Abstract
Objectives
Adrenergic and serotonergic (ADR-SER) mechanisms alter gut (GI) sensorimotor functions. We aimed to determine whether candidate ADR-SER genes affect GI responses to low dose clonidine (CLO) in humans.
Methods
Forty healthy and 120 irritable bowel syndrome (IBS) participants received CLO, 0.1mg or 0.15mg b.i.d., for 6 days. At baseline and post-clonidine, we measured: gastric volume (GV); satiation volume; rectal compliance, sensation thresholds and ratings with distensions. Genetic variations tested were: α2A (C-1291G), α2C (Del 332-325), GNβ3 (C825T) and SLC6A4 (5-HTT-LPR).
Results
CLO reduced volume to satiation (p=0.002), postprandial GV (p<0.001), sensation threshold for pain (<0.001); CLO increased rectal compliance (p=0.024). There were significant associations between post-CLO responses and gene variations for Δ GV (α2A and SLC6A4), rectal sensation of gas (α2A, GNβ3), urgency (α2A); and pain (GNβ3 and SLC6A4); and rectal compliance (SLC6A4).
Conclusion
α2A, GNβ3 and SLC6A4 genotypes significantly modify responses to clonidine on sensory and motor GI functions in health and IBS.
Keywords: adrenergic, serotonergic, GNβ3, SLC6A4, G protein, receptor
INTRODUCTION
Adrenergic and serotonergic mechanisms alter gastrointestinal functions, including gastrointestinal propulsion, contractility and tone, fluid absorption and secretion, and sensation (1, 2). The adrenergic system interacts with other effector mechanisms such as the serotonergic system (3). Serotonergic and noradrenergic systems interact to modulate pain perception in the spinal cord, brain, and peripherally in the gut (4); interaction of these systems activate the hypothalamo-pituitary-adrenal axis (5). The pathophysiology of irritable bowel syndrome (IBS) includes abnormalities of motility, sensation, psychosocial and mucosal defense (6).
At relatively high doses (e.g. 0.3mg b.i.d), the α2 receptor agonist, clonidine, reduces intestinal fluid and electrolyte secretion (2), and inhibits gastric and colonic tone, phasic contractility and sensation in the rectum and colon in response to balloon distension in healthy humans (7-9). Studies of colonic distension in healthy volunteers showed that 0.3 mg clonidine resulted in median scores of pain of zero on a 100 mm visual analog scale, and halved the scores of gas to distension at 8 and 16 mmHg above baseline operating pressure (7). Doses of 0.2 mg (9) and 0.3 mg (7) clonidine caused somnolence and hypotension.
In healthy volunteers, clonidine did not significantly alter gastrointestinal or colonic transit, but it significantly increased colonic compliance and reduced sensation to distensions (9); effects on pain during distensions were due to effects of the 0.3 mg dose (9). However, in a randomized, placebo-controlled trial, clonidine, 0.1 mg b.i.d., increased satisfactory relief in IBS and improved stool scores with minimal somnolence and hypotension (10). If clonidine is to be considered as a treatment for IBS, it would be of interest to identify subgroups that may demonstrate superior responses.
Genetics influence response to therapy in IBS, e.g., 5HTTLPR (genetic variation in promoter of SLC6A4 or SERT) influences the response to alosetron in IBS-D (11) and tegaserod in IBS-C (12). Given interaction of adrenergic and serotonergic control mechanisms and the fact that most ligand-receptor interactions result in cellular G protein translation, we assessed the effect of these three systems on the responses to low doses of clonidine (10). We have previously demonstrated significant interactions between α2A C1291T and GNβ3 genotype and gastric emptying (13) and between 5HTTLPR genotype and increased pain sensation during rectal distension in humans (14) in the absence of treatment.
Our hypotheses are: low dose clonidine affects sensory and motor functions; and genetic variation in control of α2 mechanisms impacts the pharmacological actions of clonidine in health and IBS. This could potentially lead to selection of individuals with conditions like IBS for treatment with clonidine, based on candidate genetic variation.
Our aims were to test the dose-response effects of low dose clonidine on motor and sensory functions of the stomach and rectum in IBS and to test whether pharmacodynamic effects of clonidine are modulated by genetic variations in α2AR, 5HTTLPR, and GNβ3. These variations alter the function of the α2A-adrenoceptor, the reuptake of serotonin, and the G protein translation.
MATERIALS AND METHODS
Study Strategy
The strategy followed was: first, to randomize two groups to treatment with either 0.1mg or 0.15mg clonidine, b.i.d., and to confirm that, as would be expected due to the randomization, there were no statistically significant or important clinical differences in characteristics at baseline; second, to appraise the overall effect of clonidine on gastrointestinal functions of interest; third, to compare effects of 0.1mg and 0.15mg clonidine doses; fourth, to study whether the variations in the candidate genes were associated with differences in gastrointestinal functions in response to clonidine (“pharmacogenetics”).
Study Design
All participants underwent studies at baseline, prior to treatment. The data at baseline are published elsewhere (15). They were then randomized to 6 days of double-blind treatment with clonidine, 0.1mg or 0.15mg, b.i.d., using concealed allocation. Satiation, gastric volumes and rectal compliance and sensation were reassessed following treatment.
