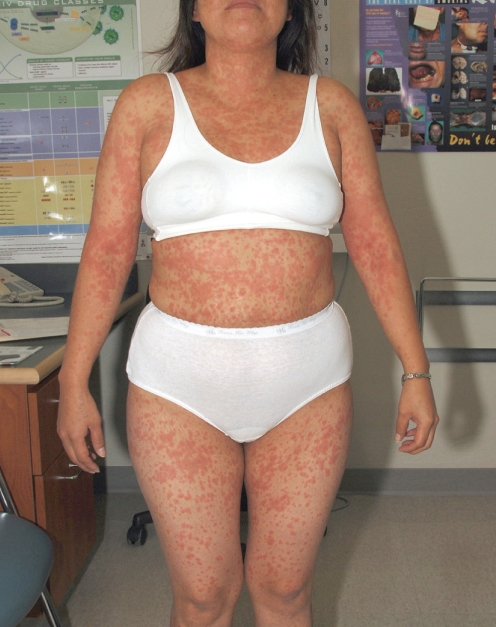
On physical examination, she was afebrile and hemodynamically stable. A maculopapular rash was observed over most of her body (Figure 1). Some mild oral mucosa erosions were appreciated, as was some slight swelling to her lips. The remainder of the examination was unremarkable. Renal function, liver enzymes and peripheral eosinophil count were all normal. Her total bilirubin level was elevated at 66 μmol/L (related to atazanavir). The patient received 50 mg of prednisone and 50 mg of diphenhydramine as therapy for a presumed medication allergy. Within 4 h, she was subjectively feeling much better. The antiretrovirals and trimethoprim-sulfamethoxazole were discontinued.
Figure 1).
Case 1: Atazanavir-associated rash
When evaluated 10 days later in follow-up, her rash had resolved. Trimethoprim-sulfamethoxazole was restarted without incident. Patch testing, as described by Phillips et al (9), was subsequently performed to assess whether the rash may have been related to abacavir. The patient did not demonstrate any evidence of abacavir hypersensitivity with this test. Antiretroviral therapy was resumed on October 17, with a combination of Kaletra (Abbott Laboratories, USA; lopinavir and ritonavir) and Truvada (Gilead Sciences Inc, USA; tenofovir and emtricitabine). The patient continues to do well. Her most recent CD4 count (December 6, 2007) was 531 cells/μL, with a corresponding viral load of less than 40 copies/mL.
Case 2
A 57-year-old African woman, who was diagnosed with HIV in 1992, had a change made to her antiretroviral therapy on July 25, 2006. She had previously been receiving lamivudine, stavudine and saquinavir. A decision was made to alter her antiretroviral therapy because of a persistently elevated viral load (viral load of 11,300 copies/mL, and CD4 count of 120 cells/μL). She was started on a combination of atazanavir 300 mg orally daily, ritonavir 100 mg orally daily, tenofovir 300 mg orally daily and emtricitabine 200 mg orally daily. This combination was selected on the basis of convenience, tolerability and resistance testing.
Eleven days after starting her new antiretroviral medications, the patient developed a pruritic rash. She also complained of some swelling of her lips and eyelids. She did not have any dyspnea. In addition to HIV, her medical history was significant for hypercholesterolemia and a previous episode of shingles. She was not receiving any other medications at the time that she developed the rash.
Her physical examination was significant for slight conjunctivitis, mild lip swelling and a diffuse maculopapular rash. No oral lesions were documented, nor was fever. Blood tests were not performed at this time. Atazanavir was thought to be the most likely culprit, and this medication was discontinued. The patient was treated symptomatically with diphenhydramine.
The rash was observed to improve within three days of atazanavir discontinuation. Kaletra (lopinavir and ritonavir) was started eight days after atazanavir was stopped. No recurrence of the rash was observed. The patient continues to do well. Her most recent CD4 count (September 14, 2007) and corresponding viral load were 204 cells/μL and less than 40 copies/mL, respectively.
Case 3
A 36-year-old Aboriginal Canadian woman, who was diagnosed with HIV in 1983, was started on antiretroviral therapy on January 2, 2006, for a falling CD4 count. Her most recent CD4 count (December 5, 2005) was 260 cells/μL, with a corresponding viral load of 33,200 copies/mL. Therapy was initiated with Combivir (GlaxoSmithKline USA; zidovudine 300 mg and lamivudine 150 mg) one tablet twice a day and atazanavir 400 mg orally once daily, chosen on the basis of convenience and adverse effect profile.
Ten days after beginning antiretroviral therapy, the patient presented to the clinic with new onset of a pruritic rash. She also reported feeling feverish. She denied symptoms of nausea, vomiting, diarrhea and dyspnea. Apart from HIV, her medical history was significant for Bosma syndrome, hypothyroidism and autoimmune hepatitis. At the time she developed the rash, she was receiving levothyroxine in addition to the antiretrovirals. She had been on this medication for over one year.
On physical examination, the patient had a temperature of 38.6°C. A raised, bright red, maculopapular rash was observed on her face, trunk and extremities. Tiny, painful, deep ulcers on the buccal mucosa were appreciated on inspection of the oral cavity. Swelling of the right upper eyelid and right lip was also noted. Laboratory investigations demonstrated a normal eosinophil count. The patient received hydroxyzine for her rash, and her antiretrovirals were discontinued.
The rash improved significantly over the following two weeks. Antiretroviral therapy was restarted on April 5, 2006, with a combination of Kaletra (lopinavir and ritonavir), tenofovir and lamivudine. The patient continues to do well on these medications. Her most recent CD4 count was 302 cells/μL, with a corresponding viral load of less than 40 copies/mL.
