Abstract
In blastocyst chimeras, embryonic stem (ES) cells contribute to embryonic tissues but not extra-embryonic trophectoderm. Conditional activation of H-Ras61L in ES cells in vitro induces the trophectoderm marker Cdx2 and enables derivation of trophoblast stem (TS) cell lines that, when injected into blastocysts, chimerize placental tissues. Erk2, the downstream effecter of Ras-Mitogen Activated Protein Kinase signaling, is asymmetrically expressed in the apical membranes of the 8-cell stage embryo just prior to morula compaction. Inhibition of MapK signaling in cultured mouse embryos compromises Cdx2 expression, delays blastocyst development, and reduces trophectoderm outgrowth from embryo explants. These data show that ectopic Ras activation can divert ES cells towards extraembryonic trophoblastic fates and implicate Ras-MapK signaling in promoting trophectoderm formation from murine embryos.
The blastocyst is the first embryonic stage with anatomical distinction of more than one cell type—the inner cell mass (ICM) and trophectoderm (TE). ICM and TE cells have distinct fates and do not transdifferentiate when transplanted to ectopic positions in the embryo 1. Embryonic Stem (ES) cells derive from the ICM and can differentiate into all tissue lineages of the adult. The TE-derived Trophoblastic Stem (TS) cells 2 contribute exclusively to the extraembryonic trophoblastic tissues of the placenta. The hypoblast of mature blastocysts gives rise to extraembryonic endoderm (XEN) stem cells, which generate parietal and visceral endoderm 3. These three stem cell types each express transcription factors that mark the segregation of these lineages: Oct4 and Nanog in ES cells; Cdx2 in TS cells; and Gata6 in Xen cells. The signaling pathways that segregate these lineages remain poorly defined. Here we show that expression of an activated Ras allele and growth in selective culture conditions diverts ES cells from embryonic to trophoblastic fates. Furthermore, inhibition of MAP kinase compromises trophectoderm function in murine embryos and outgrowth of trophoblastic tissue in explant cultures, implicating Ras-Map kinase signaling in regulating the emergence of extra-embryonic cell lineages in early development.
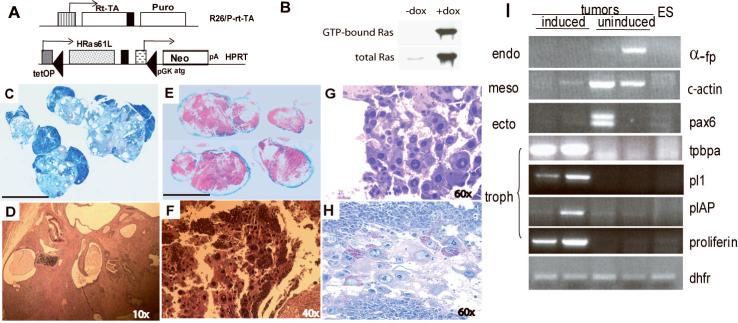
We originally set out to test the hypothesis that expression of an activated Ras gene might complement myc and telomerase function to induce malignant transformation in ES cells, in agreement with classical oncogene cooperation models 4 5. We engineered the mouse ES cell line Ainv15 6 to express an activated Ras allele (H-RasQ61L) in a doxycycline-inducible manner (iRasES cells; Fig. 1A &1B). We tested the effect of Ras activation on tumor formation of iRasES cells in immune-deficient mice (Rag2-/-γc-/-), by adding doxycycline to the drinking water 7. In control animals not given doxycycline, the iRasES cells form large well-differentiated benign teratomas (Fig. 1C and 1D). In contrast, animals fed doxycycline succumbed rapidly (beginning 12 days after cell inoculation) to aggressive tumors with massive internal hemorrhage (Fig. 1E). Tumor histology revealed giant cells with glycogen-containing inclusion bodies 8 (Fig. 1F, G and H), consistent with choriocarcinoma, a malignancy of proliferating trophoblast. Interestingly, developing mouse embryos that express H-Ras have previously been shown to induce tumors of extra-embryonic trophectodermal tissue 9.
