Abstract
Objective
Cerebral ischemia can activate endogenous reparative processes, such as proliferation of endogenous neural progenitor cells (NPCs) in the subventricular zone (SVZ). Most of these new cells die shortly after injury. The purpose of this study was to examine a novel strategy for treatment of stroke at one week after injury by enhancing the survival of ischemia-induced endogenous NPCs in SVZ.
Methods
Adult rats were subjected to a 90-min middle cerebral artery occlusion (MCAo). A p53 inhibitor pifithrin-α (PFT-α) was administered to stroke rats from days 6 to 9 after MCAo. Locomotor behavior was measured using an activity chamber. Proliferation, survival, migration, and differentiation of endogenous NPCs were examined using qRT-PCR, TUNEL, and immunohistochemistry.
Results
PFT-α enhanced functional recovery as assessed by a significant increase in multiple behavioral measurements. Delayed PFT-α treatment had no effect on the cell death processes in the lesioned cortical region. However, it enhanced the survival of SVZ progenitor cells and promoted their proliferation and migration. PFT-α inhibited the expression of a p53-dependent pro-apoptotic gene, termed PUMA (p53-upregulated modulator of apoptosis), within the SVZ of stroke animals. The enhancement of survival/proliferation of NPCs was further found in SVZ neurospheres in tissue culture. PFT-α dose-dependently increased the number and size of new neurosphere formation.
Interpretation
Delayed treatment with a p53 inhibitor PFT-α is able to modify stroke-induced endogenous neurogenesis and improve the functional recovery in stroke animals.
Introduction
Current treatment strategies for stroke primarily focus on reducing the size of ischemic damage and on rescuing dying cells early after occurrence. Treatments, such as the use of thrombolytic agents, are often limited by a narrow therapeutic time window, and no pharmacological agent has been shown to enhance the recovery process when therapy is initiated in the chronic or subacute phase, i.e. 6 days, after stroke in patients1, 2. Recent studies have indicated that transplantation of neural stem cells (NSCs) and progenitor cells (NPCs) is beneficial in animal models of stroke. For example, grafted human NSCs survived and migrated to the lesioned site one month after transplantation in stroke rats3. The clinical application of this approach may be limited by ethical and logistic concerns. On the other hand, cerebral ischemia can also activate endogenous repair processes. De novo neurogenesis was found in the subventricular zone (SVZ) of adult mammalian brain after stroke4. Most of these cells die within weeks after injury 5 and their ability to restore behavioral deficits in the stroke animals are minimal.
p53 is a major mediator of ischemia-induced cell death in brain. Cerebral ischemia activates p53 expression. Administration of the small synthetic p53 inhibitor, pifithrin-α (PFT-α) 6, 30 min before7 or 1 hour after middle cerebral artery occlusion (MCAo) reduced p53 activity, TUNEL labeling, cerebral infarction in the ischemic brain, and motor disability in rodents8. The protective effects of PFT-α were not significant if given 3 hours after MCAo. These data suggest that programmed cell death at the lesioned site is initiated rapidly after ischemia and the effectiveness of a p53 inhibitor to rescue these injured cells is limited to a narrow therapeutic window (i.e. hours after ischemia), as the cell death biochemical cascade quickly passes beyond the point of p53’s involvement 9.
Besides its involvement in early cell death in the ischemic penumbra, p53 also plays important roles in other non-ischemic regions. A recent study indicated that p53 protein is highly expressed in the NPC area in SVZ, compared to surrounding brain regions. Neurospheres from the lateral ventricle wall of adult p53 null animals proliferated faster and incorporated more BrdU than wild type controls10. These data suggest that the survival/proliferation of adult NPCs from the SVZ is influenced by p53. It is also possible that inhibition of p53 may extend the proliferation and survival of endogenous NPCs and alter the biological outcomes days after stroke.
In this study, instead of rescuing cells in the penumbra, we attempted to enhance the survival of ischemia –induced endogenous NPCs in SVZ using a p53 inhibitor. Since activation of NPC proliferation in SVZ occurs at a later stage11, compared to the cell death occurring in ischemic core or penumbra, pharmacological intervention to promote NPC survival may be effective late, i.e. a week, after the onset of stroke.
Methods
Transient focal ischemia model
Adult male Sprague–Dawley rats were anesthetized with chloral hydrate (400 mg/kg, i.p.). The right MCA was ligated and bilateral common carotids (CCAs) were clamped for 90-min to generate focal ischemia in the cerebral cortex12, 13, 14. Core body temperature was maintained at 37 °C.
Administration of PFT-α or vehicle
Rats were anesthetized 6 days after MCAo. PFT-α (0.4 μg/μl ×20 μl) or vehicle (20 μl, 10% DMSO in saline) was administered intracerebroventricularly (AP: 0.8 mm, Lat: 1.5 mm to bregma, DV: −3.7 mm). Animals then received PFT-α (0.2 mg/100g, i.p., b.i.d.) or vehicle for three consecutive days.
Injection of BrdU
Three injection protocols were used: (1) The kinetics of proliferation of NPCs after injury. BrdU was injected systemically on days 2, 4, 6, 8, 10, 12, or 14 after MCAo at the dose of 50 mg/kg ×4 at 2-hr interval. (2) Proliferation of NPCs induced by PFT-α (Fig 3A). BrdU was administered daily for 3 days (50 mg/kg ×2) after PFT-α injection (i.e. days 7 to 9 after MCAo). (3) Migration and differentiation of NPCs (Fig 4A). BrdU (50 mg/kg ×4) was given before PFT-α injection (i.e. day 5 after MCAo).
