Abstract
Study Objective:
To assess the cost-effectiveness of treatment with eszopiclone for chronic primary insomnia in adults.
Methods:
A model using patient-level data from a 6-month, double-blind, placebo-controlled, clinical trial (n = 824), combined with data from a claims database and published literature, was used to assess the quality-adjusted life years (QALYs) gained and costs associated with eszopiclone versus placebo in adults with primary insomnia. Quality of life data were collected during the trial via the SF-36, from which preference-based utility scores were derived using published algorithms. Medical and absenteeism costs, estimated via a retrospective analysis of a claims and absenteeism database, were assigned to patients based on the degree of severity of their insomnia, assessed via the Insomnia Severity Index collected in the clinical trial. Presenteeism costs (lost productivity while at work) were estimated from responses to the Work Limitation Questionnaire collected during the trial. Six-month gains in QALYs and costs for each treatment group were calculated to derive cost-effectiveness ratios. Uncertainty was addressed via univariate and multivariate sensitivity analyses.
Results:
Over the 6-month period, eszopiclone use resulted in a net gain of 0.0137 QALYs over placebo at an additional cost of $67, resulting in an incremental cost per QALY gained of slightly less than $5,000. When absenteeism and presenteeism costs were excluded, the cost-effectiveness ratio increased to approximately $33,000 per QALY gained, which is below the commonly used threshold of $50,000 used to define cost-effectiveness. Extensive sensitivity analyses indicate the results are generally robust.
Conclusion:
Our model, based on efficacy data from a clinical trial, demonstrated eszopiclone was cost-effective for the treatment of primary insomnia in adults, especially when lost productivity costs were included.
Citation:
Snedecor SJ; Botteman MF; Bojke C; Schaefer K; Barry N; Pickard AS. Cost-effectiveness of eszopiclone for the treatment of adults with primary chronic insomnia. SLEEP 2009;32(6):817-824.
Keywords: Insomnia, Costs and Cost Analysis, Public Policy, Models, Economic, Eszopiclone
INSOMNIA IS ASSOCIATED WITH REDUCTIONS IN PRODUCTIVITY, PHYSICAL AND SOCIAL FUNCTIONING, AND HEALTH-RELATED QUALITY OF LIFE (HRQoL), as well as increased health care costs and risk of occupational and vehicular accidents.1–8 Direct medical costs of insomnia in the United States have been estimated to be as high as $13.9 billion annually (in 1995), and indirect costs are estimated to range from $77 to $92 billion annually (in 1990).9 In the coming decade, health care spending is projected to increase from 16% of the gross domestic product (GDP) in 2006 to 19.5% of GDP by 2017.10 For the same period, prescription drug spending growth is projected to accelerate as well.
In this context, it has been argued that drug coverage decisions should be made on both clinical evidence and cost-effectiveness criteria (i.e., does an effective drug therapy produce sufficient benefits given its costs?).11 Therefore, coverage and formulary decisions would be guided by information about a drug's overall value,12 which can be measured in a number of ways. The most universally accepted, recommended, and, in some countries, mandated method to assess value is the cost utility analysis, a form of cost-effectiveness analysis in which health effects (the effectiveness) are measured in terms of quality-adjusted life years (QALYs) gained.12,13
In order to make coverage decisions based on value, payers and policy makers must have access to reliable cost-effectiveness information. However, as highlighted by Neumann et al. (2006), there is a paucity of published cost-utility data available to formulary committees.12 This lack of information is particularly striking in the field of insomnia treatment. Despite numerous publications over the past decade on the economic4,6,7,14–19 and humanistic1,3,5,8,14,20–23 burden of insomnia, almost no information is available on the cost utility of insomnia therapies. In the United Kingdom, the National Institute for Clinical Excellence (NICE) Assessment Group, which advises the National Heath System on the economic value of medical interventions and drugs, was unable to identify any comparative evaluations of insomnia drugs in the health economics literature.24 The 2005 National Institutes of Health State of the Science Conference Statement25 also recognized this fact and pointed out that the societal and economic benefits of managing insomnia have not been fully estimated. Thus, historically, coverage decisions in the area of insomnia treatment have been made largely on the basis of efficacy, rather than value arguments.
To the best of our knowledge, only one cost-utility analysis of insomnia pharmacotherapy has been published to date.26 While that analysis, based on an initial clinical trial of eszopiclone in adult patients with chronic primary insomnia,27 provided evidence that the pharmacologic treatment of insomnia with eszopiclone for 6 months was cost-effective, it suffered from a number of methodological limitations: (1) there was no direct validated measure of the severity of insomnia collected in the study; (2) there was no direct measure of quality of life collected in the study; (3) there was no direct measure of work productivity collected; and (4) the assessment of uncertainty surrounding the study results was limited. Thus, this initial analysis relied on a number of limiting assumptions based on the efficacy results of the Krystal et al.27 clinical trial and the existing literature.
Since that publication, a second, 6-month eszopiclone study28 was published. This second trial provided an opportunity to confirm the validity of the original model results and further refine the economic evaluation of insomnia pharmacotherapy. In particular, this study included an assessment of the impact of therapy on insomnia severity, quality of life, resource use, and work limitations. Therefore, rather than using assumptions about the effect of therapy on some of the key outcomes, as was done in the first economic analysis,26 this trial allowed for a significantly more definitive economic analysis based on patient-level data, including an extensive assessment of the degree of uncertainty associated with the results. It is hoped that the results from this new analysis can in turn be used to help make more informed coverage decisions.
METHODS
Overview
The cost utility of eszopiclone treatment was estimated via an economic model developed using patient-level data from a 6-month clinical trial28 and supplemented with cost data obtained from analyses of nationally representative medical and absenteeism claims databases.
