Abstract
AIM: To evaluate the effects of antiviral agents and HBV genotypes on intrahepatic covalently closed circular DNA (ccc DNA) in HBeAg-positive chronic hepatitis B patients.
METHODS: Seventy-one patients received lamivudine (n = 35), or sequential therapy with lamivudine- interferon alpha 2b (IFN-α 2b, n = 24) for 48 wk, or IFN-α 2b (n = 12) for 24 wk. All subjects were followed up for 24 wk. Intrahepatic ccc DNA was measured quantitatively by PCR. HBV genotypes were analyzed by PCR-RFLP.
RESULTS: Sequential lamivudine- INF-α therapy, lamivudine and INF-α monotherapy reduced ccc DNA of 1.7 log, 1.4 log and 0.8 log, respectively (P < 0.05). Seventeen out of the 71 patients developed HBeAg seroconversion, the reduction of ccc DNA in the HBeAg seroconversion patients was more significant than that in the HBeAg positive patients (3.0 log vs 1.6 log, P = 0.0407). Twenty-four weeks after antiviral therapy withdrawal, 16 patients had a sustained virological response, the baseline intrahepatic ccc DNA in the patients with a sustained virological response was significantly lower than that in the patients with virological rebound (4.6 log vs 5.4 log, P = 0.0472). HBV genotype C accounted for 85.9% (n = 61), and genotype B for 14.1% (n = 10), respectively, in the 71 patients. There was no significant difference in the change of ccc DNA level between HBV genotypes C and B (2.1 log vs 1.9 log).
CONCLUSION: Forty-eight week sequential lamivudine-INF-α therapy and lamivudine monotherapy reduce ccc DNA more significantly than 24-wk INF-α monotherapy. Low baseline intrahepatic ccc DNA level may predict the long-term efficacy of antiviral treatment. HBV genotypes C and B have no obvious influence on ccc DNA load.
Keywords: Covalently closed circular DNA, Hepatitis B virus, Sequential therapy, Lamivudine, Interferon
INTRODUCTION
Hepatitis B virus (HBV) is one of the major causes for liver disease, more than 350 million people are infected with HBV, and eventually 15% to 25% of these individuals will progress to liver cirrhosis and hepatocellular carcinoma[1]. Interferon-alpha (IFN-α)[2–4] and nucleoside analogue agents[5–8] are the currently available antiviral drugs for the treatment of patients with chronic hepatitis B. Long-term anti-viral treatment can decrease the occurrence rate of hepatocellular carcinoma in patients with chronic hepatitis B, thus improving their survival. Nonetheless, after withdrawal of treatment, relapse occurs in the great majority of patients. Furthermore, relapse may lead to severe exacerbation of the disease, causing hepatic failure and even death. The main reason for the rebound of HBV DNA to its pretreatment level after antiviral treatment withdrawal is that these agents have a profound effect on relaxed circular DNA (rc DNA) and almost no effect on covalently closed circular DNA (ccc DNA)[9–11]. ccc DNA provides the template for the transcription of all viral genes. After hepatocytes are infected with HBV, covalently closed circular DNA is formed through DNA repair of the relaxed circular replicative HBV DNA inside the nuclei of hepatocytes[12]. DNA-containing nucleocapsids can either recycle back to the nuclei to amplify and maintain the pool of ccc DNA or become enveloped and are secreted into the blood[13]. Since ccc DNA is required for the maintenance of HBV infection, it must be eliminated for viral clearance to present. Currently, ccc DNA in human peripheral blood mononuclear cells and liver biopsies can be quantitated [14–16]. Thus, it is possible to measure the intrahepatic ccc DNA level for monitoring antiviral treatment.
The aim of the present study was to analyze the effects of sequential lamivudine-INF-α therapy, lamivudine monotherapy and INF-α monotherapy on intrahepatic ccc DNA. The relationship between intrahepatic ccc DNA and HBV genotypes was also observed.