Fig 3.
Delayed PFT-α treatment promotes the proliferation of NPCs and inhibits apoptosis in the SVZ in stroke rats. (A). Rats received PFT-α or vehicle from days 6 to 9 after MCAo. BrdU was administered daily for 3 days after PFT-α injection. (B) PFT-α treatment increased the density of BrdU immunoreactivity (optical density) in SVZ (Δ, p<0.05, 2-way ANOVA). The increase in BrdU labeling was more prominent in the anterior SVZ (* p<0.05, post-hoc Newman-Keuls test, 2-way ANOVA). (C) Representative photomicrographs of the anterior SVZ indicate that BrdU labeling was enhanced in the stroke animals treated with PFT-α. Calibration = 100 μm. (D). Representative photomicrographs of the anterior subependymal zone indicate that density of PCNA positive cell increased in the stroke animals treated with PFT-α. (Panels 2 and 4, from the far left) compared to vehicle-treated animals (Panels 1 and 3) on day 10 after MCAo. Panels 3 and 4 are images at higher magnification. Scale bar = 50 μm. (E) PFT-α significantly enhanced the number of PCNA positive cells at the anterior portion of the subependymal zone (*p<0.05, t test). (F) Delayed PFT-α treatment significantly suppressed TUNEL labeling in the SVZ on day 10 after MCAo (Δ, p<0.05, 2-Way ANOVA). (G) PFT-α suppresses the expression of PUMA. SVZ tissues were harvested from PFT-α or vehicle treated stroke rats on the 4th day of PFT-α treatment. p53-regulated genes (mRNA levels) were determined by qRT-PCR. All the genes were normalized to the housekeeping gene PGK1 mRNA level. PFT-α did not change the level of Bcl2 or Bax genes. However, it decreased the mRNA levels of PUMA (*p<0.05, t test).
Fig 4.
Delayed PFT-α treatment increased the survival and migration of newly generated NPCs in the lesioned cortex. (A) Rats were subjected to MCAo on day 0. Newly generated cells were pre-labeled with BrdU on day 5 after stroke. PFT-α or vehicle was given 1 day after the BrdU labeling. Density of BrdU immunoreactivity in (B) SVZ or (C) cortex on days 10 and 21 after MCAo was normalized to the level of BrdU activity in vehicle group on Day 10. (B) In the SVZ, BrdU-labeled cells were mainly present on day 10 after MCAo. Few BrdU cells were found on day 21. PFT-α treatment significantly increased the density of BrdU cells on day 10 in SVZ. (C) In the lesioned cortex, fewer BrdU cells were found on day 10. Significantly increased BrdU labeling was found on day 21. PFT-α treatment enhanced BrdU labeling in the lesioned cortex both on days 10 and 21 after MCAo. (D) Correlation of the functional outcomes with migration of NPCs to the lesioned cortex in stroke rats. The behavioral improvement in horizontal activity (HACTV) on days 14 and 21 was calculated using the following equation. % improvement = (HACTV on day 14 or 21 – HACTV on day 2)/(HACTV on day 2) *100%]. BrdU (+) cells in the lesioned side cortex was examined on day 21 after MCAo. There is liner correlation between the number of BrdU cells in cortex and HACTV improvement at 14 days (r=0.828, p=0.002, black tracing) and 21 days (r=0.644, p=0.032, red tracing) after MCAo. (E) Photomicrographs indicate that PFT-α treatment increased BrdU-labeled cells in the lesioned cortex on day 21 after MCAo. Calibration= 50 μm.
Behavioral measurements
Each animal was individually placed in a 42×42×31 cm activity monitor for 24 hours (Omnitech Electronic Inc, Columbus, OH). Water and food were provided in the chambers. The monitor contained 16 horizontal and 8 vertical infrared sensors spaced 2.5 cm apart. Locomotor activity was calculated using the number of infrared beams broken by the animals (Supplement 3).
Immunohistochemistry (for details, please see supplement 1)
BrdU and PCNA immunostaining was carried out as described15, 16. For double labeling immunostaining, brain sections were incubated in solutions of antibodies against various markers (Nestin, MAP2, NeuN, or GFAP). For double labeling immunocytochemistry, confocal images were obtained by using UltraView confocal microscopic system (PerkinElmer).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) histochemistry
A standard TUNEL procedure for frozen tissue sections was performed as previously described17 and detailed in Supplement 1.
Quantitative reverse transcription-PCR (qRT-PCR)
The SVZ was dissected out. Total RNA (1μg) was treated with RQ-1 Rnase-free Dnase I and reverse transcribed into cDNA using random hexamers by AMV reverse transcriptase (Roche). cDNA levels for hypoxanthine guanine phosphoribosyl transferase (HPRT), phosphoglycerate kinase 1 (PGK1), Bax, Bcl2, apoptotic peptidase activating factor 1 (Apaf1), p21, and PUMA were determined by specific universal probe Library primer probe sets (Roche) by quantitative RT-PCR18. Primers and FAM-labeled probes for each gene are listed in Table 1 (Supplement 2).