Participants and Questionnaires
This study randomized 120 IBS patients (Rome II positive, 3 male) and 40 healthy controls; all except 4 participants (2 IBS and 2 healthy controls) provided DNA for genotyping. The study was approved by Mayo Clinic Institutional Review Board. All participants signed informed consent. The validated Bowel Symptom Questionnaire [including somatic symptoms (16)], review of the electronic medical record (SM), or direct physician interview and examination (MC) were used to characterize the subtype of IBS.
Participants were allowed to continue stable doses of thyroid replacement, estrogen replacement, low dose aspirin (81 mg/day), and birth control pills or depot estrogen injections. Medications for IBS or constipation were stopped 7 days prior to baseline and throughout the study.
All completed Hospital Anxiety and Depression Inventory (17) and a Symptom Check List instrument [SCL-90 (18)]. On the day of testing rectal sensation, we assessed state of anxiety, relaxation and fear of pain using the 30-item Fear of Pain Questionnaire (19) and a revised version of the 36-item Anxiety Sensitivity Index (20).
Baseline observations, as well as full gastrointestinal transit prior to clonidine have been reported elsewhere (20). In this paper, baseline results were only used as covariates in the pharmacogenetics studies.
Satiation by the Nutrient Drink Test
A standardized Ensure® (1 Kcal/mL, 11% fat, 73% carbohydrate and 16% protein), drink test (21) was used to measure satiation and fullness, nausea, bloating, and pain 30 minutes after the meal.
Gastric Volume by 99mTc-SPECT
We used this validated measurement (22) of gastric volume during fasting and post-300ml liquid nutrient (300 kcal).
Rectal Compliance and Sensation by Barostat
Rectal Barostat Equipment and Procedure
The method and performance characteristics have been extensively described elsewhere (7,23). Ascending method of limits was used to measure rectal compliance and sensory thresholds. Random order phasic distensions above baseline operating pressure were used to assess sensory ratings, as in prior studies (8,9). The methods and experimental protocol, including setting the baseline operating pressure (BOP), are detailed in Figure A in Appendix (7,23).
Methods for Measuring Rectal Compliance and Sensation Thresholds and Ratings
Bowel preparation (Fleet® phosphate enema) was self-administered at least 1 hour before reporting to the center after an overnight fast. An initial “conditioning” distension of the rectum to 20 mmHg was applied, as this renders subsequent assessments of compliance and perception more reproducible (24).
Thresholds were indicated by pressing a button at the distension pressure at which first sensation, urgency, discomfort, and pain were perceived. In order not to bias responses, patients were instructed to press the buttons in whatever order the sensations were perceived as the pressure in the balloon was increased stepwise. We did not assess the threshold for gas sensation.
The bag was then deflated to BOP and subjects were allowed to rest for 10 minutes.
Next, phasic distensions of 12, 24, 30 and 36 mmHg above BOP were each applied once in random order; each distension was maintained for 60 seconds with an inter-stimulus interval of 2 minutes, during which the balloon was deflated to BOP. Thirty seconds after onset of the distension, subjects marked four separate 100 mm visual analog scales (VAS, anchored at each end by the descriptions ‘unnoticeable’ and ‘unbearable’) for the sensations of gas, urgency, discomfort and pain.
Data Analysis
The following measurements were obtained: (i) the sensory thresholds for first sensation, urgency, discomfort and pain during ascending method of limits, (ii) the three individual sensation scores (gas, urgency, and pain) in response to the four random phasic distensions, and (iii) rectal compliance.
Rectal compliance was analyzed using linear interpolation, as recently validated (25). For each subject, we calculated the pressure observed at one-half of the maximum observed volume (Pr1/2), where a smaller Pr1/2 corresponds to higher compliance.
Candidate Genes Tested via Single Nucleotide Polymorphisms (SNPs)
SNPs tested were: α2A (C-1291G), α2C (Del 322-325), 5HTTLPR, and GNβ3 (C825T). The methods have been published in prior reports from this laboratory (11,26). Since α2C (Del 322-325) was rarely encountered, no further analyses were performed with this genotype.
Data and Statistical Analyses
The primary endpoints were: fasting and postprandial gastric volume (GV), maximum tolerated nutrient drink volume (MTV) and symptoms, colonic compliance (Pr1/2), sensory thresholds and ratings for gas, pain and urgency in response to distensions. These endpoints were assessed for overall clonidine effects (i.e., irrespective of the assigned dose) by comparing baseline values with the values measured on day 7 after starting treatment (20).
Overall Effect of Clonidine
Paired t-test (or Wilcoxon signed rank test, as warranted) was used to compare MTV, gastric volumes, and rectal compliance. A multivariate version of this test was used for the multiple sensory ratings over the set of phasic distensions. The sensory thresholds were assessed (separately, first sensation, gas, urgency and pain) using a proportional hazards regression model with “drug” (baseline, post-treatment day) as the predictor variable and a robust variance estimate to account for the paired nature of the data (27). Separate assessments also examined the potential association of subject status (healthy control vs. IBS subtype) with the overall clonidine effects relative to baseline.
Dose Effect of Clonidine
Effects on the primary endpoints were assessed using analysis of covariance (ANCOVA) models with subject group (health vs. IBS), anxiety and depression scores (HAD), somatic symptom score, BMI, and corresponding pretreatment response as covariates. For the multiple sensation rating scores (at each distending pressure), a repeated measures ANCOVA was used (compound symmetry covariance structure) to assess dose effects (separately for gas, urgency, and pain using the same covariates [including the corresponding per subject mean (over distending pressures) baseline rating score], as well as specific interactions between subject group and clonidine dose, and distending pressure and clonidine dose.
