Abstract
This article is to offer a concise review on the use of cytoreductive surgery (CRS) plus intraperitoneal hyperthermic chemotherapy (IPHC) for the treatment of peritoneal carcinomatosis (PC). Traditionally, PC was treated with systemic chemotherapy alone with very poor response and a median survival of less than 6 mo. With the establishment of several phase II studies, a new trend has been developed toward the use of CRS plus IPHC as a standard method for treating selected patients with PC, in whom sufficient cytoreduction could be achieved. In spite of the need for more high quality phase III studies, there is now a consensus among many surgical oncology experts throughout the world about the use of this new treatment strategy as standard care for colorectal cancer patients with PC. This review summarizes the current status and possible progress in future.
Keywords: Peritoneal carcinomatosis, Cytoreductive surgery, Intraperitoneal hyperthermic chemotherapy, Gastric cancer, Colorectal cancer, Ovarian cancer, Peritoneal mesothelioma
INTRODUCTION
The loco-regional progression of gastrointestinal and gynecological cancers usually result in peritoneal carcinomatosis (PC), which is characterized by the presence of tumor nodules of various size, number and distribution on the peritoneal surface, with very poor prognosis and a median survival of less than 6 mo[1–3]. The most widely accepted therapies for such PC are systemic chemotherapy, best support care and palliative treatment, without any hope of cure. Moreover, surgery alone can only remove the bulky visible tumor nodules. For the micrometastases, invisible free cancer cells and those tumor masses not suitable for resection, surgery can not achieve any effect. Therefore, neither surgery nor chemotherapy alone can make obvious positive impact on the survival and quality of life in patients with PC. In order to tackle this difficult problem, a new treatment modality called cytoreductive surgery (CRS) plus intraoperative peritoneal hyperthermic chemotherapy (IPHC) has been developed over the past two decades, which has the advantages of surgery to reduce the visible tumor burden and regional hyperthermic chemotherapy to eradicate micrometastses and free cancer cells[4]. Over the past decade, this treatment modality has gained increasingly wide acceptance in clinical practice and in some cancer centers in Europe, America, Japan and Australia, and has been adopted as the treatment of choice for PC from gastrointestinal tract or pelvic malignancies. This paper summarizes the biological basis, the indications and contraindications, the techniques, the efficacy and safety issues and future directions of this new treatment.
INCIDENCE, MORBIDITY AND MORTALITY OF PC
PC is a direct consequence of loco-regional progression of gastrointestinal and gynecological cancers including gastric cancer (GC), colorectal cancer (CRC), ovarian cancer (OC), appendiceal cancer and malignant peritoneal mesothelioma.
For GC, 10%-20% of patients being explored for potentially curative resection are found to have peritoneal seeding at the time of abdominal exploration. This occurs most frequently in patients with signet ring cell type of GC as opposed to intestinal type pathology[5]. Furthermore, peritoneal recurrence develops in 60% of patients with T3 or T4 tumor after curative resection[6]. In T3 or T4 tumor, invisible micrometastases are already present in the peritoneal cavity at the time of curative resection, and peritoneal recurrence is the only site of the first recurrence in 40%-60% of patients[7–9]. Therefore, peritoneal dissemination alone usually results in death of 20%-40% of patients with GC[10].
For CRC, peritoneal seeding of cancer cells leading to PC is rather common. It is estimated that, at the time of diagnosis, the peritoneal surface is already involved in 8%-15% of CRC cases, and that initial recurrence in the peritoneum occurs in up to 50% of patients after curative surgery[11–14]. In approximately 25% of patients with recurrent CRC, the peritoneal cavity seems to be the only metastatic site of the disease, even after a detailed diagnostic workup of the liver and lungs[15].
OC is another malignancy prone to peritoneal metastasis. About 50%-75% of women with OC will develop persistent or recurrent disease with a long survival rate in only 25% patients[16,17]. In about 82% of the cases, the recurrence is within the peritoneal cavity while in 12% it occurs in the retroperitoneal lymph nodes[18–20]. For early stage OC, the median recurrence time ranged between 11 mo and 29 mo with much more involvement of the pelvis or abdomen than retroperitoneal lymph nodes[21–28]. For advanced OC, if the initial treatment achieves meaningful clinical response, the median recurrence time ranges from 18 mo to 24 mo, again with peritoneal metastasis as the most common site[16,19,29–34].
MECHANISMS OF PC DEVELOPMENT
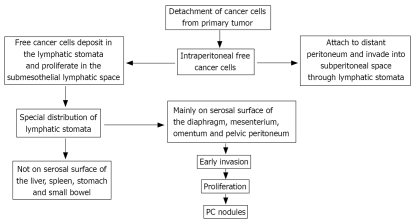
PC is formed through a multi-step process (Figure 1): (1) detachment of cancer cells from primary tumor. The origin of PC in GC, for example, is considered to be the intraperitoneal free cancer cells, which are exfoliated from the serosal surface of the primary tumor; (2) intraperitoneal free cancer cells attach to the distant peritoneum, and invade into the subperitoneal space; (3) invasion into subperitoneal space; and (4) proliferation with vascular neogenesis[35,36].
Figure 1.
Mechanism of PC development.
Recent studies have revealed special anatomical basis for peritoneal metastasis. Yonemura et al[9,37] found special peritoneal lymphatic orifices, which are referred to as the lymphatic stomata and connect with subperitoneal lymphatic channel and milky spots. Milky spots are the minute organelles which contain lymphatic vessels, lymphocytes, and peritoneal macrophages. Milky spots distribute mainly on the greater omentum and pelvic peritoneum. Intraperitoneal free cancer cells specifically deposit in the lymphatic stomata and proliferate in the submesothelial lymphatic space. The lymphatic stomata showed special distribution on the peritoneal surface. Numerous stomata are detected on the undersurface of the diaphragm, small bowl mesentery, greater omentum, appendix epiploicae of the large bowel and the pelvic peritoneum. In contrast, there are no lymphatic stomata on the liver capsule, the surface of the spleen and the serosal surface of the small bowel and stomach. Accordingly, the serosal surface of these organs is involved only at the late phase of peritoneal dissemination.
