Abstract
The rest/activity circadian rhythm (CircAct) reflects the function of the circadian timing system (CTS). In a prior single-institution study, the extent of CircAct perturbation independently predicted for survival and tumor response in 192 patients receiving chemotherapy for metastatic colorectal cancer (MCRC). Moreover, the main CircAct parameters correlated with several quality of life (HRQoL) scales. In this prospective study we attempted to extend these results to an independent cohort of chemotherapy-naïve MCRC patients participating in an international randomized phase III trial (EORTC 05963).
Patients were randomized to receive chronomodulated or conventional infusion of 5-fluorouracil, leucovorin and oxaliplatin as first line treatment for MCRC.
Patients from nine institutions completed the EORTC QLQ-C30, and wore a wrist-accelerometer (actigraph) for 3 days before chemotherapy delivery. Two validated parameters (I<O and r24) were used to estimate CircAct.
Of 130 patients with baseline CircAct assessments, 96 had baseline HRQoL data. I<O was confirmed to correlate with global quality of life, physical functioning, social functioning, fatigue and appetite loss (r>|0.25|, p<0.01). I<O further independently predicted for overall survival with a Hazard Ratio of 0.94 (p<0.0001).
The associations between CircAct parameters, HRQoL and survival that were demonstrated in this international study involving previously untreated MCRC patients, confirm prior single-institution findings in mostly pretreated MCRC patients. The circadian timing system constitutes a novel therapeutic target. Interventions that normalize circadian timing system dysfunction may impact quality of life and survival in cancer patients
Keywords: Circadian, Actigraphy, Health-related Quality of Life, Colorectal Cancer, Chronotherapy, Survival
INTRODUCTION
Most activities of daily living, such as locomotor activity, eating behaviour, sleep/wakefulness, psychophysical performance and mood are regulated along the 24-hour period by the circadian timing system (1-3). This system is constituted of molecular circadian clocks in peripheral tissues, whose coordination is ensured by the suprachiasmatic nuclei, a central pacemaker located in the anterior hypothalamus (1-3). The alternation of light and darkness, social routine and feeding schedules calibrate the circadian timing system to precisely 24 hours by resetting the central pacemaker and the peripheral clocks respectively (4). The suprachiasmatic nuclei coordinate the peripheral clocks through polysynaptic neuroanatomical pathways as well as through blood-borne cytokines and hormones (5, 6). The circadian rhythm in locomotor activity (CircAct) is a well-established marker of the function of the central pacemaker (7, 8) and can be continuously and non-invasively assessed in an objective, reliable and validated manner with wrist-actigraphy (9, 10).
The affective and constitutional symptoms, that tend to cluster in cancer patients, may be partly due to circadian disruption (5). Disrupted circadian function, objectively estimated with wrist actigraphy, has been found to correlate with subjective parameters, such as performance status (11-13) and self-reported symptoms, particularly fatigue, poor sleep, appetite loss and depression (12-15). Jet-lag and shift-work are associated with the same symptoms (16-19).
Abnormal circadian rhythms have been associated with higher risk of cancer development and more rapid cancer progression in both rodents and humans. In tumor bearing rodents, disruption of the circadian rest-activity rhythm, resulted in faster tumor growth and shorter survival (20, 21). Shift work, with the associated disruption of the circadian rhythm, is a significant and independent risk factor for the development of breast, colorectal, endometrial and prostate cancer (22-26).
Abnormal circadian rhythms have been demonstrated in cancer patients (27-29) and associated with poorer outcome. In women with metastatic breast cancer, a disrupted cortisol rhythm was associated with poorer survival (30). In a prior single-institution study, the extent of CircAct perturbation independently predicted for survival and tumor response in 192 patients receiving chemotherapy for metastatic colorectal cancer (31). Moreover, CircAct correlated with several health-related quality of life (HRQoL) scales (31, 32). In the present study we attempted to extend these previous results to an independent cohort of chemotherapy-naïve patients with metastatic colorectal cancer participating in an international randomized phase III trial.
