Abstract
The basal forebrain complex, which includes the nucleus basalis magnocellularis (NBM), provides widespread cholinergic and γ-aminobutyric acid-containing projections throughout the brain, including the insular and pyriform cortices. A number of studies have implicated the cholinergic neurons in the mediation of learning and memory processes. However, the role of basal forebrain activity in information retrieval mechanisms is less known. The aim of the present study is to evaluate the effects of reversible inactivation of the NBM by tetrodotoxin (TTX, a voltage-sensitive sodium channel blocker) during the acquisition and retrieval of conditioned taste aversion (CTA) and to measure acetylcholine (ACh) release during TTX inactivation in the insular cortex, by means of the microdialysis technique in free-moving rats. Bilateral infusion of TTX in the NBM was performed 30 min before the presentation of gustative stimuli, in either the CTA acquisition trial or retrieval trial. At the same time, levels of extracellular ACh release were measured in the insular cortex. The behavioral results showed significant impairment in CTA acquisition when the TTX was infused in the NBM, whereas retrieval was not affected when the treatment was given during the test trial. Biochemical results showed that TTX infusion into the NBM produced a marked decrease in cortical ACh release as compared with the controls during consumption of saccharin in the acquisition trial. Depleted ACh levels were found during the test trial in all groups except in the group that received TTX during acquisition. These results suggest a cholinergic-dependent process during acquisition, but not during memory retrieval, and that NBM-mediated cholinergic cortical release may play an important role in early stages of learning, but not during recall of aversive memories.
Keywords: conditioning taste aversion, free-moving microdialysis, gustatory cortex, cholinergic basal forebrain
The cerebral cortex has been considered as the ubiquitous place for the storage of long-term memory. In this regard, a great deal of investigation into brain health has focused on a particular central nervous system disorder, Alzheimer’s disease. This disease results, at least in part, from a deficit in acetylcholine (ACh) neurotransmission caused by a degeneration of large basal forebrain (BF) cholinergic neurons and a deficit in choline acetyltransferase, the enzyme that synthesizes ACh (1).
Accumulative evidence supports the role of cholinergic neurons in the BF in processes such as arousal, attention, learning, and memory. Behavioral deficits associated with lesions produced by injections of excitatory amino acid agonists into the nucleus basalis magnocellularis (NBM) have been demonstrated in a variety of tasks (2, 3). However, the behavioral deficits of these lesions might be caused not only by the resulting cholinergic deafferentation, because 30–35% of the population of BF projections neurons to the cortex are γ-aminobutyric acid-containing neurons (4–6).
In this regard, there are several experiments trying to study more directly the modulatory role of ACh in the activity of cortical neurons, which have brought their observations from electrophysiological and biochemical studies. It has been reported that ACh is released in the rat neocortex in response to a variety of behavioral and environmental conditions, including wakefulness (7), motor activity (8), restraint, or handling-induced stress (9) and by exposure to auditory, visual, and gustatory stimulation (10–12). In addition to these findings, which demonstrate differences in ACh release related to different states of arousal, other evidence suggests that associative conditioning (13–16) also may modify ACh release in the neocortex. Thus, it has been demonstrated that a significant enhancement of ACh release is associated with appetitive conditioning procedures. Training for tactile discrimination causes a specific enhancement in ACh release in the somatosensory cortex that is related to acquisition of discrimination performance (17). Additionally, Shimura and coworkers (12), using in vivo microdialysis, have shown that ACh release in the insular cortex (IC) is influenced by behavioral expression of aversive taste stimuli.
Conditioned taste aversion (CTA) has been widely used for studying the neurobiology of learning processes; the animal acquires aversion to a taste cue when it is followed by digestive malaise. The CTA model contains clearly defined anatomical substrates (18, 19), and several studies have demonstrated that the insular gustatory neocortex is strongly involved in the mnemonic representation of taste and can be disrupted by NBM lesions (20) or cortical cholinergic antagonists (21). Recently, experiments of NBM lesions have brought a differential role of cholinergic activity during learning and memory. For example, bilateral cortical administration of antibodies for nerve growth factor produced a nearly total loss of cortical ACh levels, and a significant decrement of cells in the NBM, as well as impaired acquisition, but not retrieval, of conditioned taste aversion and inhibitory avoidance (22). However, the role of the cortical ACh release, mediated by NBM afferents, during acquisition and retrieval of taste aversion memory, has not been assessed directly.
