Abstract
AIM: To evaluate the performance of commercially available immunochromatographic (ICT) and immunoblot tests covering the current infection marker CIM and conventional ELISA for the diagnosis of H pylori infection in adult dyspeptic patients.
METHODS: Consecutive non-treated dyspeptic patients undergoing diagnostic endoscopy were tested for H pylori infection by culture, rapid urease test, and histology of gastric biopsy specimens. Serum from 61 H pylori infected and 21 non-infected patients were tested for anti-H pylori IgG antibodies by commercial ELISA (AccuBindTM ELISA, Monobind, USA), ICT (Assure® H pylori Rapid Test, Genelabs Diagnostics, Singapore), and immunoblot (Helico Blot 2.1, Genelabs Diagnostics, Singapore) assays. ICT and immunoblot kits cover CIM among other parameters and their performance with and without CIM was evaluated separately.
RESULTS: Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of ELISA were 96.7%, 42.8%, 83.1%, 81.8%, and 82.9%, of ICT were 90.1%, 80.9%, 93.2%, 73.9%, and 87.8%, of ICT with CIM were 88.5%, 90.4%, 96.4%, 73.0%, and 89.0%, of immunoblot were 98.3%, 80.9%, 93.7%, 94.4%, and 93.9%, and of immunoblot with CIM were 98.3%, 90.4%, 96.7%, 95.0%, and 96.3%, respectively.
CONCLUSION: Immunoblot with CIM had the best performance. ICT with CIM was found to be more specific and accurate than the conventional ELISA and may be useful for non-invasive diagnosis of H pylori infection.
Keywords: H pylori, ELISA, Immunochromatographic test, Immunoblot, Current infection marker
INTRODUCTION
H pylori causes peptic ulcer disease[1,2] that can be cured by antimicrobial treatment[3–5]. ‘Test and treat’ strategy that involves non-invasive testing without endoscopy and eradication therapy in young patients[6] is effective in management of dyspepsia[7–9]. This calls for a simple, reliable and non-invasive diagnostic test for H pylori infection in clinical practice. At present there is no single test for H pylori that can be used as the ‘gold standard’[10]. Culture, rapid urease test, and histology require endoscopic biopsy of gastric mucosal tissue that is expensive, inconvenient for the patient and available only at specialized centers. Moreover, because of a patchy distribution of H pylori in the gastric mucosa, biopsy tissue examination may yield false negative results[11]. Serological tests that detect anti-H pylori IgG antibodies are non-invasive, less expensive, not influenced by sampling error, and less likely to be confounded by suppression of H pylori infection by colloidal bismuth, proton pump inhibitors, or antibiotics[12]. Serological tests are widely used[13] but they cannot differentiate a current infection from a past exposure[10]. Performance of serological tests depends on the antigen preparation used[12,14], and as H pylori strains differ among geographic locations, local validation of the test is necessary[13,14]. A recent development in H pylori diagnosis is a commercial immunochromatographic test (ICT) and an immunoblot test covering the current infection marker CIM. CIM is an antigenic protein synthesized by recombinant DNA technology. It is homologous to a conserved secreted protein of H pylori. According to its manufacturer (Genelabs Diagnostics, Singapore), presence of anti-CIM IgG antibody is highly predictive for active H pylori infection. If so, it should be helpful for diagnosis of H pylori infection where facility for endoscopy is not available. Several studies have found the immunoblot test Helico Blot 2.0, its newer version Helico Blot 2.1, and ICT kit Assure® H pylori Rapid Test, all of which are able to detect anti-CIM antibodies, effective for diagnosis of H pylori infection in adults and children[15–20]. Bangladesh is a developing country with a high prevalence of H pylori infection and peptic ulcer[21,22]. Different tests have been evaluated for diagnosis of H pylori infection in Bangladesh[23–26]. Two studies involved in-house immunoblot assays[25,26]. To the best of our knowledge standardized commercial immunoblot or ICT tests with CIM have not been evaluated in Bangladesh. The aim of this study was to evaluate the performance of three commercially available serological tests based on three methods to find a reliable serological test for non-invasive diagnosis of H pylori infection in adult dyspeptic patients: ELISA (AccuBindTM, ELISA, Monobind, USA), ICT (Assure®, H pylori Rapid Test, Genelabs Diagnostics, Singapore), and immunoblot (Helico Blot 2.1, Genelabs Diagnostics, Singapore). ICT and immunoblot assays cover CIM. The performance of ICT and immunoblot tests was evaluated separately with and without CIM to see whether CIM improves performance of these tests.
