Abstract
Study Objective:
The best characterized marker of sleep homeostasis is the amount of slow wave activity (SWA, 0.5–4 Hz) during NREM sleep. SWA increases as a function of previous waking time and declines during sleep, but the underlying mechanisms remain unclear. We have suggested that SWA homeostasis is linked to synaptic potentiation associated with learning during wakefulness. Indeed, studies in rodents and humans found that SWA increases after manipulations that presumably enhance synaptic strength, but the evidence remains indirect. Here we trained rats in skilled reaching, a task known to elicit long-term potentiation in the trained motor cortex, and immediately after learning measured SWA and cortical protein levels of c-fos and Arc, 2 activity-dependent genes involved in motor learning.
Design:
Intracortical local field potential recordings and training on reaching task.
Setting:
Basic sleep research laboratory.
Patients or Participants:
Long Evans adult male rats.
Interventions:
N/A
Measurements and Results:
SWA increased post-training in the trained cortex (the frontal cortex contralateral to the limb used to learn the task), with smaller or no increase in other cortical areas. This increase was reversible within 1 hour, specific to NREM sleep, and positively correlated with changes in performance during the prior training session, suggesting that it reflects plasticity and not just motor activity. Fos and Arc levels were higher in the trained relative to untrained motor cortex immediately after training, but this asymmetry was no longer present after 1 hour of sleep.
Conclusion:
Learning to reach specifically affects gene expression in the trained motor cortex and, in the same area, increases sleep need as measured by a local change in SWA.
Citation:
Hanlon EC; Faraguna U; Vyazovskiy VV; Tononi G; Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. SLEEP 2009;32(6):719-729.
Keywords: Rat, sleep, learning, Fos, Arc, motor cortex
SLEEP IS PRESENT AND HOMEOSTATICALLY REGULATED IN ALL ANIMAL SPECIES CAREFULLY STUDIED SO FAR. IN MAMMALS, THE BEST CHARACTERIZED marker of sleep homeostasis is slow wave activity (SWA), the power density in the electroencephalogram (EEG) between 0.5 and 4 Hz during NREM sleep. SWA is high at sleep onset and declines during sleep, suggesting that it may reflect the accumulation of sleep pressure as a function of duration and/or intensity of prior waking.1,2 Why and how SWA should reflect sleep homeostasis, however, is still unclear.
We hypothesized that SWA is high at sleep onset because it reflects the occurrence, during waking, of widespread synaptic potentiation in cortical and subcortical areas.3,4 This increase would be detrimental in the long term, because stronger synapses need more energy, space, and cellular supplies, and may lead to saturation of the ability to learn. Sleep would thus be crucial to re-normalize synaptic strength to an energetically sustainable level. Molecular and electrophysiological markers of synaptic potentiation increase after waking in rat cortex and hippocampus, while markers of synaptic depression do so after sleep.5,6 Moreover, in rats and humans, procedures presumably leading to synaptic potentiation or depression result in SWA increases and decreases, respectively.7–12 More specifically, a study in humans using high-density EEG found that performing a visuomotor learning task produced a local increase in SWA during subsequent sleep.7 The increase was restricted to the right parietal cortical areas presumably modified by learning.13 Moreover, it was specific for NREM sleep, reversible within the first 90 minutes of sleep, and correlated with the improvement in performance after sleep.7 However, there is no direct evidence that the motor adaptation task used in that study activates neurons in parietal cortex, nor that it causes synaptic potentiation specifically in that region.
Previous studies have suggested that activity-dependent genes activated during a specific waking experience may be reactivated during sleep, perhaps to potentiate the same synapses previously engaged during waking.14 Ribeiro and colleagues found that the exposure to a new environment for 3 hours15 results in the immediate cortical and hippocampal induction, during waking, of plasticity-related gene zif-268,16 followed by its downregulation during NREM sleep, and then again by its increased expression during REM sleep (relative to NREM sleep). Intriguingly ∼ 3 min of NREM sleep were enough to downregulate waking-induced zif-268 expression, and ∼ 2 min of REM sleep were sufficient to detect its selective reinduction, even if both NREM and REM rats were awake during the last 30 min before sacrifice. Another study17 recently found, using fluorescent in situ hybridization, that the number of hippocampal CA1 neurons showing induction of Arc and/or Homer1a was similar during “rest” periods before and after the exploration of a new environment. However, more cells were active during both exploration and post-task rest than during exploration and pre-task rest. Based on these findings, it has been suggested that the induction of immediate early genes is one of the mechanisms by which sleep consolidates waking experience, perhaps by potentiating the same synapses previously engaged during waking.14 While intriguing, this hypothesis remains untested. Specifically, the first study left unclear whether NREM and REM rats shared the same sleep/waking history (similar intensity of waking, or amount of NREM sleep, which can both affect gene induction10,18), and the second study did not test whether the rest period was actually quiet waking or sleep. Moreover, both studies measured only mRNA levels, but proteins, rather than DNA or RNA, carry out most of the cellular functions.
Here we used the single pellet reaching task, which has been demonstrated to increase the strength of the horizontal connections in layers II-III of the trained motor cortex, and saturate the ability of this area to undergo further long-term potentiation.19–21 We asked whether SWA shows a local increase in the trained motor cortex after learning to reach. Moreover, we examined whether skilled reaching increases the expression of Arc and Fos, 2 activity-dependent proteins involved in motor learning,22–24 and whether this activation is specific for the trained motor area relative to other cortical areas.
MATERIAL AND METHODS
Animals
Long Evans rats (Charles River, Wilmington, MA) 3-4 months of age were maintained on a 12hr:12hr light-dark cycle (lights on at 10:00; room temperature 22-24°C) and were given food (Harlan Teklad Rat Diet, Madison, WI) and water ad libitum prior to habituation to the behavioral paradigm. All animal procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and facilities were reviewed and approved by the IACUC of the University of Wisconsin-Madison, and were inspected and accredited by AAALAC.
1. Electrophysiological Experiments: Local Field Potentials for Regional Analysis of SWA
Under deep isoflurane anesthesia (1.5% to 2% volume), rats were implanted for chronic electroencephalographic (EEG) recordings with bipolar concentric local field potential (LFP) electrodes (inter-electrode distance ranged from 0.5–1 mm; PlasticsOne Inc., Wallingford, CT; Rhodes Medical Instruments, Summerland, CA) bilaterally in frontal cortex (from bregma: anteroposterior (AP) +2 mm, mediolateral (ML) ± 3 mm) and parietal cortex (from bregma: AP −2.5 mm, ML ± 5.5 mm), along with 2 reference epidural electrodes over the cerebellum (Figure 2A). Electrodes were connected to stainless steel wires soldered to a plug and fixed to the skull with dental cement. Two stainless steel wires (diameter 0.4 mm) inserted into the neck muscles served to record the electromyogram (EMG). At least 1 week after surgery, rats (n = 33) were continuously recorded while undergoing the experimental protocol shown in Figure 1A. Rats were trained and tested on the behavioral paradigm on sequential days (up to post-training day 4) and then sacrificed for histological analysis.