SVZ cultures
SVZ cells were collected from mouse embryos and dissociated by triturating. Cells were plated in Neurobasal medium (Invitrogen) supplemented with B27 (Invitrogen), 2mM L-glutamine (Invitrogen), antibiotic/antimycotic (Invitrogen), and heparin (Sigma). Equal numbers of cells were plated and kept in medium containing 20 ng/ml epidermal growth factor (EGF). Cells were plated at 10,000 cells/cm2. PFT-α or vehicle was added at different concentrations at DIV1 or DIV3. The number and size (diameter) of neurospheres were counted 7 days after plating.
Statistical analysis
Statistical analysis was performed using Student’s t-test, and one way or two way ANOVA as appropriate, with Newman –Keuls post hoc tests. P values <0.05 were considered significant.
Results
Kinetics of SVZ progenitor cell proliferation and cortical cell apoptosis following ischemia
Depending on the stroke model used, the activation of NPC proliferation in the SVZ and cell death in the ischemic core/penumbra varies after ischemia11. To examine the kinetics of NPC proliferation in the stroke model used in this study, a one day BrdU injection (50 mg/kg ×4, every 2 hr) was given systemically on days 2, 4, 6, 8, 10, 12, or 14 after MCAo in 45 rats. The density of immunoreactive BrdU (+) cells was examined 12 hours following the last injection. We observed a robust increase of BrdU immunoreactivity in SVZ as early as 2 days after MCAo (Fig 1A and 1B). The increase of BrdU immunoreactivity was sustained through 4 days after MCAo, started to decline between day 6 to day 8, and returned to basal levels around day 10 (Figs 1 A & 1B). The anterior SVZ (AP +0.2 mm to bregma) showed more BrdU incorporation than the posterior SVZ (AP −0.4 mm; Fig 1A and 1B, p<0.001, 2-way ANOVA). In another set of rats (n=10), the kinetics of apoptosis/necrosis in the ischemic side cortex was examined on days 2, 6 and 9 after MCAo. The density of TUNEL labeling peaked at day 2 after MCAo; it returned to basal levels (compared to sham surgery) on day 6 (Fig 1C, 1D), suggesting differential temporal windows of cell proliferation in the SVZ and cell death in the ischemic cortex. Furthermore, PFT-α, given at days 6–9 after MCAo, had no effect on the cell death processes in cortical regions (Fig 1E & 1C, p=0.266, t test). These data suggest that, for “therapeutic efficacy”, the suppression of apoptosis in the ischemic cortex should take place earlier after MCAo while rescue of the NPCs in SVZ can occur after day 6.
Figure 1.
The kinetics of proliferation of NPCs in SVZ and apoptosis in the lesioned cortex following ischemia. (A and B) BrdU was injected systemically on days 2, 4, 6, 8, 10, 12, and 14 after a transient focal ischemia induced by MCAo at the dose of 50 mg/kg × 4 at 2-hr interval in 45 adult rats. Rats were sacrificed 12 hours after the last dose of BrdU. Two sections (anterior, AP +0.2 mm from bregma; posterior, AP −0.4 mm from bregma) from each animal were counted. (A) Line graph represents the BrdU immunoreactivity (optical density) of SVZ in all animals studied. Ischemia/reperfusion induced BrdU labeling both in the anterior and posterior SVZ. The anterior is more active in BrdU incorporation than the posterior SVZ. In the anterior SVZ, the increase in BrdU immunoreactivity was sustained through 4 days after MCAo, started to decline at between days 6 to 8, and returned to basal levels around day 10. (B) Photomicrographs demonstrate that BrdU immunoreactivity is differentially enhanced in the anterior and posterior SVZ from days 2 to 14 after MCAo. (C, D, and E) Levels of apoptosis were measured by TUNEL staining at the ischemic site in cortex on days 2, 6 and 9 after MCAo. (C) TUNEL labeling was enhanced on day 2 and returned to basal level less than 6 days after MCAo. Data is normalized to the max response of TUNEL staining (2 days after MCAo); dotted line represents the level of TUNEL activity in sham-operated rats (n=3). Administration of PFT-α from days 6 to 9 did not alter TUNEL activity in the ischemic cortex on day 9 (p=0.266, t test). (D) Representative histology of TUNEL labeling in the lesioned cortex after MCAo or sham surgery at 2 or 6 days after MCAo. An increase in TUNEL density was found at 48 hours after MCAo. (E) Photomicrographs demonstrate that PFT-α, given from days 6 to 9, did not alter TUNEL activity in the ischemic cortex on day 9. Bar represents 200 μm in (B) and 100 μm in (D and E).