The purpose of the present experiment was to measure the effects of the bilateral blockage of the NBM on the ACh release in the IC of rats during acquisition and retrieval of CTA. To determine the in vivo effects, we used a microdialysis technique for freely moving animals to measure ACh release in the IC during a baseline period and during presentation of a gustatory stimulus. The rats were infused bilaterally via microdialysis probes in the NBM with tetrodotoxin (TTX; a reversible sodium channel-dependent-activity blocker) or buffer, 30 min before the presentation of a novel taste paired with gastric malaise (CTA acquisition) and, days later, during a second presentation of the taste (CTA retrieval).
MATERIALS AND METHODS
Animals.
Twenty male Wistar rats, weighing 275–325 g at the time of surgery, were used. They were housed under a 12-h light-dark cycle, with food and water ad libitum, except during behavioral tests.
Guide Cannula Implantation.
All the animals were anesthetized with pentobarbital (50 mg/kg) and were implanted with three microdialysis guide cannulae (CMA/12) by using standard stereotaxic procedures; two of them were bilaterally implanted into the NBM [anteroposterior (AP) = −0.5 mm, lateral (L) = ±2.5 mm; ventral (V) = −4.0 mm from Bregma] and the third cannula into the right IC (AP = +1.2 mm,; L = +5.5 mm from Bregma; V = −5.5 mm from dura). The guide cannulae were kept in place with three skull screws and dental acrylic cement.
Behavioral Procedures.
Two or three days after surgery, the animals were deprived of water for 24 h and then habituated to the microdialysis chamber, once a day during 45-min trials, and to drink water from a graded bottle during 15 min for 5 days, or until a stable water consumption baseline was reached. During the next day, the first microdialysis assay and the acquisition of CTA was performed. For the CTA acquisition, water in the graded tube was substituted with 0.1% saccharin solution, and 30 min later, when the microdialysis procedure was finished (see below), the animals were injected i.p. with a LiCl (0.4 M, 7.5 ml/kg) solution to induce digestive malaise (22). For the next 3 days, water baselines were recorded inside the microdialysis chamber, and, on the 10th day, microdialysis was carried out during retrieval (test) of CTA, the water was substituted for 0.1% of saccharin solution to test taste aversion. The saccharin consumption volume was taken as the taste aversion score (see ref. 23). ANOVA with repeated measures for taste aversion was done for acquisition and test aversion days and post-hoc paired t test analysis to compare groups.
Microdialysis Procedure.
Dialysis was started by connecting the probe inlet (dialysis probes CMA/12 from CMA/Microdialysis (Acton, MA), with a 3 mm total length of membrane) to the micro-infusion pump system (CMA/Microdialysis), which circulated the probe continuously at a rate of 2 μl/min with Ringer’s solution (118 mM NaCl/4.7 mM KCl/2.5 mM CaCl2). To achieve detectable amounts of ACh in the dialysate, we added 10 μM neostigmine bromide (Sigma) to the Ringer’s solution. Once the three probes were connected to the guide cannulae, the first 30-min sampling was discarded, and nine samples were collected every 15 min (30 μl/sample) and immediately frozen at −80°C or analyzed by HPLC (Beckman). The general microdialysis procedure in the bilateral NBM probes was as follows: during the first 1 h, 15 min the infusion medium contained only the Ringer’s solution; then TTX (1.0 μM previously diluted in buffer citrate 0.05 M) or Ringer’s solution plus buffer citrate (79.9 μM) was added to the infusion medium for the next 30 min. After this, the perfusion was changed again with Ringer’s solution alone and was perfused for an additional hour (see Fig. 1). The unilateral IC probe was perfused all the time (2 h, 45 min) with the Ringer’s solution alone. The graded tube with the gustatory stimuli (saccharin 0.1%) was placed in the microdialysis chamber 2 h after the placement of the probe and was retained 15 min during acquisition and test trials.