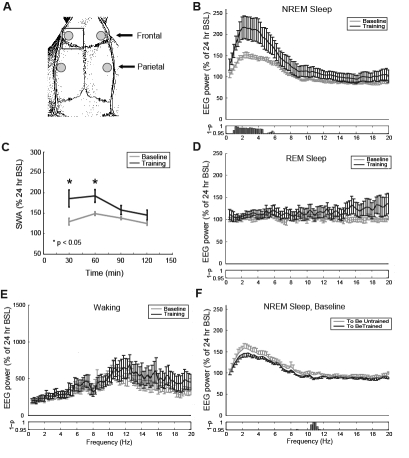
Figure 2.
A. Electrodes placement centered over frontal and parietal cortices (grey circles; not drawn to scale). The motor-sensory forelimb region is delimited by a box. B-E. Effects of motor learning on the EEG power spectrum. B. EEG power spectrum during NREM sleep in the trained motor cortex during baseline (grey line) and after training (black line). All data are mean ± SEM (n = 15 rats) for the first hour after sleep onset. For each frequency bin (0.25 Hz), values are expressed as % of the NREM EEG power spectrum relative to 24 h of baseline (BSL). C. Time course of post-training change in SWA relative to baseline in the trained motor cortex: a significant increase was observed in the first 30 (P = 0.006) and 60 (P = 0.01) min of sleep. D-E. EEG power spectra during REM sleep and waking in the trained frontal cortex during the first 3 hours after sleep onset (n = 14 rats; one rat was excluded due to the high number of EEG artifacts). Three hours after sleep onset were used for this analysis, to obtain sufficiently reliable data for each of these behavioral states. Values are expressed as % of REM or waking EEG power spectrum relative to 24 h of baseline. F. NREM power spectrum in motor cortex during baseline (to be trained, black line; to be untrained, grey line). All data are mean ± SEM (n = 8 rats) for the first hour after sleep onset. For each frequency bin (0.25 Hz), values are expressed as % of the respective NREM EEG power spectrum across the 24 h of baseline.
Figure 1.
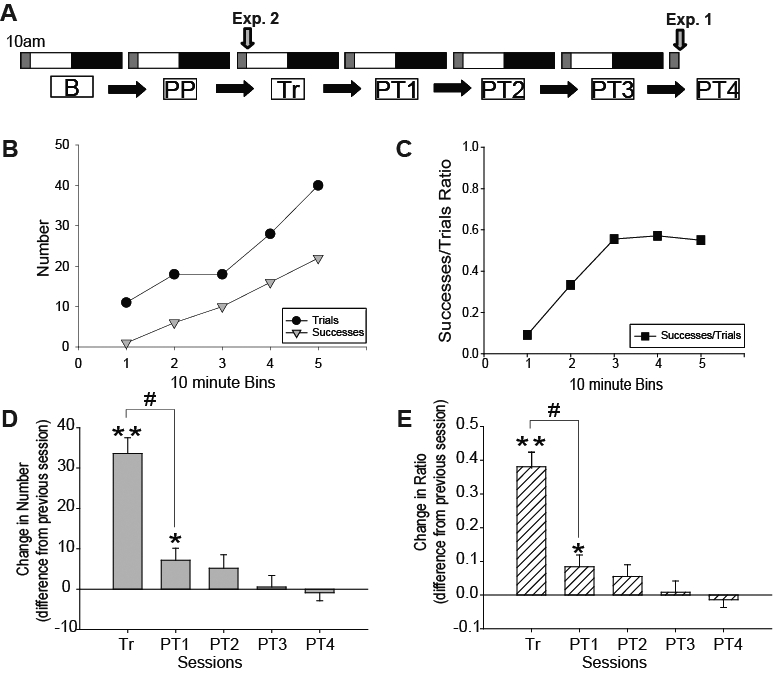
A. Schematic of the experimental protocol. Rats were kept in a 12:12 light-dark schedule with lights on at 10:00. White and black bars indicate the light and dark period, respectively. The grey area after light onset indicates the ∼1-hour period each day when rats were exposed to the behavioral paradigm. For electrophysiological experiments (Exp. 1), EEG recordings were performed continuously during all 7 experimental days (from B to PT4), while rats used for molecular biology experiments (Exp. 2) were killed after the first training session. Grey arrows denote time of sacrifice. Abbreviations are as follows: B, Baseline; PP, Preferred Paw; Tr, Training Day; PT1-PT4, Post-Training Days 1-4. B-C. Learning the single pellet reaching task. Example of one rat that learned the task in 50 min (see Methods for definition of trials and successes). At the beginning of the session the rat was only obtaining 1 pellet in the first 10 min, but the number increased to ∼ 20 pellets/10 min later in the session. All animals used for EEG analysis in experiment 1 (n = 15) learned the task, as measured by a significant increase in successes (D) or in the successes/ trials ratio (E) on the training day relative to baseline. Note that most learning occurs on the first training day. Values are mean ± SEM, expressed as the difference from the previous session. *P < 0.05, **P < 0.001 (relative to baseline); # P < 0.001.
2. Molecular Biology Experiments: Epidural Screw Electrodes
For molecular studies rats were implanted for chronic polygraphic recordings as for the electrophysiological experiments, but, to limit the risk of tissue damage, epidural EEG screw electrodes were used (bilaterally over parietal cortex, along with 2 reference epidural electrodes over the cerebellum). At least 1 week after surgery, rats (n = 19) were continuously recorded (monopolar EEG recordings referenced to one of the 2 available “neutral” cerebellar screw electrodes) while undergoing the experimental protocol shown in Figure 1A. All animals were sacrificed following training on the single pellet reaching task either immediately (waking group: n = 8) or after approximately 1 hr of sleep (sleep group: n = 11, average sleep time = 65.2 ± 3.32 min).
EEG Analysis
After surgery all rats were housed individually in transparent Plexiglas cages (36.5 × 25 × 46 cm), and kept in soundproof recording boxes for the duration of the experiment. At least 7 days were allowed for recovery after surgery, and the training protocol began only after the sleep/waking cycle had fully normalized. Rats were connected by means of a flexible cable to a commutator (Airflyte, Bayonne, NJ) and recorded continuously for 2–4 weeks using a Grass electroencephalograph (mod. 8, Grass Instruments, West Warwick, RI). Video recordings were performed continuously with infrared cameras (OptiView Technologies Inc., Potomac Falls, VA) and stored in real time (AVerMedia Technologies Inc., Milpitas, CA).
EEG (LFP and screw electrodes) and EMG signals were filtered (EEG, high-pass filter at 0.1 Hz; low-pass filter at 35 Hz; EMG, high-pass filter at 5 Hz; low-pass filter at 70 Hz). All signals were sampled and stored at 128 Hz resolution. EEG power spectra were computed by a fast Fourier transform (FFT) routine for 4-s epochs within the frequency range of 0.25 to 20 Hz. Sleep stages were scored off-line by visual inspection of 4-s epochs (SleepSign, Kissei, Matsumoto, Japan). Wakefulness was characterized by a low-voltage, high-frequency EEG pattern and high EMG activity. NREM sleep was characterized by the occurrence of high-amplitude slow waves, spindles, and low tonic EMG activity. During REM sleep, instead, the EEG was similar to that during waking, but only heart beats and occasional twitches were evident in the EMG signal. Sleep scoring was performed based on a minimum of 2 LFP derivations. Epochs containing artifacts, predominantly during active waking, were excluded from spectral analysis. Vigilance state could always be determined.