CURRENT TREATMENT STRATEGIES AGAINST PC
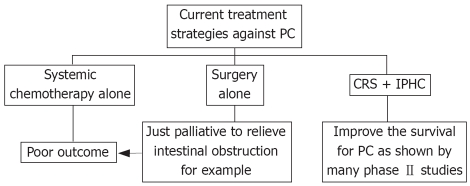
For a long time, PC has been considered as a preterminal condition and treated with systemic chemotherapy alone with poor outcome; and the role of surgery is just palliative to relieve intestinal obstruction. But at least 1 phase III and many phase II studies have shown that the use of CRS with IPHC can improve survival for PC. Currently, such method of treatment has evolved into a novel approach for PC and might represent the standard care in selected patients (Figure 2)[38–40].
Figure 2.
Rationale of CRS plus IPHC in treating PC.
Rationale
Intraoperative use of chemotherapeutic agents to maximize the efficacy of both surgery and chemotherapy has many advantages. First, surgery can separate the adhesions and remove the bulky tumor, leaving microscopic residual tumors much more susceptible to the killing effect of chemotherapeutic drugs. Second, intraperitoneal chemotherapy has distinct pharmaceutical advantages. In a recent phase I study by Morgan et al[41], 36 patients with PC of different origin were treated with intraperitoneal gemcitabine chemotherapy. It was found that the median peak peritoneal concentration was 1116 folds (range, 456-1886) higher than the peak plasma level. This could increase the local drug concentration, thus intensifying its direct antitumor effect while reducing the systemic adverse effects. Such preferential high concentration of drugs in the peritoneal cavity also occurs in many other drugs such as doxorubicin, melphalan, mitomycin C, cisplatin, gemcitabine, mitoxantrone, oxaliplatin, etoposide, irinotecan, paclitaxel, docetaxel, 5-fluorouracil, floxuridine and carboplatin[42]. Third, heat itself has direct detrimental effect on the growth of cancer cells. Cell membrane, cytoskeletons, synthesis of macromolecules, and DNA repair mechanisms are all affected by hyperthermia[38]. Temperature over 43°C has direct cytostatic effect on human breast cancer cell line MCF-7, ovarian SKOV-3 and hepatocarcinoma HEpG2 cells[43]. Fourth, the synergistic effect of hyperthermia and chemotherapy has also been well-documented. It has been shown that hyperthermia increases the cytotoxicity of some chemotherapeutic agents[43–45]. Because of these advantages, CRS plus IPHC has been increasingly used in the treatment of PC from GC[46], malignant mesothelioma[47], appendiceal cancer[48], CRC and OC[49–60].
Indications, contraindications and patient selection
IPHC is commonly indicated for the treatment of PC with GC, CRC, appendiceal cancer, OC, peritoneal mesothelioma, pseudomyxoma peritonei and malignant ascites, if the patients can stand CRS and IPHC[38]. The contraindications are as follows: (1) patients are medically unfit to undergo the rigors of CRS and IPHC e.g. those with renal and myocardial impairments; (2) there is an extra-abdominal disease; (3) parenchymal hepatic metastases; (4) bulk retroperitoneal disease; (5) the peritoneal tumor is incompletely resected or can not be significantly reduced; and (6) patients aged over 70 years[38]. A clear preoperative staging of PC is necessary for such treatment. Preoperative abdominopelvic CT, MRI, positron emission tomography (PET) or PET/CT, or laparoscopy are adequate procedures[39].
Techniques
Complete CRS is vital in improving the survival of patients with PC, although in some patients with limited PC, partial removal of peritoneum is enough to eliminate macroscopic disease[61]. The goal of complete CRS is to remove all macroscopic peritoneal dissemination. Peritonectomy consists of two big surgical components (parietal and visceral peritonectomy). A midline incision extending from xiphoid process to pubis is sometimes necessary, complete parietal peritonectomy can be achieved by stripping off the parietal peritoneum at the abdominal incision, then ascending colon, spleen, pancreatic tail, and descending sigmoid colon are mobilized, and all peritoneum lining the lower abdominal wall and pelvis is removed. Visceral peritonectomy includes subtotal colectomy, omentectomy, partial gastrectomy, and resection of mesentery to the extent that they are involved[10].
It is mandatory to examine the greater omentum, undersurface of diaphragm and Douglas pouch as free cancer cells can lodge there due to presence of lymphatic stomata[62]. Micrometastasis on the preserved peritoneum can be managed by intraperitoneal chemotherapy, so complete cytoreduction includes the removal of peritoneum with gross nodules[10,61,62].
After finishing CRS, IPHC is initiated. It has been shown that hyperthermia augments the penetration of anticancer drugs[63], in addition to its synergistic cytotoxicity on cancer cells when combined with some chemotherapeutic drugs such as mitomycin C (MMC) and cisplatin[64]. It is performed either with open or closed techniques, with more advantages of the former due to uniform distribution of the chemotherapeutic agents within peritoneal cavity[10]. In either technique, large volume (5 L-10 L) of saline heated at 42°C-43°C is introduced into the peritoneal cavity and circulated at a high flow rate of about 10 L/min[10]. The total dose of MMC and CDPP should be 30 mg and 300 mg, respectively with an optimal duration of 60-90 min[10].
ASSESSMENT OF COMPLETENESS OF CRS
There is agreement among many treatment centers that the extent of disease prior to CRS and the completeness of cytoreduction (CCR) score are the principal prognostic indicators for survival[14,38,66–85]. In CCR, the extent of residual disease after CRS is classified into 4 categories: CCR-0 indicating no visible residual tumor; CCR-1, residual tumor ≤ 2.5 mm in diameter; CCR-2, residual tumor between 2.5 mm and 2.5 cm; and CCR-3, residual tumor > 2.5 cm or confluence of disease present at any site[86]. But those with more diffuse disease (more than 2 quadrants) become worse no matter how good a cytoreduction procedure is. The understanding of a complete cytoreduction may vary according to the disease process, for example, in colorectal PC, complete cytoreduction needs a CCR-0 score[14,66–85] while in pseudomyxoma peritonei, a complete cytoreduction may involve both CCR-0 and CCR-1[39,87].