MATERIALS AND METHODS
Subjects
Between March 2003 and March 2005, seventy-one patients with HBeAg -positive chronic hepatitis B were recruited, including 59 males and 12 females aged 19-47 (mean 32 ± 9) years. Informed consent was obtained from all involved patients. The inclusion criteria for the patients were as follows: positive for HBsAg and HBeAg, serum HBV DNA ≥ 1 × 105 copies/L, serum alanine aminotransferase (ALT) levels at least two-fold higher than the normal range (normal range = 0-667 nkat/L) for more than 6 mo. Exclusion criteria were as follows: patients with alcoholism, pregnancy, cirrhosis, chronic renal failure, concurrent autoimmune disease, severe neurological disorders, human immunodeficiency virus (HIV) infection and viral hepatitis A, C, delta or E. Patients treated with interferon or other antiviral therapies for 6 mo prior to enrollment in this study were also excluded. Patients were randomized (at the ratio 3:2:1) to receive 48 wk of 100 mg lamivudine once daily (n = 35), or lamivudine from the first to the 8th mo and IFN-α from the 7th to the 12th mo (n = 24), or 24 wk of 5 million units of interferon-alpha (IFN-α) three times per week (n = 12). All subjects were followed up for 24 wk after antiviral therapy. Presence of serum HBV DNA levels ≤ 1 × 103 and normalization of serum ALT levels were assessed as treatment response.
Measurement of HBV markers and genotypes and biochemical tests
Blood samples were obtained before, immediately after treatment and at wk 24 of follow-up. Liver biochemistry and HBV marker test (using commercial assays, Abbott Laboratories, USA) were performed on these samples.
Liver biopsy specimens were collected by needle liver biopsies (0.5-1.5 cm) before and immediately after treatment. The tissue was washed several times in cold phosphate-buffered saline (PBS) and stored at -70°C. Total DNA was extracted from liver tissue with the Qiaamp DNA tissue Mini DNA kit (Qiagen, Germany). Serum and intrahepatic HBV DNA were measured quantitatively by real-time polymerase chain reaction (PCR) (Model 5700, ABI Company, USA) with a lower limit of detection of 1 × 103 HBV DNA copies/mL and 1 × 103 copies/μg total DNA, respectively. HBV genotypes were determined by PCR restriction fragment length polymorphism (PCR- RFLP) analysis as previously described[17].
PCR amplification for CCC DNA
The primers were synthesized for amplifying ccc DNA according to the published consensus sequence. Sequences of the primers and the probe are as follows: 5’-ATACGGGTCAATGTCCATGC-3’ (nt 1551, sense), 5’-CCGTCTGTGCCTTCTCATCT-3’ (nt 1991, antisense), and 5’-CCAAAGCCACCCAAGGCACA-3’ (nt 1880, probe).
PCR was performed at 94°C for 2 min, followed by 45 cycles at 94°C for 20 s, at 60°C for 1 min.
Histology measurement
Histologic inflammation was scored with a modified Knodell scoring system[18]. Histologic response was defined as a decrease by at least two points in the Knodell scoring system.
Statistical analysis
Data were analyzed with the Statistical Program for Social Sciences (SPSS 13.0 for Windows). Categorical variables were tested using chi-square test or Fisher’s exact test. Normally distributed variables were tested using t-test or ANOVA, whereas continuous variables with skewed distributions were tested using the Kruskal Wallis test. Correlation between two variables was tested using Pearson’s correlation analysis after logarithmic transformation of data with skewed distributions. P < 0.05 was considered statistically significant.
RESULTS
There was no significant difference in all the parameters among the three groups before treatment. The antiviral outcome of 48-wk sequential lamivudine - INF-α therapy was similar to that of 48-wk lamivudine monotherapy, but slightly better than that of 24-wk INF-α monotherapy. The reduction in serum HBV DNA, intrahepatic HBV DNA and ccc DNA after sequential lamivudine - INF-α therapy and lamivudine monotherapy was greater than that after INF-α monotherapy (P < 0.05) (Table 1).
Table 1.