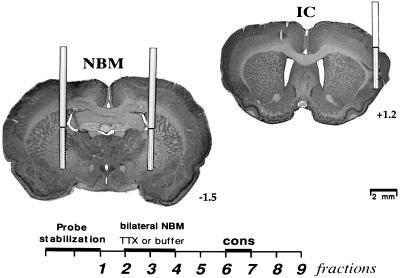
Figure 1.
Microdialysis procedure showing the number of fractions, 15 min each (Lower), and photomicrographs of cresyl violet staining of brain coronal sections, showing an schematic diagram of the probes localization in the IC and bilateral NBM (Upper).
During the first day of microdialysis, in the acquisition trial, the rats were separated in two groups (see Table 1). One group of animals was perfused in the bilateral NBM with buffer (as control group, BA n = 10), and the other group was perfused with TTX (TA, n = 10) during the third and fourth microdialysis fractions (see Fig. 1). Three days later, in the test trial, the two groups were divided again. The BA group was divided into two groups, one infused with buffer (as control group, BA-B n = 4), and the other infused with TTX (BA-T, n = 6). The TA group was divided into one infused with buffer (as control group, TA-B, n = 5), and the other infused with TTX (TA-T, n = 5).
Table 1.
NBM bilateral infusions procedure during third and fourth fractions of microdialysis in either acquisition or test trial of CTA
| Acquisition | Test |
|---|---|
| Buffer (BA) | Buffer (BA-B) |
| TTX (BA-T) | |
| TTX (TA) | Buffer (TA-B) |
| TTX (TA-T) |
The groups are shown in parentheses.
Measurement of ACh.
The collected samples were assayed for ACh content by using HPLC (Beckman) with electrochemical detection (BAS, West Lafayette, IN). The samples were injected into a polymeric reversed-phase column (BAS). ACh and choline then were converted into hydrogen peroxide and betaine in a post column enzyme reactor containing immobilized acetylcholinesterase and choline oxidase (BAS). The hydrogen peroxide was detected electrochemically by a platinum electrode set at 500 mV (vs. Ag/AgCl). The mobile phase consisted of a 50 mM sodium phosphate buffer (pH 8.5) and Kathon reagent (BAS). The detection limit was approximately 0.1 pmol.
Histology.
One day after microdialysis, the rats were deeply anesthetized with pentobarbital and perfused transcardially with a 4% solution of paraformaldehyde in phosphate buffer (0.15 M, pH 7.4). The brains were placed overnight in paraformaldehyde and then transferred to a 20% buffered sucrose solution and stored at 4°C until they were cut. Coronal sections (50 μM thick) were taken through the areas of the probe. The slide sections were stained for cresyl violet.
RESULTS
Verification of Probe Placement.
In all groups, the localization of the guide cannulae and probes was within the IC and in both NBMs. Fig. 1 shows a schematic diagram of the location of the probes from the NBM and IC used for the in vivo microdialysis analysis. One animal from the group TA-T was discarded from further analysis because of a misplaced cannula.
Behavioral Results.
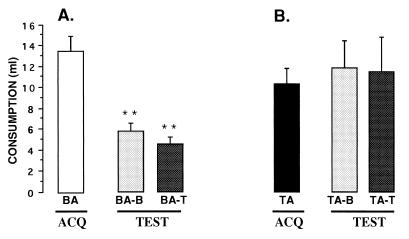
The results of the TTX effects on CTA are shown in Fig. 2. ANOVA with repeated measures was carried out for the groups that received buffer during acquisition (Fig. 2A). The analysis showed no significant differences among groups [F(1,8) = 2.135, P > 0.1] and significant differences between days of consumption [F(1,8) = 44.15, P < 0.001] and no interaction [F(1,8) = 0.722, P = > 0.420]. The saccharin consumption for groups BA-B and BA-T during the test was significantly lower when compared with their saccharin consumption on the acquisition day (paired t test P < 0.01). The TTX treatment during the test had no effect on conditioning, whereas the BA-T group presented a significant decrement in saccharin consumption, indicating that both groups showed strong taste aversions.
Figure 2.