Behavioral Testing: the Single Pellet Reaching Task
Following recovery from surgery, rats were food-restricted to 85% to 90% of their free feeding weight (water was provided ad libitum). Rats were first habituated to 45 mg dustless precision sucrose pellets (Bioserve Inc., Frenchtown, NJ) in their home cage to familiarize them with the novel food, and then familiarized with the reaching chamber. During baseline and training the reaching chamber was placed inside the home (recording) cage, so that rats were never removed from their cages and EEG recordings were never interrupted. The reaching chamber (34 × 13 × 24cm) had a 1-cm wide opening in the front wall through which a 2-cm wide shelf, mounted 3 cm from the bottom of the chamber on the outside of the front wall, could be accessed. The shelf had 2 indentations centered on the 1-cm opening in the front wall of the reaching chamber for placement of sucrose pellets. The shelf indentations were placed such that only the contralateral paw could access the sucrose pellet (as previously described by Whishaw et al., 1990).
Baseline, determination of preferred paw, training (Exps. 1 and 2), and post-training sessions (Exp.1 only) occurred on successive days (Figure 1A). During baseline, sucrose pellets were sprinkled on the floor and shelf of the chamber and baseline LFP recordings were collected. Once animals were familiarized to the chamber, sucrose pellets were placed only in the indentations on the shelf and the preferred limb for reaching was determined (20 left and 13 right pawed). Training consisted of shaping a rat to approach the opening in the front of the chamber, determine whether a sucrose pellet was available on the shelf and, if one was present, to reach through the opening and retrieve it with its preferred paw. Following an attempt to obtain a sucrose pellet, whether successful or not, rats were required to go to the back of the chamber and approach the front again before another pellet was presented. This sequence of behaviors, defined as a trial, forced the rat to reposition its body to obtain a sucrose pellet. A trial was considered completed every time a rat made an attempt to retrieve a sucrose pellet, whether or not it was successful. Thus, consuming a sucrose pellet or knocking it off the shelf would both be considered the endpoint of a trial. The training session ended after a rat performed 20 trials in 10 minutes twice, or in any case after 1.5 hours. For Experiment 1, on subsequent post-training days each rat was only allowed to perform the same number of trials as during the training day.
As in previous studies,25,26 multiple behavioral outcomes were scored, including trials, reaches, and successes. Trials were defined as above. A reach was defined as the use of the preferred paw to reach through the opening in the front wall in an attempt to obtain a sugar pellet. Successes were defined as a successful retrieval and consumption of a sucrose pellet with the preferred paw, regardless of the number of reach attempts. Since results using each of the 3 behavioral outcomes were similar, we only show the data expressed as absolute amount of trials and successes, or the ratio of successes/trials performed during each session.
Data Analysis
Comparisons in EEG power spectrum (Exp. 1 only) were assessed by paired t-tests for single frequency bins (bin size 0.25 Hz) and frequency bands (e.g., 0.5–4 Hz). Behavioral outcomes and gene expression differences were tested with paired t-tests. Regression analysis was performed with the Pearson correlation method.
Histology
At the end of behavioral testing, all animals were deeply anesthetized with isoflurane anesthesia (3% in oxygen) and perfused transcardially with saline immediately followed by 4% solution of paraformaldehyde (PFA) in 0.1 M sodium phosphate, pH 7.2. Brains were post-fixed overnight in 4% PFA, cryoprotected in increasing concentration of sucrose (15%, 20%, 30%) in phosphate buffered saline, and rapidly frozen on dry ice. For Experiment 1, brains were cut into 40-μm serial coronal sections, stained with cresyl violet, and placement of LFP electrodes was determined using light microscopy. In all cases, electrodes were in the targeted cortical regions and did not extend to the white matter below layer VI. For Experiment 2, brains were cut into 40-μm serial coronal sections and processed for Fos and Arc immunohistochemistry. No excessive or unexpected tissue damage was noted.
Fos and Arc Immunolabeling
Sections were washed in Tris buffered saline (TBS) and incubated in 0.3% hydrogen peroxide in TBS for 30 min to block endogenous peroxidase activity, and then in blocking solution (TBS, 0.25% Triton, normal blocking serum) for 2 h. Next, sections were incubated with the respective primary antibody; anti-Fos (rabbit polyclonal antibody,1:15,000, Calbiochem, San Diego, CA) at 4°C for 48 h; anti-Arc (goat polyclonal antibody, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature overnight. After 6 rinses in TBS, sections were incubated with the secondary antibody (anti-rabbit for Fos, anti-goat for Arc) in TBS and 0.25% Triton for 2 h at room temperature, rinsed 3 times in TBS, and then placed in the avidin-biotin-horseradish complex for 1 h (ABC kit, Vector Laboratories, Burlingame, CA). After 3 rinses in TBS, the peroxidase was visualized with a 3,3′ –diaminobenzidine substrate (DAB) with nickel chloride enhancement (Vector Laboratories, Burlingame, CA). To limit variability due to background staining all sections were treated with DAB for the same amount of time and sections from a waking animal and a sleep animal were always processed simultaneously. Sections were then washed in TBS, mounted onto slides, air dried, dehydrated through graded alcohols, cleared in Xylene, and coverslipped with Permount.
Analysis for both Fos and Arc was performed on at least 12 frontal sections per animal (collected at ∼ 500 μm intervals), spanning an area from +3.7 to −1.88 mm anterior/posterior to bregma. This area includes the motor cortex as defined both functionally and cytoarchitecturally,27,28 and its boundaries were confirmed in a subset of sections stained with cresyl violet (10x magnification): the medial/lateral borders of the rostral half of the motor cortex, which closely corresponds to the frontal agranular field, were easy to identify based on the lack of a granular layer IV, while those of caudal half, which also includes granular cortex, were manually delimited as spanning from +1 mm to +4 mm medial/lateral from bregma.29 Two to 4 parietal sections spanning an area from −2.3 to −4.52 mm anterior/posterior and +1 mm to +4 mm medial/lateral from bregma, were also examined in 7 of the 8 waking animals. Cell counting was performed manually under 20x magnification. Cresyl violet staining following Fos staining clearly showed that very few Fos positive cells were present in layers I and IV, making it easy to group stained cells as belonging to layers II/III or layers V/VI. Fos positive cells were identified as dark punctuate staining within the nucleus of the cell; since the great majority of Fos positive cells were darkly stained and easy to identify from background staining, all cells were counted and included in the analysis. In each section Fos positive cells were manually counted from the medial to the lateral border of the motor cortex, first in layers II-III, and then in layers V/VI. Analysis for these 2 groups was kept separate because it has been previously reported that plastic changes can occur to different extent in supra- and infragranular layers.19–21,30–32 Arc positive cells were clearly identifiable as a strong cytoplasmic staining that occasionally extended into the most proximal dendrites, and in most cases were observed in layers V/VI. All sections were manually counted at least 2 times (reported values are the average), with a margin of error of less than 5% between the 2 measurements, giving us confidence that the counting procedure was reproducible. In all cases the experimenter was blind to the origin of the samples (behavioral state and trained hemisphere).
