Abstract
p63, a homolog of the tumor suppressor p53, is critical for the development and maintenance of complex epithelia. The developmentally regulated p63 isoform, ΔNp63, can act as a transcriptional repressor, but the link between the transcriptional functions of p63 and its biological roles is unclear. Based on our initial finding that the mesoderm-inducing factor activin A is suppressed by ΔNp63 in human keratinocytes, we investigated the role of ΔNp63 in regulating mesoderm induction during early Xenopus laevis development. We find that down-regulation of ΔNp63 by morpholino injection in the early Xenopus embryo potentiates mesoderm formation whereas ectopic expression of ΔNp63 inhibits mesoderm formation. Furthermore, we show that mesodermal induction after down-regulation of ΔNp63 is dependent on p53. We propose that a key function for p63 in defining a squamous epithelial phenotype is to actively suppress mesodermal cell fates during early development. Collectively, we show that there is a distinct requirement for different p53 family members during the development of both mesodermal and ectodermal tissues. These findings have implications for the role of p63 and p53 in both development and tumorigenesis of human epithelia.
Keywords: Activin A, p53, p63, mesoderm, differentiation, EMT, Xenopus
Introduction
p63 is a homolog of the tumor suppressor p53 (Augustin et al., 1998; Osada et al., 1998; Yang et al., 1998; Yang and McKeon, 2000). In contrast to p53, which is inactivated in a majority of human cancers but is largely dispensable for normal development, p63 is critical for the development and maintenance of stratified epithelial tissues. The most striking developmental abnormality of p63−/− mice is a complete lack of stratified epithelia and its derivatives (Mills et al., 1999; Yang et al., 1999). This phenotype is recapitulated in zebrafish, in which disruption of p63 results in a lack of epidermal morphogenesis (Bakkers et al., 2002b; Lee and Kimelman, 2002a). Immunolocalization studies of p63 indicate that it is primarily expressed in the basal compartment of epithelia in the epidermis, oral mucosa, cervix, vaginal epithelium, urothelium, prostate, and breast (Dellavalle et al., 2001; Di Como et al., 2002; Yang et al., 1998) where epithelial stem cells have been postulated to reside (Pellegrini et al., 2001). p63 is essential for the proliferative potential and “stemness” of epithelial progenitor cells (Senoo et al., 2007; Yi et al., 2008), and appears to be required for epidermal stem cells to initiate a program of terminal differentiation (Truong et al., 2006; Koster et al., 2007). Taken together, these data suggest that p63 plays critical roles in development, maintenance, and differentiation of stem cells in stratified epithelia.
The p63 gene is expressed as six different transcripts in mammalian cells, all of which encode a DNA-binding domain with significant homology to that of p53 (Harms et al., 2004; Westfall et al., 2003). Gene expression and whole genome chromatin immunoprecipitation studies have shown that p63 isoforms and p53 have overlapping and distinct target gene specificities (Vigano et al., 2006; Birkaya et al., 2007). Of the six isoforms of p63, ΔNp63α and ΔNp63α are predominantly expressed in early development (Bakkers et al., 2002a; Lee and Kimelman, 2002b; Lu et al., 2001). These ΔNp63 isoforms encode proteins lacking the amino terminus and act as transcriptional repressors in vitro. In addition, the use of alternative promoters can also produce an isoform containing a transactivation domain, denoted TAp63. ΔNp63 isoforms potently oppose p53- and TAp63-mediated transactivation (Bakkers et al., 2002b; Lee and Kimelman, 2002a; Westfall et al., 2003; Yang et al., 1998), raising the possibility that this p63 isoform acts in a dominant-negative manner towards p53 and/or TAp63 isoforms during development and tumorigenesis.
One of the most critical events during early embryogenesis is the induction of the germ layers. In vertebrates, germ layer formation occurs at the onset of zygotic transcription and is initiated when signals from the vegetal half of the embryo induce the formation of mesodermal tissue in the overlying animal half (reviewed in Kimelman, 2006). The TGF-β superfamily of growth factors has been shown to play prominent roles in mesoderm induction (Kimelman, 2006). Among the TGF-β superfamily, activin was one of the first and most potent mesoderm inducers to be identified (Smith et al., 1990), although the main role as mesoderm inducers in vertebrates is thought to be covered by Nodal-related TGF-β ligands (Jones et al., 1995; Agius et al., 2000; Ramis et al., 2007). Studies in Xenopus laevis demonstrate that induction of mesodermal tissues by the TGF-β signaling cascade is in part mediated by p53 through its interaction with Smad2 (Cordenonsi et al., 2003; Takebayashi-Suzuki et al., 2003). Loss and gain-of-function studies indicate that p53 is both necessary and sufficient to induce mesoderm formation in Xenopus (Cordenonsi et al., 2003).
Herein, we investigate the role of a p63 isoform, ΔNp63, in Xenopus laevis early development. We show that ectopic expression of ΔNp63 inhibits mesoderm formation in Xenopus embryos and that this effect is dependent on the ability of ΔNp63 to bind DNA. Conversely, morpholino-induced down-regulation of endogenous ΔNp63 potentiates mesoderm induction in Xenopus embryos. Also, we show that under conditions of ΔNp63 down-regulation, induction of mesodermal tissues is p53-dependent, suggesting that ΔNp63 can antagonize the mesoderm-inducing functions of p53. Collectively, we show a requirement for different p53 family members in germ layer specification during Xenopus laevis development.
Materials And Methods
Cell culture
The human keratinocyte cell lines HaCaT and HaCaT-RG were generously provided by P. Boukamp (Boukamp et al., 1988). Human epidermal keratinocytes (NHEKs) were obtained from the Vanderbilt Skin Disease Research core. HaCaT and HaCaT-RG, cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and 1% penicillin-streptomycin. The A-549 human lung adenocarcinoma cell line (ATCC) was cultured in DMEM supplemented with 10% fetal calf serum, 10 μg/mL insulin, and 1% penicillin-streptomycin. All cells were cultured at 37°C with 5% CO2.
Cell transfection/infection and siRNA
Targeting oligonucleotides for p63 and GFP were designed as previously described (Barbieri et al., 2006; Brummelkamp et al., 2002). pCEP-H1 φ, pCEP-H1 GFP, and pCEP-H1 p63 expression vectors were generated as previously described (Barbieri et al., 2003). HaCaT, HaCaT-RG, and A-549 cells were transfected using Fugene 6 (Roche, Indianapolis, IN). Cells were selected with hygromycin B 48 h after transfection and harvested for RNA isolation as described below.
RNA isolation and Quantitative RT-PCR
HEKs were harvested and total RNA was isolated using the Aurum Total RNA Mini Kit (Biorad). HaCaT, HaCaT-RG, and A-549 cells were harvested and mRNA was isolated as previously described (Flatt et al., 2000). cDNA was made from the purified mRNA using TaqMan Reverse Transcription Reagents (Applied Biosystems) according to the manufacturer’s protocol; 500 ng of total RNA was used for each reaction. A 1:5 dilution of the resulting cDNA was used for all real-time RT -PCR experiments. Real-time PCR was conducted using an icycler (Biorad) with the iQ SYBR Green Supermix (Biorad) according to the manufacturer’s instructions. Primers specific for each gene were designed using the Beacon Designer 3 software (Premier Biosoft, Palo Alto, CA). Primer sequences are available upon request. All primers were used at a concentration of 200 nM. The cycling conditions for all genes were: 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and annealing at 55–61°C for 45 s, with data acquisition during each cycle. Melting curve analysis was conducted following PCR cycling to verify purity and quality of the PCR product.
Chromatin immunoprecipitation (ChIP)
Formaldehyde crosslinking, chromatin preparation and immunoprecipitation (ChIP) were carried out as described previously (Szak et al., 2001). For semi-quantitative ChIP experiments, cells were crosslinked and submitted to GenPathway, Inc. (San Diego, CA) according to their FactorPath protocol.