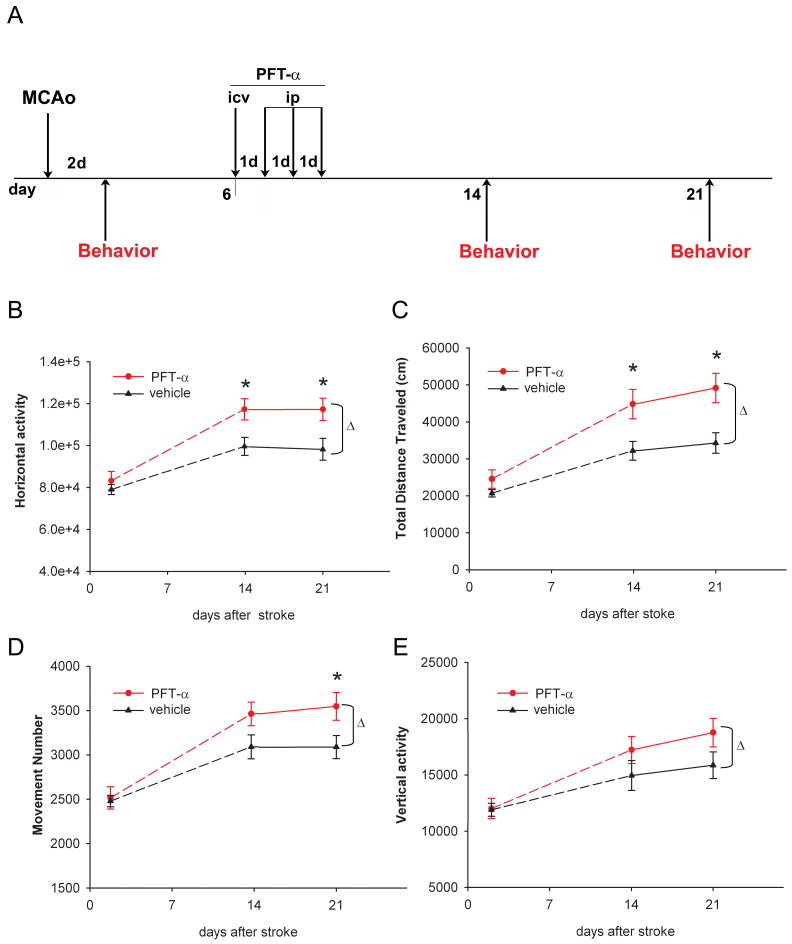
Delayed treatment with PFT-α improves the behavioral recovery after stroke
PFT-α or vehicle was given from days 6 to 9 after MCAo or sham surgery (Fig 2A). Administration of PFT-α did not alter locomotor activity in non-stroke rats (n=12, data not shown). In the stroke animals (n=29), PFT-α treatment significantly enhanced motor function, as demonstrated by increases in horizontal activity (Fig 2B, p<0.001, 2-Way ANOVA), total distance traveled (Fig 2C, p<0.001, 2-way ANOVA), number of movements (Fig 2D, p=0.009, 2-Way ANOVA), and vertical activity (Fig 2E, p=0.045, 2-Way ANOVA) on days 14 and 21 after MCAo (Table 2, Supplement 3). These results demonstrate that delayed PFT-α treatment is effective in improving the functional outcome in stroke rats.
Fig 2.
Delayed PFT-α treatment improved locomotor behavior in stroke rats. (A) Time line indicates that rats were subjected to a 90- min MCAo surgery on day 0. Vehicle or PFT-α was given from day 6 for four consecutive days. Behavioral measurements were carried out in automated activity chambers for 24 hours at 2 (before administration of PFT-α or vehicle), 14 and 21 days after MCAo. No difference was found before PFT-α or vehicle injection on day 2. Rats receiving post-stroke PFT-α treatment show enhanced recovery in motor function demonstrated by significant increases in (B) horizontal activity (total number of beam interruptions in the horizontal sensors), (C) total distance traveled, (D) number of movements, and (E) vertical activity (total number of beam interruptions in the vertical sensors), compared to the control group. (Δ, p<0.05, 2-way ANOVA; * p<0.05, post-hoc Newman-Keuls test).
Delayed treatment with PFT-α enhances the proliferation/survival of progenitor cells in SVZ in stroke rats
Since p53 negatively regulates self-renewal of NPCs in SVZ of adult brain10, the behavioral improvement induced by p53 antagonist may thus be attributed to the up-regulation of endogenous NPCs in SVZ,. We next tested whether PFT-α altered the density of NPCs in SVZ in 18 stroke rats. PFT-α (n=10) or vehicle (n=8) was administered daily from days 6 to 9; BrdU was given from days 7 to 9 after MCAo (Fig 3A). Animals were sacrificed at 12 hrs after the last injection of BrdU. Most of the BrdU-labeled progenitor cells were present in the SVZ. PFT-α significantly enhanced the number of BrdU positive cells in SVZ (Fig 3B, p=0.008, 2-way ANOVA) in stroke rats (Fig 3C).
Since p53 can regulate cell proliferation and/or survival, we next examined if the increase in NPCs density in SVZ after delayed PFT-α treatment is mediated through these two mechanisms. Proliferating cell nuclear antigen (PCNA), a marker for proliferating cells, was used to study the effect of proliferation19 in 5 vehicle-treated and 7 PFT-α-treated rats (see timeline in Fig 3A). Significant increase in PCNA (+) cell density (Fig 3E, p=0.003, t-test) was found in the rostal SVZ (AP:0.2 to bregma) in PFT-α–treated rats on day 10 after MCAo. Typical PCNA immunostaining is demonstrated in Fig 3D. These data suggest that PFT-α increases the proliferation of NPCs in SVZ of stroke rats. The effect of PFT-α on cell survival was examined in 7 rats. Delayed PFT-α treatment significantly suppressed TUNEL labeling, a marker for apoptosis/necrosis, in the SVZ on day 10 after MCAo (Fig 3F, p=0.010, 2-way ANOVA). Taken together, these data suggest that delayed PFT-α treatment enhanced both proliferation and survival of NPCs in SVZ in stroke rats.
