Summary
Aspergillus fumigatus causes serious and frequently fatal infections in immunocompromised patients. To investigate the regulation of virulence of this fungus, we constructed and analyzed an A. fumigatus mutant that lacked the transcription factor Ace2, which influences virulence in other fungi. The Δace2 mutant had dysmorphic conidiophores, reduced conidia production, and abnormal conidial cell wall architecture. This mutant produced an orange pigment when grown on solid media, although its conidia had normal pigmentation. Conidia of the Δace2 mutant were larger and had accelerated germination. The resulting germlings were resistant to hydrogen peroxide, but not other stressors. Non-neutropenic mice that were immunosuppressed with cortisone acetate and infected with the Δace2 mutant had accelerated mortality, greater pulmonary fungal burden, and increased pulmonary inflammatory responses compared to mice infected with the wild-type or Δace2∷ace2 complemented strains. The Δace2 mutant had reduced ppoC, ecm33, and ags3 mRNA expression. It is known that A. fumigatus mutants with absent or reduced expression of these genes have increased virulence in mice, as well as other phenotypic similarities to the Δace2 mutant. Therefore, reduced expression of these genes likely contributes to the increased virulence of the Δace2 mutant.
Introduction
The incidence of invasive aspergillosis has risen substantially as a result of the increasing number of immunosuppressed patients (Marr et al., 2002). Furthermore, the mortality of patients with invasive aspergillosis is approximately 35%, even with advances in antifungal therapy (Upton et al., 2007; Patterson et al., 2000). Risk factors for this disease include hematopoietic and solid organ transplantation, use of corticosteroids, chronic pulmonary disease, and chronic granulomatous disease. Although neutropenia is also a risk factor for invasive aspergillosis, the majority of patients who develop this disease are not neutropenic (Pegues et al., 2001; Patterson et al., 2000).
Most cases of invasive aspergillosis are caused by Aspergillus fumigatus (Morgan et al., 2005; Latge, 1999). Conidia of A. fumigatus are ubiquitous in nature and small enough to be deposited in the alveoli after they are inhaled (Latge, 1999). In immunocompromised patients, these conidia germinate and form hyphae that can penetrate the lung parenchyma and invade blood vessels. A characteristic feature of invasive pulmonary aspergillosis is the formation of pulmonary infiltrates that subsequently cavitate (Fraser, 1993). This pulmonary damage is likely caused by both the organism itself as well as the host inflammatory response to infection. Local hypoxia due to thrombosis of the pulmonary blood vessels that have been invaded by A. fumigatus may also contribute to lung damage.
Currently, the factors that enable A. fumigatus to cause invasive disease are incompletely understood. One approach to identifying virulence factors is to investigate the transcription factors that govern their expression. The advantage of this approach is that a single transcription factor frequently controls the expression of multiple virulence genes. As a result, disruption of one transcription factor gene has a greater probability of altering virulence than disrupting a single gene that encodes a putative virulence factor. In addition, orthologs of the same transcription factor often govern virulence in diverse fungal species. For example, orthologs of the C2H2 zinc finger transcription factor, Ace2, influence the virulence Candida albicans, Candida glabrata, and Saccharomyces cerevisiae in mouse models of hematogenously disseminated infection (MacCallum et al., 2006; Kamran et al., 2004; Kelly et al., 2004). In neutropenic mice, mutants of C. albicans and S. cerevisiae that lack Ace2 have attenuated virulence. In contrast, a Δace2 mutant of C. glabrata is hypervirulent in these mice (MacCallum et al., 2006; Kamran et al., 2004). The effect of disrupting ACE2 on virulence is also influenced by the immune status of the host. For example, the virulence of the C. albicans Δace2 mutant is much more attenuated in immunocompetent mice compared to neutropenic mice, whereas the C. glabrata Δace2 mutant is hypervirulent in immunosuppressed mice, but not in immunocompetent mice (MacCallum et al., 2006).
Filamentous fungi, such as Magnaporthe grisea, and A. fumigatus and related species contain orthologs of Ace2. However, the function of Ace2 in filamentous fungi has not been studied previously. We investigated the role of A. fumigatus Ace2 in the regulation of virulence and development. The results of these investigations indicate that this transcription factor is essential for normal conidiation, cell wall architecture, and pigment production. Importantly, a Δace2 mutant that lacked this transcription factor was hypervirulent in non-neutropenic mice that were immunosuppressed with cortisone acetate.
Results
Construction of a Δace2 mutant and Δace2∷ace2 complemented strain
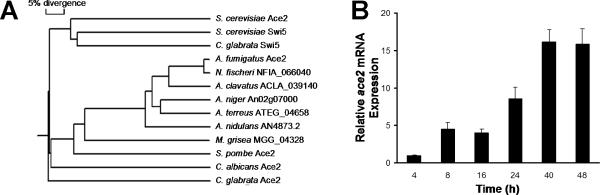
A. fumigatus Ace2 (encoded by gene Afu3g11250) was identified by BLAST searches as sharing significant homology to C. albicans Ace2 and Schizosaccharomyces pombe Ace2 (Fig. 1A). An ortholog of Ace2 was also identified in other molds, including Neosartorius fischeri, other species of Aspergillus, and Magnaporthe grisea. Ace2 and Swi5 in S. cerevisiae and C. glabrata formed a distinct group that was less closely related to the Ace2 of these other organisms.
Fig. 1.
Ace2 phylogeny and gene expression. (A) Rooted phylogeny tree of A. fumigatus Ace2 and its orthologs in other fungi. (B) Time course of ace2 expression in wild-type A. fumigatus. Conidia of strain Af293 were inoculated into Sabouraud broth and incubated at 37°C in a shaking incubator. At the indicated time points, an aliquot of the organisms was removed for RNA extraction. The expression of ace2 was determined by real-time PCR using tef1 as the reference gene. Results are the mean ± SD of two biological replicates, each measured in duplicate.
The time course of ace2 expression in A. fumigatus grown in Sabouraud broth at 37°C was investigated using real-time PCR. This gene was expressed at low levels in swollen conidia and expressed at progressively higher levels as the conidia germinated and formed hyphae (Fig. 1B).
To investigate the function of Ace2 in A. fumigatus, we constructed a mutant in which the entire protein coding region was deleted. Deletion of ace2 was confirmed by PCR and Southern blotting (data not shown). To confirm the specificity of the phenotype of the Δace2 mutant, a Δace2∷ace2 complemented strain was constructed in which a wild-type allele of ace2 was reintegrated at its native chromosomal locus. Using real-time PCR, we verified that ace2 mRNA expression was undetectable in the Δace2 mutant and similar to that of the wild-type strain in the Δace2∷ace2 complemented strain (data not shown).
The Δace2 mutant had abnormal pigmentation and conidiation, and accelerated germination
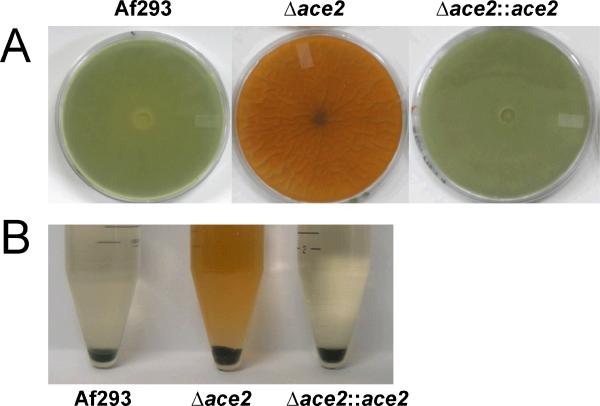
When the Δace2 mutant was grown on Sabouraud agar, it produced a yellow-orange pigment (Fig. 2A). This pigment was much less prominent when the Δace2 mutant was grown on other solid media (data not shown). The hyphae of this mutant were the normal white color of the wild-type strain. Conidia of the Δace2 mutant were also the normal grey-green color (Fig. 2B). Some of the orange pigment was recovered from the agar when the conidia were harvested using phosphate buffered saline (PBS) containing 0.1% Tween 80, indicating that the pigment is soluble (Fig. 2B). The abnormal pigment production was due to the absence of ace2 because the Δace2∷ace2 complemented strain had wild-type pigmentation.
Fig. 2.
Disruption of ace2 resulted in abnormal pigmentation. (A) Photographs of the underside of Sabouraud agar plates that had been inoculated with the indicated strains and incubated at 37°C for 7 days. (B) Photograph of conidia harvested from the indicated strains by rinsing the colonies with PBS containing 0.1% Tween. Note the normal grey-green color of the conidia from the Δace2 mutant and the orange color of the PBS-Tween above these conidia.