Xenopus embryo injections and manipulations
Xenopus laevis embryos obtained by in vitro fertilization were dejellied in 2% cysteine (pH 7.8) and cultured at 14–20°C in 10% Marc’s Modified Ringer (MMR) as previously described (Newport and Kirschner, 1982). Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop P.D., 1967). Morpholino oligonucleotides p63MO-A (5′-gagcattgttttccagatacaacat-3′; Genbank Accession Number NM_001085638), p63MO-B (5′-agcactgttttccagatacaacatc-3′; Genbank Accession Number AY459428), and a control morpholino targeting β-globin (5′-cctcttacctcagttacaatttata-3′) were obtained from Gene-Tools, LLC (Philomath, OR). Synthetic human ΔNp63α and ΔNp63α-R304W mRNA were transcribed from linearized pCS2(+) templates using the SP6 Message Machine kit (Ambion, Austin, TX); mRNA and morpholinos were injected into the dorsal blastomeres of four-cell embryos. Embryos were fixed and scored at stage 26. The observed phenotypes represent results from at least 3 independent experiments. Ectodermal explants (animal caps) dissected from stage-9 embryos after morpholino, ΔNp63α, and ΔNp63α-R304W mRNA injections were cultured in 75% MMR in the presence or absence of human activin A (R&D Systems, Minneapolis, MN). Explants were then fixed and photographed at sibling stage 18.
RNA extraction and RT-PCR analysis in embryos and explants
Whole embryos were injected at the 4-cell stage in their prospective dorsoanterior mesoderm with ΔNp63α mRNA (2 ng) or ΔNp63α-R304W mRNA (2 ng; Fig. 2,3). They were cultured until stage 10, when they were lysed and RNA was extracted for RT-PCR. Animal caps (Fig. 3) were injected with p53MO (30 ng), p63MO-A (200 ng), p63MO-B (200 ng), p63MO-A/B (100 ng of each) or controlMO (β-globin; 200 ng). Caps were dissected at stage 9 and cultured in the presence or absence of activin (10 ng/ml) until stage 11, when RNA was extracted for RT-PCR. RT-PCR primers and conditions were as described in http://www.hhmi.ucla.edu/derobertis/index.html. For each primer set, the number of cycles was determined by testing serial dilutions of reverse-transcribed cDNA, so that amplification would fall within the linear range. Ornithine decarboxylase (ODC) mRNA was used as control for RNA extraction and reverse transcription.
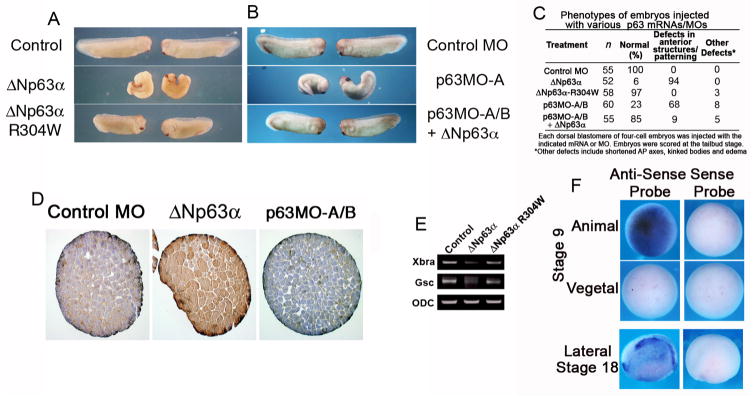
Figure 2. ΔNp63 loss and gain of function results in anterior patterning defects in Xenopus laevis.
(A) Four-cell Xenopus embryos were injected dorsally with ΔNp63 α or ΔNp63 -R304W mRNA (2 ng each) and scored at the tailbud stage. (B) Four-cell Xenopus embryos were injected dorsally with p63MO-A (200 ng) in the presence or absence of ΔNp63 α mRNA (2 ng), and scored at the tailbud stage. (C) Table summarizing results from embryo injections in (A) and (B). (D) Animal caps injected with control or p63MO, or ΔNp63-encoding RNA were fixed, sectioned, and stained for p63 (4A4 antibody). (E) Whole embryo RT-PCR shows that the pan-mesodermal marker Xbra and the anterior mesodermal marker Gsc are down-regulated in ΔNp63 α-injected but not ΔNp63 α-R304W-injected embryos. (F) In situ hybridization analysis shows that ΔNp63 is expressed in the animal pole of late blastula (stage 9) embryos and in most of the epidermis of mid-neurula (stage 18) embryos (anterior is on the left).
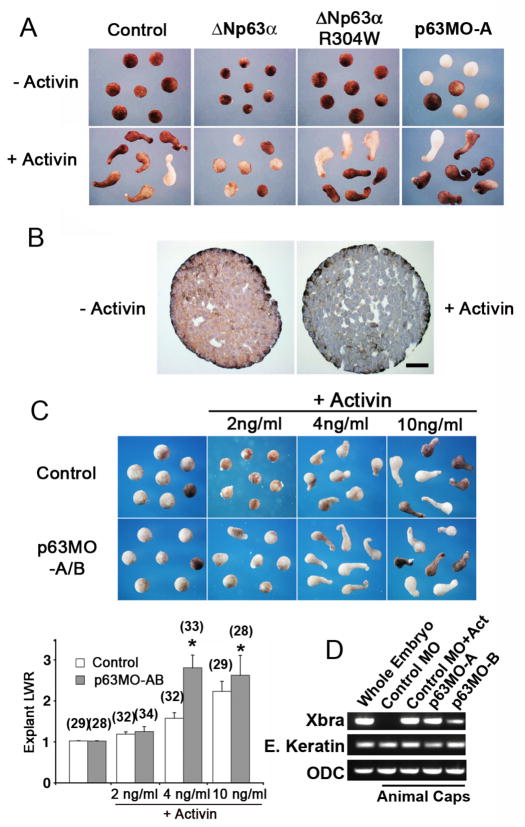
Figure 3. ΔNp63 suppresses activin-mediated mesoderm induction.
(A) ΔNp63 α suppresses activin-mediated explant elongation. Animal caps from embryos injected with ΔNp63α mRNA (2 ng), ΔNp63 α-R304W mRNA (2 ng), or p63MO-A (200 ng) were dissected at stage 9 and cultured overnight in the presence or absence of activin A (10 ng/ml). Embryos were then fixed and photographed at sibling stage 18. (B) Activin treatment of animal caps results in down-regulation of ΔNp63 expression. Caps were treated with activin (+Act, 10 ng/ml) for 2 hours or left untreated, fixed, sectioned, and stained for p63 (4A4 antibody). Scale bar is 50 μm. (C) Animal caps injected with p63MO-A and B (100 ng each) were cultured in the presence of the indicated concentrations of activin, fixed, and photographed at sibling stage 18. Length-to-width (LWR) ratios for these explants were determined as previously described (Tahinci et al., 2007). Numbers in parentheses indicate numbers of explants scored. Asterisks indicate significant difference between p63MO injected and the corresponding uninjected explants (p<0.01). (D) Animal caps injected with p63MO-A or p63-MO-B (200 ng) or a morpholino against -globin (control MO, 200 ng) were cultured in the presence or absence of activin (10 ng/ml). RNA was isolated and levels of the mesodermal marker brachyury (Xbra), the ectodermal marker epidermal keratin, and ODC (control for RNA extraction) were assayed by RT-PCR.
Whole embryo and animal cap sectioning and staining
Animal caps were dissected at stage 9 from uninjected or DNp63a (2 ng) or p63MO-injected embryos (200 ng; Fig. 3B). They were cultured in the presence or absence of activin A (10 ng/ml) until sibling stage 26, when they were fixed in MEMFA, embedded in paraffin, and sectioned. For p63 immunostaining, antigen retrieval was done by microwaving slides in 0.1 mol/L citrate buffer for 10 minutes. Slides were incubated 10 minutes in 3% hydrogen peroxide in methanol to exhaust endogenous peroxidase activity then incubated with a 1:40 dilution of p63 4A4 antibody (Santa Cruz, Santa Cruz, CA) for 1 hour at 25°C. The LSAB2 kit (Dako, Carpinteria, CA) was used to develop the slides. The slides were counterstained with hematoxylin. For whole embryos, mRNA and morpholinos were injected into the dorsal blastomeres at the four-cell stage, where indicated, and allowed to develop until stage 26. Embryos were formalin fixed, processed, embedded into paraffin and stained with hematoxylin and eosin for histological analysis.