Demographic, histological, biochemical, virological data obtained from three antiviral therapies (mean ± SD)
| Parameter | Sequential lamivudine -INF-α therapy | Lamivudine monotherapy | Interferon monotherapy |
| Number of patients | 24 | 35 | 12 |
| Sex ratio (male/female) | 21/3 | 29/6 | 9/3 |
| Mean age (y) | 33 ± 6 | 32 ± 5 | 31 ± 8 |
| Mean Knodell score | |||
| pretreatment | 8.9 ± 3.6 | 8.2 ± 4.5 | 7.1 ± 4.0 |
| post-treatment | 6.6 ± 4.0a | 5.6 ± 3.2a | 5.0 ± 2.8 |
| Mean ALT level (nkat/L) | |||
| Pretreatment | 3423 ± 2388 | 4390 ± 2454 | 3006 ± 1720 |
| Post-treatment | 668 ± 400a | 667 ± 384a | 1686 ± 1286a |
| Serum HBV DNA (log10) | |||
| Pretreatment | 7.5 ± 1.3 | 7.7 ± 0.8 | 7.8 ± 1.1 |
| Post-treatment | 3.8 ± 1.0a | 3.7 ± 1.2a | 5.0 ± 1.5ac |
| Intrahepatic HBV DNA (log10) | |||
| Pretreatment | 5.8 ± 1.2 | 6.2 ± 0.9 | 6.3 ± 0.9 |
| Post-treatment | 4.7 ± 1.1a | 4.6 ± 1.5a | 5.6 ± 1.5ac |
| Intrahepatic ccc DNA (log10) | |||
| Pretreatment | 5.1 ± 1.0 | 5.2 ± 1.2 | 5.8 ± 1.2 |
| Post-treatment | 3.4 ± 1.3a | 3.8 ± 1.1a | 5.0 ± 1.5c |
| HBeAg seroconversion | |||
| rate at the treatment completion | 8/21 (38.1%) | 6/32 (18.6%) | 3/12 (25%) |
| Sustained virological response | |||
| rate at 24 wk off treatment | 4/16 (25%) | 13/31 (41.9%) | 1/1 (100%) |
P < 0.05 vs post-treatment,
P < 0.001 vs sequential lamivudine - INF-α therapy and lamivudine monotherapy. HBeAg seroconversion rate at the end of treatment: some post-treatment data were lost, ALT: Alanine aminotransferase, ccc DNA: Covalently closed circular DNA.
Data on histology, biochemistry and virology in HBeAg seroconversion patients and HBeAg positive patients
After antiviral treatment, the means of Knodell score, serum HBV DNA levels, intrahepatic HBV DNA levels, intrahepatic ccc DNA and serum ALT levels in all the 71 patients were significantly decreased (P < 0.05). Of the 71 patients, 17 developed HBeAg seroconversion. There was no significant difference in the parameters mentioned above between the HBeAg seroconversion patients and HBeAg positive patients before treatment, except for the baseline intrahepatic HBV DNA levels (5.6 log vs 6.3 log, P = 0.02). The HBeAg seroconversion group had greater improvements than the HBeAg positive group. The mean of intrahepatic HBV DNA decreased to (4.1 ± 0.8) log10 in the seroconversion group (P = 0.0124) and to (5.1 ± 1.5) log10 (P = 0.0872) in the HBeAg positive group. The mean of intrahepatic ccc DNA decreased from (5.8 ± 0.6) log10 to (2.8 ± 1.3) log10 in the HBeAg seroconversion group, and from (5.5 ± 1.1) log10 to (3.9 ± 1.1) log10 in the HBeAg positive group (P = 0.0407, Table 2).
Table 2.