The clinical trial used in the present economic analysis has been described previously.28 In brief, 830 adult subjects, ranging from 21 to 64 years, meeting Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria for chronic primary insomnia, and reported sleep onset > 30 minutes and/or total sleep time ≤ 6.5 hours, were randomized to receive eszopiclone 3 mg (n = 550) or placebo (n = 280) nightly for 5 months. Exclusion criteria included unstable medical conditions; DSM-IV Axis I or personality disorder diagnosis; insomnia associated with known medical diagnosis or any condition that could affect sleep (e.g., sleep apnea, restless leg syndrome, chronic pain, benign prostatic hyperplasia); history of substance abuse or dependence; and women who were pregnant, lactating, or less than 6 months postpartum. Six subjects were excluded from the economic analysis due to missing baseline data. Sleep-related measures were collected at baseline and months 1, 3, and 6 using the Insomnia Severity Index (ISI),29 which assesses the severity and impact of insomnia symptoms. Health-related quality of life was measured at baseline and months 1, 3, and 6 via the Medical Outcomes Study Short-Form Health Survey (SF-36).30 The Work Limitations Questionnaire (WLQ),31 which provides a measure of the percentage of lost productivity while at work (i.e., presenteeism), was also administered at baseline and months 1, 3, and 6 to capture the degree to which insomnia symptoms interfere with ability to perform job roles and to calculate the monetary value of this loss irrespective of whether this loss is borne by the responder or society. Finally, non-protocol health care used by patients after each visit was assessed using the Health Utilization Questionnaire. Assessments included the number of times the patient visited a physician, a mental health professional, an emergency room, etc.
The economic analysis was developed from a United States societal perspective and included direct medical and indirect (productivity) costs incurred (or avoided) by the patients, in addition to quality of life measures (i.e., QALYs, the measure of effectiveness used in cost-utility evaluations, derived from the SF-36 using standard algorithms). Productivity measures included absenteeism and presenteeism costs, which were assessed separately using the WLQ. Because there is a debate among health economists as to whether productivity costs should be included along with utility, we examined the impact of their exclusion in an alternate analysis.
Other types of costs not considered for this analysis included: direct costs not covered by a third-party payer, such as over-the-counter medication/sleep aids, herbal supplements, alternative medicines, and prescription co-pays. Finally, the impact of insomnia and its treatment on family members, significant others, and/or caregivers of the patients were also not considered. All costs in the analysis were adjusted to 2006 US dollars using the medical care component of the Consumer Price Index.
Economic Analysis
The economic analysis estimates the average total cost of treatment with eszopiclone (Ce) and placebo (Cp), based on published data sources, and average QALYs gained for eszopiclone (Ee) and placebo (Ep), based on the clinical trial data using the ISI, the SF-36, and the WLQ. Next, an incremental cost-utility ratio (ICUR) was calculated to estimate the additional costs (over placebo) associated with the use of eszopiclone to achieve a gain of one additional QALY (over placebo).
This ICUR was given by the following formula:
Whether eszopiclone is cost-effective compared to placebo depends on whether its ICUR exceeds or remains below the societal maximum willingness to pay (WTP). That is, eszopiclone was considered cost-effective relative to placebo if:
In the US, a given therapy is usually considered cost-effective if its ICUR is ≤ $50,000 (i.e., WTP = $50,000), a somewhat arbitrary, but generally accepted threshold.32,33
Estimating the QALYs and costs of each treatment was accomplished by using the actual recorded outcomes of each individual patient, i, in the trial (where i = 1, 2, … , 824). As its name suggests, a QALY can be decomposed into 2 components: survival time and the quality of life during that survival (i.e., the utility component). A utility score theoretically ranges from zero, representing death, to one, representing perfect health. Thus, a patient who lives one year in a health state with a utility of 0.50 accumulates only 0.50 QALYs (i.e., 1 year × 0.50 utility). A therapy that improves that patient utility for the whole year from 0.50 to 0.75 would increase QALYs by 0.25 units (i.e., 1 year × (0.75-0.50 utility)). The total QALYs associated with each individual in the analysis is therefore a function of these 2 components and can be expressed as the sum of the utilities for each model month divided by 12:
Next, each trial patient's drug, direct medical, and indirect (productivity) costs were estimated. Productivity costs included presenteeism (lost productivity while at work) and absenteeism (lost productivity due to work missed altogether) costs. Thus, the total cost, Ci, of therapy can be expressed as follows:
Using the QALY and cost estimates for each trial subject, we then calculated the average QALYs and average costs for each treatment group as a whole. Using these averages, the ICUR was calculated as described in Eq. (1).
Utility
Items and/or scores based on patient assessments of the SF-36 recorded at baseline and months 1, 3, and 6 were transformed into a utility score using published algorithms. A simple linear interpolation was used to estimate the utility of each patient for each model month. Specifically, the average utility during the 1st month was the average of the baseline and month 1 utility estimates, the utility of the 2nd and 3rd months were the average of the month 1 and month 3 utility estimates, and so on. The QALYs were then calculated as shown in Eq. (3).
The base case analysis relied on the transformation method of Franks et al.,34 which maps a 13,000 US-patient sample of the National Medical Expenditure Survey (MEPS) to the EQ-5D health-related quality of life measure (which is a measure of utility) and to the SF-12, which is a subset of the SF-36 instrument. Because there are a number of transformation methods of the SF-36 into utility formats to choose from, a sensitivity analysis (see electronic Appendix A at www.journalsleep.org) was conducted in which these alternate transformations methods were tested. For each treatment group, the difference between the QALYs as calculated by Eq. (3) and the QALYs estimated on the basis of the baseline utility, represents the QALYs gained as a result of the placebo or eszopiclone treatment.