In situ hybridization analysis
Embryos were injected with mRNA on one side only (two cells of the 4-cell stage). They were then fixed at mid-gastrula (stage 10.5) and processed for β-gal staining (Sanes et al., 1986; Red-Gal substrate, Research Organics, Cleveland, OH) and whole-mount in situ hybridization as described by (Harland, 1991). Digoxigenin-labeled cRNA probes for the pan-mesoderm marker brachyury (Xbra) (Smith et al., 1991) were used to assess the extent of mesoderm induction in these embryos. The relative extent of Xbra staining (as determined by comparing the thickest width of the Xbra domain of the injected versus the uninjected sides of the embryo) was measured using Image J. At least 3 independent sets of experiments were performed for each experimental condition. Induction of dorsal mesoderm and ventral mesoderm was assessed by using antisense probes for chordin and Xwnt 8, respectively. An antisense probe for p63 was obtained by cloning a partial Xenopus ΔNp63γ cDNA sequence from a mid-neurula embryo by RT-PCR, using standard procedures. cDNAs for Xp53 and Dlx3 were obtained from Open Biosystems (Huntsville, AL) and used to in vitro transcribe antisense in situ hybridization probes.
Whole-mount immunohistochemistry
Detection of somitic mesoderm with the monoclonal antibody 12/101 (Developmental Studies Hybridoma Bank; Kintner, 1984) was performed as previously described (Lane and Keller, 1997). Injected embryos were fixed in MEMFA at stage 26 and stained for β-gal activity (Sanes et al., 1986; X-gal substrate, Research Products International, Mt. Prospect, IL). Immunostaining was performed with horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibodies (Jackson Immunoresearch, West Grove, PA), and diaminobenzidine tetrahydrochloride (Sigma Aldrich, St. Louis, MO) was used for color development. The relative extent of somitic mesoderm formation (as determined by comparing the thickest width of the domain stained by the 12/101 antibody of the injected versus the uninjected sides the embryo) was calculated using Image J. At least 3 independent sets of experiments were performed for each experimental condition.
Results
ΔNp63 α inhibits activin signaling
Recently, we performed microarray expression analysis following RNAi-mediated depletion of p63 in a panel of human squamous epithelial cells (Barbieri et al., 2006). Analysis of the resulting expression data revealed that components of the activin pathway were transcriptionally upregulated (data not shown). Activins are members of the TGF-β family of growth factors that regulate gene transcription through Smad2 and Smad3 proteins (Chang et al., 2002). To our knowledge, a potential link between ΔNp63α and activin signaling has not been previously described; thus, we examined the expression of activin A and its target genes following down-regulation and over-expression of ΔNp63α protein.
To test our hypothesis that p63 regulates the activin pathway, we first assessed activin A levels following p63 down-regulation in cultured cells. Human HaCaT keratinocytes and HaCaT-RG (a rapidly growing derivative of HaCaT cells that have lost the ability to differentiate) cells were transfected with control and p63 siRNA expressing vectors. Disruption of p63 by siRNA in HaCaT and HaCaT-RG cells led to a 2 to 3-fold increase in expression of activin A (Fig. 1A). The increase in activin A expression in HaCaT and HaCaT-RG cells was accompanied by a similar increase in the expression of the activin-regulated genes CDKN2B and BAMBI (Ho et al., 2004; Tsang et al., 2000; Fig. 1A). A-549 human lung epithelial cells, which do not express detectable p63 protein, were also transfected with p63 siRNA to control for off-target effects (Barbieri et al., 2005). Significant increases in expression of activin A, CDKN2B, or BAMBI were not detected in A-549 control cells transfected with p63 siRNA, suggesting that the observed increases in the HaCaT cells were not due to off-target effects of the p63 siRNA, but rather directly due to specific depletion of p63.
Figure 1. ACTIVIN A is a direct target of transcriptional repression by ΔNp63α.
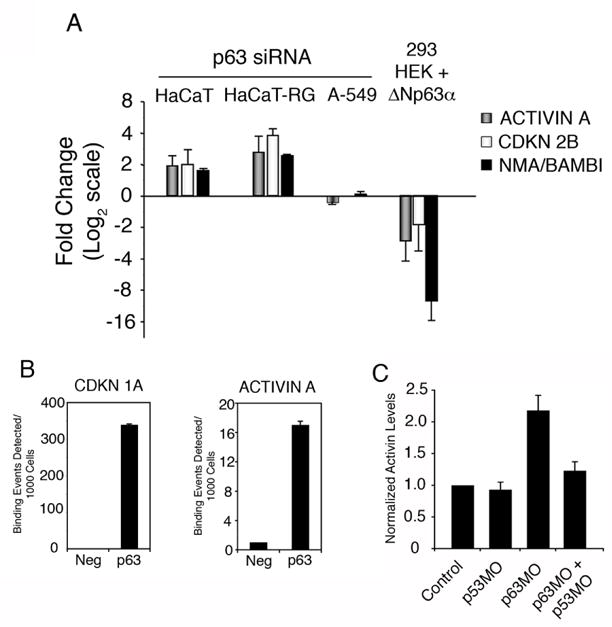
(A) GAPDH-normalized mRNA levels of ACTIVIN A, CDKN 2B, and BAMBI were determined by quantitative PCR in HaCaT, HaCaT-RG and A-549 cells expressing p63 siRNA (normalized to GFP siRNA) as well as HEK 293 cells infected with a ΔNp63 α-expressing adenovirus (normalized to control adenovirus). Data from three independent experiments are presented with standard deviation; data is presented as fold change with a base-2 logarithmic scale. (B) Human keratinocytes (NHEKs) were crosslinked and p63-bound chromatin was sonicated and purified. Semi-quantitative ChIP analysis was performed with primers flanking the known p63 response element in the CDKN1A gene or the candidate site in the INHBA (Activin A) gene as described in Materials and Methods. (C) Xenopus animal caps were injected with morpholino oligonucleotides targeting p63 and p53 singly, or in combination, or with a control morpholino targeting β-globin. RNA was isolated and the levels of activin was measured using quantitative real time PCR and normalized to the expression of ornithine decarboxylase (ODC). Data is representative of at lease three individual experiments.
To determine if the most abundant p63 isoform, ΔNp63α, has a similar effect on activin signaling, 293 human embryonic kidney (HEK) cells that do not express detectable levels of p63 were infected with adenovirus encoding this isoform. Levels of activin A, CDKN2B, and BAMBI were significantly decreased in 293 HEK cells ectopically expressing ΔNp63α as assessed by quantitative RT-PCR (Fig. 1A). These results demonstrate that ΔNp63α negatively regulates the activin signaling pathway in human squamous epithelial cells.
Activin A is a direct transcriptional target of ΔNp63α in human epithelia
Because p63 primarily regulates biological processes by modulating the transcriptional state of target genes, we examined whether activin A expression is directly controlled by p63. Based on the canonical p63 response element that we and others previously described (Perez et al., 2007; Osada et al., 2005), we identified a candidate p63 binding site located up-stream of the activin A promoter region. To test if the p63-mediated regulation of activin was direct, we employed quantitative chromatin immunoprecipitation for p63 in primary human keratinocytes (NHEK). As a positive control, we were able to successfully detect p63 binding to a known response element located in the CDKN1A (p21) gene (Westfall et al., 2003; Fig. 1B). Additionally, quantitative ChIP analysis revealed that p63 binding is robustly detected at the response element located up-stream of the activin A gene (Fig. 1B). ΔNp63α is the predominant isoform expressed in primary human keratinocytes, where it is present in ratios of at least 100:1 to TAp63 isoforms (Barbieri et al., 2006). While the antibody used can immunoprecipitate all p63 isoforms, the majority, if not all, of the signal detected in our ChIP experiment is a result of ΔNp63α binding to the activin site. Taken together, these data are consistent with activin A being a direct transcriptional target of repression by ΔNp63α in human epithelial cells.