MATERIALS AND METHODS
Patient selection
Consecutive adult dyspeptic patients attending the Department of Gastrointestinal, Hepatobiliary and Pancreatic Diseases (GHPD) of Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM) for diagnostic endoscopy during June 2004 to January 2005 were selected. Informed consent was obtained from each patient before endoscopy, and sample collection and approval of the Ethical Review Committee of BIRDEM was taken prior to initiation of the project work. Patients who underwent partial or complete gastrectomy, or those with a prior H pylori eradication therapy, or those who were treated with any antibiotics, colloidal bismuth compounds, proton pump inhibitors, or H2 receptor blocker within the last four weeks were excluded.
Endoscopy and gastric biopsy
Endoscopy was done with an Olympus EVIS 160 video endoscope (Olympus Optical Company, Japan) after an overnight fast. Six gastric biopsies (three from antrum and three from corpus) were taken from each patient.
Collection of serum
After endoscopy 3 mL of venous blood was collected from each patient. Serum was separated after 1 h and kept at -70°C until serological tests were performed.
Culture
Two gastric biopsy specimens, one from the antrum and one from the corpus, were taken up into Stuart’s transport media. Cooled samples were transported to the H pylori Laboratory of Laboratory Sciences Division of International Centre for Diarrhoeal Diseases Research, Bangladesh (ICDDR,B) within 3-4 h where culture was done as described previously[27]. Positive cultures were identified by colony and Gram stain morphology and positive catalase, oxidase, and urease tests.
Rapid urease test (RUT)
Two gastric biopsy specimens, one from the antrum and one from the corpus, were taken up in Christensen’s urea agar media (pH adjusted at 7.0) in screw-capped bottles. A change of color from yellow to pink by any specimen within 2 h was considered as positive.
Histology
Two gastric biopsy specimens, one from the antrum and one from the corpus, were fixed in 10% formalin in separate containers and were sent to the Histopathology Laboratory of Ibrahim Medical College. Samples were embedded in paraffin wax, cut at 5 μm thickness and were stained by modified Giemsa and hematoxylin & eosin (HE) dye. H pylori was identified from its characteristic appearance and distribution. The histopathologist (Nasim Ahmed) was unaware of patients’ clinical conditions and other test results.
‘Gold standard’ definition of H pylori infection
As there is no single ‘gold standard’ test for H pylori, an operational ‘gold standard’ definition of H pylori infection was used. The definition was as follows: Patients with positive culture result were considered as infected. In the case of a negative culture, patients positive by both RUT and histology were considered as infected. Patients negative by all three gastric biopsy specimen based tests were considered as non-infected. Patients negative by culture and positive by either RUT or histology were considered as indeterminate[16,17].
ELISA
ELISA test for anti-H pylori IgG antibody was performed using a commercial test kit, AccuBindTM ELISA (Monobind, USA), according to instructions of the manufacturer.
ICT
ICT test was performed by the commercial test kit Assure® H pylori Rapid Test (Genelabs Diagnostics, Singapore) according to the instructions of the manufacturer. When control (A) and test line (C) were visible the ICT test was regarded positive, when control (A), test (C), and CIM line (B) were visible it was regarded ICT with CIM positive. When only control line (A) was visible, the test was regarded negative (Figure 1). When control line (A) was absent or control line (A) and CIM line (B) present but test line (C) absent the test was regarded to be invalid.
Figure 1.

Photograph of serum ICT by Assure® H pylori Rapid Test. Left: ICT positive; Middle: Negative; Right: ICT with CIM positive (73, 74, and 78: Patients’ identification numbers).
Immunoblot
Immunoblot test was done using the commercial kit Helico Blot 2.1 (Genelabs Diagnostics, Singapore). Helico Blot 2.1 consists of a Western Blot made from bacterial lysate of H pylori strain ATCC 49503 and a recombinant antigen called CIM[18]. The test strip contains H pylori antigens with molecular weights of 116 kDa (CagA), 89 kDa (VacA), 37 kDa, 35 kDa, 30 kDa (Urease A), and 19.5 kDa as separate lines. CIM is located at the bottom of the strip as an independent band. The test was done and interpreted according to instructions of the manufacturer (Figure 2). The manufacturer’s recommended criteria for determining H pylori positivity by Helico Blot 2.1 was as follows: (1) 116 kDa (CagA) positive, where CagA has to be present with at least one of the following bands - 89 kDa (VacA), 37 kDa, 35 kDa, 30 kDa (UreA), or 19.5 kDa, or with CIM, (2) presence of any one band at 89 kDa, 37 kDa, or 35 kDa, with or without CIM, (3) presence of both 30 kDa and 19.5 kDa band with or without CIM.
