Abstract
AIM: To analyze the Hepatitis C virus (HCV) genotype distribution and transmission risk factors in a population of unselected patients in Luxembourg.
METHODS: Epidemiological information (gender, age and transmission risks) were collected from 802 patients newly diagnosed for hepatitis C and living in Luxembourg, among whom 228 patients referred from prison. Genotyping using 5’noncoding (5’NC) sequencing was performed. We compared categorical data using the Fisher’s exact F-test and odds ratios (OR) were calculated for evaluating association of HCV genotype and risk factors.
RESULTS: The sex ratio was predominantly male (2.2) and individuals aged less than 40 years represented 49.6% of the population. Genotype 1 was predominant (53.4%) followed by genotype 3 (33%). Among risk factors, intravenous drug usage (IVDU) was the most frequently reported (71.4%) followed by medical-related transmission (17.6%) including haemophilia, transfusion recipients and other nosocomial reasons. Genotype 3 was significantly associated to IVDU (OR = 4.84, P < 0.0001) whereas genotype 1 was significantly associated with a medical procedure (OR = 2.42, P < 0.001). The HCV genotype distribution from inmate patients differed significantly from the rest of the population (Chi-square test with four degrees of freedom, P < 0.0001) with a higher frequency of genotype 3 (46.5% vs 27.5%) and a lower frequency of genotype 1 and 4 (44.7% vs 56.8% and 5.3% vs 9.6%, respectively). IVDU was nearly exclusively reported as a risk factor in prison.
CONCLUSION: We report the first description of the HCV genotype distribution in Luxembourg. The repartition is similar to other European countries, with one of the highest European prevalence rates of genotype 3 (33%). Since serology screening became available in 1991, IVDU remains the most common way of HCV transmission in Luxembourg.
Keywords: Hepatitis C virus, Genotypes, Luxembourg, Risk factors, Substance abuse, Prisons
INTRODUCTION
Hepatitis C virus (HCV) is a major cause of chronic liver disease and hepatocellular carcinoma worldwide. The most recent WHO estimate of the prevalence of HCV infection is 2%, ranging from 0.6% to 2.3% in North America, Northern and Western Europe and Australia[1]. HCV has a high molecular diversity worldwide. A recent international standardization of the nomenclature proposed a classification into 6 genotypes (1 to 6) and more than 70 subtypes (termed a, b, c, d,…)[2].
Although genotypes 1, 2 and 3 are responsible for more than 90% of the infections in North and South America, Europe and Japan[3], prevalence and distribution peculiarities of HCV genotypes are linked to geographical location and mode of transmission[4]. Three broad patterns of genotype distribution have been identified to date[4]. One pattern, characterized by high genetic diversity, involves geographically discrete areas where HCV has been endemic for a long time such as West Africa with types 1 and 2, Central Africa with type 4, and Asia with types 3 and 6. Another pattern involves areas with a few subtypes circulating in specific risk groups, e.g. subtype 3a in drug addicts. The third pattern involves areas where a single subtype is present, such as in Egypt with subtype 4a and South Africa with subtype 5a.
Beside epidemiological considerations, HCV genotyping also has a clinical impact. Treatment for chronic hepatitis C includes standard or pegylated (PEG) interferon-α (IFN-α) in combination with ribavirin[5,6]. HCV genotype is a strong independent predictor of sustained virological response, which is defined by an undetectable HCV RNA level 24 wk after the end of treatment[7]. Genotypes 2 and 3 are significantly associated with a better therapeutic response, and knowledge on the HCV genotype is now used for tailoring therapeutic modalities: the duration of treatment is longer (48 wk) for genotype 1, 4, 5 and 6 than for genotype 2 and 3 (24 wk).
Up to now, no population-based study has been undertaken in Luxembourg and the precise prevalence rate of HCV infected people is not known. The National Council of Communicable Diseases in 2006 estimated the prevalence of HCV infection to be between 0.5% and 1%. Therefore, between 2250 and 4500 individuals might be infected in Luxembourg. Identifying risk factors is important to plan preventive activities and is necessary to target the higher risk population. HCV spreads in humans mainly through parenteral transmission. While uncontrolled medical procedures or transfusion were major causes of HCV transmission before serology screening was available, intravenous drug use (IVDU) has now become the predominant source of infections in developed countries[8]. Transmission of HCV also occurs through occupational, perinatal or sexual exposures, albeit with much less efficiency. Practices such as non-injection drug use (particularly via sharing of non-injecting drug equipment), tattooing or body piercing have also emerged as additional causes of HCV transmission[9].