Direct Health Care Costs
Numerous studies have shown a correlation between inpatient and outpatient utilization and the presence of insomnia.6,9,14,15,19,35–37 Because of its demonstrated efficacy in treating insomnia, eszopiclone would be expected to reduce health care costs in the clinical trial population. To attempt to quantify this reduction, medical resource utilization data were collected from all trial participants using the Health Utilization Questionnaire. However, no difference in resource utilization between the 2 treatment groups was observed during the trial, and utilization was generally low overall. These low utilization patterns may not be surprising given the controlled nature of the clinical study. Per the study protocol, physician visits occurred at regular monthly intervals during the trial period, and as such patients would have been expected to utilize few, if any, additional physician visits.
To impute the physician visit utilization that would have likely occurred in a non-experimental clinical setting, each study subject was categorized according to whether s/he had sustained remission from insomnia during the trial (defined as no clinically significant insomnia, ISI ≤ 7, at 1, 3, and 6 months). Patients with remission were assumed to experience fewer physician visit costs than patients who did not achieve remission. The assumed excess physician visit costs of non-remitters were derived from a retrospective claims database analysis of over 5 million patients comparing the cost of care during a 6-month period prior to a diagnosis of insomnia or a prescription for insomnia compared with a control group.19 Based on this analysis, the additional cost of not achieving remission of insomnia was $542.00 over a 6-month period. This cost was added to the total costs for each patient having at least one ISI score > 7.
Absenteeism Costs
Absenteeism (i.e., time missed from work) information was not collected during the clinical trial. Therefore, the same procedures described for the physician visit cost estimation were used to assign absenteeism costs to subjects who remitted or not from insomnia. Specifically, the excess cost of absenteeism ($231.20 for 6 months) in non-remitters was obtained similarly by retrospectively analyzing actual days of absences, short-term disability, and workers' compensation among patients in the MarketScan Health and Productivity Management Database (Thomson Medstat Inc, Ann Arbor, MI).
Presenteeism Costs
The method used to estimate utilities was again used to estimate the percentage of lost productivity while at work (i.e., presenteeism). WLQ values were observed at baseline and months 1, 3, and 6 for each patient enrolled in the trial. The percentage of work loss (or gain) for each patient was calculated as the 6-month average lost productivity minus the baseline lost productivity. By subtracting each patient's baseline value, the change in productivity directly attributable to the interventions in the clinical trial can be estimated.
Each subject's percentage of total work loss was multiplied by the average hourly wage of US workers ($26.86, total compensation per hour worked38), the average number of hours worked per week (33.9 hours39), and finally the percent of patients expected to be employed (78.72% of those between 18-65 years of age).40
Eszopiclone-Related Costs
The cost of eszopiclone was based on the average wholesale price as of January 1, 2006 ($403.81 per 100-day supply),41 reduced by 18.3% to reflect the over-estimate of actual pharmacy acquisition costs of brand name drugs42 plus a monthly dispensing fee of $1.98.43 It was conservatively assumed that all patients would receive one 10-minute physician visit for an established patient ($60.1844) and that those patients still using eszopiclone at month 3 would require one additional visit.
Handling of Missing Data
The economic analysis estimates the costs and QALYs on the basis of the observed outcomes of each individual patient enrolled in the trial. However, some patients enrolled in the trial had either missing observations at some time points or discontinued from the trial prematurely, which meant that their outcomes were not observed from the time of discontinuation to the end of the trial. Therefore, it was necessary to impute some of these missing observations using a variety of standard procedures described in the electronic Appendix B.
Sensitivity Analyses
Because economic analysis requires the reliance on assumptions, it is important to test their effects by using alternate scenario analyses and univariate sensitivity analyses. (The term univariate refers to the fact that only one of the key parameters is varied at a time, holding constant the values of all other parameters.) The range of values of the key parameters tested in these sensitivity analyses are presented in Table 1.
Table 1.
Medical Cost Parameter Values and Ranges Used in Sensitivity Analyses
| Parameter | Value | Range/Distribution | Source |
|---|---|---|---|
| Cost per physician visit | $60.18 | Triangular distribution, Min: $51.02, Max: $69.34 | Ingenix 2006 CPT Codebook44 |
| Difference in 6-month physician visits expenditure between having and not having remitted from insomnia | $542.00 | Triangular distribution, Min: $271.00, Max: $813.00 | Medstat Marketscan Database |
| Difference in 6-month absenteeism cost between patients having and not having remitted from insomnia | $231.20 | Triangular distribution, Min: $87.20, Max: $374.91 | Medstat Health & Productivity Management Database |
| Pharmacy dispensing fee per 30-day supply | $1.98 | Triangular distribution, Min: $1.49, Max: $2.48 | Novartis43 |
| Drug acquisition discount | 18.3% | Triangular distribution, Min: 13.73%, Max: 22.88% | Office of the Inspector General Medicaid Pharmacy42 |
| Percent of subjects working | 78.72% | Triangular distribution, Min: 59.04%, Max: 98.40% | Bureau of Labor Statistics40 |
| Average hours/week worked | 33.9 | Triangular distribution, Min: 25.43, Max: 42.38 | Bureau of Labor Statistics39 |
| Average hourly wage | $26.86 | Triangular distribution, Min: $10.15, Max: $33.58 | Hirth RA, et al.33 |
In addition to univariate sensitivity analyses, modern cost-effectiveness evaluations also incorporate some measure of uncertainty surrounding the results when all the parameters are varied simultaneously using multivariate sensitivity analysis. To test the variability and uncertainty of the clinical trial data on the results, an additional technique called bootstrapping was used. Bootstrapping involves simulating the clinical trial 5,000 times by randomly selecting a new population of the same size—with replacement—from the original population. Multivariate sensitivity analysis combined with bootstrapping provides an indication of degree of confidence associated with the results (e.g., one can estimate the percentage of the 5,000 simulations in which eszopiclone treatment saves costs compared to placebo) (see electronic Appendix C for more detail of these methods).