Figure 1. Induction of expression of activated Ras promotes formation of trophoblastic tumors from ES cells.
(A) Schema for generating inducible HRasQ61L ES cells. Ainv15 mouse embryonic stem cells 6 have the rtTA integrated at the ROSA 26 locus on chromosome 6. HRasQ61L was inserted by Cre-mediated recombination of a targeting vector (plox) into the region of the HPRT locus so that it is expressed from the tetracycline response element (tetOP). Successful recombination regenerates an ATG-truncated neomycin (G418) resistance gene (Neo) driven by the pgk promoter (PGK-ATG). (B) Ras activation assay (Upstate Bioscience). Upper blot: Co-precipitation with Raf indicates expression of active GTP-bound Ras. Lower blot: Expression of total Ras can be detected by immunoblot in doxycycline-induced cells with anti-Ras antibody. Lane 1—Un-induced (no doxycycline); lane 2—induced with doxycycline. (C-H) tumors obtained from iRasES cells implanted in Rag2-/-γc-/- mice. Scale bars in C&E: 1cm. Magnification is indicated in each panel. (C) Cross-section of teratoma from control mice (no doxycyline); (D) Histology of teratoma in C showing tissue complexity (Hematoxylin and Eosin Staining); (E) Hemorrhagic tumors isolated from mice fed doxycycline; (F, G) Histology of tumors in E, showing clusters of giant cells (H&E staining). (H) Periodic Acid Schiff (PAS) staining of sections of tumors from doxycycline-induced animals, showing glycogen-rich granules. (I) RT-PCR analysis of gene expression of two teratomas from control animals (un-induced) and hemorrhagic tumors from two mice fed doxycycline (induced). Dihydrofolate reductase (DHFR) is used as a loading control for this analysis. α-fp(a-feto protein), c-actin (cardiac-actin) and pax6 are markers of differentiation toward endoderm, mesoderm and ectoderm, respectively. Markers for trophectoderm: for trophoblastic giant cells, pl-1 (placenta lactogen 1) and plAP (placenta alkaline phosphatase); for spongiotrophoblasts, tpbpα (trophoblastic specific protein alpha) and proliferin.
Teratomas formed from un-induced iRasES cells expressed markers of the embryonic germ layers but not markers of differentiated trophectodermal tissues, whereas the tumors that formed following Ras induction lacked markers of embryonic germ layers and instead expressed markers of spongiotrophoblast (trophoblast specific protein alpha, tpbpa; and placental lactogen 1, pl1) and primary and secondary giant cells (placental alkaline phosphatase, plap; and proliferin, plfr; Fig. 1I). These data confirm that Ras gene activation in ES cells promotes differentiation into trophectodermal lineages that typically are not observed in teratomas formed from ES cells. We failed to observe tumor formation following injection of embryo-derived TS cells into immune deficient mice (N=10), thereby demonstrating that TS cells behave differently than ES cells with Ras activation.
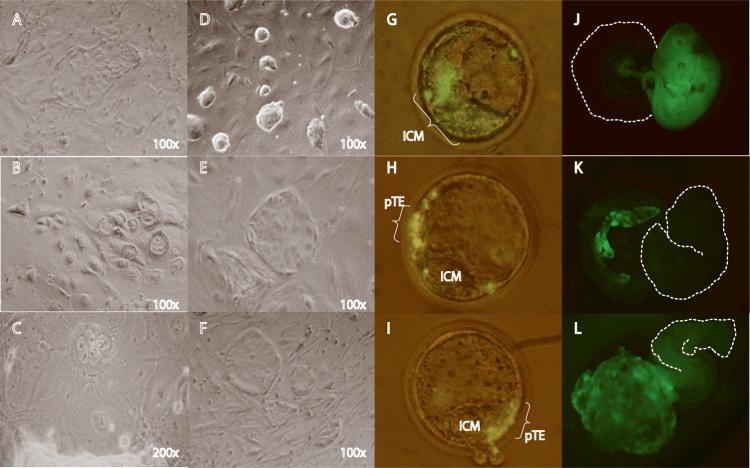
Given the multiple trophoblast cell types in Ras-induced tumors, we reasoned that activation of Ras signaling might prompt ES cells to transdifferentiate through a TS cell intermediate. When cultured in medium containing leukemia inhibitory factor (LIF), iRasES cells expressing activated Ras eventually formed flattened colonies of epithelial-like cells (Fig. 2A). When LIF was removed, the cells differentiated into trophoblastic giant cells (Fig. 2B) and syncytial trophoblasts (Fig. 2C). Replacing LIF with Fgf4 in ES cell culture maintained the colony morphology of ES cells (Fig 2D). However, Ras induction coupled to culture in Fgf4 and heparin, media conditions that promote isolation of TS cells from blastocysts, produced flat colonies (Fig. 2D) that closely resembled blastocyst-derived TS cells (which we call ES-TS cells; Fig. 2F).