The relation between incarceration and the high transmission of blood-borne viruses has been known for several years[10–12]. The HCV prevalence rate in prison is higher than in the general population because of the concentration of injecting drug users. HCV screening and genotyping was introduced in prisons of Luxembourg in 2002. These patients are usually referred to the Centre Hospitalier of Luxembourg (CHL) for medical treatment.
Because of its impact on patient management and treatment, we made a retrospective analysis of the HCV genotype distribution in a population of 802 unselected patients living in Luxembourg using 5’noncoding (5’NC) sequencing. We also analysed transmission risks related to infection. As almost 30% of the patients live in prison, we distinguished this population in our analysis. Our survey gives the first estimation of the distribution of HCV genotypes amongst unselected HCV patients in Luxembourg.
MATERIALS AND METHODS
Patients
Genotype and epidemiological information (gender and age) was collected from 802 patients newly diagnosed for hepatitis C and referred to the Centre Hospitalier of Luxembourg from 1991 to 2006. Among the overall population of 802 patients, a subset of 228 (28.4%) referred from prison (Centre Pénitentiaire of Luxembourg or CPL) has been distinguished from the remaining 574 patients, the latter being considered as general population. Among the 802 patients, 60 (7.6%) were also chronically co-infected with HIV-1 and 7 (0.9%) with HBV. Information regarding risk factors [intravenous drug usage (IVDU), medical reason (including transfusion and haemophilia or other nosocomial reasons), sexual transmission, mother-to-child transmission, occupational exposure, tattooing and body piercing] was available for 437 of the 802 patients (54.5%), and for 103 of the 228 patients (45.2%) of the CPL.
Methods
Detection of sera specific antibodies to HCV (ABBOTT Axsym HCV v3.0, Abbott, Louvain-la-Neuve, Belgium), antigen/antibodies to HIV (HIV Combo v4.0, Abbott) and surface HBV antigens (Ag HBs v2.0, Abbott) was performed with commercial kits. HCV viral load was determined with the quantitative COBAS AMPLICOR® HCV test v2.0 (Roche Diagnostics, Mannheim, Germany). Only samples with a viral load superior to 20 000 IU/mL were considered for genotyping. PCR products generated with the COBAS AMPLICOR® HCV test were collected for subsequent genotyping using the TRUGENE® HCV 5’NC methodology (Bayer s.a., Brussels, Belgium).
HCV genotype incidence
The incidence of HCV genotypes in our cohort has been calculated, per year, as the proportion of the number of new included genotypes divided by the total number of new included genotypes. Results are expressed as percentages. Patient inclusion in our cohort is based on the date of the first viral load analysis. Determination of the incidence has been performed from 1999 to 2006.
Statistical analysis
The Fisher’s exact test was used to compare categorical data such as genotype, age and gender. Odds ratios were calculated for evaluating association of HCV genotype with risk factor. Stratified analyses were conducted using the Mantel-Haenszel estimator for adjusting odds ratios with age and gender.
RESULTS
Gender and age
The sex ratio (SR) of the 802 investigated patients was predominantly male (2.2) (Table 1). Individuals aged less than 40 years, for whom a more recent time point of infection could be assumed, represented 49.6% of all patients. The population of HCV-infected patients at CPL was almost exclusively male (94.7%, SR = 17.8), with a significantly larger proportion of younger patients (77.6% patients aged less than 40 years, compared to 38.5% in the rest of the population, P < 0.0001).
Table 1.