Histological, biochemical and virological data on different therapy responses (mean ± SD)
| Parameter | HBeAg positive | HbeAg serocon -version | Sustained virological response | Virological flares |
| Number of patients | 48 | 17 | 18 | 30 |
| Mean Knodell score | ||||
| Pretreatment | 8.4 ± 4.1 | 7.1 ± 4.2 | 8.0 ± 4.0 | 8.4 ± 3.9 |
| Post-treatment | 6.0 ± 3.6 | 5.1 ± 3.1a | 6.1 ± 3.0 | 5.2 ± 2.7a |
| Mean ALT level (nkat/L) | ||||
| Pretreatment | 3206 ± 2338 | 3841 ± 2371 | 3340 ± 2638 | 4576 ± 2505 |
| Post-treatment | 1018 ± 985 | 618 ± 384a | 517 ± 300 | 601 ± 400 |
| 24 wk off- treatment | 851 ± 200 | 380 ± 183e | ||
| Serum HBV DNA (log10) | ||||
| Pretreatment | 7.9 ± 0.9 | 7.5 ± 1.0 | 7.3 ± 1.3 | 7.6 ± 0.8 |
| Post-treatment | 4.4 ± 1.4a | 3.0 ± 0.2ac | 3.1 ± 0.3a | 3.3 ± 0.4a |
| 24 wk off-treatment | 3.2 ± 0.3 | 6.1 ± 0.8ac | ||
| Intrahepatic HBV DNA (log10) | ||||
| Pretreatment | 6.3 ± 0.8 | 5.6 ± 1.2 | 5.9 ± 0.8 | 6.0 ± 1.1 |
| Post-treatment | 5.1 ± 1.5 | 4.1 ± 0.8a | 4.5 ± 1.6 | 4.3 ± 1.0a |
| Intrahepatic ccc DNA (log10) | ||||
| Pretreatment | 5.5 ± 1.1 | 5.8 ± 1.0 | 4.6 ± 1.0 | 5.4 ± 0.8e |
| Post-treatment | 3.9 ± 1.5 | 2.8 ± 1.3ac | 4.0 ± 0.9 | 3.8 ± 0.8a |
P < 0.05 vs post-treatment,
P < 0.05 vs HBeAg positive,
P < 0.05 vs sustained virological response. ALT: Alanine aminotransferase; ccc DNA: Covalently closed circular DNA.
Data on histology, biochemistry and virology in patients with different long-term responses
In the 24-wk follow-up period, of the 48 patients with virological response at the end of therapy, 18 achieved a sustained virological response, and 30 presented virological rebound. The means of intrahepatic and serum HBV DNA loads, histology and serum ALT level in the patients with a sustained virological response were similar to those in the patients with virological rebound before and after treatment. The baseline intrahepatic ccc DNA level in the patients with a sustained virological response was, however, significantly lower than that in the patients with virological rebound (P = 0.0472), but no significant difference was found at the end of treatment between the two groups (Table 2).
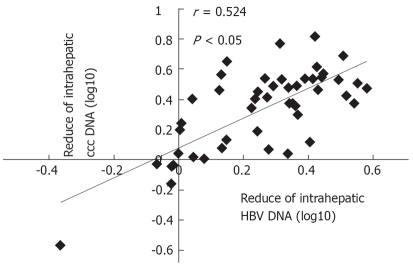
The mean of HBV ccc DNA in the 71 patients before and after treatment was (6.83 ± 0.37) log and (5.72 ± 0.23) log, respectively (P > 0.05). The change in intrahepatic ccc DNA had a positive correlation with a similar reduction in intrahepatic HBV DNA (r = 0.525, P < 0.05, Figure 1). The ccc DNA level was correlated with the serum HBeAg titer at the end of treatment (r = 0.432, P = 0.002, Figure 2).
Figure 1.
Correlation between the reduction in intrahepatic ccc DNA and a similar reduction in intrahepatic HBV DNA.
Figure 2.
