Abstract
Context
Findings from previous studies of the effects of exercise training on patient-reported health status have been inconsistent.
Objective
To test the effects of exercise training on health status among patients with heart failure.
Design, Setting, and Patients
Multicenter, randomized controlled trial among 2331 medically stable outpatients with heart failure with ejection fraction ≤ 35%. Patients were randomized from April 2003 through February 2007.
Interventions
Usual care plus aerobic exercise training, consisting of 36 supervised sessions followed by home-based training, vs usual care alone. Randomization was stratified by heart failure etiology, which was a covariate in all models.
Main Outcome Measures
Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary scale and key subscales at baseline, every 3 months for 12 months, and annually thereafter for up to 4 years. The KCCQ is scored from 0 to 100 with higher scores corresponding to better health status. Treatment group effects were estimated using linear mixed models according to the intention-to-treat principle.
Results
Median follow-up was 2.5 years. At 3 months, usual care plus exercise training led to greater improvement in the KCCQ overall summary score (mean, 5.2; 95% confidence interval, 4.4–6.0) compared with usual care alone (3.3; 95% confidence interval, 2.5–4.1). The additional 1.9-point increase in the exercise training group was statistically significant (P < .001). After 3 months, there were no further significant changes in KCCQ score for either group (P = .85 for the difference between slopes), resulting in a sustained, greater improvement overall for the exercise group (P < .001). Results were similar on the KCCQ subscales, and no subgroup interactions were detected.
Conclusions
Exercise training conferred modest but statistically significant improvements in health status compared with usual care without training. Improvements occurred early and persisted over time.
Trial Registration
clinicaltrials.gov Identifier: NCT00047437
Introduction
Heart failure is a syndrome characterized by dyspnea and fatigue; however, patients with heart failure often also experience diminished health status, including reductions in physical and social functioning and other dimensions of health-related quality of life.1,2 Pharmacological and device interventions and disease management programs for heart failure have provided little or modest improvements in health-related quality of life.3,4 The extent to which exercise training in addition to optimal evidence-based therapy improves patients’ health status is unknown.
Among previous studies of exercise training in patients with heart failure, few have assessed quality-of-life outcomes.5 These studies have had a variety of limitations, including recruitment at single centers, small sample sizes, lack of randomization or adequate controls, and limited follow-up. Moreover, previous studies have yielded conflicting results regarding the benefits of exercise for patients’ health status. Coats et al6 reported an improvement in patient-reported symptoms among 11 highly selected patients who undertook exercise training during a period of 8 weeks. A randomized trial among 99 patients by Belardinelli et al7 demonstrated improvements in the 21-item Minnesota Living With Heart Failure Questionnaire (MLHFQ) after 2 months of exercise training compared with usual care, ratings that remained stable at 12 months. In contrast, the Exercise Rehabilitation Trial among 181 patients randomly assigned to 3 months of supervised exercise training followed by 9 months of home-based training or usual care showed no differences in MLHFQ scores.8 Further underscoring the confusion surrounding the association of training-induced improvement in exercise capacity and quality of life are 2 studies by Keteyian et al,9,10 in which 24 weeks of exercise training improved peak oxygen uptake (ie, peak VO2) but not MLHFQ scores. Most of these studies were conducted prior to current guideline recommendations for pharmacologic and device therapies, including β-blockers, biventricular pacemakers, and implantable cardioverter-defibrillators.
Thus, critical questions remain about whether exercise training can improve patient-reported health status. The principal aim of Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) was to examine the effects of exercise training on clinical outcomes in patients with heart failure. A total of 759 patients (65%) in the exercise group experienced a primary end point (ie, all-cause mortality or all-cause hospitalization), compared with 796 (68%) in the usual care group (hazard ratio 0.93; 95% confidence interval [CI], 0.84–1.02; P = .13; see the accompanying manuscript by O’Connor et al). A key secondary goal was to examine the effects of exercise training on patient-reported health status, as assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a well-accepted instrument for clinical trials in heart failure.11,12 HF-ACTION compared scores on the KCCQ overall summary scale and key subscales (ie, physical limitations, symptoms, quality of life, and social limitations) between randomized groups. We hypothesized that patients with New York Heart Association (NYHA) class II to IV heart failure who were randomly assigned to usual care plus exercise training would show greater improvement in health status than patients assigned to usual care alone.
Methods
Study Design
A complete description of the study design and exercise training protocol has been published previously.13 In brief, HF-ACTION was a multicenter, randomized controlled trial designed to test the long-term safety and efficacy of aerobic exercise training plus evidence-based medical therapy vs usual care with evidence-based medical therapy alone in medically stable outpatients with left ventricular dysfunction and NYHA class II to IV heart failure. Patients were recruited from 82 centers in the United States, Canada, and France. Enrollment criteria included ejection fraction ≤ 35%, NYHA class II to IV heart failure symptoms, and ability and willingness to undergo exercise training. Patients were excluded from consideration if they were unable to exercise, were already exercising regularly (more than once per week), or had experienced a major cardiovascular event in the previous 6 weeks. The relevant institutional review boards, research ethics boards, and ethics committees of the participating centers and the coordinating center approved the protocol, and all patients provided written consent to participate.