MATERIALS AND METHODS
Study outline
The focus of this study was to correlate the circadian rest/activity rhythms of patients with advanced colorectal cancer with quality of life and survival. This open prospective study was a companion to an international, multicenter, randomized two-arm phase III trial (EORTC 05963) that compared two biweekly schedules of the combination of Oxaliplatin (l-OHP), 5-Fluorouracil (5FU) and Leucovorin (LV), in 564 chemotherapynaïve patients with metastatic colorectal cancer (33). Patients were randomized to receive either a conventional 2-day regimen (FOLFOX2), or a 4-day chronomodulated schedule (chronoFLO4). Inclusion criteria were: Performance Status (PS) of 2 or less (World Health Organization scale), age from 18 to 76 years, adequate haematological, renal and hepatic functions, no overt brain metastases and no prior chemo- or radio-therapy for metastatic disease. The primary trial registered 564 patients at 36 institutions in 10 countries. The nine institutions (in Belgium, Canada, France and Italy) participating in this companion study entered 191 patients on the main trial from August 1999. This group of patients was eligible for the current study that was approved by the local Ethics Review Boards. Clinical outcomes (overall survival, progression-free survival, tumor response and toxicity grading) were evaluated as previously described (33).
CircAct and HRQoL assessments
To assess individual circadian rest/activity rhythm, a Mini-Motionlogger actigraph (Ambulatory Monitoring Inc., Ardsley, NY, USA) was used. The actigraph is similar to a watch and is worn on the non-dominant wrist. It contains a piezoelectric linear accelerometer to detect wrist movements and a memory chip for data storage. The user-defined time interval for the recording and count of activity level was 1 minute. The actigraph was worn for at least 72 hours continuously before the beginning of the first or second course of chemotherapy (9, 10, 31). All actigraphy time series were analyzed using a specific program (Action 4 v1.10, Ambulatory Monitoring Inc., USA) by one investigator. Two robust and well characterized parameters were used to estimate the circadian activity pattern: the dichotomy index (I<O), which integrates the circadian regulation of sleep and takes into account the relative difference in activity between the rest and wakeful spans, and the autocorrelation coefficient at 24 hours (r24), a measure of the regularity and reproducibility of the activity pattern over a 24-hour period from one day to the next (31). In case of a prominent circadian rhythm, I<O reaches 100% and r24 reaches 1. Average activity (meanAct) was calculated, as the average number of wrist movements per minute throughout the recording time. All parameter values were computed for the whole monitoring period (72 continuous hours).
The EORTC Quality of Life Questionnaire QLQ-C30 (version 2.0) was completed prior to the first or second course of chemotherapy (34). All scores were calculated using the standard recommended EORTC procedures.
Statistical analyses
Means, medians, standard deviations (SD) and inter-quartile ranges were used as summary statistics for the raw distribution of CircAct parameters. The normality of the CircAct parameters distribution was assessed with the Shapiro-Wilk test. The Wilcoxon two-sample test was used to assess the association between CircAct parameters and binary factors or outcomes. The Jonckheere-Terpstra test was computed to assess the association between CircAct parameters and ordinal factors. Logistic regression was used to test whether each quantitative CircAct parameters predicted for the occurrence of severe toxic events.
The correlations among CircAct parameters, between CircAct parameters and continuous variables, as well as the correlation between CircAct parameters and baseline HRQoL scores were examined using the Spearman's rank correlation coefficient. Partial correlation coefficients were computed between CircAct parameters and HRQoL scores using age, body mass index and haemoglobin concentration separately as controlling variables. Correlations were considered as relevant if r > |0.25| and two-sided p≤0.01. Moreover, CircAct parameters were dichotomized according to their median (above and below median), and the HRQoL indices were compared in these two subgroups with the Mann-Whitney U test.
The Effect Size (ES) of the difference in HRQoL scores between the two CircAct subgroups was calculated as the difference in subgroup means divided by the pooled standard deviations. The threshold for clinical relevance was set at ES ≥ 0.4.
Since multiple analyses were performed, and since the sample size of the current study was not computed with these endpoints in mind, the threshold for statistically significant differences was set at p≤0.01.
Progression-free and overall survival functions were estimated by the Kaplan-Meier's method. The survival curves for each CircAct parameter were drawn in groups of patients defined according to the quartiles of the distribution of the considered parameter. The survival curves were compared using the Logrank test. The association between each CircAct parameter as quantitative variable and progression-free or overall survival was also assessed with a simple Cox's regression model. Each CircAct parameter found significantly prognostic with the simple regression analysis at the 5% significance level was included in a multiple Cox's regression model and adjusted for age, gender, body mass index, study center, primary tumor site, prior adjuvant chemotherapy and treatment arm, together with clinically relevant prognostic factors (31, 35). Stepwise regressions at the 5% significance level were performed, and the bootstrap resampling technique was used as an internal validation tool.
RESULTS
Study compliance
A total of 130 patients (23% of the whole trial population) had their CircAct evaluated: 103 patients before treatment onset, 24 patients after the first course and 3 after the second course of treatment. Considering the 191 potential patients available for study, the true baseline compliance was 68%. Of the 130 patients with baseline actigraphy monitoring, 96 patients (74%) also completed the EORTC QLQ-C30.