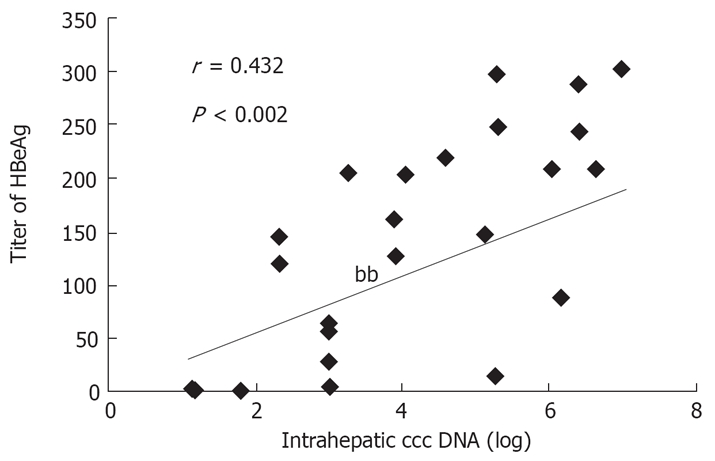
Relationship between intrahepatic ccc DNA level and HBeAg titer at the end of treatment.
Intrahepatic ccc DNA level and HBV genotype
HBV genotype C accounted for 85.9% (n = 61), and genotype B for 14.1% (n = 10) in the 71 patients. The mean of intrahepatic HBV DNA loads was (6.1 ± 0.9) log10 and (4.9 ± 1.4) log10, respectively (P < 0.05), and that of intrahepatic ccc DNA loads was (5.6 ± 1.1) log10 and (3.5 ± 1.5) log10, respectively, in the genotype C patients before and after treatment (P < 0.05). The baseline and post-treatment intrahepatic HBV DNA level was (5.6 ± 1.5) log10 and (4.7 ± 1.2) log10, respectively (P > 0.05), and that of intrahepatic ccc DNA loads was (5.8 ± 0.6) log10 and (3.9 ± 1.9) log10, respectively, in the genotype B patients (P = 0.064). No statistically significant difference was found in serum intrahepatic HBV DNA loads and ALT levels between the two groups after treatment. There was no significant difference in serum HBV DNA and alanine aminotransferase levels at 24 wk after treatment withdrawal, the sustained virological response rate and ALT normalization rate were also similar (Table 3).
Table 3.
Histological, biochemical and virological data on HBV genotypes C and B (mean ± SD)
| Parameter | HBV Genotype C | HBV Genotype B |
| Number of patients | 61 | 10 |
| Sex ratio (male/female) | 50/11 | 9/1 |
| Number of Sequential therapy | 20 (32.8%) | 4 (40%) |
| Number of Lamivudine therapy | 31 (50.8%) | 4 (40%) |
| Number of Interferon therapy | 10 (16.4%) | 2 (20%) |
| Mean Knodell score | ||
| Pretreatment | 8.1 ± 4.1 | 5.7 ± 3.2 |
| Post-treatment | 6.0 ± 3.4a | 5.4 ± 3.1 |
| Mean ALT level (nkat/L) | ||
| Pretreatment | 3390 ± 2438 | 2438 ± 2296 |
| Post-treatment | 901 ± 918 | 551 ± 233 |
| 24 wk off-treatment | 1503 ± 1336 | 1987 ± 1720 |
| Serum HBV DNA (log10) | ||
| Pretreatment | 7.7 ± 1.2 | 7.6 ± 0.8 |
| Post-treatment | 4.05 ± 1.3a | 4.9 ± 1.2a |
| 24 wk off-treatment | 5.1 ± 1.6 | 5.3 ± 1.3 |
| Intrahepatic HBV DNA (log10) | ||
| Pretreatment | 6.1 ± 0.9 | 5.6 ± 1.5 |
| Post-treatment | 4.9 ± 1.4a | 4.7 ± 1.2a |
| Intrahepatic ccc DNA (log10) | ||
| Pretreatment | 5.6 ± 1.1 | 5.8 ± 0.5 |
| Post-treatment | 3.5 ± 1.2a | 3.9 ± 1.9 |
| Sustained virological response rate at | ||
| 24 wk off-treatment | 29.4% (15/51) | 30% (3/10) |
| ALT normalization rate at | ||
| 24 wk off-treatment | 39.3% (24/51) | 30% (3/10) |
P < 0.05 vs post-treatment. ALT: Alanine aminotransferase; ccc DNA: Covalently closed circular DNA.