Figure 2. Trophoblastic stem cell establishment from iRasES cells.
A-E: in vitro culture of iRas ESCs in various conditions: (A) culture in doxycycline (dox) and leukemia inhibitory factor (LIF) for 5 days. (B and C) Formation of giant cells (B) and syncytium (C) in iRasES culture when dox was present, without Fgf4 and LIF. (D) Colony morphology of iRas ES cells cultured in ES media supplemented with Fgf4 (without dox). (E) Colony morphology of iRasES cultured in Fgf4 and dox for two weeks. (F) Colonies from blastocyst-derived trophoblastic stem cells 2. G-L, iRasES cell derived trophoblastic stem cells (ES-TS) chimerize the trophectoderm and placenta of the developing embryo. (G) Parental iRasES cells labeled with the lipophilic dye pKH26 (Sigma), injected into 4-cell embryos, and examined by fluorescence microscopy at the blastocyst stage. Note green cells within ICM (representative image from 8 out of 8 injected embryos). (H) ES-TS cells derived from culture of iRasES cells in Fgf4 and dox, labeled with pKH26, injected into 4-cell embryos, and examined at the blastocyst stage. Note green cells in polar trophectoderm. (representative image from 5 out of 8 embryos injected. Green cells were undetectable in the other 3 embryos) (I) Blastocyst-derived TS cells2 labeled with pKH26, injected into 4-cell embryos, and examined at the blastocyst stage. (representative image from 3 out of 3 embryos injected) (J-L) Fluorescent images of embryos resulting from blastocysts chimerized by iRasES cells and iRasES cell-derived ES-TS cells. Cells were transduced by a lentivirus carrying Green Fluorescent Protein (GFP). (J) Chimeric embryo injected with parental iRasES cells. (K, L) Chimeric placental tissues of embryos injected with iRasES cell-derived ES-TS cells. Margins of embryo or dissected placental tissues are outlined.
We observed that trans-differentiation of ES into ES-TS is robust, occurring in essentially all cells (supplemental note1). In contrast, reversion of established TS cells back to an ES-like phenotype by withdrawal of Ras induction occurs rarely: we obtained one revertent clone out of 10e7 cells seeded in doxycycline-free media, suggesting that the differentiation of ES cells into TS cells involves mechanisms of tight and largely irreversible epigenetic restriction.
We labeled the parental iRasES cells or the clonal ES-TS cells with the fluorescent dye pKH26, and injected them into 4-8 cell stage murine embryos in order to follow their fate in blastocyst chimeras. At day 3.5, iRasES cells were found within the ICM (Fig. 2G) whereas ES-TS cells (Fig. 2H) and blastocyst-derived TS cells (Fig 2I) were excluded from the ICM and found exclusively within the polar trophectoderm. For tracing ES-TS cell fate beyond the blastocyst stage, ES-TS cells were transduced with a GFP-expressing lentivirus 10, injected into embryos, and transferred into pseudo-pregnant foster mothers. At E13.5, uninduced iRasES cells contribute widely to fetal tissues (Fig. 2J), whereas progeny of the ES-TS cells are excluded from the embryo proper and found exclusively in the placenta (Figs. 2K and 2L). These results suggest that ES-TS cells resemble TS cells in their ability to differentiate into trophoblastic cell lineages and reconstitute placental tissue during development. Importantly, because the foster mothers are not fed doxycycline, the ES-TS cells do not generate tumors in the chimeric animals. Instead, the cells respond to the placental and fetal microenvironment of the developing embryo so that trophoblast cell growth and differentiation proceeds normally (supplemental note2).