From April 2003 through February 2007, eligible patients were randomized 1:1 using a block randomization scheme, with random permutations formed by the sorting of numbers randomly generated uniformly on the unit interval, to either usual care alone, which included optimal medical therapy and a recommendation for regular physical activity, or to usual care plus aerobic exercise training, consisting of 36 sessions of supervised aerobic exercise training at 60% to 70% of heart rate reserve 3 times per week followed by prescribed home-based training at the same intensity 5 times per week. Randomization was stratified by center and heart failure etiology.
Patient-Reported Measures
We used the KCCQ to measure health status. The KCCQ is a 23-item self-administered disease-specific questionnaire.11 In addition to an overall summary score, we report scores from the subscales for physical limitations, symptoms, quality of life, and social limitations. The KCCQ is scored from 0 to 100, with higher scores representing better health status. We handled missing values in each KCCQ domain by using the standard scoring algorithms to assign the average of the completed items within the domain, assuming that a domain-specific threshold number of items in that domain (usually half) were answered. The KCCQ was self-administered at the baseline clinic visit, at 3-month intervals during clinic visits for the first 12 months, and annually thereafter for up to 4 years. When the trial started, the KCCQ had not been validated for use by telephone,14 and we did not collect KCCQ data during telephone visits. French-speaking participants in France and Quebec used certified French-language versions of the KCCQ.
To aid interpretation of results, we considered a 5-point change in the KCCQ overall summary score to be the minimally noticeable clinical difference, based on a study by Spertus et al12 in which a 5-point change corresponded to cardiologists’ ratings of small changes in health status during an observational 6-week period among 476 patients with heart failure. This 5-point cutoff is appropriate for interpreting change within individuals. Ongoing research continues to improve our understanding of the clinical meaning of changes in KCCQ scores, especially for interpreting mean changes between groups and correlations with other functional measures.
Statistical Analysis
We used a 2-tailed significance level of α = 0.05 for all assessments. Based on an expected SD for the KCCQ clinical overall score of 24.30 (J.A. Spertus, personal communication), the effective expected sample size of 1323 patients per group provided 90% statistical power for detecting a 2.16-point or greater difference between treatments. We used SAS version 9.1 (SAS Institute, Inc, Cary, North Carolina) for all analyses. We estimated the models according to the intention-to-treat principle.
Given the complexities of analyzing longitudinal patient-reported outcome data – including correlations among serial observations of the KCCQ score, the ability of health status scores to move in both directions (ie, improve or deteriorate), and the potential for group differences in mortality or missing data – we examined treatment group effects on the KCCQ score using a longitudinal linear mixed-effects model.15 This prespecified approach models the underlying health trajectory for each patient that gives rise to the observed KCCQ scores at various time points, then compares the average trajectories between treatment groups. This method minimizes the effects of measurement error from any one assessment.16
We used full maximum likelihood estimation to model all available data from each patient without the need to impute missing values or omit patients with missing data from the analysis. This method provides unbiased estimates when the mechanism responsible for missing data can be ignored, that is, when unobserved variables do not explain the probability of missingness over and above what is explained by observed variables.17 In a sensitivity analysis, we examined whether treatment effects were the same after increasing the number of observed variables in the model. This was done by adding 28 baseline covariates, each of which had less than 1% missing data (n = 2212). The variables were age, sex, NYHA class, Canadian Cardiovascular Society angina class, heart failure etiology, left ventricular ejection fraction, previous revascularization (ie, coronary artery bypass graft surgery or percutaneous coronary intervention), history of myocardial infarction, history of diabetes mellitus, history of chronic obstructive pulmonary disease, history of peripheral vascular disease, atrial fibrillation or atrial flutter, hospitalization for heart failure in the previous 6 months, receipt of a biventricular pacemaker, receipt of an implantable cardioverter-defibrillator, use of β-blockers, use of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, use of angiotensin-converting enzyme inhibitors, use of both loop and nonloop diuretics, use of an aldosterone antagonist, smoking status, systolic and diastolic blood pressure, body mass index, resting heart rate, exercise duration on a functional exercise tolerance test, depression as measured by the Beck Depression Inventory II, and perceived social support as measured by the Perceived Social Support Scale.
The trajectory of health status over time was not linear. Graphical examination of the observed changes in the KCCQ overall summary score showed an initial steep increase from baseline to 3 months rather than a strictly linear change across all time points. Therefore, to improve model fit, we modeled time using a piecewise linear model with a joint point at 3 months. For each model, the KCCQ score was the dependent variable, and we estimated the fixed effects of ischemic etiology, the jump from baseline to 3 months, and the slope (in months) after 3 months. We specified 3 random effects: an overall intercept, a random jump at 3 months (ie, a random new intercept starting at 3 months), and a random slope starting at 3 months. To test whether the mean trajectories over time differed by treatment group, we used an omnibus likelihood ratio test of the joint significance of 2 interaction effects: treatment by the jump from baseline to 3 months and treatment by the slope after 3 months. We report the model estimates for the fixed effects.