DISCUSSION
It was reported that lamivudine reduces serum ccc DNA levels by magnitude of 2 log[19]. Forty-eight weeks after adefovir dipivoxil therapy resulted in a significant decrease of 0.8 log in ccc DNA copies/cell and changes in ccc DNA are correlated with a similar reduction in serum HBsAg titer[20]. A clinical study from Hong Kong reported that 48 wk treatment with entecavir (n = 21) and lamivudine (n = 19) can reduce serum viral load, intrahepatic total HBV DNA, and ccc DNA of about 4.8 logs, 2 logs, and 1 log, respectively[21]. In our study, 48 wk after sequential lamivudine - INF-α therapy (n = 24) and lamivudine monotherapy (n = 35), and 24 wk after INF-α monotherapy (n = 12) reduced serum HBV DNA of about 3.7 logs, 4 logs, and 2.8 log, intrahepatic total HBV DNA of about 1.1 logs, 1.6 logs and 0.7 log, ccc DNA of about 1.7 logs, 1.4 logs and 0.8 log, respectively (P < 0.05), indicating that either sequential lamivudine - INF-α therapy or lamivudine therapy significantly reduces HBV DNA and ccc DNA than INF-α monotherapy, and the relatively short duration of INF-α therapy may be responsible for such a difference.
Since serum HBV DNA and alanine aminotransferase levels often flare up after antiviral therapy even if the patients develop HBeAg seroconversion, it is important to determine what the parameters help assess the success of treatment and decide how long the therapy should be. The present data indicate that 17 out of the 71 patients developed HBeAg seroconversion after antiviral therapy, the change of ccc DNA in the HBeAg seroconversion patients was more significant than that in the HBeAg positive patients (3.0 log vs 1.6 log, P = 0.0407), suggesting that reduction in ccc DNA is crucial for chronic hepatitis patients to achieve better antiviral efficacy. Twenty-four weeks after antiviral therapy withdrawal, 18 patients achieved a sustained virological response. The baseline and post-treatment intrahepatic HBV DNA and serum HBV DNA levels in these patients were similar to those in the patients with virological rebound, except for the baseline level of intrahepatic ccc DNA (4.6 log vs 5.4 log, P = 0.0472), showing that a lower baseline intrahepatic ccc DNA helps chronic hepatitis patients to maintain a sustained virological response.
As previously reported[15,20], the data obtained from our study show that the reduction in intrahepatic ccc DNA was positively correlated with the change in intrahepatic HBV DNA (r = 0.525, P < 0.05), and the ccc DNA level was correlated with the serum HBeAg titer at 48 wk after treatment (r = 0.432, P = 0.002). Since liver tissue is not accessible to obtain for measuring intrahepatic ccc DNA, serum HBeAg titer may be used for assessing the intrahepatic ccc DNA load.
Eight genotypes (A-H) have been found in HBV, each showing a distinct geographical distribution and disease progression. HBV genotype C is believed to be associated with a higher risk of reactivation and progression to cirrhosis compared to HBV genotype B[22,23]. Whether hepatitis B virus (HBV) genotypes influence the response to antiviral treatment remains controversial, and the relationship between HBV genotypes and ccc DNA is still unknown. It was reported that HBV genotypes do not influence the development of resistance to lamivudine, but the severity of liver disease[24]. In the present study, 85.9% of patients were infected with HBV genotype C and 14.1% with HBV genotype B. There was no significant difference in serum HBV DNA level, intrahepatic HBV DNA and ccc DNA load, as well as in biochemical and histological findings before and after treatment between the two groups. Twenty-four weeks after treatment withdrawal, sustained virological response rate and ALT normalization rate were very similar (P > 0.05), indicating that HBV genotypes (B and C) have almost no effect on intrahepatic HBV DNA, ccc DNA load and the long-term efficacy of antiviral therapy.