DISCUSSION
We have presented three cases of severe rash that developed in patients receiving atazanavir. All three cases occurred in patients from minority populations (two Aboriginal Canadians and one of African ethnicity). Rash onset occurred between eight and 11 days after initiation of therapy. In all cases, the rash was maculopapular and associated with pruritis. Resolution of the rash was evident within seven to 14 days of atazanavir discontinuation. None of the patients described were rechallenged with atazanavir. In the first case, abacavir was started at the same time as atazanavir. An abacavir hypersensitivity reaction cannot be entirely excluded; this is associated with HLA-B*5701 (10,11). Patch testing has been used to immunologically confirm abacavir hypersensitivity (9,10). Our patient was both HLA B*5701-negative and abacavir patch test-negative. These factors argue against abacavir as a cause of the rash in this patient (9–11). No medication other than atazanavir could be implicated as a cause of the rash in the second patient. In the third case, zidovudine was initiated and stopped concurrently with atazanavir. It is possible that the rash in this case may have been related to zidovudine. However, serious dermotological toxicity attributable to zidovudine has been reported very infrequently in the literature despite over 20 years of use in HIV therapy (12). This argues in favour of atazanavir as the cause of the rash in this patient.
Moderate to severe dermatological reactions (grade 2 to grade 4) have been reported in 1% to 6% of atazanavir-treated patients in clinical trials (4–6). Details concerning the specific reactions observed were not provided in these publications. Additionally, in all cases atazanavir was coadministered with other antiretrovirals. This makes attributing a rash to any one drug difficult. The product monograph (13) for atazanavir reports that rash of all grades of severity, regardless of causality, has been observed in 21% of atazanavir clinical trial participants. It is also stated in the monograph that cases of Stevens-Johnson syndrome and erythema multiforme have been documented (13). The frequency of these serious events is not quantified. It is not reported whether the patients who developed these reactions were concomitantly receiving other medications that may have been temporally associated with rash onset.
To date, there are few published cases describing atazanavir-associated dermatological toxicity. Ouagari et al (7) previously reported three cases of atazanavir-associated rash. Similar to our cases, the rash was described as macular or maculopapular. The mean interval between rash onset and atazanavir initiation was 10 days. Pruritis was documented for two patients. In one case, the rash was mild and disappeared spontaneously despite continued treatment with atazanavir. Atazanavir was discontinued in the other two cases, although in one of these cases, the rash improved while the patient was still receiving therapy (7). Herzmann et al (8) described a single patient who developed a generalized, nonpruritic maculopapular eruption 12 days after initiating therapy with atazanavir. The rash subsided two days after atazanavir was discontinued (8).
It is interesting to note that all three of the cases presented occurred in patients from minority populations. This raises the question of whether patients from certain ethnic groups may have a genetic predisposition for this particular adverse effect. Patient ethnicity was only reported in one of four other cases in the literature (7,8). In that particular case, the patient was Caucasian (8).
SUMMARY
Atazanavir may be associated with the infrequent development of severe dermatological toxicity. Clinicians prescribing atazanavir should be aware of this potential adverse effect, such that it may be recognized and managed in a timely manner.
REFERENCES
- 1.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection 2008 recommendations of the international AIDS society-USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 2.Busti AJ, Hall II RG, Margolis DM. Atazanavir for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2004;24:1732–47. doi: 10.1592/phco.24.17.1732.52347. [DOI] [PubMed] [Google Scholar]
- 3.Gatell J, Salmon-Ceron D, Lazzarin A, et al. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: The SWAN study (AI424-097) 48-week results. Clin Infect Dis. 2007;44:1484–92. doi: 10.1086/517497. [DOI] [PubMed] [Google Scholar]
- 4.Squires K, Lazzarin A, Gatell JM, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004;36:1011–9. doi: 10.1097/00126334-200408150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 6.Malan DR, Krantz E, David N, et al. Efficacy and safety of atazanavir, with or without ritonavir, as part of once-daily highly active antiretroviral therapy regimens in antiretroviral-naïve patients. J Acquir Immune Defic Syndr. 2008;47:161–7. doi: 10.1097/QAI.0b013e31815ace6a. [DOI] [PubMed] [Google Scholar]
- 7.Ouagari Z, Tubiana R, Mohand HA, et al. Skin rash associated with atazanavir: Report of three cases. AIDS. 2006;20:1207–16. doi: 10.1097/01.aids.0000226965.17966.3c. [DOI] [PubMed] [Google Scholar]
- 8.Herzmann C, Kinloch S, Johnson M. Rash in an HIV-positive patient. HIV Med. 2005;6:379. doi: 10.1111/j.1468-1293.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 9.Phillips EJ, Sullivan JR, Knowles SR, Shear NH. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002;16:2223–5. doi: 10.1097/00002030-200211080-00017. [DOI] [PubMed] [Google Scholar]
- 10.Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 11.Waters LJ, Mandalia S, Gazzard B, Nelson M. Prospective HLA-B*5701 screening and abacavir hypersensitivity: A single centre experience. AIDS. 2007;21:2533–49. doi: 10.1097/QAD.0b013e328273bc07. [DOI] [PubMed] [Google Scholar]
- 12.Rotunda A, Hirsch RJ, Scheinfeld N, Weinberg JM. Severe cutaneous reactions associated with the use of human immunodeficiency virus medications. Acta Derm Venereol. 2003;83:1–9. doi: 10.1080/00015550310002611. [DOI] [PubMed] [Google Scholar]
- 13.Bristol-Myers Squibb. Princeton, New Jersey: 2008. Reyataz (atazanavir sulfate) package insert. [Google Scholar]