Representativeness of the current study population
The main clinical characteristics of the 130 patients in this companion study were comparable to those of the 564 patients registered in the main trial (Table 1). The current study population had baseline HRQoL scores, overall survival, progression-free survival, response rate and grade III-IV toxicity rate that did not significantly differ from those of the remaining trial population (p ≥ 0.10, data not shown).
Table 1.
Main clinical features of the whole EORTC 05963 trial population according to the availability or not of actigraphy assessment at baseline.
| Variable | CircAct assessment available |
Total (33) |
|
|---|---|---|---|
|
Yes (n=130)* |
No (n=434) |
n=564 |
|
| |
n (%) |
n (%) |
n (%) |
| Treatment arm | |||
| FOLFOX2 | 67 (51.5) | 215 (49.5) | 282 (50) |
| ChronoFLO4 |
63 (48.5) |
219 (50.5) |
282 (50) |
| WHO PS | |||
| 0 | 70 (53.8) | 203 (46.8) | 273 (48.4) |
| 1 | 45 (34.6) | 186 (42.9) | 231 (41.0) |
| 2 |
15 (11.5) |
45 (10.4) |
60 (10.6) |
| Age (years) | |||
| Median | 60 | 63 | 62 |
| Range |
22−76 |
24−76 |
22−76 |
| Gender | |||
| Male | 74 (56.9) | 264 (60.8) | 338 (59.9) |
| Female |
56 (43.1) |
170 (39.2) |
226 (40.1) |
| Body Mass Index (kg/m2) | |||
| ≤ 18.4 (underweight) | 4 (3.1) | 19 (4.4) | 23 (4.1) |
| 18.5 − 24.9 (normal) | 57 (43.8) | 220 (50.7) | 277 (49.1) |
| 25.0 − 29.9 (overweight) | 51 (39.2) | 134 (30.9) | 185 (32.8) |
| ≥ 30.0 (obese) | 17 (13.1) | 59 (13.6) | 77 (13.7) |
| Missing | 0 | 2 (0.5) | 2 (0.4) |
| Median | 25.2 | 24.5 | 24.6 |
| Range |
16.7−39.1 |
14.9−42.9 |
14.9−42.9 |
| Site of primary tumor | |||
| Colon | 96 (73.8) | 329 (75.8) | 425 (75.4) |
| Rectum |
34 (26.2) |
105 (24.2) |
139 (24.6) |
| Number of metastatic sites | |||
| 0 | 0 ( 0.0) | 4 ( 0.9) | 4 ( 0.7) |
| 1 | 58 (44.6) | 224 (51.6) | 282 (50.0) |
| 2 | 52 (40.0) | 139 (32.0) | 191 (33.9) |
| ≥3 | 20 (15.4) | 66 (15.2) | 86 (15.2) |
| Unknown |
0 ( 0.0) |
1 ( 0.2) |
1 ( 0.2) |
| Organs involved | |||
| Liver | 106 (81.5) | 377(86.9) | 483 (85.6) |
| ≤ 25 % | 62 (47.7) | 204 (47.0) | 266 (47.2) |
| > 25% | 44 (33.8) | 173 (39.9) | 217 (38.5) |
| Lung |
47 (36.2) |
162 (37.3) |
209 (37.1) |
|
Analgesics at entry |
26 (20.0) |
56 (12.9) |
82 (14.5) |
|
Previous adjuvant treatment |
23 (17.7) |
79 (18.2) |
102 (18.1) |
| WBC (109 cells/L) | |||
| Median | 8.0 | 8.1 | 8.1 |
| Range |
3.6 − 25.5 |
3.1 − 27.6 |
3.1 − 27.6 |
| ≥ 10 |
30 (23.1) |
102 (23.5) |
132 (23.4) |
| Baseline HRQoL available | 96 (74) | 347 (80) | 443 (79) |
Current study population
WHO PS = Performance status (World Health Organization)
WBC = White blood cell count
HRQoL = Health-related quality of life
Distribution of circadian rest/activity rhythm parameters
The 24-h patterns in rest-activity displayed wide inter-individual differences. For meanAct and r24, the assumption of a normal distribution was not rejected (p=0.20 and 0.19, respectively). Mean and median meanAct were 105 and 106 counts per minute, respectively (SD 27, interquartile range 41). For r24, mean and median values were 0.38 and 0.37, respectively (SD 0.17, interquartile range 0.24). Conversely, the distribution of I<O was non-normal (p<0.0001) and skewed. Thus median I<O was 97.0, whereas mean I<O was 94.3 (SD 8.6, interquartile range 6.8). The CircAct parameters I<O and r24 were positively correlated with each other (r = 0.74, p < 0.001) and with meanAct (r = 0.47, p < 0.001 and r = 0.46, p < 0.001, respectively).