All model calculations were performed using Microsoft Excel (Microsoft Co., Redmond, WA) and sensitivity analyses were performed using @RISK (Palisade Co, Ithaca, NY).
RESULTS
Base Case Analysis
The analysis shows that the use of eszopiclone treatment resulted in an average QALY gain over baseline of 0.0105 for the eszopiclone group compared to a reduction in average QALY 0.0032 for the placebo group (Table 2). Thus, after 6 months, patients using eszopiclone gained an average 0.0137 QALYs over placebo.
Table 2.
Baseline and 6-Month QALYs in the Base Case Analysis
| Eszopiclone | Placebo | Difference | |
|---|---|---|---|
| Baseline utility* | 0.4020 | 0.4023 | |
| Average 6-month utility | 0.4125 | 0.3991 | |
| Net QALY gain (loss) | 0.0105 | (0.0032) | 0.0137 |
| Net 6-month cost | $495 | $428 | $67 |
| ICUR | $4,919 |
For reference, the QALYs of 6 months of perfect health are 0.5.
The corresponding estimated average 6-month costs for the eszopiclone and placebo groups were $495 and $428, respectively, resulting in a net additional cost of $67 with eszopiclone (Table 3). These included the cost of drugs, excess physician visits, and absenteeism for non-remitters, and the monetary value of productivity loss measured by the WLQ. The excess absenteeism costs of remitters versus non-remitters were higher in the placebo group than in the eszopiclone group because of the lower proportion of sustained remitters. The improvement in presenteeism measured by WLQ in the eszopiclone group was nearly twice that of the placebo group (1.00% vs. 0.57% over baseline, respectively). The estimated cost savings due to this increased productivity at work is $689 for the eszopiclone group vs. $333 for the placebo group.
Table 3.
Distribution of Per Patient Costs with Eszopiclone or Placebo Treatment
| Eszopiclone | Placebo | Difference | |
|---|---|---|---|
| (Eszopiclone minus Placebo) | |||
| Total drug-therapy related costs | $603 | $60 | +$543 |
| Mean eszopiclone treatment cost | $499 | $0 | +$499 |
| Physician visits associated with seeking therapy for insomnia | $104 | $60 | +$44 |
| Mean medical cost (outpatient physician visits for remitters and non-remitters) | $435 | $525 | −$90 |
| Absenteeism costs* | $146 | $176 | −$30 |
| Change in presenteeism costs from baseline* | −$689 | −$333 | −$356 |
| Average total cost per patient | $495 | $428 | +$67 |
The absenteeism costs were estimated as a positive number corresponding to the total costs from baseline to 6 month in absence from work. The interpretation of this outcome is that lower absenteeism costs are more desirable. In contrast to the absenteeism costs, the presenteeism costs were estimated in terms of reduced productivity while at work with poor health compared to baseline levels. Thus, this outcome could be negative or positive (depending on whether the costs were reduced or increased compared to baseline, respectively). The interpretation of this outcome is similar to the absenteeism costs, i.e., the lower the reduction, the greater the savings in presenteeism costs.
Thus, eszopiclone resulted in a cost per QALY gained of $4,919:
Alternate Scenario Analyses
When all productivity costs or outpatient visit costs were excluded (separately or simultaneously), the ICURs remained below the generally accepted cost-effectiveness threshold of $50,000 (Table 4).
Table 4.
Scenario Analyses
| Scenario | Incremental |
ICUR | |
|---|---|---|---|
| QALY | Cost | ||
| Base case | 0.0137 | $67 | $4,919 |
| Alternate assumptions | |||
| Excluding productivity | 0.0137 | $453 | $33,026 |
| Excluding excess physician visit costs | 0.0137 | $157 | $11,422 |
| Excluding productivity and excess physician visit costs | 0.0137 | $542 | $39,529 |
Sensitivity Analyses
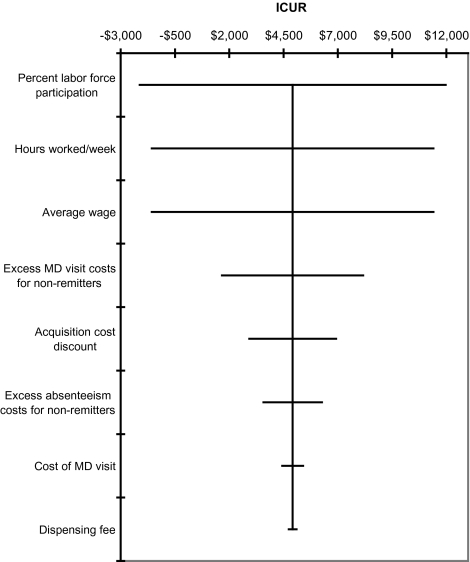
The results of univariate sensitivity analyses on model parameters listed in Table 1 show that the model is most sensitive to assumptions regarding productivity (i.e., percent labor force participation, hours worked/week, average wage) and excess physician service costs (Figure 1).
Figure 1.
Univariate sensitivity analysis of selected model parameters. ICUR = incremental cost-utility ratio.
To test the uncertainty associated with both the underlying trial data and the parameter uncertainty, a set of analyses was conducted in which both the bootstrap and the multivariate sensitivity analysis were carried out simultaneously, whereby each random draw from the parameter distributions was linked to a different bootstrap trial simulation. In these analyses, 50% of the 5,000 model replications resulted in an ICUR of $5,418 or less. In 95% of these replications, the ICUR was $34,935 or less.