Figure 2.
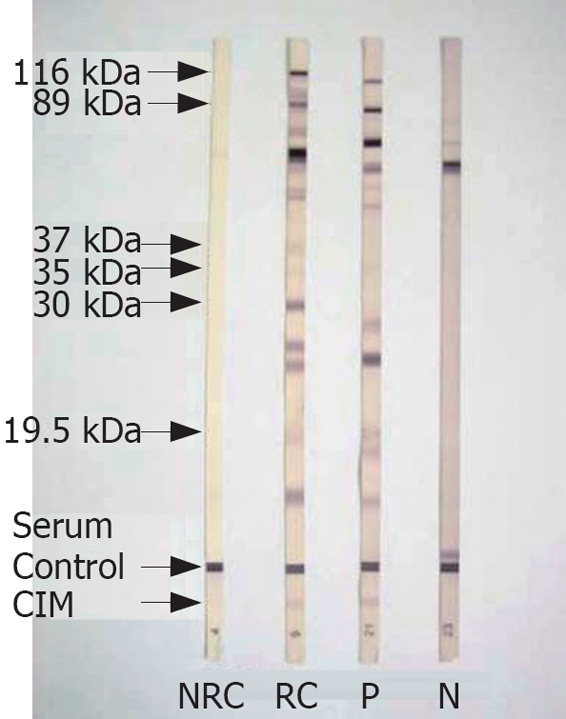
Photograph of serum Immunoblot test by Helico Blot 2.1. kDa: kilo Dalton; CIM: Current infection marker; NRC: Non-reactive control; RC: Reactive control; P: Positive Immunoblot with CIM; N: Negative.
ELISA, ICT, and immunoblot tests were carried out at the Immunology Laboratory of the Department of Immunology of BIRDEM.
Calculation of performance
Samples from 62 patients who were infected and 21 patients who were non-infected according to ‘gold standard’ definition were subjected to serological testing. Eight patients were indeterminate and excluded. Results of serological tests were plotted against H pylori infection status, and sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy with 95% confidence interval were calculated using standard formula.
RESULTS
Patients’ characteristics
A total of 82 patients were included in the study. They were aged between 18 to 75 years with a mean age of 47.6 years and standard deviation 14.0 years. Patients’ characteristics are shown in Table 1.
Table 1.
Characteristics of selected patients (n = 82)
| Characteristics | n | Percentage |
| Age group (yr) | ||
| ≤ 30 | 11 | 13.4 |
| 31-40 | 15 | 18.3 |
| 41-50 | 21 | 25.6 |
| 51-60 | 22 | 26.8 |
| ≥ 60 | 13 | 15.9 |
| Sex | ||
| Male | 49 | 59.8 |
| Female | 33 | 40.2 |
| Smoking habit | ||
| Smoker | 25 | 30.5 |
| Non-smoker | 57 | 69.5 |
| Alcohol intake | ||
| Take alcohol | 3 | 3.7 |
| Do not take alcohol | 79 | 96.3 |
| Diabetes | ||
| Diabetic | 67 | 81.7 |
| Non-diabetic | 15 | 18.3 |
| Endoscopic diagnoses | ||
| Normal | 20 | 24.4 |
| Gastritis | 24 | 29.3 |
| Duodenitis | 8 | 9.8 |
| Peptic ulcer | 25 | 30.5 |
| Gastric carcinoma | 4 | 4.8 |
| Reflux esophagitis | 1 | 1.2 |
H pylori positivity by different tests
Of the 82 patients, 53 (64.6%) were culture positive, 40 (48.8%) were rapid urease test positive, 31 (37.8%) were histology positive, 71 (86.5%) were ELISA positive, 59 (71.9%) were ICT positive, 56 (68.2%) were ICT with CIM positive, 64 (78.0%) were immunoblot positive, and 62 (75.6%) were immunoblot with CIM positive.