ΔNp63α suppresses mesoderm formation in Xenopus
Activin is a potent mesoderm-inducing factor during Xenopus laevis development (Piepenburg et al., 2004). With this in mind, we shifted to Xenopus as a model system to explore the significance of the preliminary findings of a p63/activin signaling axis first observed in HaCaT cells. To confirm an in vivo role for p63 in regulation of Xenopus activin transcription, animal caps were injected with morpholino oligonucleotides targeting ΔNp63, p53, or both morpholinos simultaneously, and analyzed for activin expression using quantitative real time PCR. Consistent with data from HaCaT cells, activin levels are significantly increased following depletion of ΔNp63 in the animal caps (Fig. 1C). Further, this increase in activin expression is p53-dependent, as coinjection with both ΔNp63 and p53 morpholinos abrogated the increase in activin transcript levels observed in caps injected with p63MO alone (Fig. 1C). To control for p63MO specificity, immunohistochemical analysis of sectioned caps revealed that ΔNp63 expression is downregulated after injection of p63MO and upregulated after ΔNp63α mRNA injection (Fig. 2D). These observations suggest that activin may be a target of ΔNp63 transcriptional regulation in Xenopus laevis.
To determine if ΔNp63α-mediated inhibition of activin signaling in Xenopus was indicative of a potential role for ΔNp63 in regulating mesoderm formation during development, we tested the effects of ectopic expression and down-regulation of ΔNp63α on Xenopus mesoderm induction. Xenopus embryos were injected dorso-anteriorly with ΔNp63α mRNA and monitored for developmental abnormalities consistent with disruption of mesodermal induction. Immunohistochemical staining of sectioned animal caps confirmed that expression of p63 is exogenously upregulated in ΔNp63α-injected embryos (Fig. 2D). Compared to controls, 94% (49/52) of ΔNp63α-injected embryos displayed defects in gastrulation and anteroposterior patterning (e.g. spina bifida, reduced anterior structures), consistent with abnormal mesoderm formation (Fig. 2A, middle panel; Fig. S3). In contrast, 97% (56/58) of embryos injected with the mutant ΔNp63α-R304W, which is defective in DNA binding (Ying et al., 2005), proceeded through gastrulation normally and did not display anterior patterning defects (Fig. 2A, bottom panel; Fig. S3). Western blot analysis demonstrated that similar levels of ΔNp63α and ΔNp63α-R304W proteins were exogenously expressed, suggesting that a functional DNA binding domain is necessary for ΔNp63α to cause patterning defects during Xenopus development (data not shown). Additionally, we observed a marked reduction in the levels of mesodermal markers, brachyury (pan-mesodermal) and goosecoid (anterior mesodermal), in embryos injected with ΔNp63α mRNA, supporting our observation that ΔNp63α can act to inhibit mesoderm induction (Fig. 2E). Embryos injected with ΔNp63α-R304W mRNA displayed levels of brachyury and goosecoid similar to control embryos (Fig. 2E), suggesting that the DNA-binding activity of ΔNp63 is required for abrogating mesoderm induction. Taken together, these data suggest that ΔNp63α transcriptionally regulates genetic programs (i.e. activin signaling) that control mesoderm induction during development.
We next tested if down-regulation of ΔNp63 by morpholino oligonucleotide injection would have any effect on mesoderm induction. Xenopus embryos serve as a useful model system to study p63 function during development, as TAp63 isoforms are not present and ΔNp63 isoforms are expressed in the prospective embryonic ectoderm by the late blastula stages (Fig. 2F; Lu et al., 2001). Thus, we are able to completely control p63 signaling by modulation of ΔNp63 isoforms. To this end, we used a p63 morpholino oligonucleotide (p63MO-A, 200 ng) directed against the translational start site of the ΔNp63 transcript to abolish translation of all ΔNp63 isoforms. To control for off-target effects, a second morpholino directed against the translation initiation site of an alternate ΔNp63 transcript (p63MO-B, 200 ng) was used and identical embryonic phenotypes were observed (data not shown). In all subsequent experiments, an equimolar mix of the two morpholino oligonucleotides was used (p63MO-A/B, 100 ng each), unless noted otherwise. Immunohistochemical staining confirmed that injection of sequence-specific morpholinos significantly decreased the expression of ΔNp63 protein (Fig. 2D).
Disruption of Xenopus ΔNp63 expression by p63MO-A and p63MO-B-injection (100 ng each) resulted in enlargement of anterior embryonic structures in 68% (41/60) of injected embryos, consistent with increased formation of dorso-anterior mesoderm (Fig. 2B, C, S3). The p63MO phenotype was rescued by co-injection of human ΔNp63α mRNA in 85% (47/55) of injected embryos, providing strong evidence that the effect of the injected p63MO is specific for disruption of ΔNp63 (Fig. 2B, C). As control, a morpholino for β-globin had no effect on embryo development (Fig. 2B, C; data not shown).
To further confirm the role of ΔNp63 in mesoderm induction and its link to activin signaling, we used the “animal cap” (ectodermal explant) assay that has been extensively used to study mesoderm-inducing processes (Symes and Smith, 1987). Treatment of animal cap explants with activin (10 ng/ml) induces formation of dorsal mesodermal tissue and results in morphogenetic movements (as visualized by pronounced elongation of the explant) that are believed to mimic the process of convergent extension. Two-cell stage embryos were injected at the animal pole with ΔNp63α and ΔNp63α-R304W mRNA, and their animal caps dissected along with caps from uninjected control embryos. Caps were then cultured in the presence or absence of activin. Animal caps from control embryos treated with activin resulted in explant elongation, whereas animal caps from embryos injected with ΔNp63α mRNA blocked elongation of the explant induced by activin treatment; injection of ΔNp63α-R304W or either of the two p63MOs had no effect on cap elongation (Fig. 3A).
Interestingly, immunohistochemistry studies on isolated animal caps showed that endogenous p63 protein was down-regulated with activin treatment (Fig. 3B), supporting the animal cap data showing that exogenous ΔNp63α expression was sufficient to block activin-induced mesoderm formation. Although injection of p63MO oligonucleotides similarly resulted in loss of p63 protein expression in animal caps (Fig. 2D), this did not appear morphologically to be sufficient to induce explant elongation in animal caps (Fig. 3A). Interestingly, injection of animal caps with either p63MO-A or p63MO-B resulted in up-regulation of the mesodermal marker brachyury (Xbra) even in the absence of activin treatment (Fig. 3D). Further, we detected a p53-dependent induction of another activin-dependent mesodermal marker, Mix.2 (Vize, 1996), following morpholino-directed p63 depletion (Fig. S1B). However, despite the observed changes in mesoderm-specific genes, these caps were unable to elongate significantly, suggesting that they had low levels of dorsal mesoderm. Animal caps injected with both p63MO oligonucleotides, however, were sensitized to the effects of activin and underwent elongation at lower concentrations of activin compared to the uninjected control caps (Fig. 3C). Finally, upregulation or downregulation of p63 activity in developing gastrulae by mRNA or morpholino injections results in small but reproducible induction or inhibition of the ectodermal marker Dlx3 (Luo et al., 2001), respectively (Fig. S1). In summary, these results are consistent with a model in which ΔNp63 acts to suppress activin-mediated mesoderm induction in Xenopus embryos while promoting differentiation into ectodermal cell fates.
Loss of ΔNp63 function induces mesoderm in a p53-dependent manner
p53 has been previously shown to directly participate in the TGF-β signaling cascades that induce mesoderm formation in Xenopus embryos by directly interacting with Smad proteins (Cordenonsi et al., 2003; Takebayashi-Suzuki et al., 2003). Our current study indicates that the related ΔNp63 inhibits mesoderm induction in Xenopus embryos. In order to determine if a link exists between the mesoderm-inducing activity of p53 and the mesoderm-inhibiting activity of ΔNp63, we assessed the activities of both proteins in early and late mesodermal tissue formation in Xenopus embryos.
As an early marker of mesoderm induction, we performed in situ hybridization analysis for the pan-mesodermal marker brachyury (Xbra, Fig. 4A,D). As a later marker for assessing mesodermal tissue formation, we performed whole-mount immunohistochemistry using the muscle-specific monoclonal antibody 12/101. This antibody stains the somitic mesoderm, one of the more prominent structures of mesodermal origin in tailbud stage embryos, revealing bilateral, symmetrical, chevron-shaped somites along the length of the developing embryo (Fig. 4C).