The effects of dose on sensation threshold responses were assessed using proportional hazards regression models, including covariates: dose level, baseline sensation threshold response, the “tired”, “worried”, “peace”, and “active” scores, somatic symptom score, BMI, and subject group.
Effect of Candidate Genes on Response to Clonidine
The genotypes were included as additional predictor variables in the ANCOVA (or proportional hazards) models along with genotype by clonidine dose interaction terms.
RESULTS
Comparison of Groups of Participants Randomized to 0.1 and 0.15 mg Clonidine
Table A in Appendix shows demographic and key clinical features (from ref. 14) and Table B in Appendix shows the key gastrointestinal physiological data at baseline in the 160 subjects [40 controls and 120 patients with IBS; 48 C-IBS, 43 D-IBS, and 29 M-IBS, characterized in detail elsewhere (14)] who were included in the study, according to the medication randomization (clonidine, 0.1 mg and 0.15 mg). Table C in Appendix shows a comparison of the covariates used in the assessment of the effects of the two doses of clonidine. As would be expected from the randomization, there were no statistically significant or clinically important differences in a wide range of psychological, physiological and genotype characteristics.
Overall Effects of Clonidine on Selected Gastric and Rectal Functions
The effects of clonidine (both doses combined) were assessed on the combined group of healthy subjects and patients with IBS. The associations of responses with subject subgroup (health vs. IBS overall and IBS subtypes) were also examined.
i. Satiation and gastric volumes
There were significant overall effects of clonidine (the two doses combined) on maximum satiation volume and postprandial gastric volumes (baseline values vs. post treatment, p=0.002 and p<0.001 respectively). Figure 1 shows changes in satiation test and gastric volume measurements after treatment with clonidine (0.1 or 0.15 mg doses). For all participants, maximum satiation and postprandial and accommodation (delta) gastric volumes were significantly lower after clonidine treatment compared to baseline, with significant changes specifically in healthy volunteers, C-IBS and M-IBS.
Figure 1.
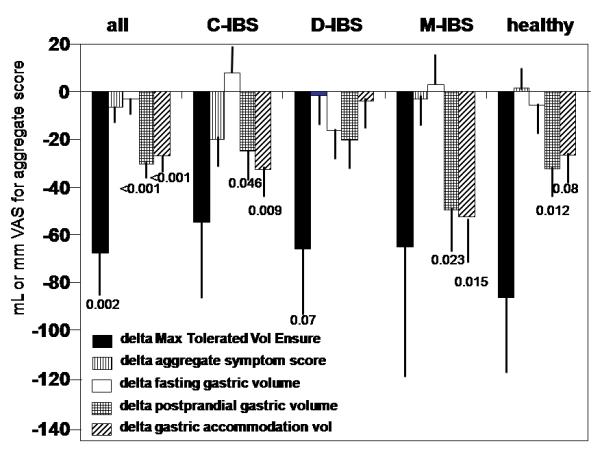
Changes in satiation test and gastric volume measurements before and after treatment with clonidine (0.1 or 0.15 mg doses) in study participants.
There were no significant effects detected on aggregate symptom scores (combined score of nausea, fullness, bloating and pain) 30 minutes following the ingestion of the maximum tolerated volume of Ensure® or on fasting gastric volumes.
ii. Rectal compliance, thresholds and sensation ratings
Overall effects of clonidine (paired t-test) on rectal compliance (Pr1/2 and maximum rectal volume achieved during the ascending method of limits) were noted (p=0.024, p=0.002, respectively). Thus, doses of clonidine used in this study significantly increased rectal compliance compared to baseline (pre-treatment), as demonstrated by the decrease in Pr1/2 with treatment (Table D in Appendix).
Clonidine had a statistically significant though numerically small effect on sensation thresholds for gas, urgency and pain (respectively p=0.006, 0.018, and <0.001), but not on first sensation (p=0.651). The thresholds tended to be slightly lower with clonidine treatment, and no associations with subgroup status (IBS vs. health) were detected. In Table D in Appendix, the effect of clonidine on thresholds, illustrated by the mean values (pre- vs. post-treatment), reflects the lower pressure to reach sensation threshold for clonidine relative to baseline, detected in the proportional hazards regression models.
There were no overall effects of clonidine on gas ratings (p=0.167), urgency ratings (p=0.329) or pain ratings (p=0.102) based on a multivariate analysis that simultaneously assessed all distension levels (Table D in Appendix).
However, there were associations (Table I) between sensation ratings for urgency and IBS subtype (overall, p=0.006). Specifically, patients with C-IBS exhibited significant changes (pre vs. post clonidine) at 36mmHg (p=0.030) and to some extent at 24mmHg (p=0.079), while the other subtypes of IBS did not. An overall association between changes in pain ratings and IBS subtype was also detected (p=0.024); the C-IBS subjects exhibited significant changes (p<0.05 at each level), while the other subtypes of IBS did not exhibit such changes.
Table I.