CircAct correlation with patient characteristics
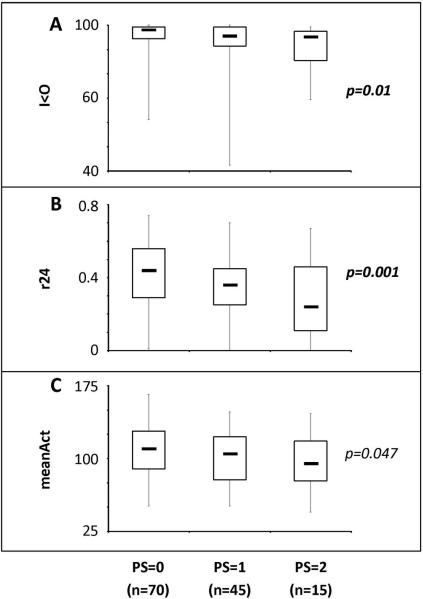
Patients with good performance status (PS) had significantly more robust CircAct in comparison to patients with poor PS (Figure 1) for I<O (p=0.01), r24 (p=0.0014) and to a lesser extent meanAct (p=0.047). Thus, median I<O and r24 respectively decreased from 98.2 and 0.44 in the patients with PS=0 (n=70), to 95.7 and 0.36 in those with PS=1 (n=45), and 95.3 and 0.24 in patients with PS=2 (n=15). Patients receiving analgesic treatment at study entry (n=26) displayed significantly more perturbed CircAct as compared to patients without painkillers for I<O (p=0.004), r24 (p=0.001) and to a lesser extent meanAct (p=0.048). Indeed, median I<O was 95.3 and median r24 was 0.26 in patients on analgesics, whereas corresponding median values were respectively 97.7 and 0.41 in patients not requiring analgesics. CircAct parameters did not correlate with age, gender, body mass index, percentage of liver involvement with tumor, number of metastatic sites, site of primary tumor, blood tests results (blood counts, liver tests, CEA) or previous adjuvant chemotherapy administration (p>0.10).
Figure 1.
Boxplots illustrating the distribution of the CircAct parameters I<O (panel A), r24 (panel B) and meanAct (panel C) according to the WHO PS. Box: median (middle line) and 1st and 3rd quartiles; thin vertical bars denote range.
CircAct Correlations with HRQoL
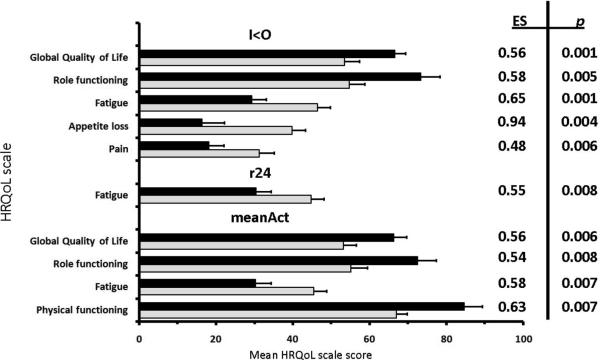
All CircAct parameters (I<O, r24, meanAct) correlated positively with global QoL and role functioning, but negatively with fatigue and appetite loss. In addition, I<O and r24 correlated positively with social functioning and negatively with pain and dyspnea. Furthermore, I<O correlated positively with physical functioning, but negatively with insomnia. MeanAct correlated positively with physical functioning. Conversely, no CircAct parameter correlated with emotional or cognitive functioning, nausea/vomiting, constipation or diarrhoea. These correlations remained statistically significant after adjustment for age, with minimal modifications in coefficient values (range: − 0.02 to + 0.04) and p values (Table 2). Controlling for body mass index or haemoglobin level did not relevantly alter any of the previously listed correlations either (results not shown). Figure 2 shows the difference in mean HRQoL scores between two subgroups of patients classified by CircAct parameters above median (black bars) vs below the median (gray bars). Based on the thresholds for clinical relevance (Effect Size ≥ 0.4 and p < 0.01), higher than median I<O was associated with better global QoL, higher role functioning, less fatigue, less appetite loss and less pain. However, higher than median r24 was only associated with less fatigue, while higher than median meanAct was associated with better global QoL, higher role functioning, less fatigue, and better physical functioning.
Table 2.