Patient details and HCV genotype distribution in the overall population, in the Centre Pénitentiaire of Luxembourg (CPL) and in the general population
| Variable | Overall | CPL | General |
| n (%) | 802 (100) | 228 (28.4) | 574 (71.6) |
| Sex ratio | 2.2 | 17.8 | 1.44 |
| Age (% less than 40 yr) | 49.6 | 77.6 | 38.5 |
| Genotype | |||
| 1 | 428 (53.4) | 102 (44.7) | 326 (56.8) |
| 2 | 38 (4.7) | 8 (3.5) | 30 (5.2) |
| 3 | 264 (33) | 106 (46.5) | 158 (27.5) |
| 4 | 67 (8.3) | 12 (5.3) | 55 (9.6) |
| 5 | 5 (0.6) | 0 | 5 (0.9) |
Genotype distribution
Genotype 1 was predominant, with 428 patients (53.4%), followed by genotype 3 (33%), 4 (8.3%), 2 (4.6%) and 5 (0.6%) (Table 1). Among patients co-infected with HIV or HBV, the genotype distribution was similar to the overall population (data not shown). Regarding prison inmates (Table 1), genotypes 1 and 3 were predominant (44.7% and 46.5%, respectively), followed by genotype 4 (5.3%) and 2 (3.5%). The HCV genotype distribution in patients from CPL differed significantly (Chi-square test with four degrees of freedom, P < 0.0001), with a higher frequency of genotype 3 (46.5% vs 27.7%) and a lower frequency of genotype 1 and 4 (44.7% vs 56.8% and 5.7% vs 9.58%, respectively).
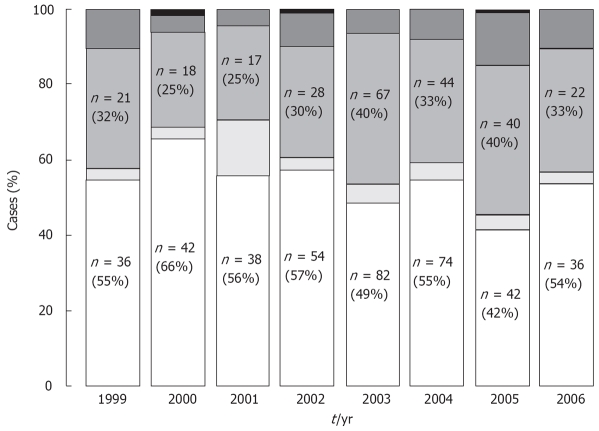
As shown in Figure 1, the analysis of HCV genotype incidence has been performed from 1999 to 2006. The rate of genotypes 1, 2, 3 and 4 was relatively constant over time, only isolated cases of genotype 5 were observed during this 7 years period.
Figure 1.
HCV genotype incidence from 1999 to 2006. The genotypes are indicated in the bars by different shades of grey, representing from bottom to top, genotypes 1, 2, 3, 4 and 5. Number of cases and percentages are mentioned for genotype 1 and 3. No significant changes in genotype distribution over time have been observed during this 7 years period.
Distribution of genotypes according to gender and age
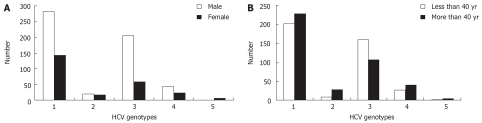
As described in Figure 2A, genotype 3 was significantly more frequent in males (69.1% vs 30.9%, P < 0.0001), while genotypes 2 and 5 were more frequent in females (P = 0.047 and P = 0.0027, respectively). The frequency of genotypes 1 and 4 were independent of gender and age (Figure 2A and B).
Figure 2.
Distribution of HCV genotypes. A: Distribution of HCV genotypes in patients according to gender. Genotype 3 is more frequent in male (P < 0.0001), genotype 2 and genotype 5 are more frequent in female (P = 0.047 and P = 0.0027 respectively); B: Distribution of HCV genotypes in patients according to age. Genotype 3 is associated to patients < 40 yr (P < 0.0001). Genotype 2 is associated to patients > 40 yr (P = 0.0064).
Genotype 3 was associated significantly with patients aged less than 40 years (60% vs 44.5% for patients aged more than 40 years, P < 0.0001) (Figure 2B). Conversely, Genotype 2 was significantly associated with patients aged more than 40 years (73% vs 49.3%, P = 0.0064).