In conclusion, 48-wk sequential lamivudine - INF-α therapy and lamivudine therapy can significantly reduce HBV DNA and ccc DNA compared to 24-wk INF-α monotherapy. Low baseline intrahepatic ccc DNA level may predict the long-term efficacy of antiviral treatment. Reduction in intrahepatic ccc DNA is positively correlated with the change in intrahepatic HBV DNA, and the ccc DNA level is correlated with the serum HBeAg titer. Intrahepatic ccc DNA level is not significantly affected by the HBV genotype. Since the sample was not large enough, well-designed and placebo-controlled studies are needed.
COMMENTS
Background
Little effect of antiviral agents on ccc DNA is the main reason for the rebound of HBV DNA after antiviral treatment. It is, therefore, important to measure intrahepatic ccc DNA for monitoring antiviral treatment. The aim of this study was to evaluate the effects of antiviral agents and HBV genotypes on intrahepatic ccc DNA in HBeAg-positive chronic hepatitis B patients.
Research frontiers
After antiviral treatment, relapse occurs in the great majority of chronic hepatitis B patients, and the main reason for the rebound of HBV DNA to its pretreatment level is that the currently available antiviral agents have little effect on ccc DNA (covalently closed circular DNA). Since ccc DNA has been found mainly in liver tissues, how it works or changes due to the action of antiviral agents remains unclear. If it could be taken as a parameter for monitoring antivirus treatment need further study.
Innovations and breakthroughs
It was a first-hand report about the effect of INF-α monotherapy and HBV genotypes on intrahepatic ccc DNA, the relationship between baseline intrahepatic ccc DNA level and the long-efficacy of antiviral treatment.
Applications
Antiviral agents can significantly reduce intrahepatic ccc DNA load, but the duration of treatment should be long enough. Low baseline intrahepatic ccc DNA level may predict the long-term efficacy of antiviral treatment, and can be used as an optimal parameter for monitoring antiviral treatment.
Terminology
ccc DNA (covalently closed circular DNA): After virion enters a susceptible hepatocyte, HBV DNA is then enters the nuclei where it forms a convalently closed circular DNA called ccc DNA. The (-) strand of such a ccc DNA is the template for transcription by cellular RNA polymerase II of a longer-than-genome-length RNA called pregenome and a shorter subgenomic transcript, all of which serve as mRNAs.
Peer review
Effects of the currently available antiviral drugs for chronic hepatitis B are not satisfactory. The main reason for the rebound of HBV DNA after therapy is the sustained existence of ccc DNA pool. The aim of this study was to evaluate the effects of antiviral agents and HBV genotypes on intrahepatic ccc DNA in HBeAg-positive chronic hepatitis B patients. The authors found that 48-wk sequential lamivudine - INF-α therapy and lamivudine monotherapy could significantly reduce ccc DNA compared to 24-wk INF-α monotherapy. Low baseline intrahepatic ccc DNA level may predict the long-term efficacy of antiviral treatment and HBV genotypes (C and B).
Acknowledgments
The authors thank Xiao-Qi Qin, Department of Medical Statistics, Peking University First Hospital, for valuable statistical advice.