Sensation Ratings in Response to Clonidine Treatment (overall) by IBS Subtypes and Healthy Controls: Association between subject status and treatment response
| Overall Effect of Clonidine on CHANGE (postRx-preRx) in |
Controls | IBS-C | IBS-D | IBS-M |
|---|---|---|---|---|
| Rectal compliance, Pr1/2, mmHg | -0.67 ± 0.67 | -0.60 ± 0.62 | -1.46 ± 0.7 | -0.12 ± 0.56 |
| Rectal maximum volume, mL | -7.1 ± 10.9 | -11.9 ± 9.8 | -40.3 ± 12.6 | -10.3 ± 12.1 |
| Sensation rating, gas 30 mmHg | -1.97 ± 4.6 | 7.1 ± 4.1 | 8.2 ± 4.3 | 0.33 ± 3.5 |
| Sensation rating, pain 30 mmHg | -0.77 ± 0.8 | 8.3 ± 3.6 | 3.7 ± 4.0 | 6.7 ± 5.4 |
| Sensation rating, urgency 30 mmHg | -5.0 ± 4.5 | 4.6 ± 3.3 | 0.74 ± 3.3 | -0.96 ± 3.5 |
| Sensation rating, gas 36 mmHg | -3.67 ± 3.1 | 6 ± 5.6 | 8.1 ± 3.6 | -0.77 ± 3.0 |
| Sensation rating, pain 36 mmHg | -0.24 ± 4.4 | 15.9 ± 5.7 | 4.8 ± 4.4 | 3.4 ± 3.3 |
| Sensation rating, urgency 36 mmHg | -3.3 ± 3.1 | 8.5 ± 3.8 * | 1.8 ± 3.0 | -2.55 ± 2.6 |
p=0.006 vs. controls
Effects of Clonidine Dose on Selected Gastric and Rectal Functions
There were no significant dose effects of clonidine on fasting and postprandial gastric volumes or on the postprandial change in gastric volume (Fig. C in Appendix). However, there was a significant effect of clonidine dose on satiation (maximum tolerated) volume (p=0.014) and aggregate symptom score (p=0.06), with the 0.15 mg dose reducing average satiation volumes approximately 100 ml compared to the 0.1 mg dose and increasing symptom scores [by an average 21 points (163±7 with 0.1 mg dose compared to 184±8 with 0.15 mg dose)]. The dose effects were not significantly different among subject subgroups (health vs. IBS).
There were no significant effects of clonidine dose on rectal sensation ratings [least square means ± SE (Table E in Appendix)]. Similarly, there were no significant dose effects of clonidine on rectal sensation thresholds, and no association with subject subgroup (health vs. 3 subtypes of IBS) was detected (data not shown).
Pharmacogenetics: Gastrointestinal Functions in Response to Clonidine
We sought to identify the potential association of the candidate genotypes with clonidine’s effects on gastric functions (Figs. 2 and 3), rectal sensation ratings (Fig. 4) and rectal compliance (Fig. 5). Included in these models were 2 two-way interactions: gene by dose, in which we assessed the potential differential effect of clonidine dose on gastric functions by genotype; and gene by subgroup (IBS vs. health) interaction, in which we assessed the post—treatment effects on gastrointestinal function response in subgroups based on genotype (i.e. pooled over both doses of clonidine).
Figure 2.
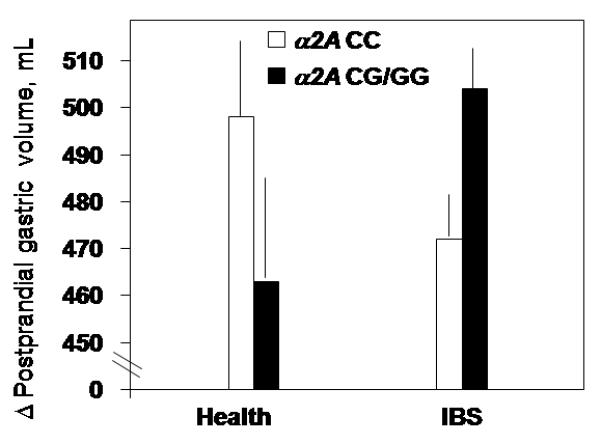
Interaction of α2A with change (PP-fasting) in gastric volume (p=0.016) post-clonidine treatment with opposite patterns by genotype observed in health versus IBS.
Figure 3.
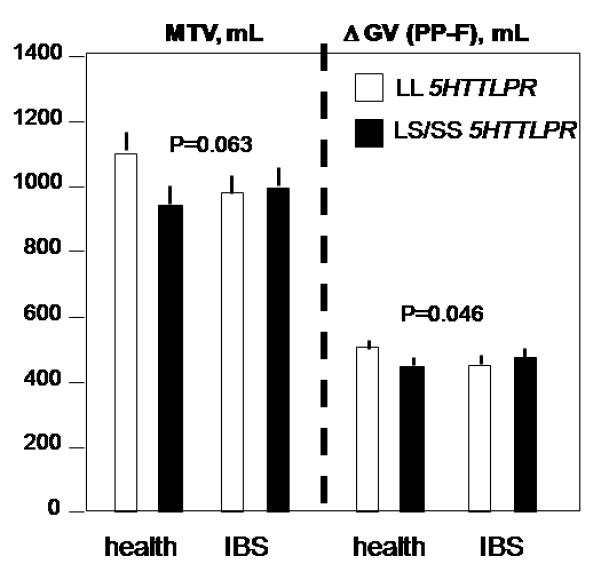
Interaction of SLC6A4 genotypes with satiation volumes (p=0.063) and change in gastric volume (p=0.046) post-clonidine treatment with opposite patterns by genotype observed in health versus IBS.
Figure 4.