Transmission risk factors
Among risk factors (Table 2), IVDU was the most frequently reported (71.4%), followed by medical-related transmission such as transfusion, haemophilia or other nosocomial reasons (17.6%, 77/437, of whom 68 cases through transfusion). 35 patients (4.7%) reported infection through sexual contact (homosexual, bisexual and heterosexual transmission). Other routes of transmission were also reported such as non-injection drug use (5 cases), occupational exposure (3 cases), tattooing (3 cases), mother-to-child transmission (1 case) and body piercing (1 case). IVDU was nearly exclusively reported as a risk factor for CPL (94.2% of the reported cases) (Table 2). Four inmate patients reported infection through medical procedure, one after sniffing and another one after tattooing. In the general population, the predominant source of infection remained IVDU (64.3%).
Table 2.
Transmission risk factors in the overall population, in the Centre Pénitentiaire of Luxembourg (CPL) and in the general population
| Risk factor | Overall | CPL | General |
| n (%) | 437 (100) | 104 (23.8) | 333 (76.2) |
| IVDU | 312 (71.4) | 98 (94.2) | 214 (64.3) |
| Medical-related | 77 (17.6) | 4 (3.8) | 73 (21.9) |
| Sexual | 35 (8) | 0 | 35 (10.5) |
| Other | 13 (3) | 2 (2) | 11 (3.3) |
Association of the genotypes and transmission risk factors
Odds ratios were determined for evaluating association of HCV genotype with risk factor in the overall population (Table 3). The odds ratio for association of IVDU and HCV genotype 3 was 4.84 (95% confidence intervals, 2.86 to 8.25; P < 0.0001). When adjusted for age and gender, IVDU patients were still significantly more likely than other patients to be infected with HCV genotype 3 (Mantel-Haenszel adjusted odds ratio = 4.54, 95% confidence intervals, 1.60 to 12.7; P = 0.0043). Conversely, IVDUs were at lower risk of being infected with HCV genotype 1 whether or not they were adjusted for age and gender.
Table 3.
Associations between genotype and transmission risk factors for the overall population
| Risk factor | IVDU | Medical-related |
| Odds ratios (OR) | ||
| Genotype 1 | 0.50 (0.33 to 0.77, P = 0.0016) | 2.42 (1.43 to 4.05, P = 0.0008) |
| Genotype 3 | 4.84 (2.86 to 8.25, P < 0.0001) | 0.17 (0.09 to 0.35, P < 0.0001) |
| MH adjusted OR1 | ||
| Genotype 1 | 0.49 (0.30 to 0.84, P = 0.0084) | 2.34 (1.35 to 4.06, P = 0.0025) |
| Genotype 3 | 4.54 (1.60 to 12.70, P = 0.0043) | 0.21 (0.07 to 0.64, P = 0.0064) |
| MH adjusted OR2 | ||
| Genotype 1 | 0.55 (0.35 to 0.85, P = 0.0081) | 2.24 (1.31 to 3.84, P = 0.0033) |
| Genotype 3 | 4.28 (2.42 to 7.57, P < 0.0001) | 0.20 (0.09 to 0.42, P < 0.0001) |
Odds ratio with confidence intervals 95%.
Mantel-Haenszel odds ratio adjusted for age and gender;
Mantel-Haenszel odds ratio adjusted for population type.
The odds ratio for association of medical procedure and HCV genotype 1 was 2.42 (95% confidence intervals, 1.43 to 4.05; P = 0.0008). When adjusted for age and gender, patients reporting infection through a medical procedure were still 2.34 as likely as other patients for contracting HCV genotype 1 (Mantel-Haenszel adjusted odds ratio = 2.34, 95% confidence intervals, 1.35 to 4.06; P = 0.0025).
Finally, when adjusted for population type, IVDU patients were still significantly more likely than other patients to be infected with HCV genotype 3 (Table 3).
DISCUSSION
We report here the first description of HCV molecular epidemiology in Luxembourg. This survey has been realized with a relatively confident case number of 802 unselected patients seen in routine practice, irrespective of their clinical background. The genotype distribution is similar to other European countries with yet one of the highest European prevalence of genotype 3 (33%). Injecting drug use has currently become the principal cause of HCV transmission.