Treatment with PFT-α promotes the survival of progenitor cells from SVZ by a PUMA-mediated pathway
Ten days after MCAo, SVZ tissues were collected from rats treated with PFT-α (n=8) or vehicle (n=9) for qRT-PCR analysis (Fig 3G). PFT-α did not alter the expression of Bcl2 (p=0.95, t test) or Bax (p=0.97, t test), but significantly reduced mRNA levels for PUMA (p=0.028, t test). The difference in p21 expression was not significant (p=0.21, t test, Fig 3G). These data suggest that PFT-α exerts an anti-apoptotic effect by inhibiting the p53-regulated pro-apoptotic gene PUMA.
Migration and differentiation of NPCs
Rats (n=29) were given BrdU systemically on day 5 and then treated with PFT-α (n=15) or vehicle (n=14) from days 6 to 9 after MCAo (Fig 4A). In the SVZ (Fig 4B), a majority of BrdU-positive cells were present on day 10 (p<0.001, 2-Way ANOVA); few cells were found on day 21 after stroke. In the lesioned cortex (Fig 4C), however, a significantly higher BrdU positive cell density was found on day 21 compared to day 10 (p=0.002, 2-way ANOVA), suggesting the migration of NPCs from SVZ to the lesioned cortex between day 10 and day 21. Almost no BrdU cells were present in the non-lesioned side cortex on days 10 and 21 in all animals (data not shown). Administration of PFT-α significantly increased density of BrdU-positive cell in the lesioned cortex on day 21 (Fig 4C, p<0.05, 2-way ANOVA) and in SVZ on day 10 (Fig 4B, p<0.05, 2-way ANOVA). Typical BrdU labeling in the lesioned cortex on day 21 is shown in Fig 4E. Taken together, our data suggest that delayed treatments with PFT-α from days 6 to 9 facilitated the proliferation of NPCs in SVZ as seen on day 10, and resulted in an increase in migration of the NPCs to the injured cortex as seen on day 21.
We found that there is a significant correlation between the functional outcomes and number of surviving BrdU positive cells in the lesioned cortex. BrdU was given to 11 rats on day 5 after MCAo. Of these animals, 5 were treated with vehicle and 6 were treated with PFT-α (Fig 4A). Horizontal activity was measured on days 14 and 21. Density of BrdU-positive cells in the lesioned cortex was counted on day 21. The improvement in horizontal activity (HACTV) on days 14 and 21 was measured using the following equation. % improvement = (HACTV on day 14 or 21 –HACTV on day 2)/(HACTV on day 2) ×100%. There is a linear correlation between the number of BrdU cells in the lesioned side cortex and HACTV improvement on day 14 (r=0.828, p=0.002, black tracing) and day 21 (r=0.644, p=0.032, red tracing) after MCAo (Fig 4D). These data suggest that NPCs migrated to, and survived in, the lesioned target (i.e., cortex), contributing to the improvement of locomotor function in stroke rats.
The differentiation of the NPCs in the lesioned cortex was examined using double label immunostaining. Stroke rats treated with PFT-α (n=6) or vehicle (n=6) were sacrificed on day 21 after MCAo. Confocal microscopic examination indicated that BrdU-positive cells in the lesioned side cerebral cortex expressed the neuronal markers nestin, NeuN or MAP2 (Fig 5). Because of the diffuse staining pattern of GFAP (Fig 5), it was difficult to determine co-localization of BrdU and GFAP. PFT-α treatment significantly increased the density of BrdU cells co-expressing nestin (p=0.011, t-test), but not MAP2 (p=0.17, t-test), in the lesioned cortex. There was a marginal increase in NeuN/BrdU cell density (P=0.075, t-test) after PFT-α administration. Using more animals may lead to a significant change.
Fig 5.
Differentiation of endogenous NPCs in the lesioned cortex in stroke rats receiving PFT-α treatment. Animals were sacrificed on day 21 after MCAo. Double immunostaining for BrdU (middle panels) and markers for NeuN, MAP2, nestin as well as GFAP (top panels) were analyzed by confocal microscopy. The newly generated cells labeled by BrdU co-expressed NeuN. BrdU positive nuclei were also surrounded by the cytosol which contains MAP2 or nestin (Arrows, bottom panels). Calibration= 100 μm.
PFT-α increases the self-renewal of SVZ progenitor cell proliferation in vitro
SVZ cells from rodent embryos were isolated and cultured in epidermal growth factor (EGF)-containing media to form neurospheres. Cells were plated at P2. PFT-α or vehicle was added either on DIV1 or DIV3 at various concentrations; the total number of neurospheres was counted on DIV7. PFT-α on DIV1 significantly (Fig 6A, p<0.05, 1-way ANOVA) and dose-dependently increased the number of neurosphere formations (p<0.001, r=0.528, linear regression). There was no significant change in the density of neurospheres when PFT-α was given on DIV3, suggesting a time window for the trophic effect of PFT-α in cultured SVZ progenitor cells (Fig 6A). A significant difference was also observed between the size of spheres receiving PFT-α and vehicle treated from DIV1 (Fig 6B and C, p<0.05, 1-way ANOVA).
Fig 6.
PFT-α increased the proliferation/survival of SVZ progenitor cells in vitro. (A) PFT-α was added to the SVZ progenitor cell culture on DIV 1 or DIV 3. Density of neurospheres was counted on DIV7. Administration of PFT-α on DIV1, but not on DIV3, dose-dependently increased number of neurospheres, as compared to the non-treatment controls (*p<0.05, 1-Way ANOVA + post-hoc Newman-Keuls test). (B) The spheres receiving PFT-α treatment on DIV1 also grew significantly larger compared to vehicle-treated sphere cultures (*p<0.05, 1-Way ANOVA + post-hoc Newman-Keuls test). (C) Representative images of neurospheres treated with PFT-α or vehicle on DIV1. Calibration= 100 μm.