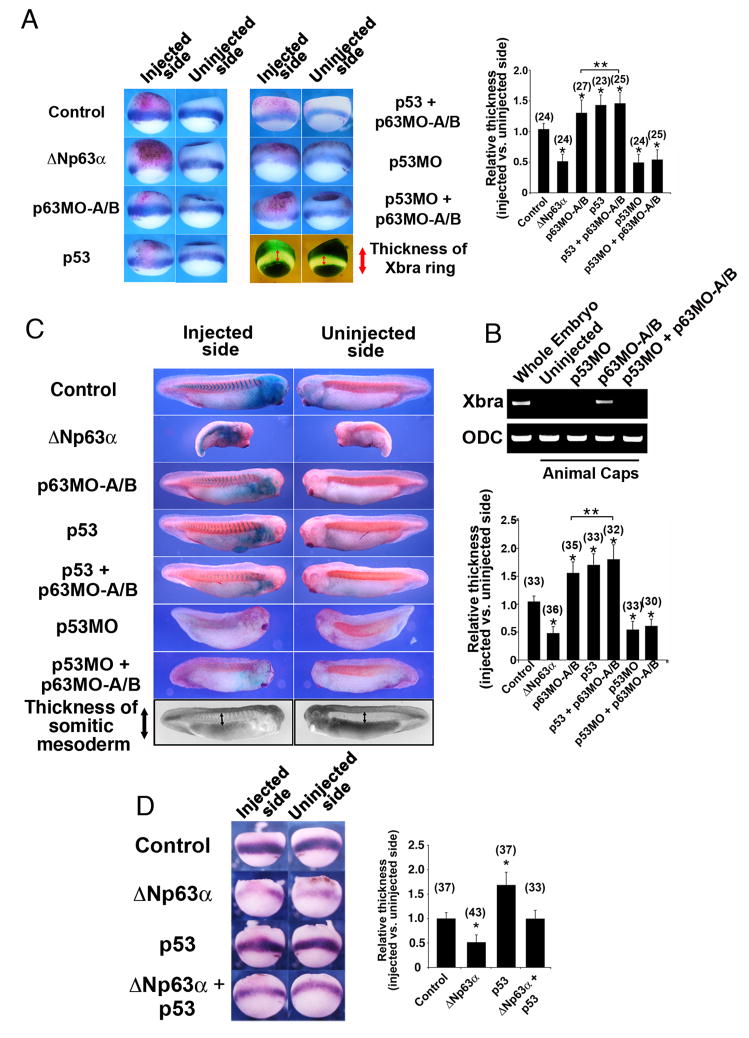
Figure 4. Mesoderm induction due to loss of p63 is p53-dependent.
(A) Embryos were injected with ΔNp63 mRNA (2 ng), p63MO-A/B (200 ng), p53 mRNA (100 pg), p53MO (30 ng), p53 mRNA + p63MO-A/B, or p53MO + p63MO-A/B. The relative extent of the Xbra expression domain between the injected and uninjected sides of each embryo was quantified as shown (two lower right image panels) and plotted on a bar graph. β-galactosidase mRNA (β-gal, 250 pg) was co-injected to mark the injection site. (B) RT-PCR analysis in animal caps injected with p63MO, p53MO, or both morpholinos confirms that down-regulation of p63 can induce mesoderm (as assessed by the pan-mesodermal marker Xbra) only in the presence of p53. (C) Embryos were injected as in (A), fixed at stage 26, stained for β-galactosidase activity (X-gal, blue color), and processed for whole-mount immunohistochemistry with an antibody specific for somitic mesoderm (12/101). The relative extent of the somitic mesoderm between the injected and uninjected sides of each embryo was measured as shown (two lower right image panels) and plotted on a bar graph. (D) Embryos were injected with ΔNp63 mRNA (2 ng), p53 mRNA (100 pg), or both, and β-galactosidase mRNA (β-gal, 250 pg) was co-injected to mark the injection site. The relative extent of the Xbra expression domain between the injected and uninjected sides of each embryo was quantified as in (A) and plotted on a bar graph. For (A) and (D), injected embryos were fixed at stage 10.5, stained for β-galactosidase activity (Red-gal, red color) and processed for in situ hybridization with probes for the pan-mesodermal marker brachyury (Xbra). Analysis of the extent of mesoderm tissue formation for (A), (C), and (D) is described in the methods section. *, **: single asterisks indicate significant changes from control embryos; double asterisks indicate significant changes between p63 MO and p63MO + p53 injected embryos (Student’s t-test, p<0.05). Numbers in parentheses indicate numbers of embryos scored.
Because variations in the extent of mesodermal induction that occur between individual embryos could confound our analysis, embryos were injected with mRNA encoding β-galactosidase to mark the injection site together with p53 mRNA, ΔNp63α mRNA, or p53/p63 morpholinos on only one side (i.e. right vs. left). Injected embryos were subsequently fixed at stage 10.5 and processed for in situ hybridization (Fig. 4A) or fixed at stage 26 and processed for immunohistochemistry (Fig. 4C). Mesodermal perturbations were simply assessed by comparing the extent of mesodermal formation in the injected versus uninjected (control) sides. Thus, mesoderm formation could be internally assessed in individual embryos, controlling for possible embryo-dependent or stage-dependent variations.
ΔNp63 down-regulation by MO injection (a 1:1 mix of A and B, 100 ng each) and ectopic expression of p53 caused significant expansion of mesodermal tissues (Figs. 4A and 4C), as measured by expansion of the Xbra expression domain (Fig. 4A) and expansion of somitic mesoderm staining (Fig. 4C). Conversely, p53 down-regulation by MO injection (Cordenonsi et al., 2003) or ectopic expression of ΔNp63α resulted in significant reduction of both Xbra expression (Fig. 4A) and the somitic mesoderm domains (Fig. 4C) compared to the uninjected side.
We next determined if mesodermal tissue induction by down-regulation of p63 could still proceed in the absence of p53. Co-injection of p53MO at concentrations that inhibit mesoderm formation (Cordenonsi et al., 2003) suppressed the mesoderm-inducing effects of p63MO injection, again suggesting that p63 acts to suppress the p53-dependent induction of mesoderm during Xenopus development (Fig. 4A, C). Further, while injection of p63MO resulted in the up-regulation of the mesodermal marker, brachyury, co-injection with a p53-specific morpholino was able to abrogate brachyury induction (Fig. 4B). These results are consistent with the ability of ΔNp63 to abrogate the p53-dependent upregulation of the mesodermal marker brachyury (Xbra) when co-injected (Fig. 4D). Statistical significance for all of these results was determined by performing the Student’s t-test (p<0.05). Collectively, these data further confirm the role of p63 in mesoderm induction in vivo and place p63 up-stream or at the level of p53 signaling in mesoderm induction.
Loss and gain of p63 affects formation of dorsal, but not ventral, mesoderm
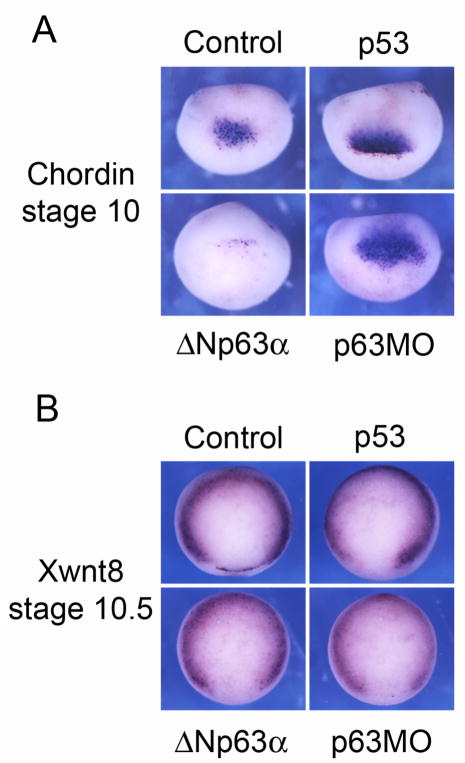
The ability of p53 to regulate dorsal mesoderm induction has been well documented (Takebayashi-Suzuki et al., 2003; Cordenonsi et al., 2003). In contrast, ventral mesodermal markers are not affected by p53 (Cordenonsi et al., 2003). To determine if p63 regulates the formation of dorsal versus ventral mesoderm, we performed in situ hybridization analysis for the dorsal mesodermal marker chordin and the ventral mesodermal marker Xwnt8 in embryos injected on one side with ΔNp63α mRNA or p63 MO and the lineage tracer β-galactosidase (to mark the injected side).