HCV genotypes 1 and 3 were the most prevalent in our patients. In Europe, there have been dynamic changes over time in the prevalence of the different HCV subtypes. The prevalence of HCV 1b and 2 have decreased, while HCV 1a , 3a and 4 have increased[13–15]. Regarding incidence of genotypes in Luxembourg, we did not notice significant changes in the emergence of genotype 3 from 1999 to 2006. The genotype proportion, as observed in our cohort, has been relatively constant overtime. No new genotype has been massively introduced in Luxembourg despite strong progression of the immigration flow from 1985 to 2001. One reason might be that the majority of immigrants are coming from the European Union. Because of the few data available, we were not able to find any association of genotypes and demographic data for the patients. As for the several genotype 4 viruses identified in our cohort, we can assume that their introduction could be related to African immigration[16]. One limitation of our study is the methodology used for genotyping: the TRUGENE® HCV 5’NC method indeed does not allow for easy discrimination between HCV subtypes. Therefore, the apparent invariability of HCV molecular epidemiology may mask significant variability in terms of subtypes.
The high prevalence of HCV genotype 3 in our population is attributed to IVDU in the overall population and in particular in the incarcerated population. In similar studies from neighbouring countries, distribution rate of genotype 3 varied from 13.42% in Germany[14], 22%-26.3% in France[13,17] to 29.3% in the Netherlands[18]. In prison, injecting drug use is the most commonly reported risk factor[19–21]. In the rest of the population, the prevalence of genotype 3 is 27.5%. This rate is comparable with other studies[13,14,17,18] in which inclusion of prisoners was not specified. Other molecular epidemiology studies have shown that HCV subtype 3a is significantly associated with transmission through injecting drug use in industrialized countries[22–24] and explain the prevalence of subtype 3a in many North and South American and European countries. HCV subtype 3a originates from Asia and has spread widely among injecting drug users[25,26]. Mechanisms of subtype 3a transmission remain poorly defined. Genotype 3 was not associated with a high level of HCV RNA as compared to the others genotypes in our cohort. Nevertheless, the relative homogeneity of NS5B sequences of injecting drug users from France, South America, Australia and California have indicated that HCV subtype 3a has been transmitted from a common origin through a unique worldwide epidemic among the drug user communities[27]. In this study, the authors reported region-specific HCV 3a variants suggesting local transmission among intravenous drug users from the same geographical area. Therefore, the intrinsic characteristics of the transmission network of IVDU (unsafe injecting practices, drug preparation materials, needle or syringe exchange) together with the importance of the social context of drug injections may explain the efficiency of HCV genotype 3 transmission in Luxembourg.
Our study shows that the main risk factor for infection by genotype 3 is IVDU whereas the main risk factor for infection by genotype 1 is medical-related transmission. These data are in agreement with other studies in Europe[15,28–30]. Serology screening is routinely performed since the early 1990s and recent infections are more likely attributable to other risk factors rather than medical-related transmission (blood, blood transfusion or surgery). Interestingly, 4 patients from the general population reported infections through tattooing and body piercing. This finding reemphasizes the fact that practices using sharps in personal services are potential sources of spread of infection.
The age of infected individuals may be a direct variable for the genotype distribution. Nevertheless, our data did not show that patients infected by genotype 1 were older than 40 years. This would mean other routes of infection by genotype 1 such as IVDU. Since serology screening is available, IVDU remains the more common route of HCV transmission in Luxembourg. Genotype 3 is significantly associated with males aged less than 40 years which is consistent with the profile of drug injecting users. The male/female ratio of the IVDU population is 3:1 and the mean age of indexed intravenous drug users is about 30 years in Luxembourg. We found that genotype 2 is significantly associated with patients aged more than 40 years. These results may indicate that the type 2 infections were not recent but probably linked to some distant event like blood transfusion. Unfortunately, we could not find any correlation of this genotype with a route of transmission. In addition, we have also shown that genotype 2 and 5 are more frequent in female. To our knowledge, this association has never been reported and merits further investigation.