Performance of serological tests
Sensitivity, specificity, PPV, NPV, and accuracy of serological tests were calculated from data presented in Table 2, and the calculated performance is shown in Table 3. ELISA was very sensitive but its specificity was low. Sensitivity and NPV of ICT were lower but specificity, PPV, and accuracy were much higher than ELISA. CIM decreased sensitivity of ICT slightly but increased its specificity, PPV, and accuracy. Sensitivity of immunoblot was higher than ELISA or ICT. Its specificity was higher than ELISA and same as ICT. Its PPV, NPV, and accuracy were much higher than ELISA and ICT. CIM increased specificity, PPV, NPV, and accuracy of immunoblot without altering its sensitivity (Table 3).
Table 2.
Comparison of serological tests with H pylori infection (n = 82)
| Test |
H pylori infection1 |
|
| Infected (n = 61) | Non-infected (n = 21) | |
| n (%)2 | n (%)2 | |
| ELISA | ||
| Positive (n = 71) | 59 (83.0) | 12 (17.0) |
| Negative (n = 11) | 2 (18.2) | 9 (81.8) |
| ICT | ||
| Positive (n = 59) | 55 (93.2) | 4 (6.8) |
| Negative (n = 23) | 6 (26.1) | 17 (73.9) |
| ICT with CIM | ||
| Positive (n = 56) | 54 (96.4) | 2 (3.6) |
| Negative (n = 26) | 7 (26.9) | 19 (73.1) |
| Immunoblot | ||
| Positive (n = 64) | 60 (93.8) | 4 (6.2) |
| Negative (n = 18) | 1 (5.6) | 17 (94.4) |
| Immunoblot with CIM | ||
| Positive (n = 62) | 60 (96.8) | 2 (3.2) |
| Negative (n = 20) | 1 (5.0) | 19 (95.0) |
Infected: Culture positive or culture negative but both RUT and histology positive; non-infected: All three tests negative;
Percentages are over row total.
Table 3.
Performance of serological tests
|
Performance (95% Confidence interval) in percentage |
|||||
| Test | Sensitivity | Specificity | PPV1 | NPV2 | Accuracy |
| ELISA | 96.7 (88.6-99.4) | 42.8 (21.8-65.9) | 83.1 (72.3-90.9) | 81.8 (48.2-97.7) | 82.9 (73.0-90.3) |
| ICT | 90.1 (79.8-96.3) | 80.9 (58.0-94.5) | 93.2 (83.5-98.1) | 73.9 (51.5-89.7) | 87.8 (78.7-93.9) |
| ICT with CIM | 88.5 (77.7-95.2) | 90.4 (69.6-98.8) | 96.4 (87.6-99.5) | 73.0 (52.2-88.4) | 89.0 (80.1-94.8) |
| Immunoblot | 98.3 (91.2-99.9) | 80.9 (58.0-94.5) | 93.7 (84.7-98.2) | 94.4 (72.7-99.8) | 93.9 (86.3-97.9) |
| Immunoblot with CIM | 98.3 (91.2-99.9) | 90.4 (69.6-98.8) | 96.7 (88.8-99.6) | 95.0 (75.1-99.8) | 96.3 (89.6-99.2) |
PPV: Positive predictive value;
NPV: Negative predictive value.
DISCUSSION
Since its discovery many tests have been designed for diagnosis of H pylori[12]. But no test is accurate enough to be the ‘gold standard’[10]. Serological tests are widely used for non-invasive diagnosis[13] but a positive serological test does not mean active infection[10]. To find a reliable non-invasive test for H pylori, we evaluated commercially available conventional ELISA, and new ICT and immunoblot assays covering the recombinant CIM.
The commercial ELISA that we evaluated was very sensitive but less specific (Table 3). Performance of the ELISA kit varies in different populations. Laheij et al reviewed a range of sensitivity of 57%-100% and a range of specificity of 31%-100% for different commercial kits in different populations[28]. A study in the Netherlands evaluated eight commercial ELISA tests and found sensitivities of 93%-98% and specificities of 95%-98%[29]. Using a further commercial kit, a study from Bangladesh found sensitivity, specificity, PPV, and NPV of 100%, 13.6%, 54.8%, and 100%, respectively[24].