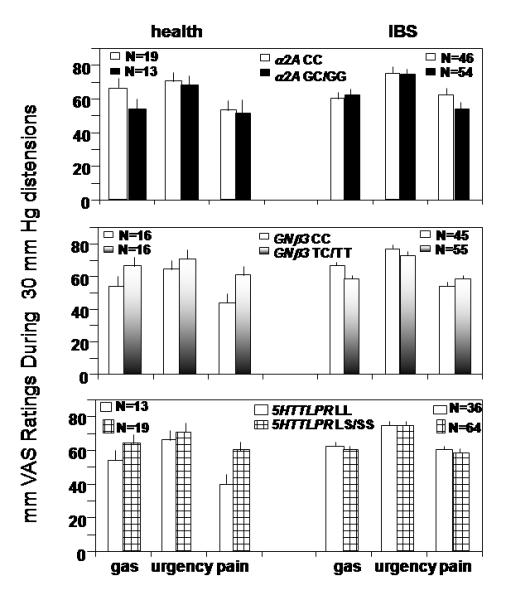
Interactions of genotype by disease group (IBS vs. health) for post-clonidine treatment rectal sensation ratings
Upper panel - Interaction effect for α2A by disease group detected for gas (p=0.053) and urgency (p=0.026), but not pain.
Middle panel - Interactions were observed between GNβ3 genotype and disease group for gas (p=0.049) and pain (p=0.022) and, to a lesser extent, urgency (p=0.068).
Lower panel - Association of disease group (health vs. IBS) with pain sensation, p=0.009, but not for gas and urgency scores.
Figure 5.
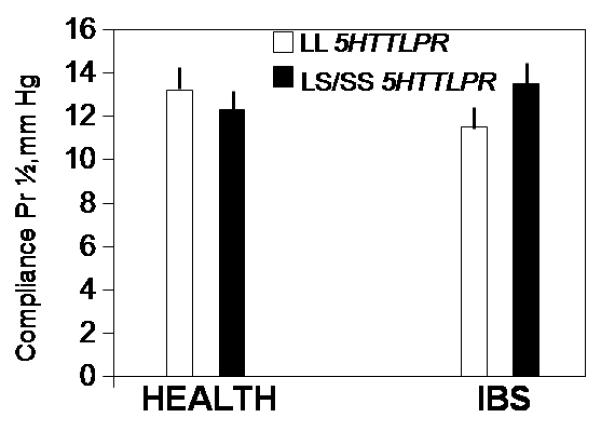
Interaction of SLC6A4 genotype by disease group for post-treatment rectal compliance: a significant SLC6A4 genotype by disease group interaction for rectal compliance (p=0.03) was detected. Thus, rectal compliance was lower (Pr1/2 higher) in IBS patients who had the LL SLC6A4 genotype, in contrast to effect of genotype in healthy controls.
In general, no significant gene by clonidine dose interactions were detected. However, we observed several interactions between overall effects of clonidine and genotype, typically manifested as a differential effect in healthy controls compared to IBS patients. These are summarized for the three genes of interest.
i. Interaction of α2A genotype and disease group in post-clonidine treatment gastrointestinal functions
There was a significant interaction effect detected for postprandial change (PP-fasting) in gastric volume between disease subgroup and genotype (Fig. 2, p=0.016). Thus, α2A CG/GG increased gastric accommodation in IBS and decreased it in health.
Significant associations were also observed between α2A genotype and IBS rectal sensory ratings for gas (p=0.054) and urgency (p=0.026), but not for pain (p=0.134, Fig. 4, upper panel). In general, scores were lower in GC/GG compared to CC genotype in healthy controls, but higher in GC/GG vs. CC genotype in IBS.
ii. Interaction of GNβ3 genotype and disease group in post-clonidine treatment rectal sensation ratings
Significant interactions were observed between GNβ3 genotype and disease group for post-clonidine sensation ratings of gas (p=0.048) and pain (p=0.022) and, to a lesser extent, urgency (p=0.066, Fig. 4, middle panel). In general, scores of gas and urgency were higher in GNβ3 TC/TT compared to CC genotype in healthy controls, but lower in TC/TT compared to CC genotype in IBS subjects. Pain scores were also higher with TC/TT compared to CC genotype in healthy controls, but they were similar in IBS patients with TC/TT or CC genotype.
(iii) Interaction of 5HTTLPR genotype and disease group in post-treatment gastrointestinal functions
a. Satiation and gastric volumes
For post-clonidine treatment, satiation volumes were modestly influenced by the 5HTTLPR genotype in health vs. IBS (Fig. 3, left panel, p=0.063). Thus, satiation volumes were similar in IBS between genotypes, but, in health, satiation volumes were reduced in LS/SS genotype. A significant 5HTTLPR genotype by disease group interaction was detected for the postprandial change in gastric volume (p=0.046), opposite patterns by genotype observed in health versus IBS (Fig. 3, right panel).
b. Rectal compliance
There was a significant 5HTTLPR genotype by disease interaction for rectal compliance (p=0.03, Fig. 5). Thus, rectal compliance was lower (Pr1/2 higher) in IBS patients who had the LL 5HTTLPR genotype, in contrast to healthy controls (Fig. 5) in whom Pr1/2 was slightly lower in those with LS/SS genotype.
c. Rectal sensation
There was an association of post-clonidine treatment pain sensation ratings with disease group status (p=0.009), but not for gas and urgency scores. A borderline significant interaction effect of 5HTTLPR genotype by disease group for pain sensation scores was detected (p=0.095), reflecting higher sensation scores in LS/SS vs. LL genotype for healthy controls, in contrast to similar scores for 5HTTLPR genotype in IBS subjects (Fig. 4, lower panel).