DISCUSSION
The present analysis was designed to assess the cost-effectiveness of eszopiclone in the management of chronic primary insomnia. The analysis combined data from the literature, retrospective database analyses, and patient-level data from a clinical trial to estimate the quality-adjusted life years gained and costs associated with treatments over a period of 6 months.
The base-case estimates of the cost per QALY gained with eszopiclone were $4,919 (including productivity costs) and $33,026 (excluding productivity costs). These cost-utility ratios compare favorably with those of other generally accepted medical interventions, and are below the commonly used (yet somewhat arbitrary) US threshold of $50,000 that designates the level below which society is willing to pay for an additional QALY. These results are also comparable to the cost utility of treatments for other generally non-life threatening conditions.45–50
The present analysis confirmed the preliminary, exploratory results obtained previously by Botteman et al.,26 who used a cruder methodology applied to a similar patient population from a different clinical trial. The previous analysis reported that 6 months of eszopiclone treatment of primary insomnia in non-elderly adults costs between $9,900 per QALY (including the impact of insomnia on productivity) and $36,900 per QALY (excluding the impact of insomnia on productivity). The current analysis is more reliable, however, because patient-level data were used to directly measure quality of life, work limitations, and remission of insomnia. The inclusion of the SF-36 allowed for the generation of utilities directly from a generic measure of HRQL. Additionally, the ability to use patient-level data allowed us to account for any within-trial uncertainty and to evaluate the degree of robustness of the estimated cost utility ratio.
Productivity costs were included in the model to capture the impairment insomnia has on daytime functioning. These productivity costs were measured directly (i.e., for presenteeism) via the WLQ and indirectly (i.e., for absenteeism, via imputation of the absenteeism costs of remitted and non-remitted insomnia). Because the inclusion of these costs together with estimates of quality of life/utilities into an analysis is somewhat controversial,51,52 we excluded these costs in sensitivity analyses and found that eszopiclone remained cost-effective even without considering productivity costs.
Limitations
Despite its methodological strengths, the current analysis also has a number of limitations.
The study of Walsh et al., on which this analysis is based, collected medical care resource use via the Health Utilization Questionnaire. However, no significant difference in resource use was found between the eszopiclone and placebo groups, and the overall level of resources was low. In the absence of a measured difference, one might argue that no such difference should be accounted for in the analysis. Several reasons discussed above justify the inclusion of at least some of these costs in the base case analysis. First, previous economic studies have demonstrated increased inpatient and outpatient utilization due to insomnia.1–8,53 Second, as patients in the trial were receiving protocol-driven care (including physician visits at monthly intervals as part of the study), it was not surprising to observe a low rate of physician visits in each treatment group. Another possible reason for the low rate of health care utilization in the trial may be that the trial populations were healthier than the general insomnia population due to the multitude of exclusion criteria common in clinical trials. In order to be conservative, the current analysis assumed no difference in medical costs for remitters and non-remitters, except for the portion attributable to physician visits. Nevertheless, even when excess physician costs were excluded in sensitivity analysis, the cost utility ratio remained below the $50,000 threshold when including ($11,422) and excluding ($39,529) productivity.
One of the advantages of the current analysis is the inclusion of the WLQ as a direct measure of productivity. However, the questionnaire was designed for employed people, and employment status was not captured in the study. We observed a high amount of missing WLQ data (10% of patients had no WLQ value for any visit, and 19% of all visits with ISI values had missing WLQ scores), and it is possible that unemployed patients completed the questionnaire, which may have affected the WLQ results.
Similarly, because we were not aware of the proportion of employed individuals in the trial population, we estimated this value to be equal to the percent employed in the general US population. This may be an overestimation of the actual percent employment because insomnia is known to interfere with work activities, perhaps indicating that an insomnia population would have a lower level of employment than the general population. On the other hand, insomnia may be perceived as a more significant economic problem in working populations. If workers are more likely to seek treatment for their insomnia, the results may underestimate the benefits of therapy. These limitations may be of particular concern because the gain in productivity constituted a large amount of the cost savings associated with eszopiclone treatment.
Despite these limitations, the results may be particularly relevant to employers in whom therapy may actually lead to net cost savings. That is, as the cost of an hour of work loss increases, the economic value of reducing the burden of insomnia increases. For instance, in the present analysis, if the cost of an hour of work loss costs $24.66 or more, then therapy with eszopiclone becomes cost saving in those subjects participating in the labor force.
Finally, the present cost utility findings reflect the experience of patients enrolled in a clinical efficacy trial, although some assumptions and modeling of external data were also required. As such, the results may have a high degree of internal validity, but a lower degree of external validity. Therefore, caution should be used in extrapolating these results to a non-clinical trial setting of day-to-day clinical practice. For instance, only one dose (3 mg) of eszopiclone was used in this trial, and the effectiveness of lower doses was not evaluated (although approximately 66% percent of prescriptions for adults are for 3 mg). In addition, there was no attempt in this analysis to model behaviors such as treatment holidays in which a patient stops taking treatment for some time (treatment switches, non-adherence, etc). In particular, physicians in non-experimental clinical setting might consider terminating or switching therapy in non-responders, or discontinuing therapy after a few months in remitted patients. This could have important implications for the economic evaluation and warrant additional research and analysis. Thus, it would be most appropriate to interpret the findings of this analysis within the context of a placebo-controlled study, a limitation also shared by many similar economic analyses.
Conclusions
For decades, private and public policy decision makers have had to rely only on efficacy and safety data to determine the intrinsic worth of insomnia therapies with relatively limited evidence of the direct and indirect economic burden of the condition. The value of our analysis to decision makers is that it provides evidence that pharmacotherapy (here with eszopiclone) for the management of primary insomnia is cost-effective.