ΔNp63α up- or down-regulation by mRNA or MO injections did not affect Xwnt8 expression in early gastrulae (Fig. 5B), suggesting that p63 does not affect the formation of ventral mesoderm; similar results have been previously reported for p53 injection (Cordenonsi et al., 2003). In contrast, the dorsal mesodermal (organizer) marker chordin is down-regulated by ΔNp63α mRNA and up-regulated by p63MO or p53 mRNA injection (Fig. 5A). These results suggest that p53 and ΔNp63 antagonize each other in regulating a subset of activin target genes that control the formation of dorsal mesoderm.
Figure 5. Loss and gain of ΔNp63 function affects dorsal but not ventral mesoderm.
(A) In situ hybridization of early gastrulae with a chordin (dorsal mesoderm) probe. Dorsal sides of representative embryos for each experimental condition are shown. (B). In situ hybridization of early gastrulae with a Xwnt8 probe (ventral mesoderm). Representative embryos are shown with their vegetal poles oriented so that the dorsal side is facing down.
Discussion
p63 is critical for the development of stratified squamous epithelia and their derivatives (e.g. mammary and prostate glands). The mechanism by which p63 executes this biological function, however, is still poorly understood. In the present study, we show that p63 has an important role in mesoderm induction in vivo and present evidence that the developmentally regulated isoform ΔNp63α negatively regulates the induction of mesodermal tissues in Xenopus embryos. Furthermore, we demonstrate that ΔNp63 and p53 can functionally cooperate to control inductive events in the early vertebrate embryo.
Activin is a member of the TGF-β family of growth factors that regulates mesoderm induction through Smad proteins, p53 signaling, as well as other pathways (Chang et al., 2002). Despite the importance of activin A in mesoderm induction (Piepenburg et al., 2004), little is known about its transcriptional control during this process. In our current study, we find that ΔNp63α negatively regulates activin A expression at the transcriptional level in human keratinocytes, and this regulation is accompanied by an increase in the activin target genes BAMBI and CDKN2B. Though likely a result of p63 regulation of activin, the possibility that p63 is acting independently of activin to also directly regulate the expression of BAMBI and/or CDKN2B cannot be excluded. Regardless, the regulation of activin signaling by ΔNp63α led us to investigate the role of ΔNp63 in mesoderm induction in Xenopus laevis, as this model system is ideal for exploring the significance of the preliminary findings of a p63/activin signaling axis first observed in HaCaT cells. Ample evidence exists to support a connection between p63 and activin levels. For instance, ΔNp63α expression is down-regulated in the epidermis during wound healing (Noszczyk and Majewski, 2001); activin A is strongly induced in keratinocytes during these events and acts as an important regulator of the wound healing process (Sulyok et al., 2004). In addition, overexpression of the p63 isoform, TAp63α, in the epidermis of transgenic mice results in hyperproliferation and dysregulation of differentiation (Koster et al., 2004). Intriguingly, expression of activin A or its down-stream signaling mediator Smad2 phenocopies the epidermal phenotype (Ito et al., 2001; Munz et al., 1999). These findings suggest that regulation of activin signaling by p63 may represent a mechanism for influencing the developmental fate of epidermal cells in vitro and in vivo.
Based on previous results and those presented herein, we propose a model in which p63 transcriptionally regulates activin A expression and subsequently mediates its effects in a p53-dependent manner (Fig. 6). p53 is an important factor for proper induction of mesoderm during early developmental stages (Cordenonsi et al., 2003; Takebayashi-Suzuki et al., 2003). Recently, the zinc-finger protein XFDL156 was shown to inhibit mesoderm induction by abrogating the transcriptional activity of p53, suggesting that inhibition of p53 is sufficient to significantly block mesoderm development (Sasai et al., 2008). Our data provide evidence that, like XFDL156, ΔNp63 also acts to functionally inhibit the mesoderm-promoting activity of p53 during Xenopus laevis development. Here, we show that loss of ΔNp63 in Xenopus results in a p53-dependent transcriptional upregulation of activin, a potent mesoderm-inducing factor (Piepenburg et al., 2004). Our data suggest that ΔNp63 is likely acting in a dominant -negative manner towards p53 during early Xenopus development to repress a mesodermal cell fate through inhibition of activin signaling. Our data are consistent with direct regulation of activin by ΔNp63, however we do not exclude the possibility that ΔNp63 may independently regulate the expression of activin target genes. Indeed, our animal cap data suggest that, in Xenopus, ΔNp63 likely regulates mesoderm induction through global regulation of both activin and its downstream target genes. Further experiments are necessary to determine if p63 also directly regulates the expression of specific activin target genes.
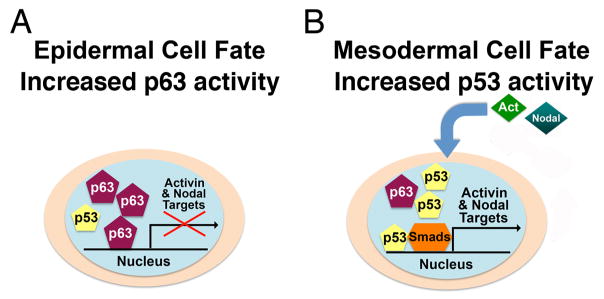
Figure 6. A proposed model for the regulation of mesoderm formation by ΔNp63, p53, activin and nodal-related proteins.
(A) ΔNp63 α activity predominates in cells that acquire epithelial cell fates and suppresses expression of activin and nodal target genes. (B) In cells that acquire mesodermal fate, activin and nodal-related proteins signal through p53 to transcriptionally up-regulate target genes.
Morphogenesis during this early time frame consists of a variety of signals and events that must be tightly regulated for proper patterning to occur. Loss of ΔNp63 expression in Xenopus embryos results in up-regulation of the mesodermal markers brachyury and Mix.2 and clearly potentiates the induction of mesoderm by activin. Additionally, mesoderm induction is abrogated by exogenous expression of ΔNp63α. It is possible that ΔNp63 acts to repress mesoderm induction in cells that do not have a mesodermal cell fate, allowing these cells to potentially become incorporated into ectodermal layers. Expressed in animal regions of the embryo (Fig. S2), ΔNp63 likely acts to antagonize the mesoderm-promoting activity of p53, ultimately directing these cells to a non-mesodermal fate (Fig. 6A). In contrast, the absence of ΔNp63 from marginal embryonic regions (Fig. S2) would allow p53 activity to be dominant, thus promoting the formation of mesoderm in these regions (Fig. 6B). Data presented in this study suggest a distinct requirement for different p53 family members during germ layer formation in Xenopus, with ΔNp63 promoting ectoderm and p53 directing the formation of non-ectodermal tissues.
In adult tissues, p63 protein is localized to sites of epithelial-mesenchymal apposition. These include the basal layer of the epidermis (and other stratified squamous tissues) and the basal cells of complex glandular structures (e.g. breast, prostate, lacrimal, and salivary glands; (Di Como et al., 2002). In addition, the phenotype of p63-deficient animals indicates that p63 may play a role in the formation of proper epithelial-mesenchymal interactions (Mills et al., 1999; Yang et al., 1999). p63 is necessary for limb formation in both mice and zebrafish, presumably due to its role in maintenance of the apical ectodermal ridge (AER), a site of complex epithelial-mesenchymal cross-talk (Bakkers et al., 2002b; Lee and Kimelman, 2002a; Mills et al., 1999; Yang et al., 1999). Morphogenesis of the palate and facial structure involves coordinated communication between epithelial and mesenchymal components, and mice and humans with defects in p63 function have craniofacial abnormalities including clefting of the lip and palate (Brunner et al., 2002; Mills et al., 1999; Yang et al., 1999).