RESULTS
Electrophysiological Experiments
Figure 1A summarizes the experimental protocol, which included 7 days (see Methods for details). Rats are nocturnal animals and thus sleep mainly during the light period. The task was performed at the same time every day, soon after light onset (at around 10:00), i.e., before the beginning of the major sleep phase. To maintain the amount of motor activity constant, rats performed the same number of trials on the training day and on each of the 4 post-training days.
All 33 animals effectively learned the task in one session (see below). Eighteen of these rats, however, were not used for EEG analysis, either because good LFP recordings from at least 2 electrodes (left and right, frontal and parietal) were not available across several experimental days, or because their individual sleep pattern was not consistent across days (i.e., varying amounts of waking for the 3-6 h preceding behavioral task performance across the experimental days). The latter is an important issue, because SWA at sleep onset is significantly affected by the sleep/waking history of the previous 3-6 h.33–35 Thus, the final analysis focused on 15 rats that on each day had similar amounts of sleep and waking in the last 3-6 h before performing the task (Table 1A). Of these 15 rats, all were used for the trained frontal SWA analysis, 8 for the untrained frontal, and 12 for both trained and untrained parietal SWA analysis (exclusion was due to the presence of recording artifacts).
Table 1.
Experiment 1
| 1A: Preceding Sleep | |||
|---|---|---|---|
| Waking | NREM | REM | |
| 3 h | |||
| Baseline | 149.9 ± 3.98 | 26.6 ± 3.40 | 1.68 ± 0.56 |
| Training | 161.6 ± 4.90 | 15.2 ± 3.82 | 1.85 ± 0.83 |
| PT1 | 156.2 ± 6.07 | 20.6 ± 5.09 | 1.73 ± 0.65 |
| PT2 | 139.2 ± 12.32 | 33.3 ± 9.47 | 5.45 ± 2.34 |
| PT3 | 146.1 ± 5.61 | 27.8 ± 4.86 | 4.19 ± 0.92 |
| 6 h | |||
| Baseline | 216.9 ± 9.11 | 115.9 ± 7.15 | 20.9 ± 2.46 |
| Training | 231.1 ± 9.34 | 103.1 ± 7.47 | 19.7 ± 2.11 |
| PT1 | 218.6 ± 9.70 | 112.8 ± 7.51 | 22.4 ± 2.41 |
| PT2 | 204.4 ± 9.52 | 124.8 ± 7.12 | 24.2 ± 2.38 |
| PT3 | 199.3 ± 13.3 | 127.4 ± 10.3 | 26.6 ± 3.35 |
| 1B: Following Sleep | |||
| 1 h | |||
| Baseline | 10.5 ± 2.46 | 42.7 ± 1.81 | 6.8 ± 0.85 |
| Training | 10.4 ± 2.27 | 45.0 ± 1.97 | 4.6 ± 0.44 |
| PT1 | 15.7 ± 2.28 | 38.9 ± 1.88 | 5.4 ± 0.78 |
| PT2 | 12.4 ± 3.35 | 41.6 ± 2.80 | 6.0 ± 0.81 |
| PT3 | 14.3 ± 4.12 | 40.4 ± 3.21 | 5.3 ± 0.99 |
| 3 h | |||
| Baseline | 33.4 ± 3.75 | 124.2 ± 3.13 | 22.4 ± 1.24 |
| Training | 35.6 ± 6.01 | 122.9 ± 5.02 | 21.4 ± 1.37 |
| PT1 | 44.0 ± 6.99 | 116.5 ± 5.68 | 19.5 ± 1.92 |
| PT2 | 38.1 ± 5.77 | 119.5 ± 5.29 | 22.5 ± 1.31 |
| PT3 | 40.1 ± 9.41 | 119.0 ± 7.80 | 20.9 ± 1.83 |
| 6 h | |||
| Baseline | 107.0 ± 5.13 | 209.9 ± 4.29 | 43.1 ± 1.70 |
| Training | 119.8 ± 11.21 | 203.7 ± 9.01 | 36.5 ± 2.51 |
| PT1 | 125.3 ± 16.44 | 198.2 ± 13.73 | 36.5 ± 3.72 |
| PT2 | 128.9 ± 14.11 | 191.33 ± 11.11 | 39.8 ± 3.33 |
| PT3 | 108.2 ± 11.24 | 211.1 ± 8.48 | 40.7 ± 3.14 |
Minutes of waking, NREM, and REM sleep during (A) the last 3 or 6 hours prior to the experimental procedure (habituation, training, or post-training), and (B) the first 1, 3, or 6 hours following performance. Values are mean ± SEM (n = 15).
Single Pellet Reaching Task
The task requires a specific region of the frontal cortex called the motor-sensory forelimb area.36–38 During training the rat learns to approach a small opening in the front of the recording chamber, determine whether a sucrose pellet is available on the shelf and, if so, reach through the opening to retrieve the pellet with its preferred paw. In some previous studies rats were trained in short (10-15 min) daily sessions across 1-2 weeks.21,39 In other studies, instead, 1-h training sessions were repeated for 3-5 days.20 In all cases, most of the learning occurred within the first 2 to 4 days. In our experiment rats were trained in a single session to concentrate most of the training in one day, and thus maximize our ability to detect SWA changes after learning. Indeed, we were able to train all 33 rats in one session, which lasted from 30 to 90 min (mean 56.3 min). The learning curve of one representative animal is presented in Figure 1B-C, which shows the number of performed trials and successful retrievals of single pellets, as well as the successes/trials ratio on the training day. Figure 1D-E shows the combined behavioral data for the 15 animals in which LFP data were analyzed (similar results were obtained for all 33 rats, data not shown). In these 15 animals, on the training day, the number of successes increased from 0 to 33.6, and the successes/trials ratio increased from 0 to 0.38 (both P < 0.001), indicating that these animals did learn the task. Figure 1D-E also shows that the improvement that occurred on post-training days 1-4 was minimal, although still significant on PT1 (successes, P = 0.0334; ratio, P = 0.029). Moreover, the increase in behavioral measures observed on the training day was significantly larger than that observed on PT1 (P < 0.001 for successes and successes/trials ratio). It should also be noted that each animal performed the exact same number of trials on each day (the training day and PT1-PT4), with the average number of trials performed across all animals being 89.2 ± 4.3. Similarly, the number of reaches performed by each rat did not significantly differ in the training day relative to PT1-PT4 (average across all rats, Tr: 174.7 ± 12.9; PT1: 165.9 ± 12.0; PT2: 154.5 ± 13.3; PT3: 144.1 ± 15.1; PT4: 154.7 ± 17.1).