Incarcerated individuals have high rates of infectious diseases. The concentration of a marginal population in prison has necessitated systematic and rigorous screening for hepatitis B and hepatitis C. Consistent with similar studies worldwide, the prevalence of HCV in prison inmates in CPL (23.7% in 2006) is higher than in the general population suggesting probable transmission in prisons through intravenous drug use and unsafe sexual behaviour[12,21,31,32]. In CPL, the prevalence rate of HCV has increased from 19.7 % in 2001 to 23.7% in 2006. This increase is consistent with the continuous increase of infection rate in the IVDU population observed in Luxembourg as well as other European countries[33]. HCV among injecting drug users is one of the European Union's major public health problems. In 2004, statistics from our laboratory provided the proportion of 74% reported HCV infections with acute and chronic HCV infections in the IVDU population. Therefore, prisons provide a good location to offer HCV screening and treatment and to prevent future transmission. Sharing injection equipment as well as tattooing and piercing during incarceration among intravenous drug users are more frequent than in the community[34]. In this respect, a syringe/needle exchange program was implemented in CPL in 2005. Exchange is conducted through the medical unit and requires an initial evaluation by the prison doctors.
In conclusion, our study gives the first estimation of the genotype distribution in Luxembourg. Like in other countries from the European Union, IVDU is the driving force for HCV transmission in Luxembourg. Therefore, increased prevention efforts targeted to drug injection are urgently needed to curb the epidemic. HCV genotype distribution has to be followed in the future in order to improve the biological tests for diagnosis and the development of preventive procedures among IVDU or the prison population. As the genotype distribution has a direct impact on medical practice and treatment, it may require new therapeutic strategies in particular with regards to vaccine development.
COMMENTS
Background
Hepatitis C virus (HCV) has a high molecular diversity worldwide and is classified into 6 genotypes. Prevalence and distribution peculiarities of HCV genotypes are linked to geographical location and mode of transmission.
Research frontiers
Data on genotypic distribution from various areas are crucial to understand the spreading mode of HCV. Identifying risk factors is important to plan preventive activities and is necessary to target the higher risk population.
Innovations and breakthroughs
This report describes for the first time the HCV genotype distribution in Luxembourg. The repartition is similar to other European countries, with one of the highest European prevalence rates of genotype 3 (33%) which is associated to intravenous drug usage.
Applications
HCV among injecting drug users is one of the European Union’s major public health problems. Mechanisms of genotype 3 transmission remain poorly defined and need to be investigated to explain the efficiency of HCV genotype 3 transmission in Luxembourg.
Peer review
This article is interesting. The authors have provided valuable information about epidemiological aspects of HCV genotype distribution in Luxembourg with nice connections to the similar studies worldwide.
Supported by The “Centre de Recherche Public-Santé” (CRP-Santé, project LRV-REC-06-01)
Peer reviewers: Natalia A Osna, Professor, Liver Study Unit, Research Service (151), VA Medical Center, 4101 Woolworth Avenue, Omaha NE 68105, United States; Yukihiro Shimizu, Dr, Kyoto Katsura Hospital, 17 Yamada-Hirao, Nishikyo, Kyoto 615-8256, Japan
S- Editor Yang RH L- Editor Alpini GD E- Editor Lu W
References
- 1.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, Halfon P, Inchauspe G, Kuiken C, Maertens G, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 3.Smith DB, Pathirana S, Davidson F, Lawlor E, Power J, Yap PL, Simmonds P. The origin of hepatitis C virus genotypes. J Gen Virol. 1997;78(Pt 2):321–328. doi: 10.1099/0022-1317-78-2-321. [DOI] [PubMed] [Google Scholar]