To test whether treatment effects differed by subgroup, we used an omnibus likelihood ratio test of the 3-way interactions of subgroup, treatment effect, and jump from baseline to 3 months, and subgroup, treatment effect, and slope (in months) after 3 months. When significant, we tested each interaction effect separately. Prespecified subgroups included age (≤ 70 or > 70 years), sex (male or female), race (black or African American, white, or other), NYHA class II or III/IV, heart failure etiology (ischemic or nonischemic), depression at baseline (scored as a continuous variable), and perceived social support (scored as a continuous variable). We also examined subgroups defined by left ventricular ejection fraction (≤ 25% or > 25%), previous revascularization (coronary artery bypass graft surgery or percutaneous coronary intervention, or no previous revascularization), history of myocardial infarction, and KCCQ overall summary score at baseline (0–50, 50–75, or 75–100).
Finally, in a post hoc analysis of individual change to assist in the clinical interpretability of the group differences, we used χ2 tests to compare the percentage of patients with a ≥ 5-point improvement in the KCCQ overall summary score by treatment group, using change-from-baseline estimates generated by the linear mixed-effects models in the primary analysis. From this comparison, we calculated the number needed to treat – namely, the estimated number of patients needed to be referred to an exercise training program to observe a clinically noticeable improvement in health status in 1 patient at 3 (or 12) months compared with usual care alone. We also report the bias-corrected and accelerated (BCa) bootstrap CIs for each of the predicted proportions. To provide additional information relevant to interpretation of the magnitude of individual changes in the KCCQ, we computed correlations between 12-month change-from-baseline scores on the KCCQ overall summary score and 3 physiologic parameters: change in exercise time on cardiopulmonary exercise test, change in peak oxygen consumption during exercise test, and change in 6-minute walk distance.
Results
The baseline characteristics of HF-ACTION participants by treatment group are shown in Table 1. Characteristics were similar between the treatment groups, and the groups had similar mean baseline scores on the KCCQ overall summary scale and key subscales. Furthermore, on the KCCQ symptom stability scale, which measures recent changes in heart failure symptoms such that a score of 50 indicates no recent changes, the mean (SD) scores were 54 (18) in the usual care group and 54 (16) in the exercise group, indicating that participants in both groups were medically stable with respect to heart failure at the time of randomization.
Table 1.
Baseline Characteristics of the Patients by Treatment Groupa
| Characteristic | Usual Care (n = 1172) | Exercise Training (n = 1159) |
|---|---|---|
| Age, median (IQR), y | 59.3 (51.1–68.2) | 59.2 (51.2–67.8) |
| Female, No. (%) | 314 (26.8) | 347 (29.9) |
| Hispanic or Latino ethnicity, No. (%) | 48 (4.1) | 40 (3.5) |
| Race, No. (%) | ||
| Black/African American | 372 (32.2) | 377 (33.1) |
| White | 728 (63.0) | 698 (61.2) |
| Other | 56 (4.8) | 65 (5.7) |
| NYHA classification, No. (%) | ||
| II | 754 (64.3) | 723 (62.4) |
| III | 409 (34.9) | 422 (36.4) |
| IV | 9 (0.8) | 14 (1.2) |
| Ischemic etiology of heart failure, No. (%) | 599 (51.1) | 598 (51.6) |
| Ejection fraction, median (IQR), % | 25 (20–30) | 25 (20–30) |
| Diabetes mellitus, No. (%) | 370 (31.6) | 378 (32.6) |
| Previous myocardial infarction, No. (%) | 499 (42.6) | 480 (41.4) |
| Hypertension, No. (%) | 676 (58.0) | 712 (61.8) |
| Renal dysfunction, No. (%)b | 14 (1.3) | 17 (1.6) |
| Anemia, No. (%)c | ||
| Normal | 741 (83.0) | 711 (80.2) |
| Mild anemia | 130 (14.6) | 150 (16.9) |
| Moderate to severe anemia | 22 (2.5) | 25 (2.2) |
| Atrial fibrillation/atrial flutter, No. (%) | 241 (20.6) | 247 (21.3) |
| Use of ACE inhibitor, No. (%) | 861 (73.5) | 875 (75.5) |
| Use of angiotensin receptor blocker, No. (%) | 260 (22.2) | 284 (24.5) |
| Use of β-blocker, No. (%) | 1112 (94.9) | 1091 (94.1) |
| Six-minute walk distance, median (IQR), m | 373.2 (300.0–432.5) | 365.8 (296.3–436.2) |
| Peak VO2, median (IQR), mL/kg/min | 14.5 (11.6–17.8) | 14.4 (11.3–17.6) |
| Beck Depression Inventory II, median (IQR) | 8 (4–15) | 9 (5–15) |
| Perceived Social Support Scale, median (IQR) | 6.0 (5.2–6.7) | 6.0 (5.2–6.7) |
| KCCQ overall summary scale, mean (SD) | 66.5 (21.0) | 65.9 (20.2) |
| KCCQ subscales, mean (SD) | ||
| Physical limitations | 69.7 (22.1) | 69.2 (21.7) |
| Symptoms | 73.2 (20.9) | 72.9 (20.7) |
| Quality of life | 60.3 (25.4) | 59.2 (23.8) |
| Social limitations | 62.6 (28.0) | 62.1 (27.0) |
Abbreviations: IQR, interquartile range; ACE, angiotensin-converting enzyme; NYHA, New York Heart Association; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Percentages may not sum to 100 because of rounding.