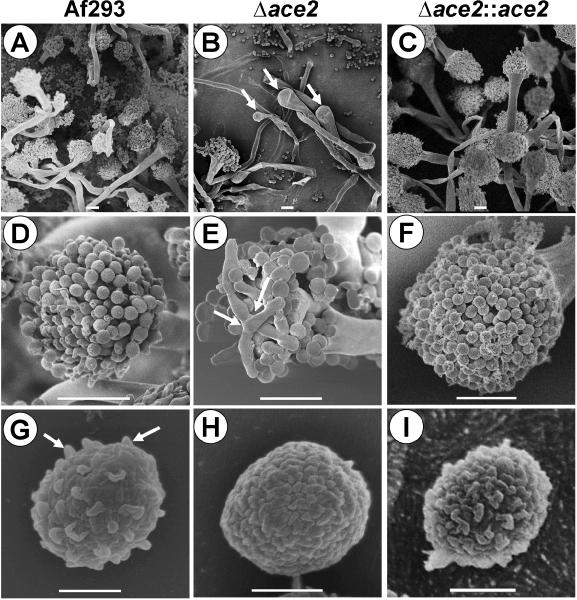
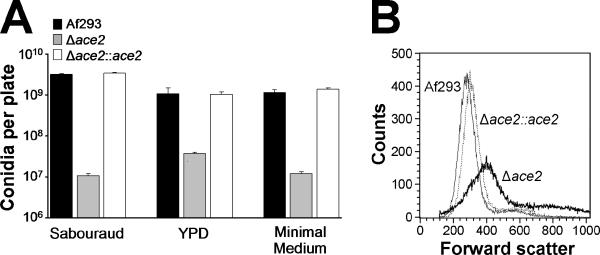
The Δace2 mutant also had abnormal conidiation compared to the wild-type and Δace2∷ace2 complemented strain. Using scanning electron microscopy, we observed that many conidiophores of the Δace2 mutant lacked phialides, whereas phialides were present on all conidiophores of (Fig. 3A-F). Other Δace2 conidiophores had phialides that were irregularly shaped and sometimes branched. In addition, some phialides were so short that conidia appeared to be emanating directly from the vesicle. As expected by the abnormal conidiophores, the Δace2 mutant produced 29- to 304-fold fewer conidia than the wild-type or Δace2∷ace2 complemented strains on three different media (Fig. 4A).
Fig. 3.
Abnormal conidiophores and conidia of the Δace2 mutant. Scanning electron micrographs of Af293 (A, D, G), the Δace2 mutant (B, E, H) and the Δace2::ace2 complemented strain (C, F, I). Arrows in (B) indicate conidiophores with absent phialides. Arrows in (E) indicate a branching phialide. Arrows in (G) indicate the knobby appearance of the surface of a wild-type conidium, in contrast to the relatively smooth surface of a Δace2 conidium. Scale bars in A-C are 10 μm; scale bars in D-I are 1 μm.
Fig. 4.
The Δace2 mutant produced fewer conidia that were larger than wild-type conidia. (A) Petri dishes containing Sabouraud, YPD, or Aspergillus minimal medium were inoculated with 5 × 103 conidia of the indicated strains and then incubated at 37°C. After 7 days, the conidia were harvested and counted. Results are the mean ± SD of a single experiment performed in triplicate. (B) Relative size of conidia of the indicated strains as determined by forward light scatter during flow cytometry. 105 conidia of each strain were analyzed.
The Δace2 conidia differed significantly from wild-type conidia. The outer surface of the Δace2 conidia was relatively smooth, whereas conidia of the wild-type and Δace2∷ace2 complemented strain had the typical knobby appearance (Fig. 3G-I). Also, while most of the Δace2 conidia were spherical in shape, a few were oval shaped or elongated (data not shown). When analyzed by flow cytometry, the size distribution of the Δace2 conidia was quite broad (Fig. 4B). In contrast, the conidia of the wild-type and Δace2∷ace2 complemented strains were much more consistent in size and generally smaller than the Δace2 conidia. To ensure that clumping of the Δace2 conidia did not contribute to their broad size distribution when analyzed by flow cytometry, the conidia were briefly sonicated beforehand. Furthermore, microscopic examination of the Δace2 conidia prior to sonication did not reveal any abnormal clumping (data not shown), a phenotype that has been seen in Δace2 yeast due to delayed mother-daughter cell separation (MacCallum et al., 2006; Alonso-Nunez et al., 2005; Bidlingmaier et al., 2001).
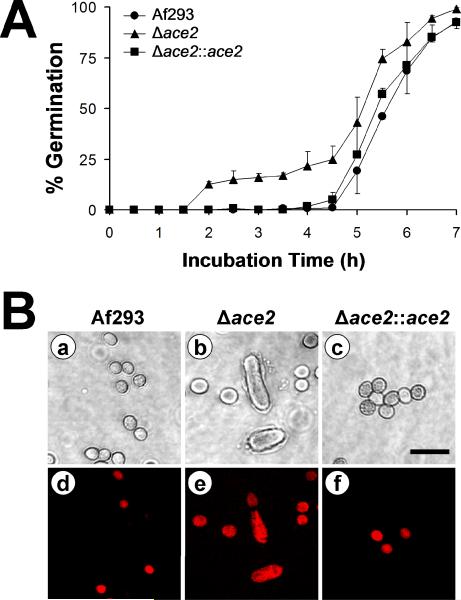
The conidia of the Δace2 mutant germinated more quickly than those of the wild-type strains. After a 2 h incubation in Sabouraud broth at 37°C, some Δace2 conidia had visible germ tubes (Fig. 5A). In contrast, conidia of the wild-type and Δace2∷ace2 complemented strains did not start to germinate until 4 h. To verify these results, we incubated freshly harvested conidia from the various strains in Sabouraud broth at 37°C to induce germination. We then stained these conidia at various time points with MitoTracker Red CM-H2 XRos. This dye fluoresces red when it is cleaved by actively respiring mitochondria, which are present in conidia that are no longer dormant. We found that after a 1 h incubation, most of the Δace2 conidia fluoresced red, indicating that they were metabolically active (Fig. 5B). Both the normal and aberrantly shaped conidia were stained with MitoTracker Red, suggesting that the accelerated germination of the Δace2 conidia was not limited to the aberrantly shape cells. In contrast, a minority of conidia from the wild-type and Δace2∷ace2 complemented strain were stained with MitoTracker Red at this time point. After 3 h of incubation virtually all conidia of all strains were stained with MitoTracker Red (data not shown). Collectively, these results demonstrate that Δace2 conidia become metabolically active and begin to germinate earlier than wild-type conidia in response to Sabouraud broth.
Fig. 5.
Early germination of the Δace2 mutant (A) Conidia of the indicated strains were incubated in Sabouraud broth at 37°C in 5% CO2 and the percentage of conidia with visible germ tubes was determined at each time point. Results are the mean ± SD of 3 experiments. (B) The Δace2 conidia had accelerated emergence from dormancy. Conidia of the indicated strains were incubated in Sabouraud broth at 37°C in 5% CO2 for 1 h, and then incubated in the same medium containing MitoTracker Red CM-H2 XRos for 20 min to stain the actively respiring mitochondria. The organisms were fixed and then imaged by differential interference contrast (panels a-c). The same microscopic fields were also imaged by confocal microscopy to visualize the cells that were stained with MitoTracker Red (panels d-f). Scale bar indicated 5 μm.
The early germination of the Δace2 conidia suggested that these conidia may have failed to become dormant. Conidia with defects in dormancy would be predicted to have reduced survival with prolonged storage in the absence of nutrients, and possibly germinate even in the absence of normal germination signals. To test these predictions, we compared the survival of Δace2 conidia with that of the wild type and Δace2∷ace2 complemented conidia when stored at room temperature in phosphate buffered saline (PBS) containing 0.1% Tween 80 (Fillinger et al., 2001). We found that the viability of the Δace2 conidia was similar to that of the wild-type and the Δace2∷ace2 complemented strain after 6 weeks of storage (data not shown). In addition, we analyzed the size distribution of these conidia by flow cytometry to determine if these conidia had begun to swell and germinate, even in the absence of germination signals. After 6 weeks of storage, the size distribution of the conidia of all strains was similar to that of freshly harvested conidia (data not shown). Collectively, these results argue that the Δace2 conidia do not have a defect in dormancy or inappropriate germination. Rather, they suggest that their accelerated germination may have been due to a more rapid response to environmental germination signals.
Despite having early germination, the radial growth rate of the Δace2 mutant was similar to that of the wild-type and Δace2∷ace2 complemented strains on Sabouraud, yeast extract peptone dextrose (YPD), and minimal medium agar (data not shown). Therefore, hyphal growth rate on solid media is independent of ace2.