Partial correlation coefficients, adjusted for age, between HRQoL indices and CircAct parameters at baseline, with significance levels.
| HRQoL scale (n = 96) | CircAct parameter | ||
|---|---|---|---|
| I<O | r24 | meanAct | |
| Global QoL | 0.39 ** | 0.32 * | 0.37 ** |
| Role functioning | 0.36 ** | 0.31 * | 0.40 ** |
| Fatigue | − 0.39 ** | − 0.36 ** | − 0.39 ** |
| Appetite loss | − 0.32 * | − 0.30 * | − 0.32 * |
| Social functioning | 0.35 ** | 0.33 ** | § |
| Pain | −0.40 ** | − 0.30 * | § |
| Dyspnoea | − 0.29 * | − 0.27 * | § |
| Physical functioning | 0.35 ** | § | 0.35 ** |
| Insomnia | − 0.33 ** | § | § |
| Emotional functioning | § | § | § |
| Cognitive functioning | § | § | § |
| Nausea/Vomiting | § | § | § |
| Constipation | § | § | § |
| Diarrhoea | § | § | § |
r<|0.25| and/or p>0.01
p≤0.01
p≤0.001
HRQoL = Health-related quality of life
CircAct = Circadian rest/activity rhythm
I<O = dichotomy index
r24 = autocorrelation coefficient at 24 hours
meanAct = average activity
Figure 2.
The difference in mean HRQoL scores (+ Standard Error of the Mean) between two subgroups of patients classified by CircAct parameters above median (black bars) vs below the median (gray bars) where the clinical relevance (Effect Size ≥ 0.4) was statistically significant (p < 0.01). Black boxes indicate CircAct parameters above median: I<O ≥ 97.0 % (n=49); r24 ≥ 0.38 (n=47); meanAct ≥ 106 counts/minute (n=49). Grey boxes indicate CircAct parameters below median: I<O < 97.0 % (n=47); r24 < 0.38 (n=49); meanAct < 106 counts/minute (n=47).
CircAct Correlations with toxicity, response and survival
Baseline CircAct parameters predicted neither for best objective tumor response (p ≥ 0.14), nor for the occurrence of at least one moderate or severe toxic event based on the NCIC-CTC v2.0 grading score (p ≥ 0.31). No statistically significant association was found between progression-free survival and any CircAct parameter (p > 0.10).
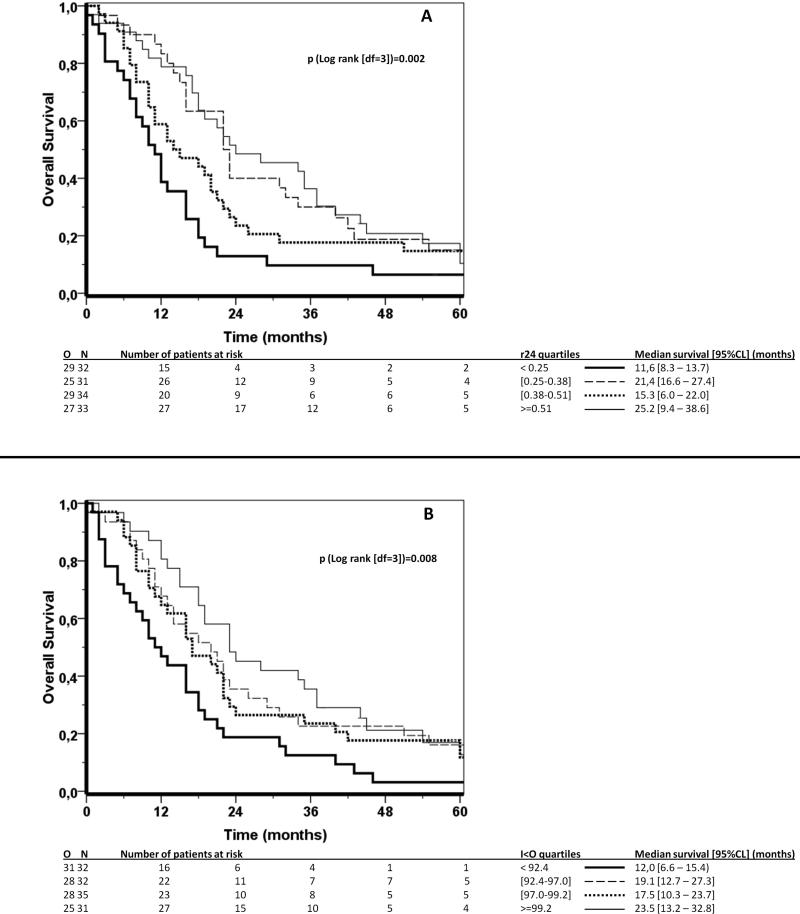
For each CircAct parameter, the comparison of the survival functions of the patient groups defined by the quartiles of the parameter showed a significant difference for r24 (logrank test, p=0.002), and for I<O (logrank test, p=0.008), but not for meanAct (logrank test, p=0.201). The corresponding Kaplan-Meier survival estimate curves for r24 and I<O were plotted in Figure 3A and 3B, respectively. No consistent trend in Kaplan-Meier survival estimates was observed as a function of r24 quartiles (Figure 3A). Conversely, a trend was evident for I<O, with the poorest survival occurring in the lowest quartile group, intermediate and overlapping survival in both middle quartiles, and a slightly better survival in the highest quartile group (Figure 3B). These observations were confirmed by plotting the log (-log) of the survival curves (not shown).