Discussion
In this study, we have demonstrated a delayed treatment strategy for stroke. Administration of PFT-α, a p53 inhibitor, starting from the 6th day after MCAo, enhanced the proliferation and migration of endogenous NPCs in the SVZ and, more importantly, it improved motor function after ischemia.
Although PFT-α can pass through the blood brain barrier when given systemically 7, we found that systemic administration of PFT-α alone, at the dose of 0.2 mg/100g (b.i.d, i.p.), was not enough to provide significant behavioral recovery in our pilot study. A loading i.c.v dose of PFT-α, which increases PFT-α levels in the brain rapidly without dramatically increasing PFT-α levels peripherally, was required to induce significant behavioral recovery.
Ischemic brain injury activates apoptotic cascades at the lesioned site 20. We found that the density of TUNEL labeling in the ischemic cortex peaked on day 2 after MCAo. Similarly, it has been reported that p53 mRNA or protein is upregulated shortly after stroke, which leads to p53-dependent programmed cell death in the penumbra 21, 22. Less focal damage is found in stroke animals pre-treated with a p53 inhibitor 7 or in p53 knockout mice 23. These data suggest that p53 is an important mediator of programmed cell death in the ischemic region. As the initiation of programmed cell death in the ischemic region occurs rapidly after stroke, Leker et al 8 reported that the effectiveness of p53 inhibition is only observed when an inhibitor is given before or shortly after MCAo. PFT-α given 3h or later after MCAo had no beneficial effect on neuronal cell loss8. Using the same treatment regimen as described by Leker et al 8, we also found that PFT-α, given at 3 hours after MCAo as a single dose, did not alter locomotor behaviors on day 2 after MCAo. There was also no difference in BrdU labeling in the SVZ between the stroke animals treated with PFT-α and vehicle at 24 hours after the last dose of BrdU injection. This temporal restriction has largely limited the clinical potential of the drug. Consistent with these observations, we also found that TUNEL labeling in the ischemic cortex returned to basal levels on day 6 after MCAo, suggesting that the window of apoptosis/necrosis in the ischemic core/penumbra is less than 6 days. Since PFT-α was given from day 6 in the current study, the improvement of motor function is not attributable to the suppression of apoptosis at the lesioned site in stroke rats; this is further supported by the finding that delayed treatment with PFT-α from days 6 to 9 did not alter TUNEL labeling in the ischemic cortex, as seen in Fig 1E.
Ischemic brain injury induces SVZ neurogenesis in the adult brain 5, 24. We observed that an increase in BrdU labeling in SVZ was sustained from day 2 to 8 after MCAo and returned to basal levels on day 10. These data suggest that the window of new cell proliferation at SVZ is longer than that of apoptosis in ischemic penumbra. In spite of the increase in new NPC proliferation in SVZ, without p53 inhibition the majority of these cells die before differentiating into a functional phenotype. It is thought that the lesioned brain tissue does not provide a suitable environment for the survival of these newly generated cells20.
We administered PFT-α from days 6 to 9 after MCAo, a temporal window when ischemia-induced NPC proliferation in SVZ, but not apoptosis in the penumbra, is still ongoing. This allows differentiation of the effect of p53 inhibition at the lesioned penumbra compared to SVZ. We found that delivering PFT-α enhanced behavioral outcome in multiple parameters of locomotor activity in the stroke animals at both 1 and 2 weeks after treatment. Since PFT-α did not augment locomotor behavior in non-stroke rats, the effect of PFT-α on promoting behavioral recovery appears specific to the stroke animals. Previous studies indicated that PFT-α, given at 3 hours after MCAo, did not reduce cell death in stroke rats 8. The behavioral improvement induced by delayed PFT-α treatment in the current study is thus probably not due to rescue of the injured cells at the penumbra but, rather, to augmenting the proliferation or survival of NPCs in the SVZ cells.
We found that BrdU labeling is enhanced in stroke animals receiving delayed PFT-α treatment, suggesting that PFT-α enhanced new cell proliferation in the SVZ after ischemic insults. It has been suggested that BrdU can be transferred to other cells over time by phagocytosis of dying cells. For example, Coyne et al reported BrdU labeling can be transferred from pre-labeled viable or control non-viable donor marrow stromal cells (MSCs), derived from GFP transgenic rats, to phagocytes, astrocytes and neurons in host brain of Sprague-Dawley rats25. Inflammation was found at the transplantation site. Numerous blood vessels in the surrounding graft regions were dilated, with cells exhibiting monocytic, lymphocytic, and granular nuclear morphology. These results suggest that exogenous MSCs transplanted to the intact adult brain were rejected by an inflammatory response. Coyne et al thus caution against the continued use of BrdU as donor cell labels. However, in this study, the increase in BrdU labeling in the SVZ observed in animals treated with PFT-a is not due to the phagocytosis of dying cells based on the following 4 reasons: (1) In contrast to the transplantation of exogenous cells, we enhanced proliferation of endogenous progenitor cells by PFT-α in this study. Since no exogenous cells were grafted, almost no inflammation was found in the SVZ. (2) Brain trauma, such as i.c.v. injection, can induce inflammation. We found that PFT-α did not alter BrdU labeling near the needle tract in cerebral cortex (unpublished observation). (3) The proliferation of SVZ was also confirmed by another marker. We found that the immunoreactivity of PCNA, an endogenous protein that is specifically expressed during the cell proliferation cycle, was enhanced after PFT-α treatment in stroke rats (Fig 3E). (4) There is a functional correlation between BrdU incorporation and behavioral recovery.