Creatinine > 3 mg/dL.
Normal, hemoglobin ≤ 12 g/dL; mild anemia, hemoglobin 10–12 g/dL; moderate to severe anemia, hemoglobin < 10 g/dL.
Median follow up was 2.5 years. Visit-level missing data are shown in Table 2, accounting for death, withdrawn consent, and visits not expected because of later enrollment in the trial. (Based on randomization date, patients were to be followed for 1 to 4 years before the trial ended.) Because the KCCQ was not administered by telephone, some participants who had telephone follow-up visits are missing KCCQ data over and above the missing visit data. The difference in missing KCCQ data by treatment group did not exceed 6% for any visit.
Table 2.
Patients With Missing Data by Visita
| Visit | Usual Care |
Exercise Training |
||||
|---|---|---|---|---|---|---|
| No. Expected | Visit Missing, No. (%) | KCCQ Missing, No. (%) | No. Expected | Visit Missing, No. (%) | KCCQ Missing, No. (%) | |
| Baseline | 1172 | 0 | 1 (< 0.1) | 1159 | 0 | 0 |
| 3 months | 1139 | 107 (9.4) | 153 (13.4) | 1142 | 52 (4.6) | 90 (7.9) |
| 6 months | 1117 | 136 (12.2) | 200 (17.9) | 1123 | 81 (7.2) | 132 (11.8) |
| 9 months | 1094 | 133 (12.2) | 193 (17.6) | 1102 | 121 (11.0) | 190 (17.2) |
| 12 months | 1045 | 127 (12.2) | 195 (18.7) | 1056 | 86 (8.1) | 150 (14.2) |
| 24 months | 725 | 99 (13.7) | 173 (23.9) | 720 | 79 (11.0) | 151 (21.0) |
| 36 months | 418 | 56 (13.4) | 138 (33.0) | 409 | 56 (13.7) | 115 (28.1) |
Abbreviation: KCCQ, Kansas City Cardiomyopathy Questionnaire.
Out of the number of patients expected at each visit, accounting for death, withdrawn consent, and rolling enrollment into the trial.
Exercise Training and Patient-Reported Health Status
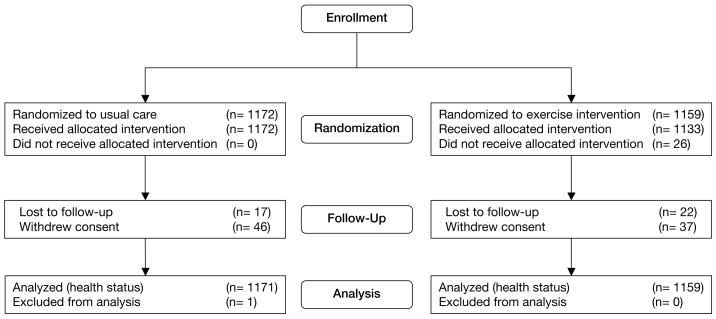
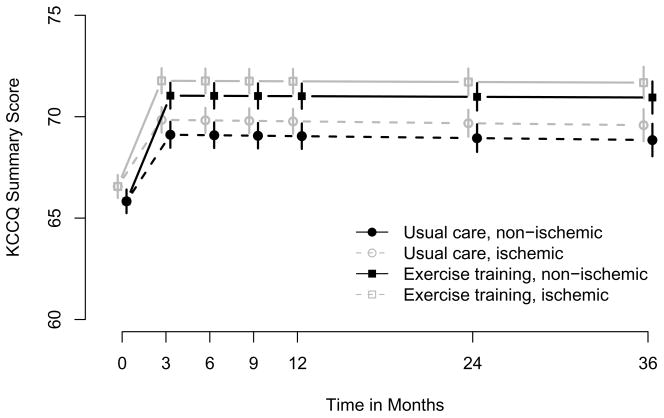
Results from the mixed models are shown in Table 3. All participants who had at least a baseline KCCQ score contributed data to this analysis (1159 in the exercise group, 1171 in the usual care group; Figure 1). After adjustment for heart failure etiology, the KCCQ overall summary score improved by 5.2 points in the exercise training group and by 3.3 points in the usual care group from baseline to 3 months. The additional 1.9-point increase in the exercise training group was statistically significant (P < .001). There was no attenuation of this early benefit over time; neither group experienced significant changes in KCCQ scores after 3 months, and there was no significant difference between groups in the slopes over time (P = .85). The overall treatment effect (ie, the difference between overall trajectories by treatment group) was statistically significant (P = .001). This relationship is displayed graphically in Figure 2. Results were similar in the analysis adjusted for the 28 baseline covariates. There were no significant subgroup interactions for age (P = .44), sex (P = .26), race (P = .97), NYHA class (P = .61), heart failure etiology (P = .75), left ventricular dysfunction (P = .06), previous revascularization (P = .84), history of myocardial infarction (P = .08), depression (P = .24), perceived social support (P = .32), or KCCQ score at baseline (P = .24).
Table 3.