Figure 3.
Kaplan Meier survival curves according to r24 (A) and I<O (B) split by the quartiles of their distribution. 1st quartile: thick solid line; 2nd quartile: thin dashed line; 3rd quartile: thick dotted line; 4th quartile: thin solid line. Logrank test (df=3): p=0.002 for r24 (A); p=0.008 for I<O (B).
A significant positive association was found between overall survival and I<O with a hazard ratio (HR) of 0.95 (95% CI: 0.93 − 0.97, p<0.0001) using a Cox's proportional hazards univariate regression model. A similar relationship was found for overall survival and r24, with a HR of 0.20 (95% CI: 0.07 − 0.60, p=0.004). No such association was found for survival and meanAct (p=0.15). The relation between I<O and survival remained roughly similar following adjustment for the main prognostic factors in metastatic colorectal cancer (31, 35) as well as age, gender, body mass index, institution, prior adjuvant chemotherapy, primary tumor site and treatment arm in a Cox's multivariate regression analysis (HR=0.94, 95% CI: 0.92 − 0.97, p<0.0001). Of those factors, the number of metastatic sites (1 vs >1) and the white blood cell count (<10 vs ≥ 10 × 10 9cells/L) were significantly related to survival (p=0.041 and p=0.01, respectively). The bootstrap analysis, showed percentages of inclusion in the multiple regression models of 89% for I<O, 74% for PS and < 60% for other parameters. I<O remained independently related to overall survival (HR: 0.93, 95% CI: 0.90 − 0.96, p<0.0001) after excluding the 23 patients who had received prior adjuvant chemotherapy. Conversely, r24 did not display an independent prognostic value in a Cox's multivariate regression analysis (p=0.13), after adjustment for the aforementioned factors.
DISCUSSION
The focus of this study was to correlate the circadian rest/activity rhythms of patients with advanced colorectal cancer with quality of life and survival. In this international study involving 130 patients with metastatic colorectal cancer, wrist actigraphy monitoring provided three objective parameters that were selected based on their clinical relevance in a previous study (31). The correlation between CircAct and selected HRQoL scales was confirmed, as well as the independent prognostic value of CircAct for survival.
Both r24 and I<O, that quantify different aspects of CircAct, were strongly correlated with each other, yet more weakly so with meanAct. The average level of activity, which does not account for any circadian rhythmicity, was not as clinically relevant, highlighting the clinical importance of circadian physiology as opposed to the mere count of physical activity. Nevertheless, meanAct correlated with the two rhythm parameters, suggesting that a dampened CircAct was associated with reduced average activity. These CircAct parameters do not constitute an objective evaluation of sleep. Other actigraphy parameters, not assessable in the current study, have been reported to correlate with subjective sleep perception, as estimated with specific sleep questionnaires (14, 36, 37). A specific assessment of sleep quality and quantity of 95 cancer patients with 42h continuous ambulatory polysomnography revealed disturbances in sleep and waking states maintenance, with features supporting a blunted sleep drive from the circadian timing system (38).
Performance status (WHO), a subjective estimate of physical performance, was significantly correlated with I<O and r24. Conversely, the extent of disease involvement did not seem to influence the rhythmic pattern of activity. Damped circadian rhythms have been described in cancer patients with no evidence of disease, for example in the adjuvant setting (14, 36), suggesting that factors other than tumor burden can account for circadian disruption.