EFFICACY AND BENEFIT OF CRS PLUS IPHC
It is now clear that CRS plus IPHC improves both the quality of life especially in patients with malignant ascitis[15,103,104], and the survival[15,67]. The results of the most significant studies about the survival rates are summarized in Table 1, which supports the use of this new modality as standard care.
Table 1.
The survival rates of CRS plus IPHC in patients with PC of different malignancies
| Authors | Pts | Year | Type of | Tumor type | Drugs, temperature | Median |
Survival rate (%) |
||||
| n | study | & duration | follow-up time | 1 yr | 2 yr | 3 yr | 4 yr | 5 yr | |||
| Pilati et al[90] | 34 | 2003 | Phase II | Colon ca | MMC 26.2 mg (20.1-31.6 mg) CDDP 193.7 mg (170-241.1 mg), 41.5°C (41.2°C-42.1°C), 90 min | 14.5 mo (6-34 mo) | 68 | 31 | - | - | - |
| Glehen et al[81] | 56 | 2003 | Phase II | CRC, OC, GC, peritoneal Mesothelioma, Pseudomyxoma peritonei, and others | MMC 0.7 mg/kg (max dose 60 mg), CDDP 1 mg/kg (max dose 80 mg), 46°C-48°C, 90 min | 544.4 d (133-1680 d) | - | R0: 79.0; R2: 44.7 | - | - | - |
| Verwaal et al[67] | 54 | 2003 | Phase III | CRC | MMC 70 mg, 41°C-42°C, 90 min | 21.6 mo | 67 | 44 | - | - | - |
| Witkamp et al[60] | 29 | 2001 | PhaseI/II | CRC | MMC 35 mg/m2, 40°C-41°C, 90 min | 38 mo (26-52 mo) | 82 | 45 | 23 | - | - |
| Shen et al[84] | 77 | 2004 | Phase II | CRC | MMC 40 mg, 40.5°C, 120 min | 15 m | 56 | - | 25 | - | 17 |
| Elias et al[91] | 24 | 2004 | Phase II | CRC | LOHP 460 mg/m2 in 2 L/m2, 43°C, 30 min | 27.4 mo (18.3-49.6 mo) | 83 | 74 | 65 | - | - |
| Yonemura et al[92] | 107 | 2005 | - | GC | MMC 30 mg, CDDP 300 mg, etoposide 150 mg, 42°C-43°C | 46 mo | - | - | - | - | 6.7 all pts; 27 R0 pts |
| Yonemura et al[93] | 48 | 2001 | Randomized study | T2-T4 GC | MMC 30 mg, CDDP 300 mg, 42°C-43°C, 60 min | 5.5 yr (2.4-10.8 yr) | - | - | - | - | 61 |
| Elias et al[94] | 30 | 2006 | Phase II | CRC | LOHP 460 mg/m2 in 2 L/m2 of iso-osmotic 5% dextrose, 43°C, 30 min | 55 mo (24-80 mo) | 97 | 73 | 53 | 49 | - |
| Yan et al[75] | 30 | 2006 | - | CRC | - | 12 mo | 72 | 64 | - | - | - |
| Glehen et al[78] | 53 | 2004 | Phase II | CRC | MMC 40-60 mg, 46°C-48°C, 90 min | 60 mo | 55 | 32 | - | - | 11 |
| Helm et al[95] | 18 | 2007 | Retrospective review | Recurrent OC | CDDP 100 mg/m2 or MMC 30-40 mg, 41°C-43°C, 90 min | 16.2 mo (6-33 mo) | - | 60 | - | - | - |
| Deraco et al[96] | 33 | 2004 | phase II | Pseudomyxoma peritonei | CDDP 25 mg/m2 per L plus MMC 3.3 mg/m2 per L, 60 min, 42.5°C | 28.6 mo (0.4-72 mo) | - | - | - | - | 97 |
| Cavaliere et al[97] | 69 | 2003 | Phase II | CRC | MMC | - | - | - | 27 | - | - |
| Glehen et al[66] | 506 | 2004 | Phase II | CRC | MMC/LOHP, 40°C-43°C, 30-90 min | 53 mo | 72 | - | 39 | - | 19 |
| Verwaal et al[98] | 117 | 2005 | Phase II | CRC | MMC 35 mg/m2, 40°C-41°C, 90 min | 46 mo | 75 | - | 28 | - | 19 |
| Sugarbaker et al[72] | 70 | 2006 | Phase II | CRC | MMC | 47 mo | 88 | - | 44 | - | 32 |
| Helm et al[99] | 5 | 2007 | Retrospective review | Recurrent endometrial ca | CDDP 100 mg/m2, 41°C-43°C, 90 min | 36 mo | 80 | 80 | 80 | 80 | - |
| Zanon et al[100] | 25 | 2006 | Phase II | CRC | MMC 15 mg/m2, 42°C, 60 min | 36 mo | 64 | 40 | - | - | - |
| Piso et al[101] | |||||||||||
| 19 | 2004 | - | OC | CDDP 75 mg/m2, or mitoxantrone 15 mg/m2, 41.5°C, 90 min | - | - | - | - | - | 15 | |
| Roviello et al[102] | 59 | 2006 | - | OC, CRC, GC, Pseudomyxoma, Mesothelioma | MMC 25 mg/m2, CDDP 100 mg/m2. (LOHP 460 mg/m2 in 4 pts with colorectal PC), 41°C-43°C, 60 min | 25 ± 21 mo | - | - | - | - | 50.8 |
GC: Gastric cancer; CRC: Colorectal cancer; OC: Ovarian cancer; MMC: Mitomycin C; CDDP: Cisplatin; LOHP: Oxaliplatin.
ADVERSE EVENTS OF IPHC
IPHC is associated with a high morbidity rate ranging from 27% to 56%[38]. The adverse events can be classified into CRS-related and chemotherapy-related morbidity[15]. Surgery-related adverse events are abscess, intestinal perforation, fistula, prolonged ileus, bile leakage, pancreatitis, pneumonia, deep vein thrombosis, pulmonary emboli, cardiac insufficiency and cerebral infarcts[11,38]. Multivariate analysis demonstrated that adverse events are related to the stage of PC, the operation duration, the number of anastomoses, and blood loss[38,88].