Effects of Motor Learning on Regional SWA
Table 1B shows that in the 15 rats used for EEG analysis the duration of total, NREM and REM sleep in the first 1, 3, and 6 hours following the task did not change significantly relative to baseline. Thus, motor learning does not affect sleep quantity. To measure the effects of training on sleep intensity we measured the EEG power spectrum using intracortical LFP electrodes. The latter, in contrast to epidural screw electrodes, record neuronal activity from a restricted brain region, and thus can potentially detect local EEG changes specific to the cortical area involved in motor learning. For each rat the “trained” motor cortex was defined as the one contralateral to the preferred forelimb (the limb used to reach), while the “untrained” motor cortex was the one ipsilateral to the preferred forelimb. In the rat, the forelimb representation within the motor cortex spans an area from + 3.7 mm anterior to −1.88 mm posterior to bregma and from the midline to +4 mm laterally.27,40 Thus, only the frontal, but not the parietal LFPs were located within the targeted forelimb region (Figure 2A).
Figure 2B shows the NREM relative power spectrum during the first hour after sleep onset (defined as the first sleep epoch after the end of training) for the trained frontal cortex, during the baseline recording (after habituation to the sugar pellets) and following the first training session. The power spectrum in all frequency bins between 1 and 4.5 Hz, which encompass SWA, was higher after training relative to baseline, and only few other frequency bins, between 5 and 6 Hz, also showed significant changes. No changes were seen in the spindle frequency range (12-15 Hz). When averaged across the entire SWA band (0.5–4.0 Hz) the relative NREM power spectrum increased significantly from 138 ± 5 during baseline (mean ± SEM, %, relative to the 24-hour value) to 195 ± 21 after training (P = 0.0103). Since all rats used for this analysis were awake for a similar amount of time prior to learning the task (Table 1A) the post-training increase in SWA cannot be due to differences in the previous sleep/wake history.
The SWA increase after learning occurred during the first 60 min after sleep onset, but no longer reached significance after 90 or 120 min (Figure 2C). The effect was specific for NREM sleep, as it did not occur during REM sleep or waking. In fact, as shown in Figure 2D-E, when the power spectra of the first 3 h after sleep onset was analyzed, no significant differences between baseline and training day, in any frequency bin, were observed during REM sleep or waking. Also, the post-training SWA increase in the trained cortex did not build upon a pre-existing asymmetry in SWA, because during baseline the relative NREM sleep power spectrum of the left and right hemisphere did not differ in the SWA frequency range (the only difference was between 10 and 12 Hz; Figure 2F). The latter analysis, however, was performed in only 8 of the 15 rats, those with artifact-free EEG signal from both left and right motor LFPs, but similar results (no relative baseline SWA asymmetry) were found in 3 additional animals not used in this study. Thus, the observed increase in SWA power is reversible, specific to NREM sleep, and only occurs after training.
The increase in SWA was significantly different from baseline after the first training session and PT1, but not on post-training days 2-3 (PT2: P = 0.1154; PT3; P = 0.0914; Figure 3A). Moreover, SWA significantly increased post-training relative to baseline in the untrained frontal cortex (P = 0.017), but the increase was smaller (Trained: +56%; Untrained: +46%; Figure 3B). There was no significant change in SWA in either left or right parietal cortex (Parietal, trained side: P = 0.1364; Parietal, untrained side: P = 0.1199; Figure 3C-D). Thus, the increase in SWA observed in the frontal cortex following training appears to be region-specific.
Figure 3.
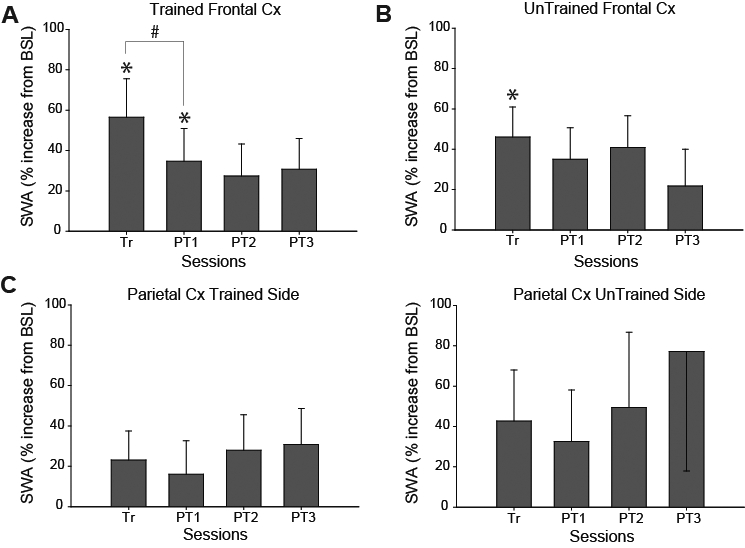
Regional changes in SWA after training. A. Changes in SWA in the trained frontal cortex during the first hour of sleep. For each testing day SWA (mean ± SEM) is expressed as percent increase relative to the 24 h of baseline (BSL). The increase is significant following the first training session and PT1 but not on the following post-training days (PT2-3). *P < 0.05 (relative to baseline); # P < 0.01 (Tr vs. PT1). B-D. Same as in A, but for the untrained motor cortex (n = 8), and for the parietal cortex of both sides (n = 12). The number of rats contributing to the analysis for each day was as follows: Trained frontal = Tr = 15, PT1 = 14, PT2 = 11, PT3 = 9; Untrained frontal = Tr + PT1 = 8, PT2 + PT3 = 6; Trained parietal = Tr = 12, PT1 = 11, PT2 = 9, PT3 = 7; Untrained parietal = Tr = 12, PT1 = 11, PT2 = 9, PT3 = 8.
Changes in SWA and Behavioral Outcomes
Correlation analyses were performed to determine whether the performance improvement in the reaching task could predict the following increase in SWA. Indeed, in the trained frontal cortex, we found a positive correlation between increase in successes and subsequent increase in SWA during the first hour of sleep post-task (n = 15, P = 0.048, r2 = 0.0796; Figure 4). By contrast, no significant correlation between these parameters was observed in the untrained motor cortex (n = 8, P = 0.548), or in the parietal cortices (n = 12, P = 0.757 and P = 0.744), and similar negative results were observed using other behavioral outcomes (i.e., ratio scoring; data not shown). Thus, animals that showed the greatest improvement in obtaining sucrose pellets on the training and post-training days also exhibited, during the following NREM sleep, the highest increases in SWA in the trained motor cortex. Importantly, rats performed the same number of trials and a similar number of reaches during the training day and each of the post-training days, suggesting that the observed increases in SWA reflect learning, as measured by the number of successes, rather than simply “use,” as measured by the number of trials or reaches. We also examined whether changes in SWA during the first hour of sleep could predict improvement in performance in the following days, but found no positive correlation. This result is perhaps not surprising, since in this task most of the learning and improvement in performance occurred during the first training day, with little change afterward.
Figure 4.