DISCUSSION
Our study has provided observations on the overall and dose-effects of low dose clonidine, 0.1 mg and 0.15 mg, b.i.d., on physiological measurements, and the pharmacogenetics of clonidine in health and IBS.
The first observation is that there were significant overall (2-dose) effects of clonidine on satiation volume and postprandial change in gastric volume (accommodation), rectal compliance, and sensation thresholds for gas, urgency and pain, but not first sensation or sensation ratings. In general, the effects of clonidine on satiation volume and postprandial gastric volumes are qualitatively similar in health and IBS (whole group and subtypes), as shown in Figure 1, with reductions in postprandial gastric volume greatest in C-IBS and M-IBS. Acute one-time administration of clonidine (0.1 mg) was previously shown to increase gastric compliance, relax fasting gastric tone and to increase gastric accommodation measured by barostat (8) in a study of nine healthy subjects (8). The current study, with repeated dosing of 0.1 mg or 0.15 mg twice a day for 6 days, involved both IBS and healthy participants and it showed reduced satiation volume and reduced gastric accommodation, as can be observed in Figure 1.
The results of the current study are contrary to what was expected, based on previous work (8). There are significant differences in the sample size (160 vs. 9), participants (IBS and health vs. 9 healthy volunteers), dose (0.1mg or 0.15mg vs. 0.1mg), design (multiple b.i.d. dosing for 6 days vs. one oral dose) and methods used to measure gastric volumes [SPECT in the current study and barostat in the earlier study (8)]. The barostat measures proximal stomach muscle compliance and tone, whereas SPECT measures whole gastric volumes. These differences may also reflect predominantly central (prejunctional) versus peripheral (postjunctional) effects of clonidine, depending on dose. Thus, a recent study from our group also demonstrated that the α2 antagonist, yohimbine (which increased colonic tone measured by barostat), was associated with augmentation of gastric volume response (increase) with GLP-1 (28).
In this study, clonidine was associated with the expected (9) increase in rectal compliance (lower Pr1/2 in Fig. B and Table C in Appendix), as measured by the barostat-controlled rectal balloon. While low dose clonidine induced the expected increase in rectal compliance, there was an unexpected reduction in sensation threshold for pain, but no change in threshold for first sensation or any sensation ratings (Table C in Appendix). The reason for and significance of the apparent reduction in pain threshold after the clonidine (0.1 mg and 0.15 mg) treatment are unclear. In fact, the literature shows that analgesic or antihyperalgesic effects of clonidine on colon pain in humans require doses of 0.3 mg (29) and administration i.t. rather than i.v. (30); these are doses and routes of administration that differ from our study. It is also worth noting that IBS subtype was significantly associated with increased rectal sensation ratings of pain and urgency. The data suggest that rectal compliance is only one determinant of sensation. Overall, the data are consistent with the hypothesis that increased compliance may actually increase circumferential tension and increase sensation, if this is mediated by tension receptors (31).
At first glance, this may seem paradoxical, since prior studies from our lab showed 0.3 mg clonidine reduced pain sensation (7). On the other hand, the absence of a reduction of pain with low dose clonidine during rectal distension is consistent with the prior dose-response studies conducted by our lab (9), in which a significant linear, dose-related sensory effect of clonidine was observed at 8mmHg and 24mmHg distensions, but the effect on pain (including dose-response relationship) was attributable to the effect of the single dose of 0.3 mg for distensions at 24mmHg (9). We had chosen to study lower doses of clonidine rather than the 0.3 mg dose because the latter dose is associated with significant hypotension and somnolence, which would reduce compliance in a multiple dosing study of 160 participants. We also postulated that lower doses would facilitate determination of effects of candidate gene variations on response to clonidine, rather than the 0.3 mg dose which has an overwhelming effect on colonic sensation (7).
The effects of clonidine on gastric volumes were similar in C-IBS and M-IBS, but they were less prominent in D-IBS (see Figure 1). This may suggest that endogenous α2-adrenergic tone is higher in D-IBS (perhaps as a homeostatic response to counteract diarrhea) and, therefore, less responsive to low dose clonidine.
A second series of observations in our study can be briefly summarized: there were no significant differences in effects of the two clonidine doses used (0.1 mg and 0.15 mg) except on satiation volume. The latter was reduced by the higher dose, 0.15 mg, and is consistent with the increased rectal sensation observed with clonidine.
The pharmacogenetic component of our study revealed significant associations between α2A and 5HTTLPR genotypes in the two disease groups (health and IBS), and effects of clonidine on postprandial change in gastric volume and between all three genetic variations and sensation in the two disease groups. On the other hand, there were no significant treatment by gene interactions with the dose of clonidine. It is important to stress that the differences in responses between the different markers in each gene are relatively small.
How might alterations in GNβ3genotype result in altered pharmacological responses to clonidine? The TC or TT genotype is associated with alternative splicing of the gene in exon 9 and the formation of a truncated splice variant Gβ3s, a protein product with 41 amino acids less than in the Gβ3 wild-type protein (32,33). This corresponds to the lack of the equivalent of one entire tryptophan—aspartate repeat domain (34). The Gβ3s protein variant is associated with enhanced G-protein activation (35) and the 825T allele corresponding to the formation of Gβ3s has been associated with cardiovascular, affective, and metabolic disorders (34,36-38). It is possible that the GNβ3genotype alters the response to clonidine, but it is unclear why there would be an increase in pain sensation rating.