- 4.Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, Beall E, Yap PL, Kolberg J, Urdea MS. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74(Pt 11):2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 5.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 7.Pawlotsky JM. Treating hepatitis C in “difficult-to-treat” patients. N Engl J Med. 2004;351:422–423. doi: 10.1056/NEJMp048068. [DOI] [PubMed] [Google Scholar]
- 8.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 9.Scheinmann R, Hagan H, Lelutiu-Weinberger C, Stern R, Des Jarlais DC, Flom PL, Strauss S. Non-injection drug use and Hepatitis C Virus: a systematic review. Drug Alcohol Depend. 2007;89:1–12. doi: 10.1016/j.drugalcdep.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalan-Soares BC, Almeida RT, Carneiro-Proietti AB. Prevalence of HIV-1/2, HTLV-I/II, hepatitis B virus (HBV), hepatitis C virus (HCV), Treponema pallidum and Trypanosoma cruzi among prison inmates at Manhuacu, Minas Gerais State, Brazil. Rev Soc Bras Med Trop. 2000;33:27–30. doi: 10.1590/s0037-86822000000100004. [DOI] [PubMed] [Google Scholar]
- 11.Heimberger TS, Chang HG, Birkhead GS, DiFerdinando GD, Greenberg AJ, Gunn R, Morse DL. High prevalence of syphilis detected through a jail screening program. A potential public health measure to address the syphilis epidemic. Arch Intern Med. 1993;153:1799–1804. [PubMed] [Google Scholar]
- 12.Stark K, Bienzle U, Vonk R, Guggenmoos-Holzmann I. History of syringe sharing in prison and risk of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection among injecting drug users in Berlin. Int J Epidemiol. 1997;26:1359–1366. doi: 10.1093/ije/26.6.1359. [DOI] [PubMed] [Google Scholar]
- 13.Payan C, Roudot-Thoraval F, Marcellin P, Bled N, Duverlie G, Fouchard-Hubert I, Trimoulet P, Couzigou P, Cointe D, Chaput C, et al. Changing of hepatitis C virus genotype patterns in France at the beginning of the third millenium: The GEMHEP GenoCII Study. J Viral Hepat. 2005;12:405–413. doi: 10.1111/j.1365-2893.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 14.Ross RS, Viazov S, Renzing-Kohler K, Roggendorf M. Changes in the epidemiology of hepatitis C infection in Germany: shift in the predominance of hepatitis C subtypes. J Med Virol. 2000;60:122–125. [PubMed] [Google Scholar]
- 15.Savvas SP, Koskinas J, Sinani C, Hadziyannis A, Spanou F, Hadziyannis SJ. Changes in epidemiological patterns of HCV infection and their impact on liver disease over the last 20 years in Greece. J Viral Hepat. 2005;12:551–557. doi: 10.1111/j.1365-2893.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 16.Simmonds P, McOmish F, Yap PL, Chan SW, Lin CK, Dusheiko G, Saeed AA, Holmes EC. Sequence variability in the 5' non-coding region of hepatitis C virus: identification of a new virus type and restrictions on sequence diversity. J Gen Virol. 1993;74(Pt 4):661–668. doi: 10.1099/0022-1317-74-4-661. [DOI] [PubMed] [Google Scholar]
- 17.Bourliere M, Halfon P, Portal I. Treatment of chronic hepatitis C in special groups. Gastroenterol Clin Biol. 2002;26 Spec No 2:B238–B247. [PubMed] [Google Scholar]
- 18.de Vries MJ, te Rijdt B, van Nieuwkerk CM. Genotype distribution amongst hepatitis C patients in The Netherlands. Neth J Med. 2006;64:109–113. [PubMed] [Google Scholar]
- 19.Alizadeh AH, Alavian SM, Jafari K, Yazdi N. Prevalence of hepatitis C virus infection and its related risk factors in drug abuser prisoners in Hamedan--Iran. World J Gastroenterol. 2005;11:4085–4089. doi: 10.3748/wjg.v11.i26.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massad E, Rozman M, Azevedo RS, Silveira AS, Takey K, Yamamoto YI, Strazza L, Ferreira MM, Burattini MN, Burattini MN. Seroprevalence of HIV, HCV and syphilis in Brazilian prisoners: preponderance of parenteral transmission. Eur J Epidemiol. 1999;15:439–445. doi: 10.1023/a:1007523027876. [DOI] [PubMed] [Google Scholar]