Main chemotherapy-related side effects are bone marrow suppression and renal insufficiency mostly with use of cisplatin[11]. The mortality rate for IPHC ranges between 0% and 11%, and the most common causes of death are bowel perforation, bone marrow suppression, respiratory failure, methicillin resistant staphylococcus aureus infection, and pulmonary embolism[38].
In spite of the above morbidities, such modality remains the suitable one if not the best at present because the patients with PC might get worse without it as systemic chemotherapy or surgery alone is associated with much poorer outcome. With careful and accurate selection of patients, excluding those who are unfit, it is possible to reduce the morbidities of CRS + IPHC and yield a significantly favorable impact on improving survival of the patients.
FUTURE DIRECTIONS
Recently a panel of 55 experts in PC has reached the consensus, which stated that systemic treatment alone may be no longer appropriate for patients with limited PC from a primary or recurrent colon cancer. CRS plus IPHC as a new treatment strategy have clear advantage in this setting[89]. Such procedure has been approved by the surgical oncology experts on PC from North America, France, Italy, Germany, Holland, Spain and Australia. The consensus also indicated that patients who have isolated PC and are likely to receive a complete cytoreduction, as determined by preoperative CT scans, should undergo CRS; and if complete cytoreduction is achieved, intraperitoneal hyperthermic MMC should be given (at 15-35 mg/m2, 39°C-42°C, for 60-120 min, either by closed or open instillation), followed by best adjuvant systemic chemotherapy[65].
Several issues have been discussed about the future of this treatment strategy, one of which is how to make such therapy standardized and available to large numbers of patients. The operative procedures required for aggressive cytoreduction are lengthy, challenging and morbid and use a great deal of hospital, blood bank, and operational theater resources[38]. Even though this treatment strategy has been confirmed to be effective in some centers, there is only one randomized clinical trial to definitely establish its efficacy in terms of improving the survival and the quality of life of patients with colorectal PC[39,67]. Currently, a large scale prospective multi-center phase II study using CRS and IPHC with MMC followed by modern adjuvant systemic chemotherapy for patients with isolated colorectal PC is in progress. This study involves 66 peritoneal surface malignancy surgeons from 46 institutions in 16 countries. Once completed, this study will provide better evidence as to the efficacy of this treatment modality[89].
It is clear that CRS plus IPHC is not indicated for all patients with PC, and the results achieved by international experts in this field may not be replicated in routine clinical practice. Patients with good performance status, low volume of peritoneal disease, and absence of extra-abdominal metastases are more likely to benefit from the combined treatment. This means early diagnosis, early referral to specialist peritonectomy centers for staging, and prompt intervention are keys to successful management. Only by collaborative efforts from medical oncologists, surgical oncologists, and peritoneal surface malignancy treatment centers can such result be achieved[39].
Supported by New-Century Excellent Talents Supporting Program of the Ministry of Education of China NCET-04-0669, Foundation for the Author of National Excellent Doctoral Dissertation of China; FANEDD-200464, Young Talents Supporting Program of Hubei Province 301161202, and National Natural Science Foundation of China No. 20675058
Peer reviewer: Kazuhiro Hanazaki, MD, Professor and Chairman, Department of Surgery, Kochi Medical School, Kochi University, Kohasu, Okohcho, Nankoku, Kochi 783-8505, Japan
S- Editor Yang RH L- Editor Ma JY E- Editor Lu W
References
- 1.Blair SL, Chu DZ, Schwarz RE. Outcome of palliative operations for malignant bowel obstruction in patients with peritoneal carcinomatosis from nongynecological cancer. Ann Surg Oncol. 2001;8:632–637. doi: 10.1007/s10434-001-0632-1. [DOI] [PubMed] [Google Scholar]
- 2.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Mori T, Fujiwara Y, Sugita Y, Azama T, Ishii T, Taniguchi K, Yamazaki K, Takiguchi S, Yasuda T, Yano M, et al. Application of molecular diagnosis for detection of peritoneal micrometastasis and evaluation of preoperative chemotherapy in advanced gastric carcinoma. Ann Surg Oncol. 2004;11:14–20. doi: 10.1007/BF02524340. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology. 2000;58:96–107. doi: 10.1159/000012086. [DOI] [PubMed] [Google Scholar]
- 6.Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256–262. doi: 10.1016/s0002-9610(99)00162-2. [DOI] [PubMed] [Google Scholar]
- 7.Iitsuka Y, Kaneshima S, Tanida O, Takeuchi T, Koga S. Intraperitoneal free cancer cells and their viability in gastric cancer. Cancer. 1979;44:1476–1480. doi: 10.1002/1097-0142(197910)44:4<1476::aid-cncr2820440442>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Koga S, Kaibara N, Iitsuka Y, Kudo H, Kimura A, Hiraoka H. Prognostic significance of intraperitoneal free cancer cells in gastric cancer patients. J Cancer Res Clin Oncol. 1984;108:236–238. doi: 10.1007/BF00402474. [DOI] [PubMed] [Google Scholar]
- 9.Yonemura Y. Hyperthermo-chemotherapy for the treatment of peritoneal dissemination. In: Yonemura Y, editor. Contemporary approached toward cure of gastric cancer. Kanazawa: Maeda Shoten; 1996. pp. 105–116. [Google Scholar]