The Δace2 mutant had increased resistance to hydrogen peroxide but not to other stressors
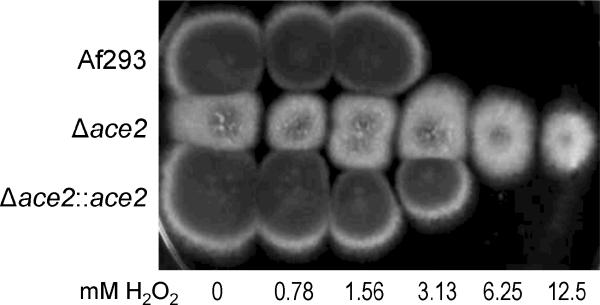
Next, we examined the role of Ace2 in governing susceptibility to environmental stressors. Both conidia and germlings of the Δace2 mutant were as susceptible as the wild-type strain to cell wall stress (Congo red and calcofluor white), plasma membrane stress (SDS), and high salt (NaCl) (data not shown). Also, Δace2 conidia had wild-type levels of susceptibility to oxidant stress induced by hydrogen peroxide (data not shown). However, germlings of the Δace2 mutant were significantly more resistant to hydrogen peroxide than those of the wild-type and Δace2∷ace2 complemented strains (Fig. 6). These results suggest that Ace2 negatively regulates hyphal resistance to oxidant stress.
Fig. 6.
Resistance to hydrogen peroxide of the Δace2 mutant. Germlings of the indicated strains were incubated for 60 min in Sabouraud broth containing the indicated concentrations of H2O2 and then transferred to Sabouraud agar and incubated at 37°C. The strains were photographed after 48 h.
Role of ace2 in endothelial cell damage
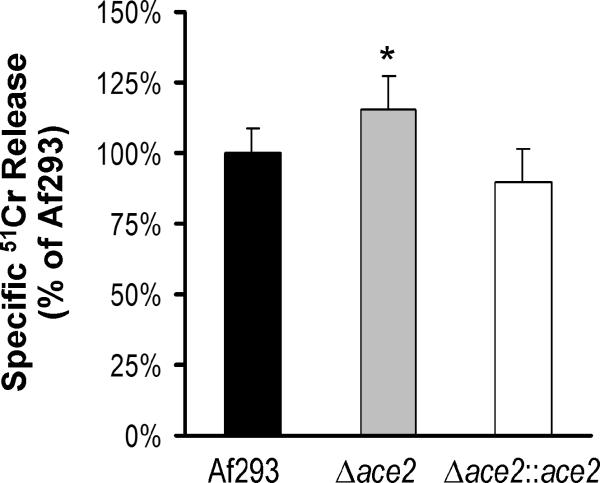
Angioinvasion is a prominent feature of invasive pulmonary aspergillosis and results in endothelial cell damage (Chiang et al., 2008; Lopes-Bezerra and Filler, 2004; Fraser, 1993). We used a 51Cr release assay to investigate the extent of endothelial cell damage caused by the Δace2 mutant in vitro. After 24 h of infection, this mutant caused 15% more damage to the endothelial cells than did the wild-type and Δace2∷ace2 complemented strains (Fig. 7). Although this difference was statistically significant, its biological significance is uncertain.
Fig. 7.
The Δace2 mutant caused more damage to endothelial cells in vitro. Endothelial cells were loaded with 51Cr and then infected with germlings of the indicated strains for 24 h. The extent of endothelial cell damage was determined by measuring the amount of 51Cr released by the infected cells. Data are expressed as a percentage the results obtained with the wild-type strain (Af293) and are the mean ± SD of 3 experiments, each performed in triplicate. *P < 0.01 compared to Af293 and the Δace2::ace2 complemented strain.
Next, we investigated the possibility that the orange pigment produced by the Δace2 mutant contributed to the increased endothelial cell damage caused by this strain. Sabouraud agar plates containing cultures of the various A. fumigatus strains were rinsed extensively with tissue culture medium to recover the pigment. We then incubated uninfected endothelial cells with filter sterilized rinses for 24 h and measured the extent of damage using a 51Cr release assay. No damage was detectable when endothelial cells were incubated with the rinses from either the wild-type or Δace2 mutant strain of A. fumigatus (data not shown), suggesting that the pigment by itself is not toxic to endothelial cells under these conditions.
The Δace2 mutant was hypervirulent in non-neutropenic mice
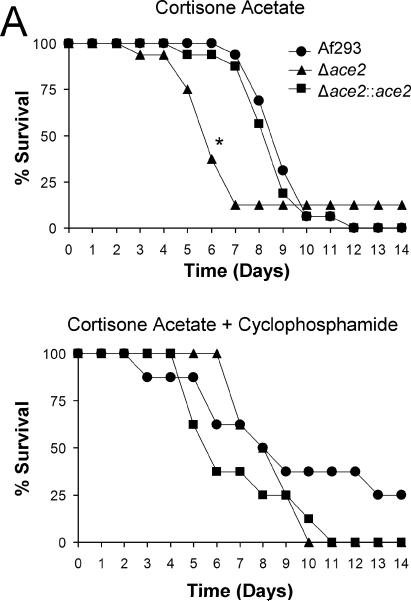
The accelerated germination, increased resistance to oxidative stress, and greater endothelial cell damage of the Δace2 mutant suggested that Ace2 may govern the virulence of A. fumigatus. To test this hypothesis, we analyzed the virulence of the Δace2 mutant in two different murine models of invasive pulmonary aspergillosis. In the first model, the mice were immunosuppressed with high-dose cortisone acetate, but were not neutropenic. In the second model, the mice were immunosuppressed with a lower dose of cortisone acetate and also rendered neutropenic with cyclophosphamide. When the non-neutropenic mice were infected via the intranasal route with the Δace2 mutant, they died significantly earlier than mice infected with either the wild-type or Δace2∷ace2 complemented strain (Fig. 8A). In contrast, the neutropenic mice had similar mortality when infected with each of the three strains of A. fumigatus (Fig. 8B). These results suggest that the increased virulence of the Δace2 mutant is dependent on the type of immunosuppression.
Fig. 8.
Increased virulence of the Δace2 mutant in non-neutropenic mice. Mice were immunosuppressed with cortisone acetate alone (A) or cortisone acetate plus cyclophosphamide (B), infected intranasally with the indicated strains of A. fumigatus, and then monitored for survival. Data are the combined results of two independent experiments, each using 8 mice per strain of A. fumigatus. *P < 0.05 compared to Af293 and the Δace2::ace2 complemented strain.
Non-neutropenic mice infected with the Δace2 mutant had higher pulmonary fungal burden and greater pulmonary inflammatory response
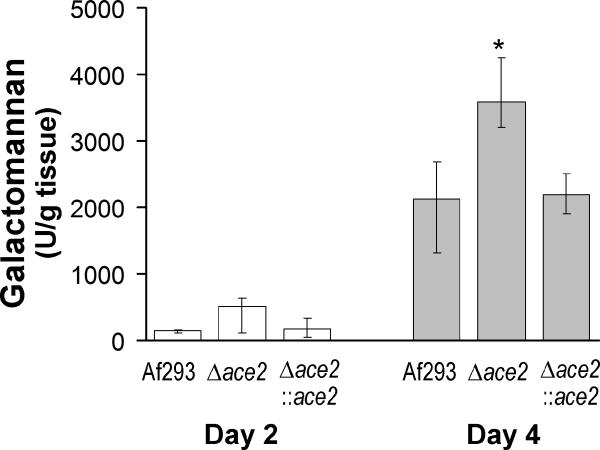
To investigate possible mechanisms for the increased virulence of the Δace2 mutant in non-neutropenic mice, we first determined the pulmonary fungal burden by measuring the level of galactomanan in the lungs. Preliminary experiments verified that the Δace2 mutant and the wild-type strain released similar amounts of galactomannan when grown in vitro (data not shown). The pulmonary galactomannan content of mice infected with the Δace2 mutant was similar to that of mice infected with either the wild-type or Δace2∷ace2 complemented strain after 2 days of infection, but significantly higher after 4 days of infection (Fig. 9). Therefore, the increased mortality of mice infected with the Δace2 mutant was likely due in part to their greater pulmonary fungal burden.
Fig. 9.
Non-neutropenic mice infected with the Δace2 mutant had a higher pulmonary fungal burden. Pulmonary galactomannan concentration after 2 and 4 days of infection in mice immunosuppressed with cortisone acetate. Results are median ± interquartile range of 7 to 8 mice per strain. *P < 0.02 compared to Af293 and the Δace2::ace2 complemented strain.