The rest-activity rhythm provides a window on the circadian timing system, which controls hormonal patterns, sleep, food intake and other rhythmic behaviors (1-3). Disturbances of the circadian timing system are associated with a cluster of symptoms, including fatigue, anorexia and sleep disturbances (16-18, 39, 40). Damped CircAct was associated with subjective impairment in the physical -, role - and social functioning dimensions of HRQoL, (with more fatigue, anorexia, pain, dyspnoea and insomnia), as well as with poor global quality of life. These correlations were barely altered by age, body mass index or haemoglobin concentration (Table 2), underpinning the hypothesis of an independent role of the circadian timing system on the well-being of patients. These findings further extended and strengthened the relevance of previous results from a single institution study (31, 32). The order of magnitude of the correlation coefficients between CircAct parameters and HRQoL scales (r ≤ 0.4, Table 2) was comparable to that reported in other studies correlating an objective and unidimensional biological parameter, such as haemoglobin, with the subjective measure of health-related quality of life (41, 42).
In the current study, CircAct parameters predicted neither for best objective tumor response, nor for progression-free survival. In our previous study, the association between CircAct parameters and progression-free survival was not investigated but I<O was found to independently predict for tumor response (31). This discrepancy could relate to differences between the two studies regarding the proportion of chemotherapynaïve patients (all vs 39%), the subsequent administration of chronomodulated chemotherapy (48% vs all) and the rate of best objective response (52% versus 35%, respectively (31, 33)).
The peripheral tissue clocks determine the temporal patterns of drug cytotoxicity (1, 43, 44). The CirAct is a marker of the output from the central circadian pacemaker and does not reflect the rhythmicity of molecular clocks in peripheral tissues. Thus, alterations of host CircAct may only marginally affect the time-dependent antitumor activity and normal tissue toxicity. This could account for the lack of correlation between CircAct parameters and tumor response, progression-free survival or toxicity.
In the present study, I<O was confirmed as an independent prognostic factor for overall survival. The lesser relevance of r24 as compared to I<O for HRQoL and survival could indicate that the regular sequence of daytime highly active spans and restful nighttime sleep spans (as measured with I<O) rather than the consistent reproducibility of the activity pattern over exactly 24 hours (as estimated by the r24) better reflect the functional status of the circadian timing system.
The trend in overall survival which differentiated the quartiles of I<O persisted with time elapsing after CircAct assessment. This observation indicated that the relationship between CircAct and overall survival was independent from immediate pre-mortality disruption in circadian rest-activity cycles (Figure 3B).
Social synchronization represents an essential time cue for the entrainment of human circadian rhythms (4, 45). A significant correlation between the social functioning dimension of HRQoL and I<O was revealed in both our previous and current studies (32). The independent prognostic value of social functioning for survival has been separately demonstrated in all patients enrolled on the EORTC 05963 trial that completed the EORTC QLQ-C30 questionnaire at baseline (46), confirming previous results (47). Impaired social functioning, perceived as interference with family life and social activities, could reflect the patient's self-sensation of cancer-related physiologic disturbances.
Taken together these data corroborate the hypothesis that the circadian rest/activity rhythm, measured with wrist actigraphy, correlates with several symptoms and domains of well-being as well as with the survival of patients with metastatic colorectal cancer.
The circadian timing system constitutes a novel potential therapeutic target for future multidisciplinary research efforts (44, 48, 49). Interventions that normalize circadian timing system dysfunction should be studied with the aim of relieving symptoms, improving health related quality of life and prolonging survival in cancer patients.
Acknowledgement
The authors sincerely thank Richard Sylvester, from the EORTC Data Center, for critically reviewing earlier versions of this manuscript.
We also acknowledge the support of the Association Internationale pour la Recherche sur le Temps Biologique et la Chronothérapie (ARTBC International), Paul Brousse hospital, Villejuif (France).
Financial support: This study was supported in part by a grant from Sanofi Pharma, which provided oxaliplatin for patients until approval of the drug in participating countries. The study was also supported by Association Internationale pour la Recherche sur le Temps Biologique et la Chronothérapie (ARTBC International), Paul Brousse hospital, Villejuif (France) and by the European Union through the Network of Excellence BioSim [LSHB-CT-2004-005137]. Ambulatory Monitoring Inc (Ardsley, NY) generously loaned actigraph units to several participating centers. This publication was supported by grants number 2U10CA11488-28 through 5U10CA11488-32 from the National Cancer Institute (Bethesda, Maryland, USA).
Footnotes
Conflict of interests: All the authors declare no conflict of interests.
Previous presentations: This study was presented in part at the 41st ASCO annual meeting (Orlando, Florida, 2005: abstracts #: 3553 & 8029).