- 10.Yonemura Y, Bandou E, Kinoshita K, Kawamura T, Takahashi S, Endou Y, Sasaki T. Effective therapy for peritoneal dissemination in gastric cancer. Surg Oncol Clin N Am. 2003;12:635–648. doi: 10.1016/s1055-3207(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 11.Portilla AG, Cendoya I, Lopez de Tejada I, Olabarria I, Martinez de Lecea C, Magrach L, Gil A, Echevarria J, Valdovinos M, Larrabide I. Peritoneal carcinomatosis of colorectal origin. Current treatment. Review and update. Rev Esp Enferm Dig. 2005;97:716–737. doi: 10.4321/s1130-01082005001000005. [DOI] [PubMed] [Google Scholar]
- 12.Sugarbaker PH, Cunliffe WJ, Belliveau J, de Bruijn EA, Graves T, Mullins RE, Schlag P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16:83–97. [PubMed] [Google Scholar]
- 13.Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24:4011–4019. doi: 10.1200/JCO.2006.07.1142. [DOI] [PubMed] [Google Scholar]
- 14.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12:65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 15.Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212–222. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadducci A, Cosio S, Conte PF, Genazzani AR. Consolidation and maintenance treatments for patients with advanced epithelial ovarian cancer in complete response after first-line chemotherapy: a review of the literature. Crit Rev Oncol Hematol. 2005;55:153–166. doi: 10.1016/j.critrevonc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ozols RF. Treatment goals in ovarian cancer. Int J Gynecol Cancer. 2005;15 Suppl 1:3–11. doi: 10.1111/j.1525-1438.2005.15351.x. [DOI] [PubMed] [Google Scholar]
- 18.Chu CS, Rubin SC. Second-look laparotomy for epithelial ovarian cancer: a reappraisal. Curr Oncol Rep. 2001;3:11–18. doi: 10.1007/s11912-001-0037-0. [DOI] [PubMed] [Google Scholar]
- 19.Gadducci A, Sartori E, Maggino T, Zola P, Landoni F, Fanucchi A, Palai N, Alessi C, Ferrero AM, Cosio S, et al. Analysis of failures after negative second-look in patients with advanced ovarian cancer: an Italian multicenter study. Gynecol Oncol. 1998;68:150–155. doi: 10.1006/gyno.1997.4890. [DOI] [PubMed] [Google Scholar]
- 20.Oei AL, Verheijen RH, Seiden MV, Benigno BB, Lopes A, Soper JT, Epenetos AA, Massuger LF. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int J Cancer. 2007;120:2710–2714. doi: 10.1002/ijc.22663. [DOI] [PubMed] [Google Scholar]
- 21.Trimbos JB, Parmar M, Vergote I, Guthrie D, Bolis G, Colombo N, Vermorken JB, Torri V, Mangioni C, Pecorelli S, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;95:105–112. [PubMed] [Google Scholar]
- 22.Young RC, Walton LA, Ellenberg SS, Homesley HD, Wilbanks GD, Decker DG, Miller A, Park R, Major F Jr. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med. 1990;322:1021–1027. doi: 10.1056/NEJM199004123221501. [DOI] [PubMed] [Google Scholar]
- 23.Vergote IB, Vergote-De Vos LN, Abeler VM, Aas M, Lindegaard MW, Kjorstad KE, Trope CG. Randomized trial comparing cisplatin with radioactive phosphorus or wholeabdomen irradiation as adjuvant treatment of ovarian cancer. Cancer. 1992;69:741–749. doi: 10.1002/1097-0142(19920201)69:3<741::aid-cncr2820690322>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Rubin SC, Wong GY, Curtin JP, Barakat RR, Hakes TB, Hoskins WJ. Platinum-based chemotherapy of high-risk stage I epithelial ovarian cancer following comprehensive surgical staging. Obstet Gynecol. 1993;82:143–147. [PubMed] [Google Scholar]
- 25.Petru E, Lahousen M, Tamussino K, Pickel H, Stranzl H, Stettner H, Winter R. Lymphadenectomy in stage I ovarian cancer. Am J Obstet Gynecol. 1994;170:656–662. doi: 10.1016/s0002-9378(94)70244-6. [DOI] [PubMed] [Google Scholar]
- 26.Malmstrom H, Simonsen E, Westberg R. A phase II study of intraperitoneal carboplatin as adjuvant treatment in early-stage ovarian cancer patients. Gynecol Oncol. 1994;52:20–25. doi: 10.1006/gyno.1994.1005. [DOI] [PubMed] [Google Scholar]
- 27.Bolis G, Colombo N, Pecorelli S, Torri V, Marsoni S, Bonazzi C, Chiari S, Favalli G, Mangili G, Presti M. Adjuvant treatment for early epithelial ovarian cancer: results of two randomised clinical trials comparing cisplatin to no further treatment or chromic phosphate (32P). G.I.C.O.G.: Gruppo Interregionale Collaborativo in Ginecologia Oncologica. Ann Oncol. 1995;6:887–893. doi: 10.1093/oxfordjournals.annonc.a059355. [DOI] [PubMed] [Google Scholar]
- 28.Gadducci A, Sartori E, Maggino T, Zola P, Landoni F, Fanucchi A, Stegher C, Alessi C, Buttitta F, Bergamin TE. Analysis of failures in patients with stage I ovarian cancer: an Italian multicenter study. Int J Gynecol Cancer. 1997;7:445–450. [Google Scholar]
- 29.Mano MS, Awada A, Minisini A, Atalay G, Lago LD, Cardoso F, Piccart M. Remaining controversies in the upfront management of advanced ovarian cancer. Int J Gynecol Cancer. 2004;14:707–720. doi: 10.1111/j.1048-891X.2004.014502.x. [DOI] [PubMed] [Google Scholar]
- 30.Podratz KC, Malkasian GD Jr, Wieand HS, Cha SS, Lee RA, Stanhope CR, Williams TJ. Recurrent disease after negative second-look laparotomy in stages III and IV ovarian carcinoma. Gynecol Oncol. 1988;29:274–282. doi: 10.1016/0090-8258(88)90226-0. [DOI] [PubMed] [Google Scholar]
- 31.Rubin SC, Hoskins WJ, Hakes TB, Markman M, Cain JM, Lewis JL Jr. Recurrence after negative second-look laparotomy for ovarian cancer: analysis of risk factors. Am J Obstet Gynecol. 1988;159:1094–1098. doi: 10.1016/0002-9378(88)90420-6. [DOI] [PubMed] [Google Scholar]
- 32.Bar-Am A, Kovner F, Lessing JB, Inbar M, Chaitchik S, Azem F, Brenner SH, Peyser MR. A second thought on second look laparotomy. Acta Obstet Gynecol Scand. 1993;72:386–390. doi: 10.3109/00016349309021119. [DOI] [PubMed] [Google Scholar]