Performance of ICT with CIM evaluated in this study was better than two other commercial ICT kits evaluated in Chinese patients[30] and similar to another commercial ICT kit evaluated in African-American, Caucasian, and Asian population with 13C urea breath test as the ‘gold standard’[31]. Its performance in our study was lower than a study that evaluated this kit in Thai children[19] and similar to a study that evaluated this kit in Portuguese children[20].
Performance of immunoblot with CIM in our study was similar to reports that evaluated the same kit in adult French population[16] and Portuguese children of different age groups[17]. Its specificity was lower in our study than a study in Japanese and American population[18]. Lepper et al compared different immunoblot kits and found sensitivity, specificity, PPV, and NPV 62.8%-95.9%, 85.7%-100.0%, 97.2%-100.0%, and 37.7%-82.4%, respectively[32].
Among the serological tests evaluated, immunoblot with CIM had the highest sensitivity, specificity, PPV, NPV, and accuracy (Table 3). It thus may be useful for non-invasive diagnosis of H pylori infection. Though conventional ELISA was highly sensitive it may not be reliable for diagnostic purpose in these patients due to its low specificity. Immunoblot is costly, takes longer time, and requires laboratory set-up and a trained staff. Though ELISA is cheaper than immunoblot, it also requires laboratory set-up and a trained staff. ICT with CIM had sensitivity and NPV lower than immunoblot or ELISA, but its specificity, PPV, and accuracy were higher than ELISA. Considering sensitivity, specificity, PPV, and accuracy, ICT with CIM yielded good performance (Table 3). ICT is a rapid test, easy to carry out, and can be done at the physician’s chamber or in the field level where well-equipped laboratory and trained staff is not available.
In conclusion, immunoblot with CIM had the best performance. Though the sensitivity of ICT with CIM was slightly lower, due to its high specificity and accuracy it would be a more reliable diagnostic tool than conventional ELISA. As ICT with CIM has good performance and it is cheap, easy and rapid, it may be useful for non-invasive diagnosis of H pylori infection in adult dyspeptic patients of Bangladesh.
COMMENTS
Background
A reliable non-invasive test for H pylori is essential in clinical practice. Commercial serological tests based on enzyme linked immunosorbent assay (ELISA), immunochromatographic test (ICT) and immunoblot are widely used. But they are unable to distinguish between active infection and a previous contact. Gastric biopsy based tests can indicate active infection but they are inconvenient for the patients and not available everywhere. A recent development is commercial ICT and immunoblot tests with CIM that may indicate active H pylori infection. In this study we evaluated performance of commercial ELISA without CIM and ICT and immunoblot with CIM for pre-treatment diagnosis of H pylori infection.
Research frontiers
CIM is an antigenic protein synthesized by recombinant DNA technology. According to its manufacturer, presence of anti-CIM IgG antibodies indicates active H pylori infection. It is interesting because usually active infection cannot be diagnosed from serum IgG response to an organism.
Innovations and breakthroughs
Though the commercial kits used in this study may not represent the methods, from this study we can see both ICT and immunoblot with CIM have better performance than conventional ELISA evaluated, and CIM improves performance of both ICT and immunoblot kits. We can also see that performance of CIM varies depending on the method in which it is used. It performed better in immunoblot than in ICT.
Applications
This study will facilitate diagnosis of H pylori infection.
Peer review
In this clinical report, Dr. Rahman et al evaluated the performance of serological tests with current infection marker and ELISA for the non-invasive diagnosis of H pylori infection. Informative results were obtained from their study which could benefit other physicians. Therefore, this study is of importance.
Acknowledgments
We wish to thank Genelabs Diagnostics, Singapore, for kindly providing Assure® H pylori Rapid test and Helico Blot 2.1 test kits free of cost.