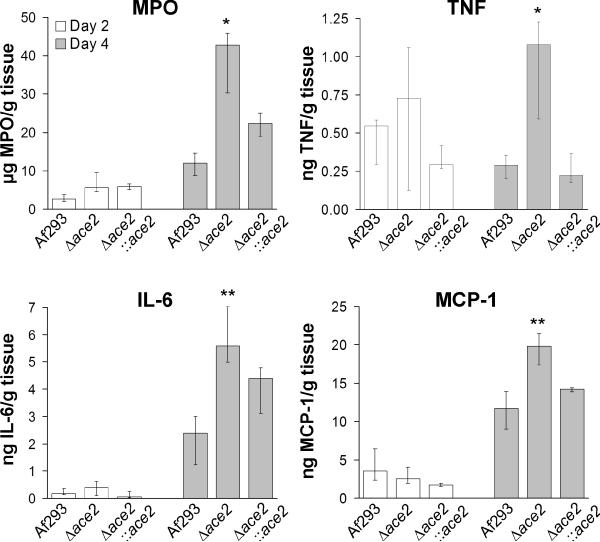
It was also possible that the mice infected with the Δace2 mutant died because of an over-exuberant host response. To investigate this possibility, the concentration of myeloperoxidase (MPO) in the lungs was analyzed as a marker of phagocyte accumulation (Glasgow et al., 2007; Tsuruta et al., 2007). MPO is made constitutively by granulocytes (Schultz and Kaminker, 1962), and is also present in lesser amounts in monocytes and some tissue macrophages (Sugiyama et al., 2001; Bos et al., 1978). After 4 days of infection, the pulmonary MPO levels of mice infected with the Δace2 mutant were significantly higher than those of mice infected with either the wild-type or Δace2∷ace2 complemented strain (Fig. 10). These results suggest that there was greater accumulation of phagocytes in the lungs of the mice infected with the Δace2 mutant.
Fig. 10.
The Δace2 mutant induced a higher pro-inflammatory response. Concentration of myeloperoxidase (MPO) and the indicated cytokines after 2 and 4 days of infection in the lungs of mice immunosuppressed with cortisone acetate. Results are median ± interquartile range of 5 to 8 mice per strain. *P < 0.05 vs. Af293 and the Δace2::ace2 complemented strain. **P < 0.05 vs. Af293.
Next, we measured the concentration of pro-inflammatory cytokines in the lungs to determine possible mechanisms for the increased phagocyte accumulation. Mice infected with the Δace2 mutant had significantly greater pulmonary tumor necrosis factor (TNF) levels compared to mice infected with the other two strains (Fig. 10). Infection with the Δace2 mutant also induced higher pulmonary levels of interleukin 6 (IL-6) and monocyte chemoattractant protein 1 (MCP-1) compared to mice infected with the wild-type strain, but not the Δace2∷ace2 complemented strain (Fig. 10). There was no difference in the pulmonary IL-12p70 and interferon-γ levels among mice infected with the different strains, and no IL-10 was detectable in the lungs of any of the mice (data not shown). Collectively, these results indicate that mice infected with the Δace2 mutant had both a higher pulmonary fungal burden and a stronger pro-inflammatory response. It is likely that both of these factors contributed to the accelerated mortality in mice infected with this mutant.
Ace2 is required for normal expression of ppoC, ecm33, and ags3
Three other A. fumigatus strains have been reported to have increased virulence in various murine models of invasive aspergillosis. These strains are a mutant in which ppoA, ppoB and ppoC are silence by RNAi, a Δecm33 null mutant, and a Δags3 null mutant (Maubon et al., 2006; Romano et al., 2006; Tsitsigiannis and Keller, 2006). PpoA, PpoB, and PpoC are fatty acid oxygenases that synthesize prostaglandins and other oxylipins (Tsitsigiannis et al., 2005). Ecm33 is a glycosylphosphatidylinositol-anchored cell wall protein that is required for normal cell wall assembly (Chabane et al., 2006; Romano et al., 2006), Ags3 is an α(1,3) glucan synthase (Maubon et al., 2006).
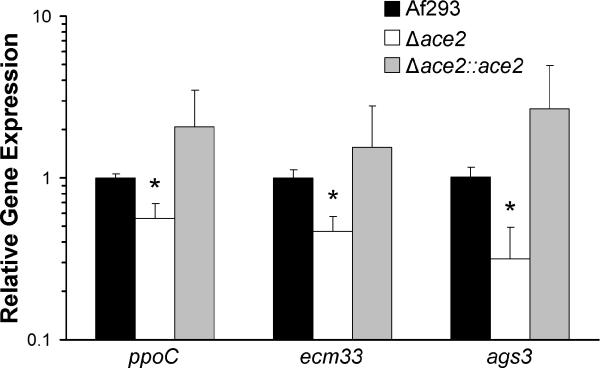
We considered the possibility that Ace2 is required for the normal expression of fatty acid oxygenase genes, as well as ecm33, and/or ags3. We chose to study ppoC as representative fatty acid oxygenase gene because its product is responsible for the greatest amount of prostaglandin synthesis in Aspergillus nidulans (Tsitsigiannis et al., 2005). Real-time PCR was used to assess the relative levels of ppoC, ecm33, and ags3 mRNA in the Δace2 mutant, as well as the wild-type and Δace2∷ace2 complemented strains. We found that all three of these genes were expressed at significantly lower levels in the Δace2 mutant compared to the other two strains (Fig. 11). These results suggest that the increased virulence of the Δace2 mutant was due at least in part to the reduced expression of ppoC, ecm33, and ags3.
Fig. 11.
Reduced expression of ppoC, ecm33, and ags3 in the Δace2 mutant. The indicated strains of A. fumigatus were incubated in Sabouraud broth at 37°C for 24 h, after which RNA was isolated. The expression of ecm33, ags3 and ppoC were measured by real-time PCR using tef1 as the reference gene. Results are the mean ± SD of four biological replicates, each tested in duplicate. P < 0.05 vs. Af293 and the Δace2::ace2 complemented strain.
Conidia of the Δace2 mutant had abnormal cell wall architecture
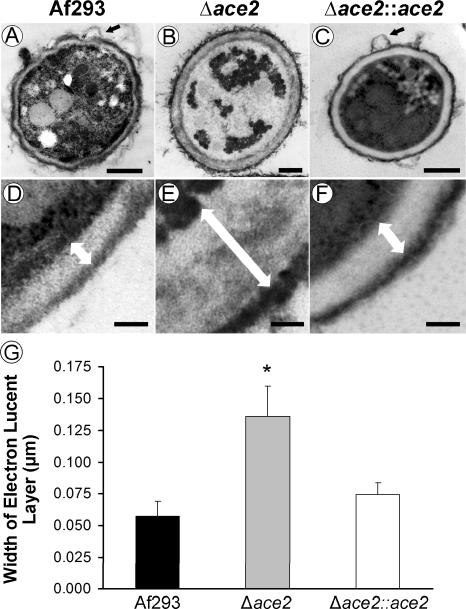
Both Ecm33 and Ags3 govern cell wall structure and composition in A. fumigatus (Chabane et al., 2006; Maubon et al., 2006; Romano et al., 2006). Therefore, we used transmission electron microscopy to investigate the ultrastructure of the cell wall of conidia of the Δace2 mutant and control strains. Consistent with scanning electron microscopy results, the Δace2 mutant lacked the knobby protuberances that were present on the surface of conidia of the wild-type and Δace2∷ace2 complemented strains (Fig. 12A-C). The cell wall of conidia of all three strains had an electron dense outer layer, which likely consists of mannoproteins and pigment, and an electron lucent inner layer, which is likely composed of chitin and 1,3 β-glucan (Fig. 12 D and F) (Chiou et al., 2001; Tronchin et al., 1995). This inner layer was approximately 2-fold thicker in the Δace2 conidia than conidia of the wild-type and Δace2∷ace2 complemented strains (Fig. 12E and G). Because deletion of ecm33 is known to result in increased cell wall chitin content (Chabane et al., 2006) and ecm33 expression was decreased in the Δace2 mutant, it is possible that the increased thickness of the electron lucent layer in the Δace2 conidia was also due to an increased amount of chitin in the cell wall.
Fig. 12.
ace2 is necessary for the normal cell wall architecture of A. fumigatus. (A-F) Transmission electron micrographs of ultrathin sections of conidia of the indicated strains of A. fumigatus. (A-C) Lower power images showing an entire conidium. Small black arrows indicate the knobby protuberances on conidia of the wild-type and Δace2::ace2 complemented strains. These protuberances were not present on Δace2 conidia. Scale bars indicate 500 nm. (D-F) Higher power images showing the thickness of the various layers of the cell wall. White, double-headed arrows indicate the thickness of the electron lucent layer of the cell wall. Scale bars indicate 100 nm. (G) Width of the electron lucent layer of the cell walls of the indicated strains of A. fumigatus. Data are the mean ± SD of 50 conidia of each strain. *P < 0.005 vs. Af293 and the Δace2::ace2 complemented strain.