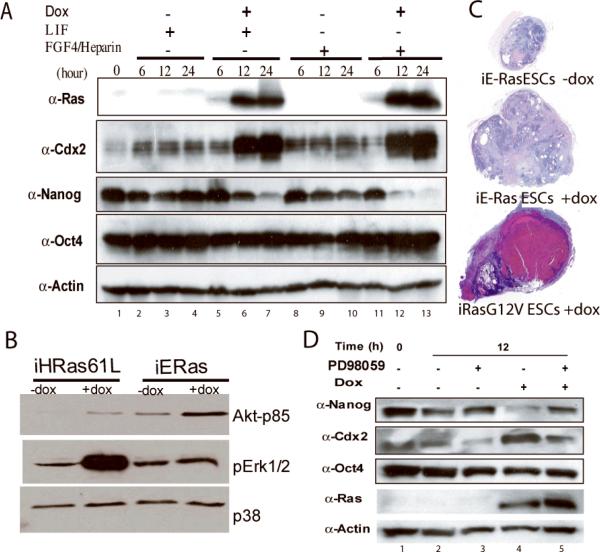
We set out to investigate the temporal relationship between Ras activation in ES cells and the expression of the cell-fate regulators Oct3/411, Cdx2 12, and Nanog 13. The iRasES cells were plated on gelatin without MEFs in four different media conditions: LIF, with and without doxycycline; and Fgf4/Heparin, with and without doxycycline. Doxycycline addition led to marked induction of Ras protein expression within 6 hours, which became maximal by 12 hours (Fig 3A, lanes 5-7, and lanes 11-13). The increase in Ras protein expression correlated with a marked decrease in Nanog and increase in Cdx2 expression, detectable after 12 hours of culture (Fig 3A, lanes 5-7, and lanes 11-13). These changes were dependent upon Ras expression, as culture in Fgf4/Heparin alone did not provoke changes in Cdx2 (Lanes 8-10). We also observed induction of Cdx2 and Hand1, another marker of trophectoderm, in two independent experimental contexts: in V6.5 ES cells expressing H-Ras61L from a retroviral vector, and in ES cells in which the K-Ras G12V allele was expressed at physiologic levels from the endogenous locus (Supplemental figureS2).
Figure 3. Expression of Cdx2, Nanog, and Oct4 in response to Ras/MapK signaling in ES cells.
(A) iRasES cells cultured as indicated for 6, 12 and 24 hours. Cell lysates were analyzed by immunoblot to detect protein levels of Ras, Cdx2, Nanog, Oct4 and Actin. (B) Cell lysates from iRasES cells (H-RasQ61L) and iE-Ras ES cells prepared after 48 hours of culture, and immunoblotted with antibody to activated phospho-proteins, as indicated. (C) Tumors formed by ES cells with or without doxycycline-induction of ERas or H-RasG12V, as indicated. (D) Cell lysates of iRasES cells treated for 12 hours with doxycycline and/or the MapK inhibitor PD98095, analyzed by immunoblot with the antibodies indicated.
Interestingly, the previously observed reciprocal inhibition between Cdx2 and Oct4 14 is not recapitulated in our system. Within the first 24 hours of Ras induction the expression level of Oct3/4 protein (Fig. 3A, lanes 5-7, 11-13) and RNA (by microarray analysis, data not shown) remained unchanged. We therefore hypothesize that a reciprocal interaction between Cdx2 and Nanog might contribute to lineage segregation. Indeed, aberrant expression of Nanog can be found in the TE of Cdx2 deleted embryos 12.