Alterations in serotonin reuptake may influence the effect of norepinephrine. This is demonstrated by the effects of SNRI in depression, as well as the effects of a prototype, venlafaxine, on gastric and colonic functions (39). We have previously shown that there are significant genotype-function associations between 5HTTLPR genetic variation and rectal compliance and sensation (14).
There are a number of limitations in our study. First, the absence of a placebo arm makes it difficult to exclude a time effect in the responses observed with clonidine; however, the consistency of the data across the groups for most endpoints makes it unlikely that an effect due simply to subsequent measurements would account for a significant component of the observations. Second, the sample size is insufficient to separately appraise the contribution at each distension level for each sensation in the 4 subgroups of patients or volunteers jointly for the three genes of interest. Hence, we cannot exclude the possibility that there is a type II error for the individual symptoms at a specific distension level. However, the sample size is sufficient to address the overall effect of clonidine (across two doses) on the functions tested and to assess whether there were differential dose effects.
In conclusion, clonidine alters gastric satiation and rectal sensations. α2A, GNβ3 and 5HTTLPR genotypes influence several gastrointestinal functions in responses to low dose clonidine in IBS. Although the magnitude of the effect of the genetic variation is small on average, these data suggest there are pharmacogenetic considerations (which are different in health and IBS) that may enhance the therapeutic potential of low dose clonidine which is generally well tolerated. For example, IBS patients with α2A CG/GG genotype have a greater change in postprandial gastric volume and this may reduce postprandial fullness and bloating; and GNβ3 TC/TT genotype may be associated with lower sensations of gas and urgency in response to clonidine. Differences observed in health and IBS also suggest that factors other than the genetic variations also influence the response to clonidine.
Supplementary Material
Acknowledgments
Dr. Camilleri is funded in part by grants RO1 DK-54681 and K24 DK-02638 from National Institutes of Health. We thank Adil E. Bharucha, M.D. for helpful discussions and Mrs. Cindy Stanislav for secretarial assistance.
Abbreviations used in manuscript
- Δ
delta (change e.g. in gastric volume postprandially)
- GV
gastric volume
- HAD
Hospital anxiety and depression inventory
- 5-HTT-LPR
serotonin transporter linked polymorphic region
- IBS
irritable bowel syndrome
- IBS-C
constipation-predominant IBS
- IBS-D
diarrhea-predominant IBS
- IBS-M
mixed bowel dysfunction IBS
- SNP
single nucleotide polymorphism
- MTV
maximum tolerated volume, also termed satiation volume
- SCL-90
symptom check list 90
- SLC6A4
solute carrier family 6 (neurotransmitter transporter, serotonin), member 4
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Weems WA, Szurszewski JH. Modulation of colonic motility by peripheral neural inputs to neurons of the inferior mesenteric ganglion. Gastroenterology. 1977;73:273–278. [PubMed] [Google Scholar]
- 2.Chang EB, Bergenstal RM, Field M. Diarrhea in streptozocin-treated rats. Loss of adrenergic regulation of intestinal fluid and electrolyte transport. J Clin Invest. 1985;75:1666–1670. doi: 10.1172/JCI111874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Mahony S, Dinan TG, Keeling PW, Chua AS. Central serotonergic and noradrenergic receptors in functional dyspepsia. World J Gastroenterol. 2006;12:2681–2687. doi: 10.3748/wjg.v12.i17.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa I, Omote K, Kitahata LM, Collins JG, Murata K. Serotonergic mediation of spinal analgesia and its interaction with noradrenergic systems. Anesthesiology. 1990;73:474–478. doi: 10.1097/00000542-199009000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M. Mechanisms in IBS: something old, something new, something borrowed. Neurogastroenterol Motil. 2005;17:311–316. doi: 10.1111/j.1365-2982.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol. 1997;273:G997–G1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- 8.Thumshirn M, Camilleri M, Choi MG, Zinsmeister AR. Modulation of gastric sensory and motor functions by nitrergic and alpha2-adrenergic agents in humans. Gastroenterology. 1999;116:573–585. doi: 10.1016/s0016-5085(99)70179-4. [DOI] [PubMed] [Google Scholar]
- 9.Viramontes BE, Malcolm A, Camilleri M, Szarka LA, McKinzie S, Burton DD, et al. Effects of an alpha(2)-adrenergic agonist on gastrointestinal transit, colonic motility, and sensation in humans. Am J Physiol. 2001;281:G1468–G1476. doi: 10.1152/ajpgi.2001.281.6.G1468. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Kim DY, McKinzie S, Kim HJ, Thomforde GM, Burton DD, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2003;1:111–121. doi: 10.1053/cgh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Nie Y, Xie J, Tang W, Liang P, Sha W, Yang H, Zhou Y. The association of serotonin transporter genetic polymorphisms and irritable bowel syndrome and its influence on tegaserod treatment in Chinese patients. Dig Dis Sci. 2007;52:2942–9. doi: 10.1007/s10620-006-9679-y. [DOI] [PubMed] [Google Scholar]