DISCLOSURE STATEMENT
This study was sponsored by Sepracor, Inc., the manufacturer of eszopiclone. Dr. Snedecor is employed by Pharmerit North America, LLC which performed the research work for Sepracor. Dr. Botteman is a health economic consultant and is the managing partner and co-owner of Pharmerit North America, LLC. Mr. Bojke is a director of Pharmerit Ltd, the UK branch of Pharmerit. Mr. Schaefer and Ms. Barry are employees of Sepracor. Dr. Pickard is an independent consultant and managing partner of Second City Outcomes Research, LLC and was retained by Pharmerit to participate in this study.
ACKNOWLEDGMENTS
The authors also wish to extend thanks to the editor and three anonymous reviewers for their constructive comments on an earlier draft of the manuscript.
SUPPLEMENTARY DATA
Electronic Appendix A
Eight algorithms were initially considered to transform the SF-36 data collected at baseline and months 1, 3, and 6 into utility formats. Four of these were excluded because they (a) excluded the SF-36 vitality domain, previously determined to be clinically relevant to insomnia,54 (b) were not considered to be a “generic algorithm” representing societal preferences because they were age- and/or sex-dependent,55,56 or (c) were based on visual analog scores, which are not considered to be a preference-based measure.57
In addition the algorithm used in the base case analysis, the use of the other 3 algorithms was tested in scenario analysis, including another mapping MEPS data to the EQ-5D (Lawrence and Fleishman),58 one using a much smaller US sample (Franks et al., 2003)59 and an algorithm based on a UK patient sample mapping to the standard gamble utility (Brazier and Roberts).60 The Brazier and Roberts algorithm may not be ideal for US decision makers because of its UK population basis, but it was included because of its methodological merit of using a direct, preference-based utility measure.
Using these alternate utility algorithms relying on US population estimates resulted in relatively constant estimates of cost per QALY (Lawrence and Fleishman: $5,420; Franks et al. 2004: $4,889).34,58 The Brazier and Roberts algorithm, based on a UK population sample and the standard gamble utility, generated a higher mean ICUR of $10,089.
Electronic Appendix B
The economic analysis estimates the costs and QALYs on the basis of the observed ISI (to estimate medical costs and absenteeism) and utilities (estimated by transformation of SF-36 scores) of each individual patient enrolled in the trial. However, some patients enrolled in the trial had either missing observations at some time points or discontinued prematurely. Therefore, it was necessary to impute some of these missing observations as follows.
Missing ISI observations for subjects who did not discontinue the trial (i.e., ISI data missing between 2 non-missing time points) were imputed using the method of last observation carried forward. More patients in the placebo group did not complete the trial than in the eszopiclone group (52.1% vs. 36.9%, respectively). Missing ISI values following trial discontinuation were imputed with the average post-treatment ISI value stratified by treatment group and remission status at discontinuation. For example, remitters at the time of discontinuation in the placebo group were assigned the average post-treatment ISI value for all placebo patients who were observed as being in remission at month 6. Similarly, placebo subjects who were not in remission at discontinuation were assigned the average post-treatment ISI for placebo patients who were non-remitters at month 6. Given the available data, this method of relating the last observed value to the first observed value after a period of no active treatment is a reasonable approximation, although there may be some level of inaccuracy in this prediction because discontinuation to blinded placebo treatment and discontinuation to no treatment would not necessarily generate equivalent results due to the possibility of a placebo effect.
The productivity loss given by the WLQ responses was assessed at baseline and months 1, 3, and 6. WLQ values missing for any reason were imputed using a two-step regression model (Table B1). The first step used a Probit model with random effects to examine the probability that any work loss has occurred (WLQ > 0) based on the patients' age, ISI, and time-invariant patient-specific random effect at the corresponding visit. This probability was multiplied by the expected WLQ determined by a generalized linear model based on the visit ISI (either observed or imputed) and a random effect term.
Table A1.
Cost-Effectiveness Using Alternative SF-36 to Utility Transformation Methods
Table B1.
Model UTILITY and WLQ Parameter Values and Ranges Used in Sensitivity Analysis
| Regression to estimate missing utility values | Regression coefficients to estimate missing WLQ values |
||
|---|---|---|---|
| Mean (SE)* | Part 1: Probability of non-zero WLQ Mean (SE)* | Part 2: Estimation of WLQ given WLQ > 0 Mean (SE)* | |
| Constant | −2.683 (0.086) | 1.571 (0.305) | 1.051 (0.114) |
| ISI | 0.041 (0.002) | 0.038 (0.006) | 0.053 (0.003) |
| Age | 0.005 (0.002) | −0.021 (0.006) | |
| Random Effect** | 0.285 (0.016) | 2.001 (0.226) | 0.289 (0.027) |
In the PSA, all parameters were assumed to have a normal distribution with standard deviation equal to the SE of the estimate.
Not included in probabilistic sensitivity analysis
Missing utility values were also imputed using a generalized linear regression model with random effects relating the expected ISI value at the missing visit and age to the expected utility generated from the utility algorithm (Table B1).
Electronic Appendix C
The bootstrapping method of uncertainty analysis entails simulating the trial population (by treatment group) a large number of times (e.g., 5,000 times) by randomly selecting a new population of the same size–with replacement–from the original trial population. Each of these “new” trials is then simulated and each leads to new outcomes. Characterizations of the distribution of the bootstrapped outcomes illustrate the amount of uncertainty contained within the clinical trial data.
Univariate and multivariate sensitivity analysis is conducted by creating new simulations by replacing the model parameters with random draws from specified probability distributions (e.g., normal, uniform, or other) reflecting the uncertainty about each parameter (Table 1 and Table B1 in Appendix B). For every combination of random draws, a new outcome results is estimated. A large number of these simulations are run to generate a distribution of outcomes, again providing an indication of the level of uncertainty associated with key model parameters. Used together, bootstrapping and multivariate sensitivity analysis offers a means to test the joint uncertainty associated with the underlying trial data and the model parameters.