Estimated Changes in Patient-Reported Health Status by Treatment Groupa
| KCCQ Scale | Usual Care (n = 1171) |
Exercise Training (n = 1159) |
||
|---|---|---|---|---|
| Parameter Estimate (95% CI) | P Valueb | Parameter Estimate (95% CI) | P Valueb | |
| Overall summary scale | ||||
| Baseline to 3-month visit | 3.28 (2.48 to 4.09) | < .001 | 5.21 (4.42 to 6.00) | < .001 |
| 3-month visit to end of study | −0.01 (−0.05 to 0.03) | .69 | 0.00 (−0.04 to 0.03) | .69 |
| Physical limitations subscale | ||||
| Baseline to 3-month visit | 1.25 (0.30 to 2.20) | .01 | 3.55 (2.62 to 4.48) | < .001 |
| 3-month visit to end of study | −0.06 (−0.02 to −0.10) | .006 | −0.05 (−0.01 to 0.10) | .01 |
| Symptoms subscale | ||||
| Baseline to 3-month visit | 2.06 (1.21 to 2.92) | < .001 | 3.58 (2.74 to 4.42) | < .001 |
| 3-month visit to end of study | −0.03 (−0.07 to 0.01) | .14 | −0.03 (−0.07 to 0.01) | .10 |
| Quality of life subscale | ||||
| Baseline to 3-month visit | 5.73 (4.70 to 6.77) | < .001 | 7.36 (6.35 to 8.38) | < .001 |
| 3-month visit to end of study | 0.08 (0.03 to 0.12) | < .001 | 0.09 (0.05 to 0.14) | < .001 |
| Social limitations subscale | ||||
| Baseline to 3-month visit | 4.50 (3.32 to 5.67) | < .001 | 6.28 (5.13 to 7.44) | < .001 |
| 3-month visit to end of study | −0.04 (−0.09 to 0.01) | .16 | 0.02 (−0.03 to 0.07) | .44 |
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionnaire; CI, confidence interval.
Estimates are derived from a longitudinal piecewise linear mixed model that adjusts for ischemic etiology of heart failure. The models estimate change from baseline to 3 months and monthly change from 3 months to the end of study for each treatment group.
P values are from F tests for the significance of each of the fixed effects.
Figure 1.
Flow of Participants Through the Trial
Figure 2.
Predicted Average Health Status Trajectories by Treatment Group Note: P = .001 for treatment effect for both ischemic and nonischemic heart failure. Error bars indicate SEs at each time point.
Results for the KCCQ subscales were similar to the results for the overall summary scale. After adjustment for heart failure etiology, there was a significant overall treatment effect on physical limitations (P < .001), symptoms (P = .02), quality of life (P < .001), and social limitations (P = .02). For these 4 subscales, additional early improvements in the exercise training group compared with usual care were significant (2.3 points on physical limitations, P < .001; 1.5 points on symptoms, P < .001; 1.6 points on quality of life, P = .02; and 1.8 points on social limitations, P = .02). After 3 months, both groups experienced similar slight decreases in physical limitations scores (0.06 points per month for usual care vs 0.05 points per month for the exercise group; difference of 0.006 not significant at P = .84) and slight increases in quality-of-life scores (0.08 points per month for usual care vs 0.09 points per month for the exercise group; difference of 0.02 not significant at P = .60). There were no significant changes in symptoms (P = .91) or social limitations (P = .12) scores after 3 months.
Exercise Training and the Proportion of Patients Benefiting
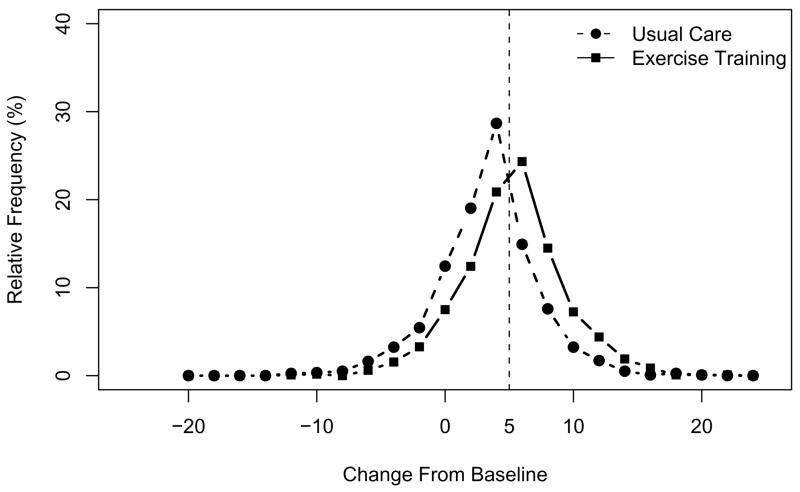
Figure 3 shows the distribution of predicted changes in the KCCQ overall summary score from baseline to 3 months. In this post hoc analysis, 54% (n = 621; 95% CI, 51%–56%) of patients in the exercise group had a clinically noticeable improvement of ≥ 5 points compared with 29% (n = 334; 95% CI, 26%–31%) of patients in the usual care group (P < .001). (In the analysis adjusted for 28 covariates, the percentages were 52% [n = 602; 95% CI, 50%–55%] and 32% [n = 378; 95% CI, 30%–35%], respectively.) Given the difference in the proportion of patients who experienced a clinically noticeable improvement in health status (26%), the number of well-medicated patients with class II to IV heart failure who would need to be referred to exercise training for 1 patient to benefit significantly more by 3 months than if all patients received usual care alone is 4 (in the adjusted analysis, 5). At 12 months, 53% (n = 618; 95% CI, 50%–56%) of patients in the exercise training group had a clinically noticeable improvement from baseline compared with 33% (n = 386; 95% CI, 30%–35%) in the usual care group (P < .001), suggesting a number needed to treat of 5. (In the adjusted analysis, the percentages were 53% (n = 614; 95% CI, 51%–56%) and 34% (n = 395; 95% CI, 31%–36%), resulting in a number needed to treat of 5.)