- 13.Grudell ABM, Camilleri M, Carlson P, Gorman H, Ryks M, Burton D, Baxter K, Zinsmeister AR. An exploratory study of the association of adrenergic and serotonergic genotype and gastrointestinal motor functions. Neurogastroenterol Motil. 2008;20:213–219. doi: 10.1111/j.1365-2982.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M, Busciglio I, Carlson P, McKinzie S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Candidate genes and sensory functions in health and irritable bowel syndrome. Am J Physiol. 2008 May 29; doi: 10.1152/ajpgi.90202.2008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, et al. Prospective study of motor, sensory, psychologic and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ., 3rd Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 19.Osman A, Breitenstein JL, Barrios FX, Gutierrez PM, Kopper BA. The fear of pain questionnaire III: further reliability and validity with nonclinical samples. J Behav Med. 2002;25:155–173. doi: 10.1023/a:1014884704974. [DOI] [PubMed] [Google Scholar]
- 20.Taylor S, Cox BJ. An expanded anxiety sensitivity index: evidence for a hierarchic structure in a clinical sample. J Anxiety Disord. 1998;12:463–483. doi: 10.1016/s0887-6185(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 21.Chial HJ, Camilleri C, Delgado-Aros S, Burton D, Thomforde G, Ferber I, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol Motil. 2002;14:249–253. doi: 10.1046/j.1365-2982.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 22.Bouras EP, Delgado-Aros S, Camilleri M, Castillo EJ, Burton DD, Thomforde GM, et al. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut. 2002;51:781–786. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremonini F, Houghton LA, Camilleri M, Ferber I, Fell C, Cox V, et al. Barostat testing of rectal sensation and compliance in humans: comparison of results across two centres and overall reproducibility. Neurogastroenterol Motil. 2005;17:810–820. doi: 10.1111/j.1365-2982.2005.00709.x. [DOI] [PubMed] [Google Scholar]
- 24.Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol. 1998;274:G584–G590. doi: 10.1152/ajpgi.1998.274.3.G584. [DOI] [PubMed] [Google Scholar]
- 25.Floyd BNI, Camilleri M, Andresen V, Esfandyari T, Busciglio I, Zinsmeister AR. Comparison of mathematical methods for calculating colonic compliance in humans: power exponential, computer-based, and manual linear interpolation models. Neurogastroenterol Motil. 2007 Oct 25; doi: 10.1111/j.1365-2982.2007.01024.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Camilleri M, Carlson PJ, Cremonini F, Ferber I, Stephens D, et al. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut. 2004;53:829–837. doi: 10.1136/gut.2003.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Statistical Assoc. 1989;84:1065–1073. [Google Scholar]
- 28.Andrews CN, Bharucha AE, Camilleri M, Low PA, Seide BM, Burton DD, Nickander KK, Baxter KL, Zinsmeister AR. Effects of glucagon-like peptide-1 and sympathetic stimulation on gastric accommodation in humans. Neurogastroenterol Motil. 2007;19:716–723. doi: 10.1111/j.1365-2982.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavand’homme P, De Kock M. The use of intraoperative epidural or spinal analgesia modulates postoperative hyperalgesia and reduces residual pain after major abdominal surgery. Acta Anaesthesiol Belg. 2006;57:373–379. [PubMed] [Google Scholar]
- 30.De Kock M, Lavand’homme P, Waterloos H. The short-lasting analgesia and long-term antihyperalgesic effect of intrathecal clonidine in patients undergoing colonic surgery. Anesth Analg. 2005;101:566–572. doi: 10.1213/01.ANE.0000157121.71808.04. [DOI] [PubMed] [Google Scholar]
- 31.Camilleri M, Coulie B, Tack JF. Visceral hypersensitivity: facts, speculations, and challenges. Gut. 2001;48:125–131. doi: 10.1136/gut.48.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgart D, Naber C, Haude M, Oldenburg O, Erbel R, Heusch G, et al. G protein beta3 subunit 825T allele and enhanced coronary vasoconstriction on alpha(2)-adrenoceptor activation. Circ Res. 1999;85:965–969. doi: 10.1161/01.res.85.10.965. [DOI] [PubMed] [Google Scholar]
- 33.Rosskopf D, Manthey I, Habich C, Kielbik M, Eisenhardt A, Nikula C, et al. Identification and characterization of G beta 3s2, a novel splice variant of the G-protein beta 3 subunit. Biochem J. 2003;371:223–232. doi: 10.1042/BJ20021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 35.Rosskopf D, Koch K, Habich C, Geerdes J, Ludwig A, Wilhelms S, et al. Interaction of Gbeta3s, a splice variant of the G-protein Gbeta3, with Ggamma- and Galpha-proteins. Cell Signal. 2003;15:479–488. doi: 10.1016/s0898-6568(02)00140-7. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Velasco V, Ikeda SR. A splice variant of the G protein beta 3-subunit implicated in disease states does not modulate ion channels. Physiol Genomics. 2003;13:85–95. doi: 10.1152/physiolgenomics.00057.2002. [DOI] [PubMed] [Google Scholar]
- 37.Avissar S, Schreiber G. Ziskind-Somerfeld Research Award. The involvement of guanine nucleotide binding proteins in the pathogenesis and treatment of affective disorders. Biol Psychiatry. 1992;31:435–459. doi: 10.1016/0006-3223(92)90257-z. [DOI] [PubMed] [Google Scholar]
- 38.Siffert W. Cardiovascular pharmacogenetics: on the way toward individually tailored drug therapy. Kidney Int. 2003;84:S168–S171. doi: 10.1046/j.1523-1755.63.s84.38.x. [DOI] [PubMed] [Google Scholar]
- 39.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol. 2003;284:G130–G137. doi: 10.1152/ajpgi.00266.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