Discussion
In fungi, C2H2 zinc finger transcription factors constitute a large family of proteins that govern numerous cellular responses. Relatively few transcription factors of A. fumigatus have been studied for their role in virulence. We identified a C2H2 zinc finger transcription factor, Ace2 that governs pigment production, conidiogenesis, resistance to oxidative stress, cell wall organization, and virulence in A. fumigatus. This transcription factor was identified by its homology to Ace2 of C. albicans, C. glabrata, and S. cerevisiae. Orthologs of Ace2 are also present in moulds other than A. fumigatus. However, the function of Ace2 in these organisms has not been reported.
A striking finding was the production of an orange pigment by the Δace2 mutant when it was grown under certain culture conditions. Presumably, the absence of Ace2 results in derepression of the production of this pigment. This pigment was not toxic to endothelial cells under the conditions tested. Therefore, the increased endothelial cell damage caused by the Δace2 mutant is unlikely to be due to the increased production of this pigment.
The Δace2 mutant had increased virulence in the non-neutropenic mouse model of invasive pulmonary aspergillosis, but not in neutropenic mice. This finding suggests that the presence of neutrophils was necessary for the increased virulence of the Δace2 mutant. There are several, non-exclusive possible mechanisms for the increased virulence of this strain. The increased pulmonary fungal burden of mice infected with this strain suggests that it is resistant to being killed by host phagocytes, particularly neutrophils. Although the Δace2 mutant was resistant to oxidant stress induced by hydrogen peroxide, recent evidence suggests that the susceptibility of A. fumigatus to oxidant stress correlates poorly with susceptibility to neutrophil killing in vitro and virulence in mice (Valiante et al., 2008; Lamarre et al., 2007; Lessing et al., 2007). Conidia of the Δace2 mutant also had accelerated germination, a trait that has been linked to increased virulence in mice (Maubon et al., 2006). However, accelerated germination is unlikely to result in resistance to neutrophil killing because swollen conidia and hyphae of A. fumigatus are killed more readily by neutrophils than are resting conidia (Levitz and Diamond, 1985).
It is also probable that the increased host inflammatory response induced by the Δace2 mutant contributed to the more rapid death of mice infected with this strain. The increase in pulmonary MPO and TNF content was proportional to the rise in fungal burden in these animals. These results suggest that the Δace2 mutant may have provoked a Th17 response, which has been suggested to cause increased inflammation, but reduced fungal clearance (Zelante et al., 2007). Alternatively, it possible that the increased inflammatory response in the lungs of mice infected with the Δace2 mutant was induced by the greater pulmonary fungal burden. Interestingly, a Δace2 mutant of C. glabrata is hypervirulent in a mouse model of disseminated candidiasis and this increase in virulence is also associated with increased serum TNF levels (Kamran et al., 2004). However, the C. glabrata Δace2 mutant is only hypervirulent in neutropenic mice (MacCallum et al., 2006), whereas the A. fumigatus Δace2 mutant is only hypervirulent in non-neutropenic mice.
It was notable that the expression of ppoC, ecm33, and ags3 was significantly lower in the Δace2 mutant compared to the wild-type and Δace2∷ace2 complemented strains. We investigated the expression of these genes because ppoA-C silenced mutants, as well as Δecm33 and Δags3 strains have increased virulence in various mouse models of invasive aspergillosis (Maubon et al., 2006; Romano et al., 2006; Tsitsigiannis and Keller, 2006). Also, the phenotype of the Δace2 mutant shared some additional similarities with the ΔppoC, Δecm33, and Δags3 mutants of A. fumigatus.
A mutant in which ppoA, ppoB, and ppoC are silenced by RNAi has increased virulence when inoculated intranasally into neutropenic mice that had also received a single dose of cortisone acetate (Tsitsigiannis et al., 2005). In contrast, the Δace2 mutant did not have increased virulence when tested in a similar model. It is possible that the hypervirulence of the triple-silenced ppoA-C mutant was magnified because there was reduced expression of three different fatty acid oxygenase genes. Recently, it was reported that a ΔppoC mutant has wild-type levels of virulence after being inoculated intranasally into either neutropenic mice, or non-neutropenic mice that were immunosuppressed with cortisone acetate alone (Dagenais et al., 2008). Therefore, reduced expression of ppoC alone is unlikely to be the explanation for the increased virulence of the Δace2 mutant. Also, because the expression of ppoC was reduced by only 43% in the Δace2 mutant compared to the wild-type strain, it is likely that expression of ppoC is governed by other factors, in addition to Ace2. Whether Ace2 is also required for maximal expression ppoA or ppoB is not yet known.
Like the Δace2 mutant, a ΔppoC mutant has reduced conidiation, reflecting the probable role of oxylipins as sporulation signals (Dagenais et al., 2008). Whether the ΔppoC mutant has dysmorphic conidiophores similar to the Δace2 mutant has not been reported. Interestingly, conidia from both ΔppoC and Δace2 mutants are larger than wild-type conidia and have accelerated germination. We found that the accelerated germination of the Δace2 conidia did not appear to be the result of abnormal dormancy. Instead, it was likely due a more rapid response to environmental germination signals. This more rapid response occurred in both normal and aberrantly shaped conidia. In S. cerevisiae, Ace2 is required for G1 delay of daughter cells during budding (Laabs et al., 2003). It is therefore possible that the more rapid germination of the Δace2 conidia was due to an abnormally short G1 period in these cells.
A Δecm33 mutant has increased virulence when inoculated intravenously into neutropenic mice (Romano et al., 2006). This model of disseminated aspergillosis is quite different from the model of invasive pulmonary aspergillosis used in the current studies. After intravenous inoculation, A. fumigatus infects virtually all organs, especially the kidney. In contrast, intranasal inoculation results in infection that is limited to the lungs and possibly the sinuses; there is little to no dissemination to other organs. Our finding that ecm33 expression is reduced in the Δace2 mutant suggests that this mutant may also be hypervirulent upon intravenous inoculation.
Ecm33 governs cell wall structure (Chabane et al., 2006; Romano et al., 2006; Martinez-Lopez et al., 2004; Pardo et al., 2004), and expression of ecm33 was reduced in the A. fumigatus Δace2 mutant. ECM33 expression is also reduced in a C. albicans Δ/Δace2 mutant (Mulhern et al., 2006). Conidia of the A. fumigatus Δace2 mutant had increased thickness of electron lucent layer of the cell wall. This abnormality is likely due to increased chitin content and is consistent with the effects of reduced expression of ecm33 (Chabane et al., 2006). Increased thickness of this layer has also been found in blastospores of a C. albicans Δ/Δecm33 mutants (Martinez-Lopez et al., 2006). In addition, like the Δace2 mutant, Δecm33 mutants have large conidia that have accelerated germination (Chabane et al., 2006; Romano et al., 2006). It is probable that both the abnormal cell wall and the accelerated germination of the Δace2 mutant contributed to its increased virulence.
A Δags3 mutant has enhanced virulence when inoculated intranasally into non-neutropenic mice that are immunosuppressed with cortisone acetate (Maubon et al., 2006). Because this model is similar to the non-neutropenic model used in the current studies, it is likely that the mechanism of increased virulence of the Δags3 mutant is similar to that of the Δace2 mutant. Conidia of the Δags3 mutant share some features with those of the Δace2 mutant. They are relatively smooth and lack the knobs that are present on wild-type conidia. In addition, Δags3 conidia have accelerated germination. However, although Δags3 conidia have an abnormally thick cell wall, this increased thickness is due to an increase in the electron-dense outer layer, rather than the electron-lucent inner layer that was present in the Δace2 mutant (Maubon et al., 2006). A common feature of Δace2 mutants of S. cerevisiae, S. pombe, C. albicans and C. glabrata is reduced glucanase expression. This decreased glucanase activity results in increased cellular α glucan content, delayed separation of mother and daughter cells, and a clumping phenotype (Mulhern et al., 2006; Alonso-Nunez et al., 2005; Bidlingmaier et al., 2001). The A. fumigatus Δace2 conidia did not have any evidence of a cell separation defect, such as abnormal clumping or formation of chains of conidia. The absence of these findings may have been due in part to the reduced expression of ags3, which would be predicted to result in a reduction in cellular α glucan content. Thus, Ace2 may have a somewhat different function in filamentous fungi compared to yeast.
While the phenotypes of the Δecm33, Δags3, and ppoA-C silenced strains of A. fumigatus share some features with the Δace2 mutant, none of them are identical. For example, none of these mutants has been reported to produce an orange pigment. Also, while ΔppoC and Δags3 conidia are resistant to oxidative stress (Dagenais et al., 2008; Maubon et al., 2006), only Δace2 hyphae, but not Δace2 conidia, are resistant to this stress. Thus, the phenotype of the Δace2 mutant is probably the combined result of the abnormal expression of multiple genes.