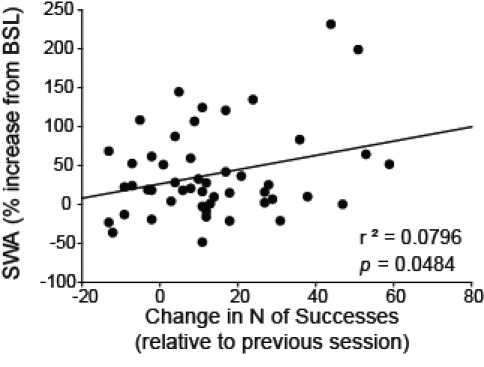
Correlation analysis. Correlation between improvement in performance (measured as difference in successes relative to the previous session), and subsequent increases in SWA (relative to the 24 h of baseline, BSL) for the first hour of sleep post-task. The plot includes 49 data points from 15 rats across 4 days, from training day to post-training day 3 (9 rats contributed 4 points, 2 rats contributed 3 points, 3 rats contributed 2 points, and one rat contributed one point).
Molecular Biology Experiments
The experimental protocol included 3 days (Figure 1A), and rats were killed immediately after training (waking group) or after ∼1 h of post-training sleep (sleep group). The immediate early gene c-fos is induced within minutes of many forms of extracellular stimulation, and its protein product Fos is amply used as a cellular marker of neuronal activation.41 The activity-regulated cytoskeleton-associated protein Arc accumulates in the portions of the dendritic tree activated by the induction of LTP,42,43 and Arc sustained expression controls LTP maintenance in vivo.44,45 Thus, Fos and Arc immunocytochemistry was used to determine the extent to which skilled reaching activates neurons within the motor cortex, whether such induction is stronger in the trained relative to the untrained motor cortex, and whether it subsides during the post-learning sleep.
Fos Staining
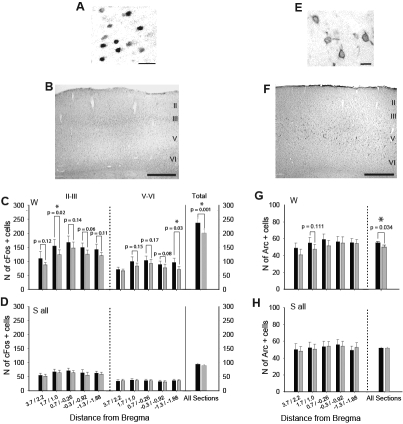
In the waking group (n = 8), as expected, Fos nuclear staining was consistently observed throughout the cerebral cortex, including the entire motor cortex as previously defined27,40 (Figure 5A). In the frontal areas of both sides Fos positive cells were mostly seen in layers II-III and V-VI, although a few cells were also present in layers I and IV (Figure 5B). Sections were first divided into 5 groups spanning the entire rostrocaudal extension of the motor cortex (4 sections/group, from + 3.7 to − 1.88 mm relative to bregma). Since previous reports have suggested that plastic changes may affect differently layers II-III than layers V/VI,19–21,50–51 Fos expression was measured separately for supra- and infragranular layers. As shown in Figure 5C, in all 5 groups of sections Fos expression in both layers II-III and layers V-VI was higher in the trained relative to the untrained motor cortex, although the difference was only significant at some levels. Overall, the asymmetry was significant when all groups of sections were pooled for layers II-III (trained vs untrained, P = 0.0003), layers V-VI (P = 0.016), and all layers (total, P = 0.001; Figure 5C). We also examined Fos expression in parietal cortex (n = 7, in one waking animal parietal sections were not available); sections were divided into 5 groups (from −2.3 to −4.52 from bregma, 2 sections/group). No asymmetry in Fos expression was observed in any group, when all groups of sections were pooled for layers II-III, V-VI, or all layers (data not shown).
Figure 5.
Fos and Arc expression after training. A. Photomicrograph of Fos staining in a representative waking animal (motor cortex, layers II/III; scale bar = 20μm) B. Photomicrograph of Fos and cresyl violet double staining used to identify cortical layers (scale bar = 500 μm). C. Number of Fos expressing cells in the trained (black) and untrained (grey) hemispheres for the waking group. Each level (e.g., 3.7/2.2) represents 4 sections. Numbers of cells is given for layers II-III, layers V-VI, and for all layers across the entire motor cortex. D. Same as B for the sleep group. All data are mean ± SEM for waking (n = 8) and sleep (n = 11) groups. E. Photomicrograph of Arc staining in a representative waking animal (motor cortex, layers V-VI; scale bar = 20μm). F. Photomicrograph of Arc and cresyl violet double staining used to identify cortical layers (scale bar = 500 μm). G-H. Number of Arc expressing cells for each level (4 sections/level as for Fos) and for all layers across the entire motor cortex. All data are mean ± SEM for the waking (n = 8) and sleep (n = 11) groups.
Sleeping animals (n = 11) slept on average 65.2 min (± 3.32 SEM), and all showed, relative to the waking group, significantly reduced Fos expression in the entire cerebral cortex, including the motor cortex. This is consistent with previous evidence that the increased expression of Fos protein observed during waking returns to low levels after one hour of sleep.46 Moreover, as seen in Figure 5D, there was no asymmetry in Fos expression between the trained and untrained side in any part of the motor cortex. Also, no asymmetry was present when data from all layers and groups of sections were combined (Figure 5D). In the same animals (n = 11) no asymmetry was observed in parietal cortex, neither in layers II-III or V-VI, nor when all layers were combined (data not shown).
Similar negative results (no asymmetry in layers II/III or V/VI) were obtained when sleeping animals were divided into 2 groups of low (< 3%) or high (> 9%) REM sleep, based on the percentage of time spent in this behavioral state during the first 30 min of sleep post-training (i.e., from 60 to 30 min before sacrifice; Table 2). Furthermore, no significant asymmetry in Fos expression was observed in any layer in the 7 rats (out of 11) that had REM bouts of 90 seconds or longer 50–60 min before sacrifice (average time 55.7 min). Of note, 4 of the sleeping rats stayed awake longer after training than the other rats (67 ± 35 vs 15 ± 3 min). These animals did show an asymmetry in Fos expression between trained and untrained cortex (across all sections, layers II/III trained vs. untrained: P = 0.05; total trained vs. untrained: P = 0.026).
Table 2.
Experiment 2: Sleep Architecture
| Waking | NREM | REM | |
|---|---|---|---|
| Entire Sleep (%) | |||
| All Sleep | 28.49 ± 4.59 | 58.58 ± 3.34 | 10.37 ± 1.41 |
| Low REM | 32.74 ± 3.91 | 56.03 ± 2.17 | 8.78 ± 1.82 |
| High REM | 14.57 ± 2.15 | 68.52 ± 2.09 | 14.33 ± 0.46 |
| First 30 min (%) | |||
| All Sleep | 41.41 ± 7.35 | 49.34 ± 5.63 | 6.74 ± 1.65 |
| Low REM | 52.72 ± 7.42 | 41.29 ± 5.89 | 3.99 ± 1.37 |
| High REM | 16.95 ± 4.36 | 67.51 ± 3.37 | 12.51 ± 1.33 |
Percentage of waking, NREM, and REM sleep during the entire post-task sleep period (65.2 ± 3.32 min), or for the first 30 min of post-task sleep. Data refer to all sleeping rats (n = 11), or to the same rats divided into 2 groups based on low (n = 6) and high (n = 4) REM sleep amount during the first 30 min of post-task sleep. Values are mean ± SEM.