Figure 3.
Distribution of Predicted Change From Baseline to 3 Months in the KCCQ Overall Summary Score by Treatment Group Note: Bin size = 2. The bin centered over zero ranges from greater than −1 to 1.
Relationship Between KCCQ and Physiologic Outcomes
Changes from baseline to 12 months in the KCCQ overall summary score were associated with changes in exercise time on the cardiopulmonary exercise test (r = 0.28; P < .001), peak oxygen consumption (r = 0.21; P < .001), and 6-minute walk distance (r = 0.18; P < .001). Based on these relationships, an individual’s change of 5 points on the KCCQ overall summary score corresponded to a 1.7-minute change in exercise time, a 1.4-mL/min/kg change in peak oxygen consumption, and a 49.7-m change in distance walked.
Comment
In HF-ACTION, health status as assessed by the KCCQ improved more during the initial 3-month period for patients in the exercise training group than for patients in the usual care group. This improvement persisted throughout follow-up and was seen not only on the overall summary scale but also on key subscales, including physical limitations, symptoms, quality of life, and social limitations. These results support our primary hypothesis that exercise training significantly improves health status for patients with heart failure compared with usual care alone. The size of the initial mean benefit was modest and may not be considered clinically significant when examining mean differences between groups. However, in the post hoc analyses comparing the distribution of the individual change scores, there was a significantly larger proportion of patients assigned to exercise training who experienced at least a 5-point improvement compared with those assigned to usual care alone. (Ancillary analyses indicated that a change of 5 points on the KCCQ was as large as or larger than corresponding changes in physiologic outcomes that are considered clinically meaningful.5,18,19)
Few studies have evaluated the effects of exercise training on health status and quality of life in patients with systolic heart failure. The present study of patients with NYHA class II to IV heart failure is the largest and longest study to evaluate this relationship. Patients with heart failure who exercise may experience dyspnea and fatigue, but it is noteworthy that patients in the exercise group showed early improvements in KCCQ scores, including the subscales for physical limitations and symptoms. Unlike previous studies suggesting that women20 and older patients21 may not respond as well to exercise training, our findings of exercise-related benefit were relatively consistent across sex, race, age, and other subgroups.
The lack of further improvement in scores after 3 months may reflect the potential influence of the social support that occurred during the supervised sessions or the possibility that the principal benefits of exercise on health status are attained early. Alternatively, it could be a result of the low adherence to home-based exercise in some patients assigned to exercise training and to increased physical activity in the usual care group. However, we did not observe a decline in KCCQ scores after 3 months in the exercise group, which we might have expected had patient-reported health status been related to declining adherence over time. There also was no decline in health status in the usual care group after 3 months, perhaps because of the support associated with participating in a clinical trial. In previous exercise studies, the failure to demonstrate improvements in quality of life has been attributed to a lack of sensitivity of measurement tools, small sample sizes, short duration of exercise training, and lack of adherence to the exercise regimen. Authors of a Cochrane review of exercise rehabilitation in patients with heart failure concluded that quality of life improved in the short term, but they cited the need for larger, more representative studies.22 In a systematic review of home-based exercise training in patients with chronic heart failure, quality of life (measured with the MLHFQ) did not improve compared with usual activity. The authors noted that initial adherence to the prescribed intensity of 60% to 70% of heart rate reserve declined progressively during the course of home training, which may explain the lack of improvement in quality of life. They suggest that future study is needed of methods to reduce barriers and improve adherence to home exercise training.23
Our ability to characterize the benefit from exercise in HF-ACTION was enhanced by the use of linear mixed-effects models that accommodated variable assessment intervals, nonlinear change, and study dropout.17 This approach offers advantages over other analytic approaches used in previous research on patient-reported outcomes in heart failure. In particular, deriving model-based estimates of change from baseline can result in less bias from measurement error and missing data.24 Future clinical studies should consider such an approach to increase the power and interpretability of treatment effects on quality-of-life outcomes.
Limitations
According to the study design, patients who were unwilling or unable to exercise were excluded, which may have reduced the generalizability of the results. Patients in the usual care group may have increased their physical activity level after enrollment or even self-referred to an exercise rehabilitation program (ie, true crossover). These factors, combined with suboptimal adherence in the exercise group, may have diminished the identified health status benefit associated with exercise training. An analysis addressing the relationship between adherence and health status is ongoing. Second, because of the nature of the intervention, it was not possible to blind patients to treatment assignment. It is impossible to ascertain whether this influenced self-reported KCCQ scores. Third, the measurement schedule of yearly assessments of the KCCQ after 12 months may have hindered our ability to detect changes in health status associated with clinical events after 1 year. Fourth, measurement of health status was based on a single instrument, the KCCQ, that was not administered during telephone visits, so the rate of missing data on health status, especially at 24 and 36 months, was higher than expected. However, censoring the data at 12 months had no effect on the results. Finally, the description of the relationship between the KCCQ and physiologic measures was restricted to patients who had complete data and may not represent these relationships in the total trial population.