In summary, our results indicate that the C2H2 transcription factor, Ace2 governs the multiple processes in A. fumigatus including conidiation, pigment production, oxidative stress, cell wall architecture, and virulence. Ace2 is also required for the normal expression of ppoC, ecm33, and ags3. However, Ace2 undoubtedly governs the expression of additional genes. Transcription profiling experiments to identify these Ace2 target genes are currently in progress.
Experimental procedures
Organisms and culture conditions
A. fumigatus strain Af293 (a generous gift from P. T. Magee, University of Minnesota, St. Paul, MN) was used as the wild-type strain in all experiments. To obtain conidia, the strains were grown on Sabouraud agar (Difco) for at 37°C for 1 week, unless otherwise specified. The conidia were harvested by rinsing with PBS containing 0.1% Tween 80 (Sigma-Aldrich). Germlings were prepared by adding freshly harvested conidia in Sabouraud broth to 150mm petri dishes and incubating them at 37°C in 5% CO2 for 6 to7 h (Lopes-Bezerra and Filler, 2004). The incubation time for each strain was adjusted so that all of the germlings were of similar length.
RNA isolation and real-time PCR
For RNA isolation, conidia were inoculated into Sabouraud broth at a final concentration of 105 conidia per ml, and grown in shaking culture at 37°C. At various time points, the organisms were harvested by filtration. They were disrupted using a bead beater, after which the total RNA was extracted using Tri reagent (Ambion) following the manufacturer's instructions. After treating the samples with DNase (Ambion), cDNA was generated using the Retroscript kit (Ambion). Real-time PCR was performed using an ABI 7000 thermocycler (Applied Biosystems) and the reaction products were detected with SYBR Green (Qiagen). The expression of each gene was determined by the ΔΔCt method, using tef1 as the reference gene (Gravelat et al., 2008; Sheppard et al., 2005). The primers used to detect ace2, ppoC, ecm33, ags3, and tef1 are listed in Table 1.
Table 1.
PCR primers used in the experiments
| Primer name | Primer sequence (5' to 3') |
|---|---|
| ACE-F4 | GAACCATGGTCGTTGAGTTTT |
| ACE-F3 | TCCTGTGTGAAATTGTTATCCGCT ATGTTGTCGATAACGCTCGTG |
| ACE-F2 | GTCGTGACTGGGAAAACCCTGGCG TCTACCTTCGAACCCGGAAT |
| ACE-F1 | AATCCAAGCTGCTCTAGCAA |
| HYG-F | CGCCAGGGTTTTCCCAGTCACGAC |
| HYG-R | AGCGGATAACAATTTCACACAGGA |
| HY | GGATGCCTCCGCTCGAAGTA |
| YG | CGTTGCAAGACCTGCCTGAA |
| ACE-VER1 | AGCTGGAGTCTCAACTTTCCC |
| ACE-VER2 | TATTGACGCATGAGACGTGG |
| HY-VER | GAGCTCACGAGTTCGTCACA |
| ACEREV-F | ACTGGCGGCCGCGTTTAAACAAGACGTAGGTGAGGTGGCACTT |
| ACEREV-R | ATCGACTAGTGTTTAAACTCCGTAAGTTCTATTGTGCCC |
| RTAGS3-F | CTTTGGAAGATGGTCCTGGT |
| RTAGS3-R | ACAAATCTATCGGCCTCCAC |
| RTECM33-F | CACCAAGGGTGTCAATGAAG |
| RTECM33-R | TTCAGCGAGATACCGTTCAG |
| RTPPOC-F | TGCTGAACAAAGAGGAGTGG |
| RTPPOC-R | ATCACCGGAGTAAGATTGCC |
| RTTEF1-F | GTGACTCCAAGAACGATCCC |
| RTTEF1-R | AGAACTTGCAAGCAATGTGG |
| RTACE-F | GACAATCACGAGAAGGCTCA |
| RTACE-R | GGTTTCGTCAAGGTGGAACT |
Strain Construction
The split-marker approach was used to disrupt the entire protein coding region of Ace2 (Afu3g11250) in A. fumigatus as described previously (Sheppard et al., 2005; Catlett et al., 2003). In short, a DNA fragment encompassing 1500 bp upstream of the ace2 protein coding region was amplified from A. fumigatus Af293 genomic DNA by PCR using primers ACE-F4 and ACE-F3 (Table 1). Next, the 5' region of the hygromycin resistance cassette was amplified from plasmid pAN7-1 using primers HYG-R and HY. Fusion PCR using primers ACE-F4 and HY was used to combine the upstream ace2 flanking region with the 5' region of the hygromycin resistance cassette. Similarly, a DNA fragment containing 1600 bp downstream of the ace2 protein coding region was amplified using primers ACE-F2 and ACE-F1. The 3' region of the hygromycin resistance cassette was amplified from plasmid pAN7-1 using primers HYG-F and YG, and the resulting fragment was combined with the downstream ace2 flanking region by fusion PCR with primers ACE-F1 and YG. The two fusion PCR products containing the upstream and downstream flanking regions of ace2 were used to co-transform protoplasts of strain Af293 (Bhabhra et al., 2004). Hygromycin-resistant transformants were screened for disruption of ace2 by colony PCR using the primers ACE-VER1, ACE -VER2 and HY-VER (Table 1). The clone of the Δace2 mutant that was used in the current experiments was analyzed by Southern blotting to confirm homologous integration of the disruption cassette and to assure that no additional fragments were integrated into the genome during transformation. The Southern blot was probed with a 412bp fragment of the hygromycin resistance cassette. (data not shown).
To complement the Δace2 mutant strain with a wild-type allele of ace2, the entire ace2 open reading frame, along with 2000 bp upstream and 1000 bp downstream flanking sequence were PCR amplified from genomic DNA of Af293 using primers ACEREV-F and ACEREV-R (Table 1). This fragment was cloned into plasmid p402, which carries the phleomycin resistance gene (Richie et al., 2007). The resulting plasmid was digested with PmlI, which cut it at nucleotide 872 of the A. fumigatus insert. The linearized plasmid was used to transform protoplasts of the Δace2 mutant strain. Proper integration of this plasmid upstream of the disrupted ace2 locus of phleomycin-resistant transformants was verified by PCR using primers ACE-VER1, ACE-VER2 and HY-VER. In addition, the expression of ace2 in several different clones of the Δace2∷ace2 complemented strain was analyzed by real-time PCR using primers RTACE-F and RTACE-R (Table 1). Clone Z, which expressed ace2 at similar levels to the wild-type strain was selected for use in the experiments.
Electron microscopy
For scanning electron microscopy, conidia of the different strains of A. fumigatus were incubated for 5 days at 28°C in separate wells of a 24-well tissue culture plate containing Sabouraud agar. The resulting colonies were fixed for 1 h in 2.5% glutaraldehyde and 4% paraformaldehyde in 0.1M cacodylate buffer pH 7.2, washed three times with the same buffer, and post-fixed with 1% osmium tetroxide, 0.8% potassium ferrocyanide and 5mM CaCl2 in 0.1M cacodylate buffer. Next, they were dehydrated in a graded ethanol series and critical point-dried with CO2. The specimens were coated with ~3 nm of gold in a Balzer's apparatus, and imaged using a JEOL JMS-6340 F field emission scanning electron microscope.
For transmission electron microscopy, samples from A. fumigatus strains cultured on Sabouraud agar were fixed as in the scanning electron microscopy experiments. The specimens were then washed in cacodylate buffer, dehydrated in a graded series of ethanol, and embedded in Spurr resin. Ultrathin sections were cut using an ultramicrotome (Supernova; Reichert), stained with uranyl acetate and lead citrate, and imaged with a Zeiss 900 transmission electron microscope. To determine the cell wall thickness of conidia of the various strains, digitally captured micrographs were processed with Adobe Photoshop CS2 for gamma correction, and cell walls were measured using SemAfore software for microscopy from JEOL. The cell wall thickness of 50 conidia of each strain was measured.
Characterization of conidial phenotype and hyphal radial growth
To determine conidial production and radial growth rate of the different strains, 5 × 103 conidia were added in the center of 100 mm Petri dishes containing Sabouraud, Aspergillus minimal medium, or YPD agar. The diameter of the colony in each dish was measured daily (Bhabhra et al., 2004). After one week of growth, the conidia were harvested from the plates in a standardized manner and then counted using a hemacytometer. The experiments were performed in triplicate on three separate occasions.
The relative size of conidia from the various strains was determined by flow cytometry. Freshly harvested conidia in PBS+0.1% Tween 80 were sonicated briefly, after which 105 cells per strain were analyzed for light sidescatter using a Becton Dickinson Facscalibur flow cytometer.
The time course of germination was determined by adding 5 × 105 conidia suspended in Sabouraud broth to 24-well tissue culture plates coated with gelatin. At half hour time intervals, individual wells were fixed with 4% of paraformaldehyde. At least 300 cells of each strain were examined using an inverted microscope and the number of conidia with visible germ tubes was determined. The experiment was performed in triplicate.