Arc Staining
Arc staining was most consistently observed in the proximal dendrites of neurons in layer V of the cerebral cortex, although some cells were detected also in layer VI (Figure 5E-F). In the waking group, when all sections across the entire motor cortex were pooled, the number of Arc positive cells was significantly higher in the trained relative to the untrained motor cortex (P = 0.034; Figure 5G). In contrast to Fos, however, this difference was mainly due to the asymmetric expression of Arc at the most rostral levels (Figure 5G). The waking-induced asymmetric expression of Arc (in favor of the trained cortex), was no longer present in the sleeping animals, either when all rats were grouped together (Figure 5H) or when they were separated into 2 groups based on amount of REM sleep during the first 30 min of sleep post-training. Similar negative results were observed when considering only the 7 animals with a 90 sec REM bout approximately 1 h before sacrifice. As for Fos, however, the 4 rats that stayed awake longer after training showed an asymmetry in Arc expression (total; P = 0.016). The overall number of Arc labeled cells was not significantly lower in the sleep group relative to the waking group, consistent with the fact that once induced, Arc expression can take up to 6 h to decline.47,48
DISCUSSION
We found that motor learning in rats induces a local increase in SWA in the cortical area directly involved in learning the task, but not in parietal areas not implicated in skilled reaching. The SWA increase in the trained motor cortex did not just reflect motor activity, but was also positively correlated with the improvement in performance during the prior training session, while no correlation was present in the untrained motor area. These results lend support to the notion that sleep is not only a global phenomenon, and that its intensity can be regulated at a regional level.49 Consistent with this, previous studies in humans found that intermittent vibration of one hand produces a transient and small increase in SWA in the contralateral somatosensory cortex,50 while whisker stimulation in rodents produces an increase in SWA in the contralateral somatosensory cortex.51,52 Increases in SWA also occur in circumscribed cortical areas after learning a rotational adaptation task,7 after TMS-induced stimulation of motor cortex,9 or following paired associative stimulation within sensorimotor cortex,11 while arm immobilization decreases SWA.8 Overall, this evidence was consistent with synaptic potentiation driving SWA increases, and synaptic depression SWA decreases.3,4 Yet, a direct test of this hypothesis was still missing, because none of the manipulations used so far had been proven to cause synaptic potentiation of cortical circuits.
To directly test the hypothesis that changes in sleep SWA are caused by synaptic potentiation during prior waking we used a skilled reaching task, because several studies in vitro and in vivo have demonstrated that learning to reach induces synaptic potentiation in the trained motor cortex, and saturates the ability of this area to undergo further potentiation.19–21,32 Specifically, training on the single pellet reaching task produces an increase in dendritic branching in layers II-III and V,30,31 enhances the strength of the horizontal intra-cortical connections, increases response size, partially occludes further LTP in layers II-III of the trained cortex,19–21 and increases dendritic spine density within layer I of the same area.32 A difference between the present and the aforementioned studies is the number and duration of the training sessions. In the previous studies, training occurred over multiple brief sessions (i.e., five 10-min sessions) and asymptotic responding occurred in the later sessions. Instead, we trained animals in one single session that lasted approximately 1 h, during which significant learning occurred (Figure 1). Thus, our data strongly suggest that the local changes in SWA are driven by synaptic potentiation and learning.
We found no SWA asymmetry between right and left frontal cortex during baseline, consistent with a recent analysis of the effects of handedness on EEG asymmetry.53 That study, however, did find a minor increase in NREM EEG power between 3.5 and 6 Hz in the frontal cortex contralateral to the preferred paw,53 while we did not. One reason may be that, before training, we did not select rats based on a strong paw preference. Also, we did not use screw electrodes, which can sample a larger area of motor cortex. Interestingly, the same study found that, after rats were forced to use their preferred paw for 2 h to reach for food, the NREM sleep EEG in the contralateral frontal cortex showed a widespread increase in most frequencies > 1.5 Hz.53 Also, the NREM EEG power between 2 and 9 Hz was positively correlated with paw use.53 Instead we found that, after learning the reaching task, increases in relative power spectra from baseline were specific for SWA, and the changes could not be accounted for by the prior use of the paw. Thus, asymmetric motor activities such as those due to handedness can widely affect the NREM sleep EEG, but the effect of motor learning seems to be more specific for SWA.
After learning the task SWA increased also in the “untrained” motor area (controlling the paw not used to reach), although the increase was smaller than in trained cortex and did not correlate with changes in performance. SWA changes in the untrained motor cortex are not entirely surprising, because during training rats learn to use the contralateral paw in a novel way, for instance to support the body during reaching. Skilled reaching is a complex task, and difficult tasks in humans are known to activate the ipsilateral motor cortex more than simple ones.54–56 Also, activation and potentiation of circuits in the untrained motor area may have resulted from the strong excitatory connections between the 2 hemispheres, via the corpus callosum, and/or from the small fraction of uncrossed fibers of the corticospinal tract.57
We also found that motor learning induces higher expression of Fos and Arc in the trained relative to untrained motor cortex, while there was no asymmetric Fos expression in parietal cortex. Overall, these molecular changes are consistent with the SWA changes described above, and provide evidence for a local and specific activation at the cellular level. Fos positive cells were mostly observed in layers II-III and V-VI, while the expression of Arc was restricted to layers V-VI. Fos expression was measured separately for supra- and infragranular layers because previous studies suggested that plastic phenomena may be layer-specific.19–21,30–32 In the current experiments, however, we observed similar levels of Fos asymmetry in layers II/III and layers V/VI. The number of Fos positive cells increases in layers II-III of the rat motor cortex after training to traverse a complex series of obstacles, and Fos expression seems to correlate more with motor learning than with motor activity per se.22 Learning a spatial water maze task, or a lever-press task, also increases Arc levels, and Arc induction is stronger in newly trained relative to overtrained or pseudotrained animals.23,24 Spatial exploration also increases Arc expression, and does so specifically in glutamatergic neurons of hippocampus and neocortex.58 Thus, the increased expression of Fos and Arc during training may reflect not just neuronal activation, but more specifically synaptic plasticity.
The higher number of Fos and Arc positive cells observed in the trained, relative to untrained, motor cortex immediately after learning was no longer present after 1 h of sleep. In our study, we found no evidence for specific reactivation of Fos or Arc during sleep, not even in those rats with more REM sleep during the first 30 min after sleep onset. Thus, to the extent that asymmetric Fos and Arc protein expression reflects synaptic plasticity triggered by motor learning, we saw no signs that this form of plasticity is reinduced during sleep post-learning. However, we only looked at one time point, and the induction of immediate early genes is an indirect measure of synaptic plasticity. Thus, whether the same synapses activated by learning during waking are reactivated during sleep post-learning remains an open question. We recognize that these data are far from conclusive, and a definitive answer will require the ability to determine in vivo, with cellular resolution, whether the same synapses potentiated during waking will undergo a second wave of potentiation during the subsequent sleep, as some suggest,14 and/or a generalized depression.3,4
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Tononi has participated in speaking engagements for Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The work was supported by the Pickwick Fellowship of the National Sleep Foundation to ECH, NIMH award 1P20MH077967 to CC, and the NIH Director's Pioneer award to GT. We thank Dr. Ian Q. Whishaw and his lab for help in implementing the reaching task.