REFERENCES
- 1.Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- 2.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–61. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 3.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 4.Roenneberg T, Daan S, Merrow M. The art of entrainment. J Biol Rhythms. 2003;18:183–94. doi: 10.1177/0748730403018003001. [DOI] [PubMed] [Google Scholar]
- 5.Rich TA. Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis. J Support Oncol. 2007;5:167–74. discussion 76−7. [PubMed] [Google Scholar]
- 6.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 7.Moore-Ede MC, Czeisler CA, Richardson GS. Circadian timekeeping in health and disease. Part 2. Clinical implications of circadian rhythmicity. N Engl J Med. 1983;309:530–6. doi: 10.1056/NEJM198309013090905. [DOI] [PubMed] [Google Scholar]
- 8.Moore-Ede MC, Czeisler CA, Richardson GS. Circadian timekeeping in health and disease. Part 1. Basic properties of circadian pacemakers. N Engl J Med. 1983;309:469–76. doi: 10.1056/NEJM198308253090806. [DOI] [PubMed] [Google Scholar]
- 9.Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological Challenges When Using Actigraphy in Research. J Pain Symptom Manage. 2008 doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 11.Levin RD, Daehler MA, Grutsch JF, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. 2005;93:1202–8. doi: 10.1038/sj.bjc.6602859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17:320–32. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 13.Roscoe JA, Morrow GR, Hickok JT, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10:329–36. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 14.Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouladiun M, Korner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res. 2007;13:6379–85. doi: 10.1158/1078-0432.CCR-07-1147. [DOI] [PubMed] [Google Scholar]
- 16.Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. 1997;350:1611–6. doi: 10.1016/S0140-6736(97)07569-7. [DOI] [PubMed] [Google Scholar]
- 17.Waterhouse J, Reilly T, Atkinson G, Edwards B. Jet lag: trends and coping strategies. Lancet. 2007;369:1117–29. doi: 10.1016/S0140-6736(07)60529-7. [DOI] [PubMed] [Google Scholar]
- 18.Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6:407–14. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- 19.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 20.Filipski E, Delaunay F, King VM, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–85. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 21.Filipski E, King VM, Li X, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–7. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 22.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 23.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 24.Schernhammer ES, Laden F, Speizer FE, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–8. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 25.Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–22. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- 26.Kubo T, Ozasa K, Mikami K, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–55. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 27.Mormont MC, Levi F. Circadian-system alterations during cancer processes: a review. Int J Cancer. 1997;70:241–7. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321–8. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee K, Cho M, Miaskowski C, Dodd M. Impaired sleep and rhythms in persons with cancer. Sleep Med Rev. 2004;8:199–212. doi: 10.1016/j.smrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 31.Mormont MC, Waterhouse J, Bleuzen P, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038–45. [PubMed] [Google Scholar]
- 32.Mormont MC, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int. 2002;19:313–23. doi: 10.1081/cbi-120002606. [DOI] [PubMed] [Google Scholar]
- 33.Giacchetti S, Bjarnason G, Garufi C, et al. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006;24:3562–9. doi: 10.1200/JCO.2006.06.1440. [DOI] [PubMed] [Google Scholar]
- 34.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 35.Kohne CH, Cunningham D, Di CF, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308–17. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- 36.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–9. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palesh O, Zeitzer JM, Conrad A, et al. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med. 2008;4:441–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Parker KP, Bliwise DL, Ribeiro M, et al. Sleep/Wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26:2464–72. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 39.Reid KJ, Zee PC. Circadian rhythm disorders. Semin Neurol. 2004;24:315–25. doi: 10.1055/s-2004-835063. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. 2002;13:965–73. doi: 10.1093/annonc/mdf122. [DOI] [PubMed] [Google Scholar]
- 42.Lind M, Vernon C, Cruickshank D, et al. The level of haemoglobin in anaemic cancer patients correlates positively with quality of life. Br J Cancer. 2002;86:1243–9. doi: 10.1038/sj.bjc.6600247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 44.Liu AC, Lewis WG, Kay SA. Mammalian circadian signaling networks and therapeutic targets. Nat Chem Biol. 2007;3:630–9. doi: 10.1038/nchembio.2007.37. [DOI] [PubMed] [Google Scholar]
- 45.Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? J Biol Rhythms. 2005;20:339–52. doi: 10.1177/0748730405277982. [DOI] [PubMed] [Google Scholar]
- 46.Efficace F, Innominato PF, Bjarnason G, et al. Validation of patient's self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: results of an international study by the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2008;26:2020–6. doi: 10.1200/JCO.2007.12.3117. [DOI] [PubMed] [Google Scholar]
- 47.Efficace F, Bottomley A, Coens C, et al. Does a patient's self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer. 2006;42:42–9. doi: 10.1016/j.ejca.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 48.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–82. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiegel D. Losing sleep over cancer. J Clin Oncol. 2008;26:2431–2. doi: 10.1200/JCO.2008.16.2008. [DOI] [PubMed] [Google Scholar]