Conclusion
HF-ACTION is the largest and most comprehensive study of exercise training in patients with NYHA class II to IV heart failure. The results demonstrate that participation in an exercise training program provides a modest but statistically significant improvement in patient-reported health status compared with usual care. The clinical meaningfulness of the magnitude of average change requires further study. The improvement associated with exercise training occurred early during supervised training and persisted over time during home-based training. The results were consistent across KCCQ subscales and key subgroups.
Acknowledgments
Funding/Support: HF-ACTION was funded by grants 5U01HL063747 (Duke University, C. O’Connor, coordinating center), 5U01HL066461 (Duke University, K. Schulman, economics and quality of life), 5U01HL068973 (Boston Medical Center, W. Colucci), 5U01HL066501 (Case Western Reserve University, I. Pina), 5U01HL066482 (Emory University, A. Smith), 5U01HL064250 (Henry Ford Hospital, S. Keteyian), 5U01HL066494 (Ohio State University, W. Abraham), 5U01HL064257 (Oregon Health & Science University, R. Hershberger), 5U01HL066497 (University of Alabama at Birmingham, V. Bittner), 5U01HL068980 (University of California, Los Angeles, G. Fonarow), 5U01HL064265 (University of Colorado Health Sciences Center, E. Wolfel), 5U01HL066491 (Wake Forest University, D. Kitzman), and 5U01HL064264 (Washington University in St. Louis, G. Ewald) from the National Heart, Lung, and Blood Institute.
Footnotes
Author Contributions: Drs Flynn and O’Connor had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Pina, Whellan, Blumenthal, Keteyian, Kitzman, Kraus, O’Connor, Weinfurt. Acquisition of data: Pina, Whellan, Howlett, Keteyian, Kitzman, Kraus, O’Connor, Weinfurt. Analysis and interpretation of data: Flynn, Pina, Whellan, Lin, Blumenthal, Ellis, Fine, Howlett, Keteyian, Houston Miller, Schulman, Spertus, O’Connor, Weinfurt. Drafting of the manuscript: Flynn, Pina, Whellan, Howlett, Keteyian, Houston Miller, O’Connor, Weinfurt. Critical revision of the manuscript for important intellectual content: Flynn, Pina, Whellan, Lin, Blumenthal, Ellis, Fine, Howlett, Keteyian, Kraus, Schulman, Spertus, O’Connor, Weinfurt. Statistical analysis: Flynn, Pina, Whellan, Lin, Ellis, Spertus, Weinfurt. Obtained funding: Whellan, Keteyian, Kitzman, O’Connor, Weinfurt. Administrative, technical, or material support: Pina, Whellan, Blumenthal, Fine, Howlett, Keteyian, Kitzman, Schulman, Spertus, O’Connor. Study supervision: Flynn, Whellan, Keteyian, Houston Miller, Schulman, O’Connor, Weinfurt.
Role of the Sponsor: The National Heart, Lung, and Blood Institute had a role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the preparation, review, and approval of the manuscript.
Publisher's Disclaimer: Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Previous Presentation: Presented as a late-breaking clinical trial at the American Heart Association Scientific Sessions; November 12, 2008; New Orleans, La.
Additional Contributions: We thank Damon M. Seils, MA, Duke University, for editorial assistance and manuscript preparation. Mr Seils did not receive compensation for his assistance apart from his employment at the study coordinating center.
Financial Disclosures: Dr Piña reports receiving grants or funding from the National Institutes of Health; receiving personal income for consulting from the Food and Drug Administration; and receiving honoraria from AstraZeneca, Innovia, Merck, Novartis, Sanofi-Aventis, and Solvay. Dr Whellan reports receiving grants or funding from GE Medical and the National Institutes of Health. Dr Blumenthal reports receiving grants or funding from the National Institutes of Health. Dr Ellis reports receiving grants from GE Medical. Dr Keteyian reports receiving honoraria for lectures to scientific, educational, and community groups; and receiving royalties from books published by Human Kinetics and McGraw-Hill. Dr Kraus reports receiving grants or funding from the National Institutes of Health. Ms Houston Miller reports receiving personal income for consulting from Triage Wireless; and receiving honoraria from Pfizer, CV Therapeutics, and AstraZeneca. Dr Schulman reports receiving research support from Actelion Pharmaceuticals, Allergan, Amgen, Astellas Pharma, Bristol-Myers Squibb, The Duke Endowment, Genentech, Inspire Pharmaceuticals, Johnson & Johnson, Kureha Corporation, LifeMasters Supported SelfCare, Medtronic, Merck & Co, Nabi Biopharmaceuticals, National Patient Advocate Foundation, North Carolina Biotechnology Center, NovaCardia, Novartis, OSI Eyetech, Pfizer, Sanofi-Aventis, Scios, Tengion, Theravance, Thomson Healthcare, and Vertex Pharmaceuticals; receiving personal income for consulting from McKinsey & Company and the National Pharmaceutical Council; having equity in Alnylam Pharmaceuticals; having equity in and serving on the board of directors of Cancer Consultants; and having equity in and serving on the executive board of Faculty Connection LLC. Dr Schulman has made available online a detailed listing of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp). Dr Weinfurt reports receiving research support from Bristol-Myers Squibb, Inspire Pharmaceuticals, Johnson & Johnson (Ortho Biotech), and Novartis; and receiving personal income for consulting from Inspire Pharmaceuticals. Dr Weinfurt has made available online a detailed listing of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp). Dr Spertus owns the copyright to the Kansas City Cardiomyopathy Questionnaire. Dr O’Connor reports receiving grants or funding, personal income for consulting, and honoraria from GE Medical, Roche, and the National Institutes of Health. No other disclosures were reported.