REFERENCES
- 1.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 2.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 3.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, et al. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 6.Lubenov EV, Siapas AG. Decoupling through synchrony in neuronal circuits with propagation delays. Neuron. 2008;58:118–31. doi: 10.1016/j.neuron.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 8.Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 9.Huber R, Esser SK, Ferrarelli F, et al. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30:129–39. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- 11.Huber R, Maatta S, Esser SK, et al. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–8. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraguna U, Vyazovskiy VV, Nelson AB, et al. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–95. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghilardi M, Ghez C, Dhawan V, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–45. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 14.Rasch B, Born J. Maintaining memories by reactivation. Curr Opin Neurobiol. 2007;17:698–703. doi: 10.1016/j.conb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro S, Goyal V, Mello CV, Pavlides C. Brain gene expression during REM sleep depends on prior waking experience. Learn Mem. 1999;6:500–8. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Marrone DF, Schaner MJ, McNaughton BL, et al. Immediate-early gene expression at rest recapitulates recent experience. J Neurosci. 2008;28:1030–3. doi: 10.1523/JNEUROSCI.4235-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassi-Zucconi G, Giuditta A, Mandile P, et al. c-fos spontaneous expression during wakefulness is reversed during sleep in neuronal subsets of the rat cortex. J Physiol Paris. 1994;88:91–93. doi: 10.1016/0928-4257(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 19.Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–4. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 20.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–6. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 21.Monfils MH, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004;125:329–36. doi: 10.1016/j.neuroscience.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 22.Kleim JA, Lussnig E, Schwarz ER, et al. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–35. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–98. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene arc differs across brain regions. J Neurosci. 2003;23:6443–51. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwaniuk AN, Whishaw IQ. On the origin of skilled forelimb movements. Trends Neurosci. 2000;23:372–6. doi: 10.1016/s0166-2236(00)01618-0. [DOI] [PubMed] [Google Scholar]
- 26.Whishaw IQ, Alaverdashvili M, Kolb B. The problem of relating plasticity and skilled reaching after motor cortex stroke in the rat. Behav Brain Res. 2008;192:124–36. doi: 10.1016/j.bbr.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Neafsey EJ, Bold EL, Haas G, et al. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fourth Edition. San Diego: Academic Press Inc.; 1998. [Google Scholar]
- 29.Wise SP, Donoghue JP. Motor cortex of rodents. In: Jones EG, Peters P, editors. Cerebral Cortex. Vol. 5. New York: Plenum Press; 1986. pp. 243–270. [Google Scholar]
- 30.Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–14. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- 31.Withers GS, Greenough WT. Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27:61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]
- 32.Harms KJ, Rioult-Pedotti MS, Carter DR, Dunaevsky A. Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J Neurosci. 2008;28:5686–90. doi: 10.1523/JNEUROSCI.0584-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–76. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 34.Tobler I, Franken P, Gao B, et al. Sleep deprivation in the rat at different ambient temperatures: effect on sleep, EEG spectra and brain temperature. Archives Italiennes de Biologie. 1994;132:39–52. [PubMed] [Google Scholar]
- 35.Huber R, Deboer T, Tobler I. Topography of EEG dynamics after sleep deprivation in mice. J Neurophysiol. 2000;84:1888–93. doi: 10.1152/jn.2000.84.4.1888. [DOI] [PubMed] [Google Scholar]
- 36.Castro AJ. The effects of cortical ablations on digital usage in the rat. Brain Res. 1972;37:173–85. doi: 10.1016/0006-8993(72)90665-8. [DOI] [PubMed] [Google Scholar]
- 37.Whishaw IQ, O'Connor WT, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine and caudate-putamen to skilled forelimb use in the rat. Brain. 1986;109:805–43. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- 38.Whishaw IQ, Kolb B. Sparing of skilled forelimb reaching and corticospinal projections after neonatal motor cortex removal or hemidecortication in the rat: support for the Kennard doctrine. Brain Res. 1988;451:97–114. doi: 10.1016/0006-8993(88)90753-6. [DOI] [PubMed] [Google Scholar]
- 39.Gharbawie OA, Gonzalez CL, Whishaw IQ. Skilled reaching impairments from the lateral frontal cortex component of middle cerebral artery stroke: a qualitative and quantitative comparison to focal motor cortex lesions in rats. Behav Brain Res. 2005;156:125–37. doi: 10.1016/j.bbr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Neafsey EJ, Sievert C. A second forelimb motor area exists in rat frontal cortex. Brain Res. 1982;232:151–6. doi: 10.1016/0006-8993(82)90617-5. [DOI] [PubMed] [Google Scholar]
- 41.Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–97. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 42.Link W, Konietzko U, Kauselmann G, et al. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proceedings of the National Academy of Science of the United States of America. 1995;92:5734–8. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyford GL, Yamagata K, Kaufmann WE, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–45. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 44.Guzowski JF, Lyford GL, Stevenson GD, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messaoudi E, Kanhema T, Soule J, et al. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–55. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basheer R, Sherin JE, Saper CB, et al. Effects of sleep on wake-induced c-fos expression. J Neurosci. 1997;17:9746–50. doi: 10.1523/JNEUROSCI.17-24-09746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace CS, Lyford GL, Worley PF, Steward O. Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence. J Neurosci. 1998;18:26–35. doi: 10.1523/JNEUROSCI.18-01-00026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazdarjanova A, McNaughton BL, Barnes CA, et al. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–71. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 50.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 51.Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–71. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 52.Vyazovskiy VV, Welker E, Fritschy JM, Tobler I. Regional pattern of metabolic activation is reflected in the sleep EEG after sleep deprivation combined with unilateral whisker stimulation in mice. Eur J Neurosci. 2004;20:1363–70. doi: 10.1111/j.1460-9568.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- 53.Vyazovskiy VV, Tobler I. Handedness leads to interhemispheric EEG asymmetry during sleep in the rat. J Neurophysiol. 2008;99:969–75. doi: 10.1152/jn.01154.2007. [DOI] [PubMed] [Google Scholar]
- 54.Sadato N, Zeffiro TA, Campbell G, et al. Regional cerebral blood flow changes in motor cortical areas after transient anesthesia of the forearm. Ann Neurol. 1995;37:74–81. doi: 10.1002/ana.410370114. [DOI] [PubMed] [Google Scholar]
- 55.Hummel F, Kirsammer R, Gerloff C. Ipsilateral cortical activation during finger sequences of increasing complexity: representation of movement difficulty or memory load? Clin Neurophysiol. 2003;114:605–13. doi: 10.1016/s1388-2457(02)00417-0. [DOI] [PubMed] [Google Scholar]
- 56.Verstynen T, Diedrichsen J, Albert N, et al. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–22. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- 57.Glees P, Cole J. Ipsilateral representation in the cerebral cortex; its significance in relation to motor function. Lancet. 1952;1:1191–2. doi: 10.1016/s0140-6736(52)91090-8. [DOI] [PubMed] [Google Scholar]
- 58.Vazdarjanova A, Ramirez-Amaya V, Insel N, et al. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–29. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]