Supported partly by SIDA Grant, No. GR-00384 to Motiur Rahman
Peer reviewers: Xian-Ming Chen, MD, Associate Professor, Dept. of Medical Microbiology and Immunology, Creighton University, 2500 California Plaza, Omaha NE 68178, United States; Siegfried Wagner, Professor, Medizinische Klinik II, Klinikum Deggendorf, Perlasberger Str. 41, Deggendorf 94469, Germany
S- Editor Zhu LH L- Editor Mihm S E- Editor Liu Y
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Marshall BJ, McGechie DB, Rogers PA, Glancy RJ. Pyloric Campylobacter infection and gastroduodenal disease. Med J Aust. 1985;142:439–444. doi: 10.5694/j.1326-5377.1985.tb113444.x. [DOI] [PubMed] [Google Scholar]
- 3.Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ Jr, Saeed ZA, Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992;116:705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 4.Hosking SW, Ling TK, Chung SC, Yung MY, Cheng AF, Sung JJ, Li AK. Duodenal ulcer healing by eradication of Helicobacter pylori without anti-acid treatment: randomised controlled trial. Lancet. 1994;343:508–510. doi: 10.1016/s0140-6736(94)91460-5. [DOI] [PubMed] [Google Scholar]
- 5.Sung JJ, Chung SC, Ling TK, Yung MY, Leung VK, Ng EK, Li MK, Cheng AF, Li AK. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori. N Engl J Med. 1995;332:139–142. doi: 10.1056/NEJM199501193320302. [DOI] [PubMed] [Google Scholar]
- 6.Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter pylori Study Group. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lassen AT, Hallas J, Schaffalitzky de Muckadell OB. Helicobacter pylori test and eradicate versus prompt endoscopy for management of dyspeptic patients: 6.7 year follow up of a randomised trial. Gut. 2004;53:1758–1763. doi: 10.1136/gut.2004.043570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane JA, Murray LJ, Noble S, Egger M, Harvey IM, Donovan JL, Nair P, Harvey RF. Impact of Helicobacter pylori eradication on dyspepsia, health resource use, and quality of life in the Bristol helicobacter project: randomised controlled trial. BMJ. 2006;332:199–204. doi: 10.1136/bmj.38702.662546.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw IS, Valori RM, Charlett A, McNulty CA. Limited impact on endoscopy demand from a primary care based 'test and treat' dyspepsia management strategy: the results of a randomised controlled trial. Br J Gen Pract. 2006;56:369–374. [PMC free article] [PubMed] [Google Scholar]
- 10.Vaira D, Gatta L, Ricci C, Miglioli M. Review article: diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16 Suppl 1:16–23. doi: 10.1046/j.1365-2036.2002.0160s1016.x. [DOI] [PubMed] [Google Scholar]
- 11.Morris A, Ali MR, Brown P, Lane M, Patton K. Campylobacter pylori infection in biopsy specimens of gastric antrum: laboratory diagnosis and estimation of sampling error. J Clin Pathol. 1989;42:727–732. doi: 10.1136/jcp.42.7.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 14.Makristathis A, Hirschl AM, Lehours P, Megraud F. Diagnosis of Helicobacter pylori infection. Helicobacter. 2004;9 Suppl 1:7–14. doi: 10.1111/j.1083-4389.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 15.Rocha GA, Oliveira AM, Queiroz DM, Carvalho AS, Nogueira AM. Immunoblot analysis of humoral immune response to Helicobacter pylori in children with and without duodenal ulcer. J Clin Microbiol. 2000;38:1777–1781. doi: 10.1128/jcm.38.5.1777-1781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteiro L, de Mascarel A, Sarrasqueta AM, Bergey B, Barberis C, Talby P, Roux D, Shouler L, Goldfain D, Lamouliatte H, et al. Diagnosis of Helicobacter pylori infection: noninvasive methods compared to invasive methods and evaluation of two new tests. Am J Gastroenterol. 2001;96:353–358. doi: 10.1111/j.1572-0241.2001.03518.x. [DOI] [PubMed] [Google Scholar]