References
- 1.Dracup K, Walden JA, Stevenson LW, Brecht ML. Quality of life in patients with advanced heart failure. J Heart Lung Transplant. 1992;11(2 Pt 1):273–279. [PubMed] [Google Scholar]
- 2.Krumholz HM, Butler J, Miller J, et al. Prognostic importance of emotional support for elderly patients hospitalized with heart failure. Circulation. 1998;97(10):958–964. doi: 10.1161/01.cir.97.10.958. [DOI] [PubMed] [Google Scholar]
- 3.Metra M, Giubbini R, Nodari S, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000;102(5):546–551. doi: 10.1161/01.cir.102.5.546. [DOI] [PubMed] [Google Scholar]
- 4.Pires LA, Abraham WT, Young JB, Johnson KM. Clinical predictors and timing of New York Heart Association class improvement with cardiac resynchronization therapy in patients with advanced chronic heart failure: results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) trials. Am Heart J. 2006;151(4):837–843. doi: 10.1016/j.ahj.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 5.McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev. 2008;13(1):3–11. doi: 10.1007/s10741-007-9052-z. [DOI] [PubMed] [Google Scholar]
- 6.Coats AJ, Adamopoulos S, Meyer TE, Conway J, Sleight P. Effects of physical training in chronic heart failure. Lancet. 1990;335(8681):63–66. doi: 10.1016/0140-6736(90)90536-e. [DOI] [PubMed] [Google Scholar]
- 7.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99(9):1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 8.McKelvie RS, Teo KK, Roberts R, et al. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT) Am Heart J. 2002;144(1):23–30. doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 9.Keteyian SJ, Brawner CA, Schairer JR, et al. Effects of exercise training on chronotropic incompetence in patients with heart failure. Am Heart J. 1999;138(2 Pt 1):233–240. doi: 10.1016/s0002-8703(99)70106-7. [DOI] [PubMed] [Google Scholar]
- 10.Keteyian SJ, Levine AB, Brawner CA, et al. Exercise training in patients with heart failure. A randomized, controlled trial. Ann Intern Med. 1996;124(12):1051–1057. doi: 10.7326/0003-4819-124-12-199606150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 12.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Whellan DJ, O’Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153(2):201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Havranek EP, Spertus JA, Masoudi FA, Jones PG, Rumsfeld JS. Predictors of the onset of depressive symptoms in patients with heart failure. J Am Coll Cardiol. 2004;44(12):2333–2338. doi: 10.1016/j.jacc.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Bryk AS, Raudenbush SW. Hierarchical Linear Models. Newbury Park: 1992. [Google Scholar]
- 16.Kraemer HC, Thiemann S. A strategy to use soft data effectively in randomized controlled clinical trials. J Consult Clin Psychol. 1989;57(1):148–154. doi: 10.1037//0022-006x.57.1.148. [DOI] [PubMed] [Google Scholar]
- 17.Schafer JL. Analysis of Incomplete Multivariable Data. Chapman & Hall/CRC; 1997. [Google Scholar]
- 18.Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289(20):2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 19.Shah MR, Hasselblad V, Gheorghiade M, et al. Prognostic usefulness of the six-minute walk in patients with advanced congestive heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2001;88(9):987–993. doi: 10.1016/s0002-9149(01)01975-0. [DOI] [PubMed] [Google Scholar]
- 20.Gary RA, Sueta CA, Dougherty M, et al. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33(4):210–218. doi: 10.1016/j.hrtlng.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Wielenga RP, Huisveld IA, Bol E, et al. Exercise training in elderly patients with chronic heart failure. Coron Artery Dis. 1998;9(11):765–770. doi: 10.1097/00019501-199809110-00010. [DOI] [PubMed] [Google Scholar]
- 22.Rees K, Taylor RS, Singh S, Coats AJ, Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev. 2004;3:CD003331. doi: 10.1002/14651858.CD003331.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien CL, Lee CM, Wu YW, Chen TA, Wu YT. Home-based exercise increases exercise capacity but not quality of life in people with chronic heart failure: a systematic review. Aust J Physiother. 2008;54(2):87–93. doi: 10.1016/s0004-9514(08)70041-2. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]