REFERENCES
- 1.Hajak G. Epidemiology of severe insomnia and its consequences in Germany. Eur Arch Psychiatry Clin Neurosci. 2001;251:49–56. doi: 10.1007/s004060170052. [DOI] [PubMed] [Google Scholar]
- 2.Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA. The relationship between chronically disrupted sleep and healthcare use. Sleep. 2002;25:289–96. [PubMed] [Google Scholar]
- 3.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–35. [PubMed] [Google Scholar]
- 4.Kuppermann M, Lubeck DP, Mazonson PD, et al. Sleep problems and their correlates in a working population. J Gen Intern Med. 1995;10:25–32. doi: 10.1007/BF02599573. [DOI] [PubMed] [Google Scholar]
- 5.Leger D, Scheuermaier K, Philip P, Paillard M, Guilleminault C. SF-36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom Med. 2001;63:49–55. doi: 10.1097/00006842-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Leger D, Guilleminault C, Bader G, Levy E, Paillard M. Medical and socio-professional impact of insomnia. Sleep. 2002;25:625–9. [PubMed] [Google Scholar]
- 7.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 8.Zammit GK, Weiner J, Damato N, Sillup GP, McMillan CA. Quality of life in people with insomnia. Sleep. 1999;22(Suppl 2):S379–S385. [PubMed] [Google Scholar]
- 9.Martin SA, Aikens JE, Chervin RD. Toward cost-effectiveness analysis in the diagnosis and treatment of insomnia. Sleep Med Rev. 2004;8:63–72. doi: 10.1016/j.smrv.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 10.National health expenditure projections 2007-2017. http://www cms hhs gov/NationalHealthExpendData/Downloads/proj2007 pdf [serial online] 2007.
- 11.Garber AM. Cost-effectiveness and evidence evaluation as criteria for coverage policy. Health Affairs (Millwood) 2004. Web Exclusive. doi: 10.1377/hlthaff.w4.284. [DOI] [PubMed] [Google Scholar]
- 12.Neumann PJ, Lin PJ, Greenberg D, et al. Do drug formulary policies reflect evidence of value? Am J Manag Care. 2006;12:30–36. [PubMed] [Google Scholar]
- 13.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 14.Hatoum HT, Kong SX, Kania CM, Wong JM, Mendelson WB. Insomnia, health-related quality of life and healthcare resource consumption. A study of managed-care organisation enrollees. Pharmacoeconomics. 1998;14:629–37. doi: 10.2165/00019053-199814060-00004. [DOI] [PubMed] [Google Scholar]
- 15.Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22(Suppl 2):S386–S393. [PubMed] [Google Scholar]
- 16.Leger D, Levy E, Paillard M. The direct costs of insomnia in France. Sleep. 1999;22(Suppl 2):S394–S401. [PubMed] [Google Scholar]
- 17.Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65(Suppl 8):13–19. [PubMed] [Google Scholar]
- 18.Godet-Cayre V, Pelletier-Fleury N, Le VM, Dinet J, Massuel MA, Leger D. Insomnia and absenteeism at work. Who pays the cost? Sleep. 2006;29:179–84. doi: 10.1093/sleep/29.2.179. [DOI] [PubMed] [Google Scholar]
- 19.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30:263–73. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 20.Chevalier H, Los F, Boichut D, et al. Evaluation of severe insomnia in the general population: results of a European multinational survey. J Psychopharmacol. 1999;13:S21–S24. doi: 10.1177/026988119901304S04. [DOI] [PubMed] [Google Scholar]
- 21.Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335–49. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 22.Byles JE, Mishra GD, Harris MA. The experience of insomnia among older women. Sleep. 2005;28:972–9. doi: 10.1093/sleep/28.8.972. [DOI] [PubMed] [Google Scholar]
- 23.Le GY, Gagnadoux F, Hureaux J, et al. Sleep disturbances and impaired daytime functioning in outpatients with newly diagnosed lung cancer. Lung Cancer. 2007;58:139–43. doi: 10.1016/j.lungcan.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Technology Appraisal Guidance 77 Guidance on the use of zaleplon, zolpidem and zopiclone for the short-term management of Insomnia. Issue date: April 2004 Review date: April 2007. www.nice.org.uk/guidance/TA077 [serial online] 2007.