The percentage of conidia that were metabolically active was determined by staining with MitoTracker Red CM-H2 XRos (Invitrogen). Freshly harvested conidia were suspended in Sabouraud broth at 3 × 106 conidia/ml. They were added to plastic 8-well chamber slides (Nunc) and incubated in a humidified chamber at 37°C in 5% CO2. At various time points, the medium was gently aspirated from the wells and replaced with fresh Sabouraud broth containing 50 nM MitoTracker Red. After a 20 min incubation, the cells were fixed with 3.7% formaldehyde, the chambers were detached from the slides, and coverslips were added. The cells were then viewed by differential interference contrast and confocal microscopy.
The viability of the various conidia during prolonged storage was determined by a minor modification of the method of Fillinger et al. (Fillinger et al., 2001). Briefly, duplicate 15 ml centrifuge tubes containing 2 × 108 conidia in 2 ml PBS plus 0.1% Tween 80 were stored at room temperature in the dark. At biweekly intervals, each tube was sonicated briefly, after which a 100 μl aliquot was removed and quantitatively cultured on Sabouraud agar.
Susceptibility to environmental stress
Conidia and germlings of the different strains were tested for their susceptibility to environmental stress by the microdilution method. To measure susceptibility to oxidant stress, 103 cells were added to individual wells of a 96-well microtiter plate containing 2-fold serial dilutions of hydrogen peroxide (0.4 to 12.5 mM for germ tubes and 12.5 to 400 mM for conidia) in Sabouraud broth. After 30, 60, and 90 min, a 10 μl aliquot was withdrawn from each well, plated onto Sabouraud agar, and incubated at 37°C for 2 days. The susceptibility of the strains to other stressors was determined by adding 5×103 conidia or germlings in Sabouraud broth to each well of a 96-well microtiter plate containing 2-fold dilutions of SDS (0.0002 to 0.2%), NaCl (0.002 to 2 M), calcofluor white (Fluorescent Brightener 28; Sigma-Aldrich; 5 to 5000 μg/ml), and Congo red (Sigma-Aldrich; 5 to 5000 μg/ml). The plates were examined after incubation at 37°C for 2 days. The minimal inhibitory concentration of each compound was defined as the concentration that caused at least an 80% reduction in growth compared to organisms grown in the absence of the compound. Each experiment was repeated 3 times.
Endothelial cell damage assay
Endothelial cells were harvested from human umbilical cord veins and grown in M199 medium (Gibco) containing 10% fetal bovine serum (Gemini Bio-Products), 10% bovine calf serum (Gemini Bio-Products), and 2 mM L-glutamine with penicillin and streptomycin (Irvine Scientific) in 5% CO2 at 37°C as previously described (Lopes-Bezerra and Filler, 2004; Jaffe et al., 1973). The capacity of the different strains to cause endothelial cell damage was determined as outlined previously (Lopes-Bezerra and Filler, 2004). Endothelial cells were grown to confluence in a 24-well tissue culture plate and then loaded with 51Cr overnight. After removing the unincorporated 51Cr by rinsing, the endothelial cells were infected for 24 h with 5 × 105 germ tubes per well in M199 medium plus serum. Next, the medium above the cells was collected and the endothelial cells were lysed with 6N NaOH. The lysed cells were aspirated and the wells were rinsed twice with RadiacWash (Biodex Medical Systems, Inc.). These rinses were added to the cell lysates. The amount of 51Cr in the medium and in the cell lysates was determined by gamma counting. To measure the spontaneous release of 51Cr, uninfected endothelial cells exposed to medium alone were processed in parallel. The percent specific release of 51Cr was calculated using the following formula: (experimental release - spontaneous release) / (total incorporation - spontaneous release) × 100. Each experiment was performed in triplicate three different times.
To determine if the orange pigment produced by the Δace2 mutant contributed to endothelial cell damage, Af293, the Δace2 mutant, and the Δace2∷ace2 complemented strain were grown on Petri dishes containing Sabouraud agar for 1 to 2 weeks at 37°C until the agar underneath the Δace2 mutant had turned orange. Each Petri dish was rinsed by passing 5 ml of M-199 medium plus serum over the dish 20 times. The rinse from each Petri dish was then filter sterilized and an aliquot was incubated with endothelial cells for 24 h, after which the specific release of 51Cr was determined as described above.
Murine models of invasive pulmonary aspergillosis
The virulence of the various A. fumigatus strains was tested in two different murine models of invasive pulmonary aspergillosis. In the first model, the mice were immunosuppressed by administering 10 mg of cortisone acetate (Sigma-Aldrich) subcutaneously every other day, starting on day -4 relative to infection and finishing on day +4, for a total of 5 doses. In the second model, the mice were immunosuppressed with cortisone acetate, 250mg/kg subcutaneously on days -2 and +3, and cyclophosphamide (Western Medical Supply), 250mg/kg intraperitoneally on day -2 and 200 mg/kg on day +3 (Sheppard et al., 2004). Tetracycline was added to the drinking water of mice treated with cortisone acetate alone, and ceftazidime was administrated intraperitoneally each day to the mice immunosuppressed with cortisone and cyclophosphamide while they were neutropenic to prevent bacterial infections. To induce infection, all animals were anesthetized with isoflurane and infected intranasally with 25 μl of a conidial suspension in PBS plus 0.1% Tween 80. The inoculum was 104 conidia for mice treated with cortisone acetate alone, and 5×105 conidia for the animals treated with cortisone acetate and cyclophosphamide. These inocula were verified by quantitative culture.
The virulence of the various mutants was first determined in survival experiments, in which 8 mice were inoculated with each strain of A. fumigatus. An additional group of 8 control mice were immunosuppressed and then PBS plus 0.1% Tween 80 without conidia was administered intranasally as a negative control. The survival experiments were performed twice and the results from both experiments were combined. The animal studies were approved by the Institutional Animal Use and Care Committee, and performed according to the National Institutes of Health guidelines for animal housing and care.
Pulmonary fungal burden, MPO, and cytokine analysis
To determine the fungal burden, MPO and cytokine content during infection, mice were immunosuppressed with cortisone acetate alone and inoculated intranasally as in the survival experiments. After 2 and 4 days of infection, 7 to 8 mice per strain of A. fumigatus were sacrificed, after which their lungs were harvested, weighed, and then homogenized in 1 ml of PBS containing protease inhibitor cocktail (Sigma-Aldrich). The homogenate was clarified by centrifugation and the resulting supernatant was stored in aliquots at -70°C for subsequent analysis.
The galactomannan concentration of the lungs was used to estimate the fungal burden. It was determined using the Platelia Aspergillus enzyme immunoassay (BioRad) according to a minor modification of the manufacturer's instructions as reported previously (Sheppard et al., 2006). Briefly, a 15 μl sample of each pulmonary homogenate was 1:10 diluted in ultra-pure water and processed according to the manufacturer's instructions. In order to determine the relative galactomannan content per gram of lung tissue, the resulting optical densities were compared with a standard curve made up of serial dilutions of a pool of lung homogenates from five heavily infected immunosuppressed mice (7 days after intranasal infection with strain Af293). In preliminary experiments, we determined that the Δace2 mutant released a similar amount of GM into the medium when grown in RPMI 1640 medium as compared to strain Af293.
The MPO content of the lungs was measured by enzyme immunoassay (Cell Sciences), and the pulmonary cytokine content was measured using the mouse inflammation cytometric bead array kit (BD Biosciences).
Statistical analysis
The results of the in vitro experiments were compared by Analysis of Variance. The survival data were analyzed using the Log-Rank test, and the pulmonary fungal burden, MPO, and cytokine data were analyzed with the Wilcoxon Rank Sum test. P values of ≤ 0.05 were considered to be significant.
Acknowledgements
We thank the perinatal nurses at the Harbor-UCLA Medical Center Pediatric Clinical Research Center for collection of umbilical cords. The Olympus phase-contrast microscope used for these studies was generously donated by Toyota U.S.A. This work was supported in part by grants M01RR00425, R21AI064511, and R01AI073829, as well as contract no. N01-AI-30041 from the National Institutes of Health, U.S.A. S.R. was supported in part by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil). D.C.S was supported in part by a Clinician-Scientist award and Operating Grant from the Canadian Institutes of Health.