We found that administration of PFT-α increased NPC proliferation and survival of NPCs in the SVZ after MCAo. Selective regulation of p53 target genes is critical for the different cellular outcomes mediated by p53 and, under physiological/pathological conditions, p53 can regulate different apoptosis-related and cell-cycle arrest genes26. It has been shown that PFT-α inhibits p53 function in vivo by blockade of the translocation of p53 to the nucleus and mitochondria8 and by subsequent suppression of the expression of pro-apoptotic genes27. In this study, the expression of pro-apoptotic genes in the SVZ is, likewise, modulated by PFT-α in stroke rats. We examined the gene expression level in SVZ for known p53-regulated genes that are involved in neuronal apoptosis, such as Bax/Bcl29, apoptotic protease-activating factor 1 (apaf1)28 and p53 upregulated modulator of apoptosis (PUMA)29. PFT-α inhibited the p53-dependent pro-apoptotic gene, PUMA, a gene which is upregulated by hypoxia30 and can lead to caspase activation29. Since deletion of PUMA attenuated cell death and improved physiological function after ischemia-reperfusion injury30, it is possible that PFT-α promotes the survival of SVZ progenitor cells, at least in part, through the inhibition of expression of PUMA.
p21 is another downstream target gene for p53. Deficiencies in p21 affect stem cell proliferation in mice31. p21 gene expression is upregulated after ischemia31, 32. Deficiencies in p21 increased SVZ progenitor cell proliferation in mice after ischemic injury31. However, in our study, there was not a significant difference in the p21 expression level in SVZ after PFT-α treatment in stroke animals.
Endogenous NPCs can migrate from the SVZ to the damaged areas after MCAo33. Our data demonstrated that the migration of NPCs to the lesioned cortex is associated with functional recovery. We also observed a differential distribution of BrdU-positive cells in the SVZ and cortex on days 10 and 21 after stroke. A higher density of BrdU cells was present in the SVZ on day 10, while increased BrdU immunoreactivity was found in the lesioned cortex on day 21. This difference may be attributed to the migration of NPCs from SVZ to the lesioned cortex between day 10 and day 21. Loss of p53 in p53 knockout(−/−) mice confers a proliferative advantage to the cells in the adult SVZ and favors the progression toward a differentiated phenotype32. We also found that PFT-α enhanced proliferation of NPCs and inhibited cell death/necrosis in the SVZ on day 10, which may secondarily facilitate the migration and differentiation of NPCs as the densities of BrdU, Nestin/BrdU, and NeuN/BrdU (this was marginal, p=0.075) cells in the lesioned cortex were increased by PFT-α on day 21 after MCAo.
We found that PFT-α dose- and time-dependently increased the number and size of new neurosphere formations in in vitro cultures. Spheres that received PFT-α treatment on DIV1 grew larger compared to vehicle-treated sphere cultures, consistent with the report that SVZ cells extracted from p53−/− mice showed an increased number of neurosphere formations as well as increased size in the spheres formed34. These data suggest that the positive effect of PFT-α on NPCs from SVZ is reflected in survival and growth in vitro.
In conclusion, our results support an effect of PFT-α in enhancing neurogenesis by promoting proliferation and survival of the endogenous NPCs in a stroke model. In contrast to previous studies using PFT-α at a much earlier and narrower time frame, our results demonstrate a significant behavioral recovery at much later time points, days after the ischemia occurs. Our results suggest a new potential treatment strategy for stroke patients, enabling a longer treatment window after onset of stroke, a disorder frequently difficult to treat immediately after occurrence.
Supplementary Material
Acknowledgments
This study is supported by the Intramural Research Programs at the National Institute on Drug Abuse and the National Institute of Aging, NIH. We thank Dr. Teruo Hayashi for his technical assistance.
Nonstandard abbreviations used
- Apaf1
apoptotic protease-activating factor 1
- Bax
BCL2-associated X protein
- Bcl2
B-cell leukemia/lymphoma 2
- BCC
bilateral common carotids
- GFAP
glial fibrillary acidic protein
- HPRT
hypoxanthine guanine phosphoribosyl transferase
- MAP2
microtubule-associated protein 2
- MCAo
middle cerebral artery occlusion
- NPCs
neural progenitor cells
- NSCs
neural stem cells
- NeuN
neuronal nuclear antigen
- PCNA
proliferating cell nuclear antigen PFT-α, pifithrin-α
- PGK1
phosphoglycerate kinase 1
- PUMA
p53-upregulated modulator of apoptosis
- qRT-PCR
Quantitative reverse transcription-PCR
- SVZ
subventricular zone
Contributor Information
Yu Luo, National Institute on Drug Abuse, IRP, Baltimore, MD 21224.
Chi-Chung Kuo, National Institute on Drug Abuse, IRP, Baltimore, MD 21224.
Hui Shen, National Institute on Drug Abuse, IRP, Baltimore, MD 21224.
Jenny Chou, National Institute on Drug Abuse, IRP, Baltimore, MD 21224.