Recently, we discovered that p63 promotes an epithelial phenotype and suppresses mesenchymal-specific genes in squamous epithelial cells (Barbieri et al., 2006). Additionally, ΔNp63α plays an important role in specifying and maintaining the integrity of the epidermal/dermal interface through regulation of specific target genes (Koster et al., 2007). These peculiar findings provide insights into the role of ΔNp63α in epithelial-mesenchymal interactions that are also critical for mesoderm induction. In Xenopus laevis, our data show a distinct requirement for different p53 family members during germ layer formation; embryonic regions where p53 is active are destined to become mesoderm, while the presence of ΔNp63 in the animal region acts to repress p53 activity and direct these cells to an ectodermal cell fate.
Further elucidation of the functional interactions between p63, activin signaling, and p53 will provide insight into the molecular events that control inductive events during early embryonic development. This information will aid us in understanding the basis of epithelial-mesenchymal interactions that are important during the development, homeostasis, and tumorigenesis of human tissues.
Supplementary Material
Modulation of p53/ΔNp63 regulates the expression of markers of cell fate. (A) In situ hybridization analysis of embryos injected with ΔNp63 mRNA and morpholino show that expression of the ectodermal marker Dlx3 is regulated by ΔNp63, indicating that the prospective ectoderm is competent to respond to ΔNp63 signaling at an early developmental stage. Animal poles of embryos are shown. (B) Xenopus animal caps were injected with morpholino oligonucleotides targeting ΔNp63 and p53 singly, or in combination, or with a control morpholino targeting β-globin. RNA was isolated and the levels of the mesodermal marker, Mix.2, was measured using quantitative real time PCR and normalized to the expression of ornithine decarboxylase (ODC). Data is representative of at lease three individual experiments
Endogenous p63/p53 in situ analysis. In situ hybridization shows that p53 is expressed in both the prospective ectoderm and mesoderm of the developing gastrula [as previously described, Hoever et al, Oncogene, 9, 109–120 (1994)], while p63 is primarily expressed in the ectoderm. This suggests that regulation of p63 expression can be endogenously controlled in a dynamic fashion between mesodermal and ectodermal tissues and that p53 and p63 interact endogenously in the prospective ectoderm. The limits of p63 expression in the equatorial region determine the extent of ectodermal (vs. mesodermal) formation.
ΔNp63 regulates the formation of anterior mesodermal and ectodermal structures. Embryos injected with ΔNp63 α mRNA (2 ng), ΔNp63 α-R304W mRNA (2 ng), or p63MO-A/B (200 ng) were fixed, embedded and sectioned anteriorly. Representative sections through corresponding anterior levels are shown for each set of injected embryos. Note the changes in the organization of the head mesenchyme in embryos injected with ΔNp63α mRNA and p63MO. EV, eye vesicle; MEV, mesencephalic ventricle; SA, stomodeal anlage; HM, head mesenchyme; CG, cement gland; NT, neural tube. Scale bar is 30 μm.
Acknowledgments
This work was supported by National Institutes of Health Grants CA70856 and CA105436 (J.A. Pietenpol), American Cancer Society Research Scholar Grant RSG-05-126-01 and National Cancer Institute Grant GI SPORE P50 CA95103 (E. Lee), NIH training grants T32CA078136-08 (C.E. Barton), GM073407 and CA009385 (C.E. Barbieri), 5F30ES016504 (A.J. Hanson), as well as ES00267 and CA68485 (Core services). E. Lee is also supported by a Pew Scholarship in the Biomedical Sciences (CA 20305301) and a National Institutes of Health Grant 1 R01 GM081635-01. We thank Dr. Matthew Westfall for construction of the ΔNp63α adenovirus. We thank members of Dr. Pietenpol’s and Dr. Laura Lee’s laboratories for critical reading of the manuscript and helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–83. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin M, Bamberger C, Paul D, Schmale H. Cloning and chromosomal mapping of the human p53-related KET gene to Chromosome 3q27 and its murine homolog Ket to mouse Chromosome 16. Mammalian Genome. 1998;9:899–902. doi: 10.1007/s003359900891. [DOI] [PubMed] [Google Scholar]
- Bakkers J, Hild M, Kramer C, Furutani-Seiki M, Hammerschmidt M. Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Developmental Cell. 2002a;2:617–27. doi: 10.1016/s1534-5807(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bakkers J, Hild M, Kramer C, Furutani-Seiki M, Hammerschmidt M. Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev Cell. 2002b;2:617–27. doi: 10.1016/s1534-5807(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Barbieri CE, Barton CE, Pietenpol JA. Delta Np63 alpha expression is regulated by the phosphoinositide 3-kinase pathway. J Biol Chem. 2003;278:51408–14. doi: 10.1074/jbc.M309943200. [DOI] [PubMed] [Google Scholar]
- Barbieri CE, Perez CA, Johnson KN, Ely KA, Billheimer D, Pietenpol JA. IGFBP-3 is a direct target of transcriptional regulation by DeltaNp63alpha in squamous epithelium. Cancer Research. 2005;65:2314–20. doi: 10.1158/0008-5472.CAN-04-3449. [DOI] [PubMed] [Google Scholar]
- Barbieri CE, Tang LJ, Brown KA, Pietenpol JA. Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res. 2006;66:7589–97. doi: 10.1158/0008-5472.CAN-06-2020. [DOI] [PubMed] [Google Scholar]
- Birkaya B, Ortt K, Sinha S. Novel in vivo targets of DeltaNp63 in keratinocytes identified by a modified chromatin immunoprecipitation approach. BMC Mol Biol. 2007;8:43. doi: 10.1186/1471-2199-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Hamel BC, Van Bokhoven H. The p63 gene in EEC and other syndromes. J Med Genet. 2002;39:377–81. doi: 10.1136/jmg.39.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell. 2003;113:301–14. doi: 10.1016/s0092-8674(03)00308-8. [DOI] [PubMed] [Google Scholar]
- Dellavalle RP, Egbert TB, Marchbank A, Su LJ, Lee LA, Walsh P. CUSP/p63 expression in rat and human tissues. J Dermatol Sci. 2001;27:82–7. doi: 10.1016/s0923-1811(01)00105-0. [DOI] [PubMed] [Google Scholar]
- Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, Pohar K, Hoos A, Cordon-Cardo C. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- Flatt PM, Tang LJ, Scatena CD, Szak ST, Pietenpol JA. p53 regulation of G(2) checkpoint is retinoblastoma protein dependent. Mol Cell Biol. 2000;20:4210–23. doi: 10.1128/mcb.20.12.4210-4223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole mount method for Xenopus embryos. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical uses in cell and molecular biology. Vol. 36. Academic Press; San Diego: 1991. pp. 685–695. [DOI] [PubMed] [Google Scholar]
- Harms K, Nozell S, Chen X. The common and distinct target genes of the p53 family transcription factors. Cell Mol Life Sci. 2004;61:822–42. doi: 10.1007/s00018-003-3304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, deGuise C, Kim C, Lemay S, Wang XF, LeBrun JJ. Activin induces hepatocyte cell growth arrest through induction of the cyclin-dependent kinase inhibitor p15INK4B and Sp1. Cell Signal. 2004;16:693–701. doi: 10.1016/j.cellsig.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ito Y, Sarkar P, Mi Q, Wu N, Bringas P, Jr, Liu Y, Reddy S, Maxson R, Deng C, Chai Y. Overexpression of Smad2 reveals its concerted action with Smad4 in regulating TGF-beta-mediated epidermal homeostasis. Dev Biol. 2001;236:181–94. doi: 10.1006/dbio.2001.0332. [DOI] [PubMed] [Google Scholar]
- Jones CM, Kuehn MR, Hogan BL, Smith JC, Wright CV. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–62. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006;7:360–72. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal Antibodies Identify Blastemal Cells Derived from Dedifferentiating Limb Regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Koster MI, Dai D, Marinari B, Sano Y, Costanzo A, Karin M, Roop DR. p63 induces key target genes required for epidermal morphogenesis. Proc Natl Acad Sci U S A. 2007;104:3255–60. doi: 10.1073/pnas.0611376104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–31. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MC, Keller R. Microtubule disruption reveals that Spemann’s organizer is subdivided into two domains by the vegetal alignment zone. Development. 1997;124:895–906. doi: 10.1242/dev.124.4.895. [DOI] [PubMed] [Google Scholar]