- 21.Taylor A, Goldberg D, Hutchinson S, Cameron S, Gore SM, McMenamin J, Green S, Pithie A, Fox R. Prevalence of hepatitis C virus infection among injecting drug users in Glasgow 1990-1996: are current harm reduction strategies working? J Infect. 2000;40:176–183. doi: 10.1053/jinf.2000.0647. [DOI] [PubMed] [Google Scholar]
- 22.Bourliere M, Barberin JM, Rotily M, Guagliardo V, Portal I, Lecomte L, Benali S, Boustiere C, Perrier H, Jullien M, et al. Epidemiological changes in hepatitis C virus genotypes in France: evidence in intravenous drug users. J Viral Hepat. 2002;9:62–70. doi: 10.1046/j.1365-2893.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 23.McCaw R, Moaven L, Locarnini SA, Bowden DS. Hepatitis C virus genotypes in Australia. J Viral Hepat. 1997;4:351–357. doi: 10.1046/j.1365-2893.1997.00060.x. [DOI] [PubMed] [Google Scholar]
- 24.Pawlotsky JM, Tsakiris L, Roudot-Thoraval F, Pellet C, Stuyver L, Duval J, Dhumeaux D. Relationship between hepatitis C virus genotypes and sources of infection in patients with chronic hepatitis C. J Infect Dis. 1995;171:1607–1610. doi: 10.1093/infdis/171.6.1607. [DOI] [PubMed] [Google Scholar]
- 25.Greene WK, Cheong MK, Ng V, Yap KW. Prevalence of hepatitis C virus sequence variants in South-East Asia. J Gen Virol. 1995;76(Pt 1):211–215. doi: 10.1099/0022-1317-76-1-211. [DOI] [PubMed] [Google Scholar]
- 26.Lole KS, Jha JA, Shrotri SP, Tandon BN, Prasad VG, Arankalle VA. Comparison of hepatitis C virus genotyping by 5' noncoding region- and core-based reverse transcriptase PCR assay with sequencing and use of the assay for determining subtype distribution in India. J Clin Microbiol. 2003;41:5240–5244. doi: 10.1128/JCM.41.11.5240-5244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morice Y, Cantaloube JF, Beaucourt S, Barbotte L, De Gendt S, Goncales FL, Butterworth L, Cooksley G, Gish RG, Beaugrand M, et al. Molecular epidemiology of hepatitis C virus subtype 3a in injecting drug users. J Med Virol. 2006;78:1296–1303. doi: 10.1002/jmv.20692. [DOI] [PubMed] [Google Scholar]
- 28.Balogun MA, Laurichesse H, Ramsay ME, Sellwood J, Westmoreland D, Paver WK, Pugh SF, Zuckerman M, Pillay D, Wreghitt T. Risk factors, clinical features and genotype distribution of diagnosed hepatitis C virus infections: a pilot for a sentinel laboratory-based surveillance. Commun Dis Public Health. 2003;6:34–39. [PubMed] [Google Scholar]
- 29.Dal Molin G, Ansaldi F, Biagi C, D’Agaro P, Comar M, Croce L, Tiribelli C, Campello C. Changing molecular epidemiology of hepatitis C virus infection in Northeast Italy. J Med Virol. 2002;68:352–356. doi: 10.1002/jmv.10210. [DOI] [PubMed] [Google Scholar]
- 30.Kleter B, Brouwer JT, Nevens F, van Doorn LJ, Elewaut A, Versieck J, Michielsen PP, Hautekeete ML, Chamuleau RA, Brenard R, et al. Hepatitis C virus genotypes: epidemiological and clinical associations. Benelux Study Group on Treatment of Chronic Hepatitis C. Liver. 1998;18:32–38. [PubMed] [Google Scholar]
- 31.Mutter RC, Grimes RM, Labarthe D. Evidence of intraprison spread of HIV infection. Arch Intern Med. 1994;154:793–795. [PubMed] [Google Scholar]
- 32.Skoretz S, Zaniewski G, Goedhuis NJ. Hepatitis C virus transmission in the prison/inmate population. Can Commun Dis Rep. 2004;30:141–148. [PubMed] [Google Scholar]
- 33.Muga R, Sanvisens A, Bolao F, Tor J, Santesmases J, Pujol R, Tural C, Langohr K, Rey-Joly C, Munoz A. Significant reductions of HIV prevalence but not of hepatitis C virus infections in injection drug users from metropolitan Barcelona: 1987-2001. Drug Alcohol Depend. 2006;82 Suppl 1:S29–S33. doi: 10.1016/s0376-8716(06)80005-0. [DOI] [PubMed] [Google Scholar]
- 34.Hilton BA, Thompson R, Moore-Dempsey L, Janzen RG. Harm reduction theories and strategies for control of human immunodeficiency virus: a review of the literature. J Adv Nurs. 2001;33:357–370. doi: 10.1046/j.1365-2648.2001.01672.x. [DOI] [PubMed] [Google Scholar]