Nigel H. Greig, National Institute of Aging, Baltimore, MD 21224.
Barry J. Hoffer, National Institute on Drug Abuse, IRP, Baltimore, MD 21224.
Yun Wang, National Institute on Drug Abuse, IRP, Baltimore, MD 21224.
References
- 1.Goldstein LB. Acute ischemic stroke treatment in 2007. Circulation. 2007;116:1504–1514. doi: 10.1161/CIRCULATIONAHA.106.670885. [DOI] [PubMed] [Google Scholar]
- 2.Gilman S. Pharmacologic management of ischemic stroke: relevance to stem cell therapy. Exp Neurol. 2006;199:28–36. doi: 10.1016/j.expneurol.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci. 2007;26:605–614. doi: 10.1111/j.1460-9568.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- 4.Abrahams JM, Gokhan S, Flamm ES, Mehler MF. De novo neurogenesis and acute stroke: are exogenous stem cells really necessary? Neurosurgery. 2004;54:150–155. doi: 10.1227/01.neu.0000097515.27930.5e. discussion 155–156. [DOI] [PubMed] [Google Scholar]
- 5.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Yu QS, Cutler RG, et al. Novel p53 inactivators with neuroprotective action: syntheses and pharmacological evaluation of 2-imino-2,3,4,5,6,7-hexahydrobenzothiazole and 2-imino-2,3,4,5,6,7-hexahydrobenzoxazole derivatives. J Med Chem. 2002;45:5090–5097. doi: 10.1021/jm020044d. [DOI] [PubMed] [Google Scholar]
- 7.Culmsee C, Zhu X, Yu QS, et al. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem. 2001;77:220–228. doi: 10.1046/j.1471-4159.2001.t01-1-00220.x. [DOI] [PubMed] [Google Scholar]
- 8.Leker RR, Aharonowiz M, Greig NH, Ovadia H. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol. 2004;187:478–486. doi: 10.1016/j.expneurol.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 10.Meletis K, Wirta V, Hede SM, et al. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 11.Felling RJ, Levison SW. Enhanced neurogenesis following stroke. J Neurosci Res. 2003;73:277–283. doi: 10.1002/jnr.10670. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Chang CF, Morales M, et al. Diadenosine tetraphosphate protects against injuries induced by ischemia and 6-hydroxydopamine in rat brain. J Neurosci. 2003;23:7958–7965. doi: 10.1523/JNEUROSCI.23-21-07958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen ST, Hsu CY, Hogan EL, et al. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- 14.Tomac AC, Agulnick AD, Haughey N, et al. Effects of cerebral ischemia in mice deficient in Persephin. Proc Natl Acad Sci U S A. 2002;99:9521–9526. doi: 10.1073/pnas.152535899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou J, Harvey BK, Chang CF, et al. Neuroregenerative effects of BMP7 after stroke in rats. J Neurol Sci. 2006;240:21–29. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 17.Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- 18.Chou J, Luo Y, Kuo CC, et al. Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neuroscience. 2008;151:92–103. doi: 10.1016/j.neuroscience.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis MA, Penney EB, Pearson AG, et al. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci U S A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiltrout C, Lang B, Yan Y, et al. Repairing brain after stroke: a review on post-ischemic neurogenesis. Neurochem Int. 2007;50:1028–1041. doi: 10.1016/j.neuint.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Chopp M, Zhang ZG, et al. p53-immunoreactive protein and p53 mRNA expression after transient middle cerebral artery occlusion in rats. Stroke. 1994;25:849–855. doi: 10.1161/01.str.25.4.849. discussion 855–846. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Chopp M, Powers C, Jiang N. Apoptosis and protein expression after focal cerebral ischemia in rat. Brain Res. 1997;765:301–312. doi: 10.1016/s0006-8993(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 23.Crumrine RC, Thomas AL, Morgan PF. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab. 1994;14:887–891. doi: 10.1038/jcbfm.1994.119. [DOI] [PubMed] [Google Scholar]
- 24.Okano H, Sakaguchi M, Ohki K, et al. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102:1459–1465. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- 25.Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- 26.Das S, Boswell SA, Aaronson SA, Lee SW. P53 promoter selection: choosing between life and death. Cell Cycle. 2008;7:154–157. doi: 10.4161/cc.7.2.5236. [DOI] [PubMed] [Google Scholar]
- 27.Duan W, Zhu X, Ladenheim B, et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- 28.Fortin A, Cregan SP, MacLaurin JG, et al. APAF1 is a key transcriptional target for p53 in the regulation of neuronal cell death. J Cell Biol. 2001;155:207–216. doi: 10.1083/jcb.200105137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 30.Toth A, Jeffers JR, Nickson P, et al. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H52–60. doi: 10.1152/ajpheart.01046.2005. [DOI] [PubMed] [Google Scholar]
- 31.Qiu J, Takagi Y, Harada J, et al. Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med. 2004;199:937–945. doi: 10.1084/jem.20031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomasevic G, Shamloo M, Israeli D, Wieloch T. Activation of p53 and its target genes p21(WAF1/Cip1) and PAG608/Wig-1 in ischemic preconditioning. Brain Res Mol Brain Res. 1999;70:304–313. doi: 10.1016/s0169-328x(99)00146-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, Zhang Z, Wang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Gil-Perotin S, Marin-Husstege M, Li J, et al. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26:1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.