Mean volume of saccharin consumption (± SEM) during acquisition and test of CTA. (A) The control group (BA) that received buffer in the NBM during acquisition in the test trial showed a significant saccharin aversion regardless of the treatment received during the test trial. That is, both the control group (BA-B) and the group that received TTX (BA-T) during the CTA test, showed taste aversion. (B) When TTX was perfused during the acquisition trial (TA), the buffer (TA-B) or TTX (TA-T) groups were not able to show taste aversion. ∗∗, paired t test; P < 0.01.
For the groups treated with TTX during acquisition (Fig. 2B), the ANOVA with repeated measures revealed that there were no significant differences between groups [F(1,7) = 1.14, P = 0.321], nor between days of consumption [F(1,7) = 0.187, P = 0.678] and no interaction [F(1,7) = 0.310, P = 0.595]. The groups that received TTX treatment during acquisition (TA) did not show any significant decrement of saccharin consumption in the taste trial (TA-B and TA-T), indicating that application of TTX into the IC during acquisition caused a significant disruption of CTA independently of the treatment applied during the test trial.
Release of ACh During CTA Acquisition.
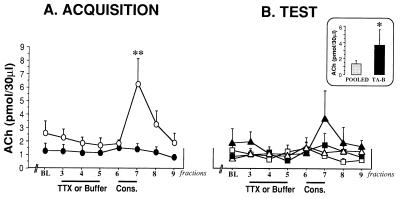
Fig. 3A shows the release of cortical ACh during saccharin consumption in the acquisition trial, from the NBM-treated groups with buffer or TTX (BA and TA groups). ANOVA with repeated measures was done with the mean of the two first samples, and then compared with the ACh release obtained during TTX and consumption fractions (3–9 fractions, Fig. 1A). The analysis revealed a borderline difference between groups [F(1,13) = 2.946, P = 0.1098], significant differences between fractions [F(1,13) = 5.3946, P < .0001], and a significant group x fraction interaction [F(1,13) = 3.125, P < 0.01]. To analyze the source of interaction, we performed a simple ANOVA during each of the fractions. Simple ANOVA of ACh levels revealed significant differences [F(1,17) = 6.079, P < 0.05] among groups in the seventh fraction taken after presentation of the gustatory stimuli (135 min after probe implantation). Fisher post-hoc analysis showed that there was a significant difference between control (BA) and the TTX-perfused group (TA) during acquisition trial (P < 0.05), indicating that saccharin consumption produced a significant increase in cortical ACh release in the control group (BA), whereas the TTX treatment produced a reliable blocking in ACh release.
Figure 3.
Mean extracellular levels of ACh in the IC during TTX or buffer perfusion in the acquisition trial (A) and during TTX or buffer perfusion in the retrieval trial (B). (Inset) The ACh release observed in the seventh fraction, after the consumption of the aversive stimuli. The group TA-B was compared with the pooled BA-B, BA-T, and TA-T groups. (BL = mean of the two first samples). ○, BA group; ●, TA group. ▵, BA-B group; ▴, TA-B group; □, BA-T group; ■, TA-T group. Values are means ± SEM. ∗∗, P < 0.01, ∗, P < 0.05.
Release of ACh During CTA Retrieval.
Fig. 3B shows the release of cortical ACh during the test trial, from the groups treated with buffer or TTX (BA-B, BA-T, TA-B, and TA-T groups) in the NBM, during saccharin consumption in the retrieval trial. ANOVA with repeated measures showed no significant differences between groups [F(3,12) = 1.609, P = 0.239], and there were no significant differences between fractions [F(3,12) = 1.621, P = 0.1405], and no group x fraction interaction [F(3,12) = 1.527, P > 0.09]. However, we found significant differences between groups when we compared the group TA-B with pooled groups BA-B-BA-T and TA-T [F(1,17) = 4.619, P < 0.05], because the last three groups did not show any differences among them (see Fig. 3B).