References
- Alonso-Nunez ML, An H, Martin-Cuadrado AB, Mehta S, Petit C, Sipiczki M, et al. Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe. Mol Biol Cell. 2005;16:2003–2017. doi: 10.1091/mbc.E04-06-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhabhra R, Miley MD, Mylonakis E, Boettner D, Fortwendel J, Panepinto JC, et al. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect Immun. 2004;72:4731–4740. doi: 10.1128/IAI.72.8.4731-4740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmaier S, Weiss EL, Seidel C, Drubin DG, Snyder M. The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:2449–2462. doi: 10.1128/MCB.21.7.2449-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos A, Wever R, Roos D. Characterization and quantification of the peroxidase in human monocytes. Biochim Biophys Acta. 1978;525:37–44. doi: 10.1016/0005-2744(78)90197-3. [DOI] [PubMed] [Google Scholar]
- Catlett NL, Lee BN, Yoder OC, Turgeon BG. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet Newsl. 2003;50:9–11. [Google Scholar]
- Chabane S, Sarfati J, Ibrahim-Granet O, Du C, Schmidt C, Mouyna I, et al. Glycosylphosphatidylinositol-anchored Ecm33p influences conidial cell wall biosynthesis in Aspergillus fumigatus. Appl Environ Microbiol. 2006;72:3259–3267. doi: 10.1128/AEM.72.5.3259-3267.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang LY, Sheppard DC, Gravelat FN, Patterson TF, Filler SG. Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease. Infect Immun. 2008;76:3429–3438. doi: 10.1128/IAI.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou CC, Mavrogiorgos N, Tillem E, Hector R, Walsh TJ. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob Agents Chemother. 2001;45:3310–3321. doi: 10.1128/AAC.45.12.3310-3321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais TRT, Chung D, Giles SS, Hull CM, Andes D, Keller NP. Defects in conidiophore development and conidium-macrophage interactions in a dioxygenase mutant of Aspergillus fumigatus. Infect. Immun. 2008;76:3214–3220. doi: 10.1128/IAI.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger S, Chaveroche MK, van Dijck P, de Vries R, Ruijter G, Thevelein J, d'Enfert C. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology. 2001;147:1851–1862. doi: 10.1099/00221287-147-7-1851. [DOI] [PubMed] [Google Scholar]
- Fraser RS. Pulmonary aspergillosis: pathologic and pathogenetic features. Pathol Annu. 1993;28(Pt 1):231–277. [PubMed] [Google Scholar]
- Glasgow SC, Ramachandran S, Blackwell TS, Mohanakumar T, Chapman WC. Interleukin-1beta is the primary initiator of pulmonary inflammation following liver injury in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L491–496. doi: 10.1152/ajplung.00009.2007. [DOI] [PubMed] [Google Scholar]
- Gravelat FN, Doedt T, Chiang LY, Liu H, Filler SG, Patterson TF, Sheppard DC. In vivo analysis of Aspergillus fumigatus developmental gene expression determined by real-time reverse transcription-PCR. Infect Immun. 2008;76:3632–3639. doi: 10.1128/IAI.01483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran M, Calcagno AM, Findon H, Bignell E, Jones MD, Warn P, et al. Inactivation of transcription factor gene ACE2 in the fungal pathogen Candida glabrata results in hypervirulence. Eukaryot Cell. 2004;3:546–552. doi: 10.1128/EC.3.2.546-552.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MT, MacCallum DM, Clancy SD, Odds FC, Brown AJ, Butler G. The Candida albicans CaACE2 gene affects morphogenesis, adherence and virulence. Mol Microbiol. 2004;53:969–983. doi: 10.1111/j.1365-2958.2004.04185.x. [DOI] [PubMed] [Google Scholar]
- Laabs TL, Markwardt DD, Slattery MG, Newcomb LL, Stillman DJ, Heideman W. ACE2 is required for daughter cell-specific G1 delay in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2003;100:10275–10280. doi: 10.1073/pnas.1833999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre C, Ibrahim-Granet O, Du C, Calderone R, Latge JP. Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet Biol. 2007;44:682–690. doi: 10.1016/j.fgb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessing F, Kniemeyer O, Wozniok I, Loeffler J, Kurzai O, Haertl A, Brakhage AA. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryot Cell. 2007;6:2290–2302. doi: 10.1128/EC.00267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz SM, Diamond RD. Mechanisms of resistance of Aspergillus fumigatus conidia to killing by neutrophils in vitro. J Infect Dis. 1985;152:33–42. doi: 10.1093/infdis/152.1.33. [DOI] [PubMed] [Google Scholar]
- Lopes-Bezerra LM, Filler SG. Interactions of Aspergillus fumigatus with endothelial cells: internalization, injury, and stimulation of tissue factor activity. Blood. 2004;103:2143–2149. doi: 10.1182/blood-2003-06-2186. [DOI] [PubMed] [Google Scholar]
- MacCallum DM, Findon H, Kenny CC, Butler G, Haynes K, Odds FC. Different consequences of ACE2 and SWI5 gene disruptions for virulence of pathogenic and nonpathogenic yeasts. Infect Immun. 2006;74:5244–5248. doi: 10.1128/IAI.00817-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- Martinez-Lopez R, Monteoliva L, Diez-Orejas R, Nombela C, Gil C. The GPI-anchored protein CaEcm 33p is required for cell wall integrity, morphogenesis and virulence in Candida albicans. Microbiology. 2004;150:3341–3354. doi: 10.1099/mic.0.27320-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Lopez R, Park H, Myers CL, Gil C, Filler SG. Candida albicans Ecm33p is important for normal cell wall architecture and interactions with host cells. Eukaryot Cell. 2006;5:140–147. doi: 10.1128/EC.5.1.140-147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubon D, Park S, Tanguy M, Huerre M, Schmitt C, Prevost MC, et al. AGS3, an alpha(1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet Biol. 2006;43:366–375. doi: 10.1016/j.fgb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Morgan J, Wannemuehler KA, Marr KA, Hadley S, Kontoyiannis DP, Walsh TJ, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43(Suppl 1):49–58. doi: 10.1080/13693780400020113. [DOI] [PubMed] [Google Scholar]
- Mulhern SM, Logue ME, Butler G. Candida albicans transcription factor Ace2 regulates metabolism and is required for filamentation in hypoxic conditions. Eukaryot Cell. 2006;5:2001–2013. doi: 10.1128/EC.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M, Monteoliva L, Vazquez P, Martinez R, Molero G, Nombela C, Gil C. PST1 and ECM33 encode two yeast cell surface GPI proteins important for cell wall integrity. Microbiology. 2004;150:4157–4170. doi: 10.1099/mic.0.26924-0. [DOI] [PubMed] [Google Scholar]
- Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 2000;79:250–260. doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Pegues CF, Daar ES, Murthy AR. The epidemiology of invasive pulmonary aspergillosis at a large teaching hospital. Infect Control Hosp Epidemiol. 2001;22:370–374. doi: 10.1086/501915. [DOI] [PubMed] [Google Scholar]
- Richie DL, Fuller KK, Fortwendel J, Miley MD, McCarthy JW, Feldmesser M, et al. Unexpected link between metal ion deficiency and autophagy in Aspergillus fumigatus. Eukaryot Cell. 2007;6:2437–2447. doi: 10.1128/EC.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano J, Nimrod G, Ben-Tal N, Shadkchan Y, Baruch K, Sharon H, Osherov N. Disruption of the Aspergillus fumigatus ECM33 homologue results in rapid conidial germination, antifungal resistance and hypervirulence. Microbiology. 2006;152:1919–1928. doi: 10.1099/mic.0.28936-0. [DOI] [PubMed] [Google Scholar]
- Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE, Jr., Ibrahim AS. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2004;48:1908–1911. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DC, Marr KA, Fredricks DN, Chiang LY, Doedt T, Filler SG. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin Microbiol Infect. 2006;12:376–380. doi: 10.1111/j.1469-0691.2005.01349.x. [DOI] [PubMed] [Google Scholar]
- Sheppard DC, Doedt T, Chiang LY, Kim HS, Chen D, Nierman WC, Filler SG. The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol Biol Cell. 2005;16:5866–5879. doi: 10.1091/mbc.E05-07-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G, Bouchara JP, Ferron M, Larcher G, Chabasse D. Cell surface properties of Aspergillus fumigatus conidia: correlation between adherence, agglutination, and rearrangements of the cell wall. Can J Microbiol. 1995;41:714–721. doi: 10.1139/m95-098. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Keller NP. Oxylipins act as determinants of natural product biosynthesis and seed colonization in Aspergillus nidulans. Mol Microbiol. 2006;59:882–892. doi: 10.1111/j.1365-2958.2005.05000.x. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Bok JW, Andes D, Nielsen KF, Frisvad JC, Keller NP. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect Immun. 2005;73:4548–4559. doi: 10.1128/IAI.73.8.4548-4559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E. Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol. 2007;179:7079–7086. doi: 10.4049/jimmunol.179.10.7079. [DOI] [PubMed] [Google Scholar]
- Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- Valiante V, Heinekamp T, Jain R, Hartl A, Brakhage AA. The mitogen-activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet Biol. 2008;45:618–627. doi: 10.1016/j.fgb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]