- 33.Thigpen JT. Current controversies in ovarian cancer: maintenance chemotherapy as standard care. Educational Book, 40th Annual Meeting; June 5-8, New Orleans, LA. Alexandria, VA. In: Perry MC, editor. American Society of Clinical Oncology; 2004. pp. USA Greves 2004: 281–284. [Google Scholar]
- 34.Gadducci A, Cosio S, Zola P, Landoni F, Maggino T, Sartori E. Surveillance procedures for patients treated for epithelial ovarian cancer: a review of the literature. Int J Gynecol Cancer. 2007;17:21–31. doi: 10.1111/j.1525-1438.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 35.Yonemura Y, Endou Y, Fujita H, Fushida S, Bandou E, Taniguchi K, Miwa K, Sugiyama K, Sasaki T. Role of MMP-7 in the formation of peritoneal dissemination in gastric cancer. Gastric Cancer. 2000;3:63–70. doi: 10.1007/pl00011698. [DOI] [PubMed] [Google Scholar]
- 36.Liotta LA. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 37.Yonemura Y, Bandou E, Kawamura T, Endou Y, Sasaki T. Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. Eur J Surg Oncol. 2006;32:602–606. doi: 10.1016/j.ejso.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Stewart JH 4th, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol. 2005;12:765–777. doi: 10.1245/ASO.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Yan TD, Sim J, Morris DL. Selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2007;14:1807–1817. doi: 10.1245/s10434-007-9350-7. [DOI] [PubMed] [Google Scholar]
- 40.De Roover A, Detroz B, Detry O, Coimbra C, Polus M, Belaiche J, Meurisse M, Honore P. Adjuvant hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) associated with curative surgery for locally advanced gastric carcinoma. An initial experience. Acta Chir Belg. 2006;106:297–301. doi: 10.1080/00015458.2006.11679896. [DOI] [PubMed] [Google Scholar]
- 41.Morgan RJ Jr, Synold TW, Xi B, Lim D, Shibata S, Margolin K, Schwarz RE, Leong L, Somlo G, Twardowski P, et al. Phase I trial of intraperitoneal gemcitabine in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Clin Cancer Res. 2007;13:1232–1237. doi: 10.1158/1078-0432.CCR-06-1735. [DOI] [PubMed] [Google Scholar]
- 42.Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist. 2005;10:112–122. doi: 10.1634/theoncologist.10-2-112. [DOI] [PubMed] [Google Scholar]
- 43.Michalakis J, Georgatos SD, de Bree E, Polioudaki H, Romanos J, Georgoulias V, Tsiftsis DD, Theodoropoulos PA. Short-term exposure of cancer cells to micromolar doses of paclitaxel, with or without hyperthermia, induces long-term inhibition of cell proliferation and cell death in vitro. Ann Surg Oncol. 2007;14:1220–1228. doi: 10.1245/s10434-006-9305-4. [DOI] [PubMed] [Google Scholar]
- 44.Sticca RP, Dach BW. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg Oncol Clin N Am. 2003;12:689–701. doi: 10.1016/s1055-3207(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 45.Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol. 2003;10:463–468. doi: 10.1245/aso.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T, Isawa E, Sumida M, Ohkubo H. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer. 1997;79:884–891. doi: 10.1002/(sici)1097-0142(19970301)79:5<884::aid-cncr3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 47.Loggie BW, Fleming RA, McQuellon RP, Russell GB, Geisinger KR, Levine EA. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg. 2001;67:999–1003. [PubMed] [Google Scholar]
- 48.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–731. doi: 10.1007/s10434-999-0727-7. [DOI] [PubMed] [Google Scholar]
- 49.Bartlett DL, Buell JF, Libutti SK, Reed E, Lee KB, Figg WD, Venzon DJ, Alexander HR. A phase I trial of continuous hyperthermic peritoneal perfusion with tumor necrosis factor and cisplatin in the treatment of peritoneal carcinomatosis. Cancer. 1998;83:1251–1261. [PubMed] [Google Scholar]
- 50.Loggie BW, Sterchi JM, Rogers AT, Lentz S, Holmesley H, Charles D, Sundberg D. Intraperitoneal hyperthermic chemotherapy for advanced gastrointestinal and ovarian cancers. Reg Cancer Treat. 1994;2:78–81. [Google Scholar]
- 51.Cavaliere F, Perri P, Di Filippo F, Giannarelli D, Botti C, Cosimelli M, Tedesco M, Principi F, Laurenzi L, Cavaliere R. Treatment of peritoneal carcinomatosis with intent to cure. J Surg Oncol. 2000;74:41–44. doi: 10.1002/1096-9098(200005)74:1<41::aid-jso10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 52.van der Vange N, van Goethem AR, Zoetmulder FA, Kaag MM, van de Vaart PJ, ten Bokkel Huinink WW, Beijnen JH. Extensive cytoreductive surgery combined with intra-operative intraperitoneal perfusion with cisplatin under hyperthermic conditions (OVHIPEC) in patients with recurrent ovarian cancer: a feasibility pilot. Eur J Surg Oncol. 2000;26:663–668. doi: 10.1053/ejso.2000.0978. [DOI] [PubMed] [Google Scholar]
- 53.Deraco M, Rossi CR, Pennacchioli E, Guadagni S, Somers DC, Santoro N, Raspagliesi F, Kusamura S, Vaglini M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion in the treatment of recurrent epithelial ovarian cancer: a phase II clinical study. Tumori. 2001;87:120–126. doi: 10.1177/030089160108700302. [DOI] [PubMed] [Google Scholar]
- 54.Panteix G, Beaujard A, Garbit F, Chaduiron-Faye C, Guillaumont M, Gilly F, Baltassat P, Bressolle F. Population pharmacokinetics of cisplatin in patients with advanced ovarian cancer during intraperitoneal hyperthermia chemotherapy. Anticancer Res. 2002;22:1329–1336. [PubMed] [Google Scholar]