DISCUSSION
The present study demonstrates that consumption of a novel taste increases release of ACh within the IC of awake, freely moving rats at control conditions. Our results show a significant ACh increase after the control animals drank a saccharin solution, and a clear inhibition of that release when the animals were infused bilaterally with TTX into the NBM. Additionally, we demonstrate that TTX blockade of the NBM results in significant impairment in acquisition, but not retrieval, of CTA. The differential effects found indicate that blocking the NBM during the acquisition phase has effects on the performance of CTA, regardless of the status of the NBM during the evocation period of the behavior. Thus, the control group, which received buffer in the NBM during acquisition, showed a significant saccharin aversion in the test trial regardless of the treatment received during the test trial. In this way, both the control group and the group that received TTX during the CTA test showed taste aversion.
Conversely, the group that received TTX during acquisition showed disrupted taste aversion because its saccharin consumption during retrieval was similar to that in the acquisition trial, regardless of the treatment received during the test trial. Neither of the groups that received buffer or TTX during the test showed a significant decrement in saccharin consumption. Moreover, the group that received TTX during the acquisition and buffer during the retrieval test had an increase of ACh release similar to that observed in the control group during the acquisition trial. This result suggests that blocking the ACh release during acquisition interrupts the information about the novelty of the stimulus and provokes a cortical ACh release during the second presentation of that stimulus (test trial). Our results show that the release of cortical ACh, immediately after saccharin consumption during the acquisition trial, could be directly related to the possibility of recognition of the novel gustatory stimulus.
The TTX dose used in the present experiment was based on previous work (9, 24) and preliminary experiments of ACh release in the cortex after TTX blockage and simultaneous KCI stimulation of NBM, where the results showed a significantly TTX-blocked cortical ACh release. TTX did not affect other behavioral parameters that have been related to cholinergic afferents, for example, attention or motivation, given that both the control and TTX-treated groups show similar saccharin consumption during the acquisition trial. In the same way, TTX and the number of guide cannulae used did not affect the sensorial and discriminatory properties of the animals to the gustatory stimulus, because the groups treated with TTX or buffer during the test showed a significant taste aversion. The TTX produced a general blockage of neurotransmission for about 4 h (25), and the behavioral effects reported here may not only depend on the cholinergic afferents. It is well known that the principal afferents of the NBM are cholinergic and GABAergic (4–6). In this regard, it recently has been postulated that both cholinergic and GABAergic projections from the BF to the cortex play an important role in the induction of long-term changes in the somatotopic organization of primary somatosensory cortex, which involve a simultaneous γ-aminobutyric acid disinhibition and increased ACh release (26).
We have demonstrated that bilateral exitotoxic lesions of the NBM prevent the acquisition of CTA and produced a significant reduction of acetylcholinesterase and acetyltransferase activities of K+-stimulated [3H]acetylcholine release after the lesion. These lesions did not have any effect on the glutamate descarboxylase activity or γ-amino[14C]butyric acid release from IC slices (20). In addition, we recently demonstrated that repeated injections of anti-nerve growth factor into the IC produce a dramatic decrease of cortical ACh and a significant decrement of NBM-cortical afferents, which impaired acquisition, but not retrieval, of conditioned taste aversion and inhibitory avoidance. However, the learning deficits observed in these experiments might not only be the result of the resulting cholinergic deafferentation, because nerve growth factor-mediated signaling in the cortex is also necessary for sustaining neuronal metabolic and functional integrity, apart from the supporting the ascending cholinergic fibers (22). Given these results, it seems that the BF pathway is involved only in the acquisition of aversively motivated conditioning, and not in retrieval of aversive memories.
The results of this research demonstrate that the temporary blockage of the NBM disrupted the acquisition, but not the retrieval, of aversive memories and seems to be related with the in vivo ACh release. It should be pointed out that ACh is required during acquisition because the ACh increase observed during learning does not occur during test retrieval, and the retrieval of the learned information is still observed. These results suggest that cholinergic NBM projections to the cortex are related to the novelty of the stimulus. Preliminary results in our laboratory show that the cortical ACh release produced by a novel stimulus decreased after several presentations of the same stimulus, reaching the same ACh levels as those induced by water, indicating the role of ACh in the recognition or encoding of the novelty of the stimulus. In this regard, a recent work by Naor and Dudai (21) has suggested that cholinergic neuromodulation participates in memory formation to aversive stimulus in the IC, either by encoding novelty at the cellular level, or by instructing the neural circuits to store the novel taste representation (27). They showed that after CTA training there is a rapid and marked increase in tyrosine phosphorylation of a set of proteins in the IC but not in other brain areas. The enhancement of protein tyrosine phosphorylation produced by unfamiliar taste during acquisition of CTA could be mimicked by micro injections of carbachol and blocked by muscarinic antagonist. Moreover, ACh increments during consumption of novel gustatory stimuli, like saccharin or quinine, have been reported by Shimura and coworkers (12).