- 25.National Institutes of Health. State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. June 13-15, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Botteman MF, Ozminkowski RJ, Wang S, Pashos CL, Schaefer K, Foley DJ. Cost effectiveness of long-term treatment with eszopiclone for primary insomnia in adults: a decision analytical model. CNS Drugs. 2007;21:319–34. doi: 10.2165/00023210-200721040-00005. [DOI] [PubMed] [Google Scholar]
- 27.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 28.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959–68. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE. SF-36 health survey: Manual and interpretation guide. Quality Metric Inc. 2003 [Google Scholar]
- 31.Lerner D, Amick BC, III, Rogers WH, Malspeis S, Bungay K, Cynn D. The Work Limitations Questionnaire. Med Care. 2001;39:72–85. doi: 10.1097/00005650-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–28. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 33.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–42. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 34.Franks P, Lubetkin EI, Gold MR, Tancredi DJ, Jia H. Mapping the SF-12 to the EuroQol EQ-5D Index in a national US sample. Med Decis Making. 2004;24:247–54. doi: 10.1177/0272989X04265477. [DOI] [PubMed] [Google Scholar]
- 36.Chilcott LA, Shapiro CM. The socioeconomic impact of insomnia. An overview. Pharmacoeconomics. 1996;10(Suppl 1):1–14. doi: 10.2165/00019053-199600101-00003. [DOI] [PubMed] [Google Scholar]
- 36.Metlaine A, Leger D, Choudat D. Socioeconomic impact of insomnia in working populations. Ind Health. 2005;43:11–19. doi: 10.2486/indhealth.43.11. [DOI] [PubMed] [Google Scholar]
- 37.Stoller MK. Economic effects of insomnia. Clin Ther. 1994;16:873–97. [PubMed] [Google Scholar]
- 38.Bureau of Labor Statistics. Employer costs for employee compensation-June 2006, Table. 1 - Employer costs per hour worked for employee compensation and costs as a percent of total compensation: Civilian workers, by major occupational and industry group, June 2006. [Accessed December 7, 2007];US Department of Labor [serial online] 2006. [Google Scholar]
- 39.Bureau of Labor Statistics. The employment situation: October 2006. Table A. Major indicators of labor market activity, seasonally adjusted. Total private hours of work. [Accessed December 7, 2007];US Department of Labor [serial online] 2007. [Google Scholar]
- 40.Bureau of Labor Statistics. Table A-13. Employment status of the civilian noninstitutional population by age, sex, and race. [Accessed December 7, 2007];US Department of Labor [serial online] 2006. [Google Scholar]
- 41.Physician's desk reference red book 2006. Oxford: Blackwell; 2006. [Google Scholar]
- 42.Office of the Inspector General Medicaid Pharmacy. Actual Acquisition Cost of Prescription Drug Products for Brand Name Drugs. [Accessed September 13, 2007];Department of Health and Human Services [serial online] 2007. [Google Scholar]
- 43.Novartis. Novartis pharmacy benefit report: facts & figures; 1999
- 44.Ingenix. CPT Expert - 2006. Thomson Delmar Learning, 2005
- 45.Thompson M, Gawel M, Desjardins B, Ferko N, Grima D. An economic evaluation of rizatriptan in the treatment of migraine. Pharmacoeconomics. 2005;23:837–50. doi: 10.2165/00019053-200523080-00008. [DOI] [PubMed] [Google Scholar]
- 46.Kobelt G, Jonsson L, Mattiasson A. Cost-effectiveness of new treatments for overactive bladder: the example of tolterodine, a new muscarinic agent: a Markov model. Neurourol Urodyn. 1998;17:599–611. doi: 10.1002/(sici)1520-6777(1998)17:6<599::aid-nau4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 47.McDonald H, Hux M, Brisson M, Bernard L, Nickel JC. An economic evaluation of doxazosin, finasteride and combination therapy in the treatment of benign prostatic hyperplasia. Can J Urol. 2004;11:2327–40. [PubMed] [Google Scholar]
- 48.Baladi JF, Menon D, Otten N. An economic evaluation of finasteride for treatment of benign prostatic hyperplasia. Pharmacoeconomics. 1996;9:443–54. doi: 10.2165/00019053-199609050-00007. [DOI] [PubMed] [Google Scholar]
- 49.Brown JS, Papadopoulos G, Neumann PJ, Friedman M, Miller JD, Menzin J. Cost-effectiveness of topiramate in migraine prevention: results from a pharmacoeconomic model of topiramate treatment. Headache. 2005;45:1012–22. doi: 10.1111/j.1526-4610.2005.05182.x. [DOI] [PubMed] [Google Scholar]
- 50.Smith KJ, Roberts MS. Antiviral therapies for herpes zoster infections. Are they economically justifiable? Pharmacoeconomics. 2000;18:95–104. doi: 10.2165/00019053-200018020-00001. [DOI] [PubMed] [Google Scholar]
- 51.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 52.Weinstein MC, Siegel JE, Garber AM, et al. Productivity costs, time costs and health-related quality of life: a response to the Erasmus Group. Health Econ. 1997;6:505–10. doi: 10.1002/(sici)1099-1050(199709)6:5<505::aid-hec294>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 53.Lichstein KL, Durrence HH, Bayen UJ, Riedel BW. Primary versus secondary insomnia in older adults: subjective sleep and daytime functioning. Psychol Aging. 2001;16:264–71. doi: 10.1037//0882-7974.16.2.264. [DOI] [PubMed] [Google Scholar]
- 54.Fryback DG, Lawrence WF, Martin PA, Klein R, Klein BE. Predicting Quality of Well-being scores from the SF-36: results from the Beaver Dam Health Outcomes Study. Med Decis Making. 1997;17:1–9. doi: 10.1177/0272989X9701700101. [DOI] [PubMed] [Google Scholar]
- 55.Lundberg L, Johannesson M, Isacson DG, Borgquist L. The relationship between health-state utilities and the SF-12 in a general population. Med Decis Making. 1999;19:128–40. doi: 10.1177/0272989X9901900203. [DOI] [PubMed] [Google Scholar]
- 56.Nichol MB, Sengupta N, Globe DR. Evaluating quality-adjusted life years: estimation of the health utility index (HUI2) from the SF-36. Med Decis Making. 2001;21:105–12. doi: 10.1177/0272989X0102100203. [DOI] [PubMed] [Google Scholar]
- 57.Shmueli A. The relationship between the visual analog scale and the SF-36 scales in the general population: an update. Med Decis Making. 2004;24:61–63. doi: 10.1177/0272989X03261562. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence WF, Fleishman JA. Predicting EuroQoL EQ-5D preference scores from the SF-12 Health Survey in a nationally representative sample. Med Decis Making. 2004;24:160–9. doi: 10.1177/0272989X04264015. [DOI] [PubMed] [Google Scholar]
- 59.Franks P, Lubetkin EI, Gold MR, Tancredi DJ. Mapping the SF-12 to preference-based instruments: convergent validity in a low-income, minority population. Med Care. 2003;41:1277–83. doi: 10.1097/01.MLR.0000093480.58308.D8. [DOI] [PubMed] [Google Scholar]
- 60.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–9. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]