Supported by Beijing Municipal Science & Technology Commission, No. H020920020690
Peer reviewer: Xin-Xin Zhang, Professor, Department of Infectious Diseases, Ruijin Hospital, 197, Ruijin Er Road, Shanghai 200025, China
S- Editor Li DL L- Editor Wang XL E- Editor Wang HF
References
- 1.Fact sheets: Hepatutus B. Geneva: World Health Organization, October. 2000 [Google Scholar]
- 2.Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001;120:1828–1853. doi: 10.1053/gast.2001.24839. [DOI] [PubMed] [Google Scholar]
- 3.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 4.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 5.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 6.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673–2681. doi: 10.1056/NEJMoa042957. [DOI] [PubMed] [Google Scholar]
- 7.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 8.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 9.Lai CL, Ching CK, Tung AK, Li E, Young J, Hill A, Wong BC, Dent J, Wu PC. Lamivudine is effective in suppressing hepatitis B virus DNA in Chinese hepatitis B surface antigen carriers: a placebo-controlled trial. Hepatology. 1997;25:241–244. doi: 10.1002/hep.510250144. [DOI] [PubMed] [Google Scholar]
- 10.Moraleda G, Saputelli J, Aldrich CE, Averett D, Condreay L, Mason WS. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9399. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaney WE 4th, Miller TG, Isom HC. Use of the hepatitis B virus recombinant baculovirus-HepG2 system to study the effects of (-)-beta-2’,3’-dideoxy-3’-thiacytidine on replication of hepatitis B virus and accumulation of covalently closed circular DNA. Antimicrob Agents Chemother. 1999;43:2017–2026. doi: 10.1128/aac.43.8.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W, Summers J. Integration of hepadnavirus DNA in infected liver: evidence for a linear precursor. J Virol. 1999;73:9710–9717. doi: 10.1128/jvi.73.12.9710-9717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoulim F. New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J Hepatol. 2005;42:302–308. doi: 10.1016/j.jhep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Mazet-Wagner AA, Baclet MC, Loustaud-Ratti V, Denis F, Alain S. Real-time PCR quantitation of hepatitis B virus total DNA and covalently closed circular DNA in peripheral blood mononuclear cells from hepatitis B virus-infected patients. J Virol Methods. 2006;138:70–79. doi: 10.1016/j.jviromet.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Wong DK, Yuen MF, Yuan H, Sum SS, Hui CK, Hall J, Lai CL. Quantitation of covalently closed circular hepatitis B virus DNA in chronic hepatitis B patients. Hepatology. 2004;40:727–737. doi: 10.1002/hep.20353. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Dicaire A, Wakil AE, Luscombe C, Sacks SL. Quantitation of hepatitis B virus (HBV) covalently closed circular DNA (cccDNA) in the liver of HBV-infected patients by LightCycler real-time PCR. J Virol Methods. 2004;118:159–167. doi: 10.1016/j.jviromet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Orito E, Yuen MF, Mukaide M, Sugauchi F, Ito K, Ozasa A, Sakamoto T, Kurbanov F, Lai CL, et al. Two subtypes (subgenotypes) of hepatitis B virus genotype C: A novel subtyping assay based on restriction fragment length polymorphism. Hepatol Res. 2005;33:216–224. doi: 10.1016/j.hepres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 19.Yuen MF, Wong DK, Sum SS, Yuan HJ, Yuen JC, Chan AO, Wong BC, Lai CL. Effect of lamivudine therapy on the serum covalently closed-circular (ccc) DNA of chronic hepatitis B infection. Am J Gastroenterol. 2005;100:1099–1103. doi: 10.1111/j.1572-0241.2005.41530.x. [DOI] [PubMed] [Google Scholar]
- 20.Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE 4th, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Wong DK, Yuen MF, Ngai VW, Fung J, Lai CL. One-year entecavir or lamivudine therapy results in reduction of hepatitis B virus intrahepatic covalently closed circular DNA levels. Antivir Ther. 2006;11:909–916. [PubMed] [Google Scholar]
- 22.Watanabe K, Takahashi T, Takahashi S, Okoshi S, Ichida T, Aoyagi Y. Comparative study of genotype B and C hepatitis B virus-induced chronic hepatitis in relation to the basic core promoter and precore mutations. J Gastroenterol Hepatol. 2005;20:441–449. doi: 10.1111/j.1440-1746.2004.03572.x. [DOI] [PubMed] [Google Scholar]
- 23.Yuen MF, Sablon E, Tanaka Y, Kato T, Mizokami M, Doutreloigne J, Yuan HJ, Wong DK, Sum SM, Lai CL. Epidemiological study of hepatitis B virus genotypes, core promoter and precore mutations of chronic hepatitis B infection in Hong Kong. J Hepatol. 2004;41:119–125. doi: 10.1016/j.jhep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Kao JH. Hepatitis B virus genotypes and hepatocellular carcinoma in Taiwan. Intervirology. 2003;46:400–407. doi: 10.1159/000074999. [DOI] [PubMed] [Google Scholar]