The involvement of the in vivo release of ACh in the associative phase of learning also has been demonstrated in the acquisition of operant behaviors. Thus, significant increments of ACh release occurred only when a steady rise in the number of lever pressings, indicating that the rats began to associate lever operation with reward (28). Conversely, when the animals were already well trained in lever pressing, the authors did not find any increase in ACh release in the cortex or in the hippocampus during performance. This finding indicates that the ACh release plays a role in the early stages of learning but not during the retrieval phase of the operant behavior.
Additionally, the role of the BF in learning-induced plasticity has been underlined by recent reports by Kilgard and Merzenich (29) and Weinberger and coworkers (30, 31). Weinberger et al. demonstrated that the convergence in the auditory cortex of acoustic frequency information and the application of cortical ACh or electrical stimulation of the nucleus basalis induced receptive field plasticity similar to that produced by behavioral learning, and this plasticity was blocked by muscarinic antagonists. Others have shown that cholinergic activation can facilitate the formation of long-lasting, experience-dependent changes in the response properties of neocortical sensory neurons in a variety of contexts (32–34). The above results suggest that the cholinergic system, as a neuromodulator, may facilitate cortical plasticity by signaling the stimulus relevance during memory formation and are in accord with the hypothesis that ACh modulates the general efficacy of the sensory cortical processing or association of information (35).
In conclusion, our results suggest that the increase in ACh release seen in the IC during acquisition of CTA in free-moving animals reflects the involvement of the cortical cholinergic system in encoding new items during the early stages of memory formation.
Acknowledgments
We acknowledge the assistance of Oreste Carbajal and Federico Jandete and give thanks to Shaun Harris for his text review, and to Yolanda Díaz de Castro for preparing the manuscript. This research was supported by Consejo Nacional de Ciencia y Technología (Mexico) Grant 3260-P-N9608.
ABBREVIATIONS
- NBM
nucleus basalis magnocellularis
- TTX
tetrodotoxin
- CTA
conditioned taste aversion
- ACh
acetylcholine
- IC
insular cortex
- BF
basal forebrain
References
- 1.Whitehouse P J, Price D L, Struble R, Clarke A W, Coyle J T, DeLong M R. Science. 1982;215:1237–1239. [Google Scholar]
- 2.Bartus R T, Flicker C, Dean R L, Pontecorvo M, Figueiredo J C, Fisher S K. Pharmacol Biochem Behav. 1985;23:125–135. doi: 10.1016/0091-3057(85)90139-x. [DOI] [PubMed] [Google Scholar]
- 3.Flicker C, Dean R, Watkins D L, Fisher S K, Bartus R T. Pharmacol Biochem Behav. 1983;18:973–981. doi: 10.1016/s0091-3057(83)80023-9. [DOI] [PubMed] [Google Scholar]
- 4.Fisher R S, Buchwald N A, Hull C D, Levine M S. J Comp Neurol. 1988;272:489–502. doi: 10.1002/cne.902720404. [DOI] [PubMed] [Google Scholar]
- 5.Freund T F, Meskenaite V. Proc Natl Acad Sci USA. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones B F, Mainville L, Mancia M, Gritti I. Soc Neurosci Abstr. 1995;21:1617. [Google Scholar]
- 7.Jiménez-Capdeville M E, Dykes R W. Brain Res. 1993;625:152–158. doi: 10.1016/0006-8993(93)90148-g. [DOI] [PubMed] [Google Scholar]