- 55.Helm CW, Randall-Whitis L, Martin RS 3rd, Metzinger DS, Gordinier ME, Parker LP, Edwards RP. Hyperthermic intraperitoneal chemotherapy in conjunction with surgery for the treatment of recurrent ovarian carcinoma. Gynecol Oncol. 2007;105:90–96. doi: 10.1016/j.ygyno.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 56.Zanon C, Clara R, Chiappino I, Bortolini M, Cornaglia S, Simone P, Bruno F, De Riu L, Airoldi M, Pedani F. Cytoreductive surgery and intraperitoneal chemohyperthermia for recurrent peritoneal carcinomatosis from ovarian cancer. World J Surg. 2004;28:1040–1045. doi: 10.1007/s00268-004-7461-x. [DOI] [PubMed] [Google Scholar]
- 57.Reichman TW, Cracchiolo B, Sama J, Bryan M, Harrison J, Pliner L, Harrison LE. Cytoreductive surgery and intraoperative hyperthermic chemoperfusion for advanced ovarian carcinoma. J Surg Oncol. 2005;90:51–56; discussion 56-58. doi: 10.1002/jso.20243. [DOI] [PubMed] [Google Scholar]
- 58.Look M, Chang D, Sugarbaker PH. Long-term results of cytoreductive surgery for advanced and recurrent epithelial ovarian cancers and papillary serous carcinoma of the peritoneum. Int J Gynecol Cancer. 2004;14:35–41. doi: 10.1111/j.1048-891x.2004.14008.x. [DOI] [PubMed] [Google Scholar]
- 59.Steller MA, Egorin MJ, Trimble EL, Bartlett DL, Zuhowski EG, Alexander HR, Dedrick RL. A pilot phase I trial of continuous hyperthermic peritoneal perfusion with high-dose carboplatin as primary treatment of patients with small-volume residual ovarian cancer. Cancer Chemother Pharmacol. 1999;43:106–114. doi: 10.1007/s002800050870. [DOI] [PubMed] [Google Scholar]
- 60.Witkamp AJ, de Bree E, Kaag MM, Boot H, Beijnen JH, van Slooten GW, van Coevorden F, Zoetmulder FA. Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer. 2001;37:979–984. doi: 10.1016/s0959-8049(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 61.Yonemura Y, Kawamura T, Nojima N, Bandou E, Keizou T, Fujita H, Michiwa Y, Fujimura T, Fushida S, Ajisaka H, et al. Postoperative results of left upper abdominal evisceration for advanced gastric cancer. Hepatogastroenterology. 2000;47:571–574. [PubMed] [Google Scholar]
- 62.Yonemura Y. Mechanisms of the formation of peritoneal dissemination. In: Yonemura Y, editor. Peritoneal dissemination. Kanazawa: Maeda Shoten; 1998. pp. 1–46. [Google Scholar]
- 63.Los G, van Vugt MJ, Pinedo HM. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br J Cancer. 1994;69:235–241. doi: 10.1038/bjc.1994.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yonemura Y. Hyperthermo-chemotherapy for treatment of peritoneal dissemination. In: Yonemura Y, editor. Peritoneal dissemination. Kanazawa: Maeda Shoten; 1998. pp. 237–260. [Google Scholar]
- 65.Yan TD. Peritoneal carcinomatosis of colorectal origin: standard of care. Ann Surg. 2006;244:632–633; author reply 633-634. doi: 10.1097/01.sla.0000239629.96036.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 68.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sugarbaker PH, Schellinx ME, Chang D, Koslowe P, von Meyerfeldt M. Peritoneal carcinomatosis from adenocarcinoma of the colon. World J Surg. 1996;20:585–591; discussion 592. doi: 10.1007/s002689900091. [DOI] [PubMed] [Google Scholar]
- 70.Pestieau SR, Sugarbaker PH. Treatment of primary colon cancer with peritoneal carcinomatosis: comparison of concomitant vs. delayed management. Dis Colon Rectum. 2000;43:1341–1346; discussion 1347-1348. doi: 10.1007/BF02236627. [DOI] [PubMed] [Google Scholar]
- 71.Carmignani CP, Ortega-Perez G, Sugarbaker PH. The management of synchronous peritoneal carcinomatosis and hematogenous metastasis from colorectal cancer. Eur J Surg Oncol. 2004;30:391–398. doi: 10.1016/j.ejso.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 72.Gomes da Silva R, Sugarbaker P. Analysis of 10 prognostic factors in 70 patients having complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. Am J Coll Surg. 2006;203:878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 73.Hadi R, Saunders V, Utkina O, Clingan P, Kam P, Links M, Morris DL. Review of patients with peritoneal malignancy treated with peritonectomy and heated intraperitoneal chemotherapy. ANZ J Surg. 2006;76:156–161. doi: 10.1111/j.1445-2197.2006.03579.x. [DOI] [PubMed] [Google Scholar]
- 74.Shehata M, Chu F, Saunders V, Kam PC, Links M, Morris DL. Peritoneal carcinomatosis from colorectal cancer and small bowel cancer treated with peritonectomy. ANZ J Surg. 2006;76:467–471. doi: 10.1111/j.1445-2197.2006.03749.x. [DOI] [PubMed] [Google Scholar]
- 75.Yan TD, Chu F, Links M, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma: non-mucinous tumour associated with an improved survival. Eur J Surg Oncol. 2006;32:1119–1124. doi: 10.1016/j.ejso.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Kecmanovic DM, Pavlov MJ, Ceranic MS, Sepetkovski AV, Kovacevic PA, Stamenkovic AB. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31:147–152. doi: 10.1016/j.ejso.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 77.Verwaal VJ, van Tinteren H, van Ruth S, Zoetmulder FA. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2004;91:739–746. doi: 10.1002/bjs.4516. [DOI] [PubMed] [Google Scholar]
- 78.Glehen O, Cotte E, Schreiber V, Sayag-Beaujard AC, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:747–754. doi: 10.1002/bjs.4473. [DOI] [PubMed] [Google Scholar]
- 79.Portilla AG, Sugarbaker PH, Chang D. Second-look surgery after cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: analysis of prognostic features. World J Surg. 1999;23:23–29. doi: 10.1007/s002689900560. [DOI] [PubMed] [Google Scholar]
- 80.Gomes da Silva R, Cabanas J, Sugarbaker PH. Limited survival in the treatment of carcinomatosis from rectal cancer. Dis Colon Rectum. 2005;48:2258–2263. doi: 10.1007/s10350-005-0189-3. [DOI] [PubMed] [Google Scholar]