- 17.Oleastro M, Matos R, Cabral J, Barros R, Lopes AI, Ramalho P, Monteiro L. Evaluation of a Western blot test, Helico blot 2.1, in the diagnosis of Helicobacter pylori infection in a pediatric population. Helicobacter. 2002;7:210–215. doi: 10.1046/j.1523-5378.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 18.Park CY, Cho YK, Kodama T, El-Zimaity HM, Osato MS, Graham DY, Yamaoka Y. New serological assay for detection of putative Helicobacter pylori virulence factors. J Clin Microbiol. 2002;40:4753–4756. doi: 10.1128/JCM.40.12.4753-4756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treepongkaruna S, Nopchinda S, Taweewongsounton A, Atisook K, Pienvichit P, Vithayasai N, Simakachorn N, Aanpreung P. A rapid serologic test and immunoblotting for the detection of Helicobacter pylori infection in children. J Trop Pediatr. 2006;52:267–271. doi: 10.1093/tropej/fmk003. [DOI] [PubMed] [Google Scholar]
- 20.Pelerito A, Oleastro M, Lopes AI, Ramalho P, Cabral J, Monteiro L. Evaluation of rapid test Assure Helicobacter pylori for diagnosis of H. pylori in pediatric population. J Microbiol Methods. 2006;66:331–335. doi: 10.1016/j.mimet.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad MM, Rahman M, Rumi AK, Islam S, Huq F, Chowdhury MF, Jinnah F, Morshed MG, Hassan MS, Khan AK, et al. Prevalence of Helicobacter pylori in asymptomatic population--a pilot serological study in Bangladesh. J Epidemiol. 1997;7:251–254. doi: 10.2188/jea.7.251. [DOI] [PubMed] [Google Scholar]
- 22.Hassan M, Ali SMK, Khan AKA. Peptic ulcer in Bangladesh, an endoscopic survey (Abstract) Gut. 1985;26:A1117. [Google Scholar]
- 23.Talukder MSI, Khan BR, Islam B, Barua AR, Hassan K, Mashud M. Comparison of rapid urease test, culture, imprint smear and histology in the detection of Helicobacter pylori. Bangladesh J Pathol. 1995;10:49–54. [Google Scholar]
- 24.Morshed MG, Jinnah F, Islam MS, Rumi MA, Ahmed S, Ahmed MM, Sadeque M, Chowdhury MF. Evaluation of culture, histological examination, serology and the rapid urease test for diagnosis of Helicobacter pylori in patients with dyspepsia in Bangladesh. Jpn J Med Sci Biol. 1997;50:55–62. doi: 10.7883/yoken1952.50.55. [DOI] [PubMed] [Google Scholar]
- 25.Casswall TH, Nilsson HO, Bergstrom M, Aleljung P, Wadstrom T, Dahlstrom AK, Albert MJ, Sarker SA. Evaluation of serology, 13C-urea breath test, and polymerase chain reaction of stool samples to detect Helicobacter pylori in Bangladeshi children. J Pediatr Gastroenterol Nutr. 1999;28:31–36. doi: 10.1097/00005176-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Sarker SA, Nahar S, Rahman M, Bardhan PK, Nair GB, Beglinger C, Gyr N. High prevalence of cagA and vacA seropositivity in asymptomatic Bangladeshi children with Helicobacter pylori infection. Acta Paediatr. 2004;93:1432–1436. doi: 10.1080/08035250410033088. [DOI] [PubMed] [Google Scholar]
- 27.Rahman M, Mukhopadhyay AK, Nahar S, Datta S, Ahmad MM, Sarker S, Masud IM, Engstrand L, Albert MJ, Nair GB, et al. DNA-level characterization of Helicobacter pylori strains from patients with overt disease and with benign infections in Bangladesh. J Clin Microbiol. 2003;41:2008–2014. doi: 10.1128/JCM.41.5.2008-2014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laheij RJ, Straatman H, Jansen JB, Verbeek AL. Evaluation of commercially available Helicobacter pylori serology kits: a review. J Clin Microbiol. 1998;36:2803–2809. doi: 10.1128/jcm.36.10.2803-2809.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijer BC, Thijs JC, Kleibeuker JH, van Zwet AA, Berrelkamp RJ. Evaluation of eight enzyme immunoassays for detection of immunoglobulin G against Helicobacter pylori. J Clin Microbiol. 1997;35:292–294. doi: 10.1128/jcm.35.1.292-294.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung WK, Chan FK, Falk MS, Suen R, Sung JJ. Comparison of two rapid whole-blood tests for Helicobacter pylori infection in Chinese patients. J Clin Microbiol. 1998;36:3441–3442. doi: 10.1128/jcm.36.11.3441-3442.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham DY, Evans DJ Jr, Peacock J, Baker JT, Schrier WH. Comparison of rapid serological tests (FlexSure HP and QuickVue) with conventional ELISA for detection of Helicobacter pylori infection. Am J Gastroenterol. 1996;91:942–948. [PubMed] [Google Scholar]
- 32.Lepper PM, Moricke A, Vogt K, Bode G, Trautmann M. Comparison of different criteria for interpretation of immunoglobulin G immunoblotting results for diagnosis of Helicobacter pylori infection. Clin Diagn Lab Immunol. 2004;11:569–576. doi: 10.1128/CDLI.11.3.569-576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