- 8.Day J, Damsma G, Fibiger H C. Pharmacol Biochem Behav. 1991;38:723–729. doi: 10.1016/0091-3057(91)90233-r. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblad C, Nilsson O G. Neuroscience. 1993;55:353–362. doi: 10.1016/0306-4522(93)90504-9. [DOI] [PubMed] [Google Scholar]
- 10.Inglis F M, Fibiger H C. Neuroscience. 1995;66:81–86. doi: 10.1016/0306-4522(94)00578-s. [DOI] [PubMed] [Google Scholar]
- 11.Kurosawa M, Sato A, Sato Y. Neurochem Intl. 1992;21:423–427. doi: 10.1016/0197-0186(92)90194-v. [DOI] [PubMed] [Google Scholar]
- 12.Shimura T, Suzuki M, Yamamoto T. Brain Res. 1995;679:221–226. doi: 10.1016/0006-8993(95)00225-f. [DOI] [PubMed] [Google Scholar]
- 13.Acquas E, Wilson C, Fibiger H C. J Neurosci. 1996;16:3089–3096. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inglis F M, Day J C, Fibiger H C. Neuroscience. 1994;62:1049–1056. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 15.Rasmusson D, Szerb J C. Brain Res. 1976;104:243–259. doi: 10.1016/0006-8993(76)90617-x. [DOI] [PubMed] [Google Scholar]
- 16.Sarter M F, Bruno J P. Trends Neurosci. 1994;17:217–221. doi: 10.1016/0166-2236(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 17.Butt A E, Testylier G, Dykes R. Psychobiology. 1997;25:18–33. [Google Scholar]
- 18.Kiefer S W. Ann NY Acad Sci. 1985;443:100–109. doi: 10.1111/j.1749-6632.1985.tb27066.x. [DOI] [PubMed] [Google Scholar]
- 19.Bures J, Bermúdez-Rattoni F, Yamamoto T. Conditioned Taste Aversion: Memory of a Special Kind. New York: Oxford Univ. Press; 1998. [Google Scholar]
- 20.López-García J C, Fernández-Ruiz J, Escobar M L, Bermúdez-Rattoni F, Tapia R. Pharmacol Biochem Behav. 1993;45:147–152. doi: 10.1016/0091-3057(93)90098-e. [DOI] [PubMed] [Google Scholar]
- 21.Naor C, Dudai Y. Behav Brain Res. 1996;79:61–67. doi: 10.1016/0166-4328(95)00262-6. [DOI] [PubMed] [Google Scholar]
- 22.Gutiérrez H, Miranda M I, Bermúdez-Rattoni F. J Neurosci. 1997;17:3796–3803. doi: 10.1523/JNEUROSCI.17-10-03796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miranda M I, López-Colomé A M, Bermúdez-Rattoni F. Brain Res. 1997;759:141–148. doi: 10.1016/s0006-8993(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 24.Bermúdez-Rattoni F, Introini-Collinson I B, McGaugh J L. Proc Natl Acad Sci USA. 1991;88:5379–5382. doi: 10.1073/pnas.88.12.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuravin I A, Bures J. Exp Brain Res. 1991;83:687–690. doi: 10.1007/BF00229849. [DOI] [PubMed] [Google Scholar]
- 26.Dykes R W. Can J Physiol Pharmacol. 1997;75:535–545. [PubMed] [Google Scholar]
- 27.Rosenblum K, Berman D E, Hazvi S, Dudai Y. NeuroReport. 1996;7:1401–1404. doi: 10.1097/00001756-199605310-00015. [DOI] [PubMed] [Google Scholar]
- 28.Orsetti M, Casamenti F, Pepeu G. Brain Res. 1996;724:89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- 29.Kilgard M P, Merzenich M M. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 30.Metherate R, Weinberger N M. Synapse. 1990;6:133–145. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- 31.Bakin J S, Weinberger N M. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butt A E, Testtylier G, Dykes R. Psychobiology. 1997;25:18–33. [Google Scholar]
- 33.Baskerville K A, Heaston N R, Schweitzer J B, Herron P. Soc Neurosci Abstr. 1995;21:123. [Google Scholar]
- 34.Bear M F, Singer W. Nature (London) 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 35.Sarter M, Bruno J P. Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]