- 81.Glehen O, Mithieux F, Osinsky D, Beaujard AC, Freyer G, Guertsch P, Francois Y, Peyrat P, Panteix G, Vignal J, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21:799–806. doi: 10.1200/JCO.2003.06.139. [DOI] [PubMed] [Google Scholar]
- 82.Loggie BW, Fleming RA, McQuellon RP, Russell GB, Geisinger KR. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of gastrointestinal origin. Am Surg. 2000;66:561–568. [PubMed] [Google Scholar]
- 83.Shen P, Levine EA, Hall J, Case D, Russell G, Fleming R, McQuellon R, Geisinger KR, Loggie BW. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg. 2003;138:26–33. doi: 10.1001/archsurg.138.1.26. [DOI] [PubMed] [Google Scholar]
- 84.Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, Levine EA. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178–186. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Culliford AT 4th, Brooks AD, Sharma S, Saltz LB, Schwartz GK, O'Reilly EM, Ilson DH, Kemeny NE, Kelsen DP, Guillem JG, et al. Surgical debulking and intraperitoneal chemotherapy for established peritoneal metastases from colon and appendix cancer. Ann Surg Oncol. 2001;8:787–795. doi: 10.1007/s10434-001-0787-9. [DOI] [PubMed] [Google Scholar]
- 86.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 87.Yan TD, Links M, Xu ZY, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg. 2006;93:1270–1276. doi: 10.1002/bjs.5427. [DOI] [PubMed] [Google Scholar]
- 88.Elias D, Goere D, Blot F, Billard V, Pocard M, Kohneh-Shahri N, Raynard B. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol. 2007;14:1818–1824. doi: 10.1245/s10434-007-9348-1. [DOI] [PubMed] [Google Scholar]
- 89.Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, Baratti D, Bartlett D, Barone R, Barrios P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14:128–133. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 90.Pilati P, Mocellin S, Rossi CR, Foletto M, Campana L, Nitti D, Lise M. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10:508–513. doi: 10.1245/aso.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 91.Elias D, Sideris L, Pocard M, Ede C, Ben Hassouna D, Ducreux M, Boige V, Cote JF, Lasser P. Efficacy of intraperitoneal chemohyperthermia with oxaliplatin in colorectal peritoneal carcinomatosis. Preliminary results in 24 patients. Ann Oncol. 2004;15:781–785. doi: 10.1093/annonc/mdh186. [DOI] [PubMed] [Google Scholar]
- 92.Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92:370–375. doi: 10.1002/bjs.4695. [DOI] [PubMed] [Google Scholar]
- 93.Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, Sugiyama K, Kawamura T, Kinoshita K, Endou Y, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology. 2001;48:1776–1782. [PubMed] [Google Scholar]
- 94.Elias D, Raynard B, Farkhondeh F, Goere D, Rouquie D, Ciuchendea R, Pocard M, Ducreux M. Peritoneal carcinomatosis of colorectal origin. Gastroenterol Clin Biol. 2006;30:1200–1204. doi: 10.1016/s0399-8320(06)73512-6. [DOI] [PubMed] [Google Scholar]
- 95.Helm CW, Randall-Whitis L, Martin RS 3rd, Metzinger DS, Gordinier ME, Parker LP, Edwards RP. Hyperthermic intraperitoneal chemotherapy in conjunction with surgery for the treatment of recurrent ovarian carcinoma. Gynecol Oncol. 2007;105:90–96. doi: 10.1016/j.ygyno.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 96.Deraco M, Baratti D, Inglese MG, Allaria B, Andreola S, Gavazzi C, Kusamura S. Peritonectomy and intraperitoneal hyperthermic perfusion (IPHP): a strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Ann Surg Oncol. 2004;11:393–398. doi: 10.1245/ASO.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Cavaliere F, Perri P, Rossi CR, Pilati PL, De Simone M, Vaira M, Deraco M, Di Filippo F. Indications for integrated surgical treatment of peritoneal carcinomatosis of colorectal origin: experience of the Italian Society of Locoregional Integrated Therapy in Oncology. Tumori. 2003;89:21–23. [PubMed] [Google Scholar]
- 98.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12:65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 99.Helm CW, Toler CR, Martin RS 3rd, Gordinier ME, Parker LP, Metzinger DS, Edwards RP. Cytoreduction and intraperitoneal heated chemotherapy for the treatment of endometrial carcinoma recurrent within the peritoneal cavity. Int J Gynecol Cancer. 2007;17:204–209. doi: 10.1111/j.1525-1438.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 100.Zanon C, Bortolini M, Chiappino I, Simone P, Bruno F, Gaglia P, Airoldi M, Deriu L, Mashiah A. Cytoreductive surgery combined with intraperitoneal chemohyperthermia for the treatment of advanced colon cancer. World J Surg. 2006;30:2025–2032. doi: 10.1007/s00268-005-0486-y. [DOI] [PubMed] [Google Scholar]
- 101.Piso P, Dahlke MH, Loss M, Schlitt HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from ovarian cancer. World J Surg Oncol. 2004;2:21. doi: 10.1186/1477-7819-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roviello F, Marrelli D, Neri A, Cerretani D, de Manzoni G, Pedrazzani C, Cioppa T, Nastri G, Giorgi G, Pinto E. Treatment of peritoneal carcinomatosis by cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion (IHCP): postoperative outcome and risk factors for morbidity. World J Surg. 2006;30:2033–2040; discussion 2041-2042. doi: 10.1007/s00268-006-0038-0. [DOI] [PubMed] [Google Scholar]
- 103.McQuellon RP, Loggie BW, Fleming RA, Russell GB, Lehman AB, Rambo TD. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65–73. doi: 10.1053/ejso.2000.1033. [DOI] [PubMed] [Google Scholar]
- 104.McQuellon RP, Loggie BW, Lehman AB, Russell GB, Fleming RA, Shen P, Levine EA. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10:155–162. doi: 10.1245/aso.2003.03.067. [DOI] [PubMed] [Google Scholar]