- Lee H, Kimelman D. A dominant-negative form of p63 is required for epidermal proliferation in zebrafish. Dev Cell. 2002a;2:607–616. doi: 10.1016/s1534-5807(02)00166-1. [DOI] [PubMed] [Google Scholar]
- Lee H, Kimelman D. A dominant-negative form of p63 is required for epidermal proliferation in zebrafish. Dev Cell. 2002b;2:607–16. doi: 10.1016/s1534-5807(02)00166-1. [DOI] [PubMed] [Google Scholar]
- Lu P, Barad M, Vize PD. Xenopus p63 expression in early ectoderm and neurectoderm. Mech Dev. 2001;102:275–8. doi: 10.1016/s0925-4773(01)00315-x. [DOI] [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Sargent TD. Distinct roles for Distal-less genes Dlx3 and Dlx5 in regulating ectodermal development in Xenopus. Mol Reprod Dev. 2001;60:331–7. doi: 10.1002/mrd.1095. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng BH, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Munz B, Smola H, Engelhardt F, Bleuel K, Brauchle M, Lein I, Evans LW, Huylebroeck D, Balling R, Werner S. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. Embo J. 1999;18:5205–15. doi: 10.1093/emboj/18.19.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982;30:687–96. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PDFJ. Normal table of Xenopus laevis (Daudin) 2. North Holland Publishing Company; Amsterdam: 1967. [Google Scholar]
- Noszczyk BH, Majewski ST. p63 expression during normal cutaneous wound healing in humans. Plast Reconstr Surg. 2001;108:1242–7. doi: 10.1097/00006534-200110000-00022. discussion 1248–50. [DOI] [PubMed] [Google Scholar]
- Osada M, Ohba M, Kawahara C, Ishioka C, Kanamaru R, Katoh I, Ikawa Y, Nimura Y, Nakagawara A, Obinata M, Ikawa S. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nature Med. 1998;4:839–843. doi: 10.1038/nm0798-839. [DOI] [PubMed] [Google Scholar]
- Osada M, Park HL, Nagakawa Y, Yamashita K, Fomenkov A, Kim MS, Wu G, Nomoto S, Trink B, Sidransky D. Differential recognition of response elements determines target gene specificity for p53 and p63. Mol Cell Biol. 2005;25:6077–89. doi: 10.1128/MCB.25.14.6077-6089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CA, Ott J, Mays DJ, Pietenpol JA. p63 consensus DNA-binding site: identification, analysis and application into a p63MH algorithm. Oncogene. 2007;26:7363–70. doi: 10.1038/sj.onc.1210561. [DOI] [PubMed] [Google Scholar]
- Piepenburg O, Grimmer D, Williams PH, Smith JC. Activin redux: specification of mesodermal pattern in Xenopus by graded concentrations of endogenous activin B. Development. 2004;131:4977–86. doi: 10.1242/dev.01323. [DOI] [PubMed] [Google Scholar]
- Ramis JM, Collart C, Smith JC. Xnrs and activin regulate distinct genes during xenopus development: activin regulates cell division. PLoS ONE. 2007;14:e213. doi: 10.1371/journal.pone.0000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Rubenstein JL, Nicolas JF. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. Embo J. 1986;5:3133–42. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai N, Yakura R, Kamiya D, Nakazawa Y, Sasai Y. Ectodermal factor restricts mesoderm differentiation by inhibiting p53. Cell. 2008;133:878–90. doi: 10.1016/j.cell.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BM, Van Nimmen K, Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990;345:729–31. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BMJ, Green JBA, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Sulyok S, Wankell M, Alzheimer C, Werner S. Activin: an important regulator of wound repair, fibrosis, and neuroprotection. Mol Cell Endocrinol. 2004;225:127–32. doi: 10.1016/j.mce.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Symes K, Smith JC. Gastrulation movements provide an early marker of mesoderm induction in Xenopus laevis. Development. 1987;101:339–349. [Google Scholar]
- Szak ST, Mays D, Pietenpol JA. Kinetics of p53 binding to promoter sites in vivo. Mol Cell Biol. 2001;21:3375–86. doi: 10.1128/MCB.21.10.3375-3386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahinci E, Thorne CA, Franklin JL, Salic A, Christian KM, Lee LA, Coffey RJ, Lee E. Lrp6 is required for convergent extension during Xenopus gastrulation. Development. 2007;134:4095–106. doi: 10.1242/dev.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi-Suzuki K, Funami J, Tokumori D, Saito A, Watabe T, Miyazono K, Kanda A, Suzuki A. Interplay between the tumor suppressor p53 and TGF beta signaling shapes embryonic body axes in Xenopus. Development. 2003;130:3929–39. doi: 10.1242/dev.00615. [DOI] [PubMed] [Google Scholar]
- Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185–97. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M, Kim R, deCaestecker MP, Kudoh T, Roberts AB, Dawid IB. Zebrafish nma is involved in TGFbeta family signaling. Genesis. 2000;28:47–57. doi: 10.1002/1526-968x(200010)28:2<47::aid-gene20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Vigano MA, Lamartine J, Testoni B, Merico D, Alotto D, Castagnoli C, Robert A, Candi E, Melino G, Gidrol X, Mantovani R. New p63 targets in keratinocytes identified by a genome-wide approach. Embo J. 2006;25:5105–16. doi: 10.1038/sj.emboj.7601375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vize PD. DNA sequences mediating the transcriptional response of the Mix.2 homeobox gene to mesoderm induction. Dev Biol. 1996;177:226–31. doi: 10.1006/dbio.1996.0158. [DOI] [PubMed] [Google Scholar]
- Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. The ΔNp63 alpha phosphoprotein binds the p21 and 14-3-3s promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol. 2003;23:2264–76. doi: 10.1128/MCB.23.7.2264-2276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillet E, Fleming M, Dotsch V, Andrews N, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000;1:199–207. doi: 10.1038/35043127. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson R, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–9. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Chang DL, Zheng H, McKeon F, Xiao ZX. DNA-binding and transactivation activities are essential for TAp63 protein degradation. Mol Cell Biol. 2005;25:6154–64. doi: 10.1128/MCB.25.14.6154-6164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Modulation of p53/ΔNp63 regulates the expression of markers of cell fate. (A) In situ hybridization analysis of embryos injected with ΔNp63 mRNA and morpholino show that expression of the ectodermal marker Dlx3 is regulated by ΔNp63, indicating that the prospective ectoderm is competent to respond to ΔNp63 signaling at an early developmental stage. Animal poles of embryos are shown. (B) Xenopus animal caps were injected with morpholino oligonucleotides targeting ΔNp63 and p53 singly, or in combination, or with a control morpholino targeting β-globin. RNA was isolated and the levels of the mesodermal marker, Mix.2, was measured using quantitative real time PCR and normalized to the expression of ornithine decarboxylase (ODC). Data is representative of at lease three individual experiments
Endogenous p63/p53 in situ analysis. In situ hybridization shows that p53 is expressed in both the prospective ectoderm and mesoderm of the developing gastrula [as previously described, Hoever et al, Oncogene, 9, 109–120 (1994)], while p63 is primarily expressed in the ectoderm. This suggests that regulation of p63 expression can be endogenously controlled in a dynamic fashion between mesodermal and ectodermal tissues and that p53 and p63 interact endogenously in the prospective ectoderm. The limits of p63 expression in the equatorial region determine the extent of ectodermal (vs. mesodermal) formation.
ΔNp63 regulates the formation of anterior mesodermal and ectodermal structures. Embryos injected with ΔNp63 α mRNA (2 ng), ΔNp63 α-R304W mRNA (2 ng), or p63MO-A/B (200 ng) were fixed, embedded and sectioned anteriorly. Representative sections through corresponding anterior levels are shown for each set of injected embryos. Note the changes in the organization of the head mesenchyme in embryos injected with ΔNp63α mRNA and p63MO. EV, eye vesicle; MEV, mesencephalic ventricle; SA, stomodeal anlage; HM, head mesenchyme; CG, cement gland; NT, neural tube. Scale bar is 30 μm.