We engineered the Ainv15 ES cell line to conditionally express E-Ras, an ES-cell specific Ras isoform (iE-RasES cells15). E-Ras expression led to an increase in PI3Kinase activity, as measured by phosphorylation of p85Akt (pAkt), without altering MapK activity, as measured by phosphorylation of Erk1/2 (pErk1/2; Fig 3B). In contrast, induction of the activated H-RasQ61L allele in iRasES cells led to a marked increase of pErk1/2 and a modest increase in pAkt. In assays of tumor formation in immune-deficient mice, the iE-RasES cells formed larger teratomas when mice were fed doxycycline, but did not induce trophoblastic differentiation, while expression of a distinct activated allele of H-Ras (G12V), known to signal through MapK preferentially over PI3Kinase, caused the hemorrhagic choriocarcinoma (Fig. 3C). The induction of trophectoderm from ES cells thus appears to require signaling through the MapK-Erk1/2 pathway rather than the PI3Kinase pathway.
Induction of H-RasQ61L is associated with changes in the expression of the transcription factors Cdx2 and Nanog (Fig. 3). Consistently, inhibition of MapK signaling with the inhibitor PD98059 maintained Nanog expression and blunted induction of Cdx2 expression (Fig 3D, lane 4&5). Inhibitors of JNK, p38 and PI3Kinase did not alter the induction of Cdx2 expression (data not shown). These data link Ras-MapK signaling to the establishment or maintenance of trophectoderm, and provide a rationale for the common practice of including PD98095 in media used during ES cell derivation 16, which is likely acting to suppress the outgrowth of trophectoderm from cultured blastocysts.
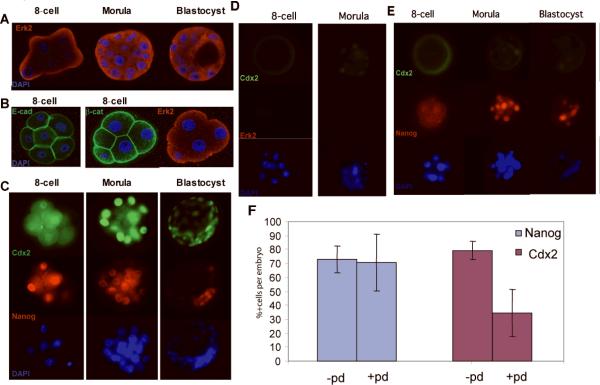
In the mouse embryo, K-Ras and other small GTP-binding proteins are activated during the 8-cell to morula transition 17. Given that our biochemical evidence that Ras-MapK signaling can divert the fate of ES cells into trophectoderm, we investigated whether Ras-MapK/Erk activity might be asymmetrically localized between the inner and outer cells of the embryo at the onset of morula compaction. We used an antibody specific for Erk2 to examine the temporal-spatial expression of this critical signaling intermediate in mouse embryos during the transition of blastomeres to morula to blastocyst. Confocal microscopy revealed polarized expression of Erk2 at the apical membrane of peripherally-localized blastomeres at the 8-cell stage, while at later stages Erk2 was distributed evenly throughout all cells of the morula and blastocyst (Fig. 4A). This pattern contrasted with and was complementary to the basolateral expression of E-cadherin (Fig. 4B, left panel), and is distinct from the pattern of β-catenin staining that appears uniform along both apical and basolateral membranes (Fig 4B, right panel). This asymmetric pattern of Erk2 localization at the 8-cell stage contrasts with the uniform distribution of Cdx2 and Nanog, which are detected equally in all blastomeres (Fig. 4C, left panels). Only later, when embryos develop further to the morula stage, do the outer cells assume stronger Cdx2 expression, while the inner cells show stronger Nanog expression (Fig. 4C, center panels). At the blastocyst stage, Cdx2 expression is restricted to the outer TE cells while Nanog is detected exclusively within the ICM (Fig. 4C, right panels). Our observation of asymmetric Erk2 expression at the apical surface of the 8 cell murine embryo, prior to the point at which blastomeres have adopted a clear fate, implicates Ras-MapK signaling as a possible upstream effecter of trophectoderm fate.
Figure 4. Ras-MapK signaling regulates Cdx2 expression and trophoblast outgrowth in mouse embryos.
(A-E) Immunofluorescence images of mouse embryos at 8-cell, morula and blastocyst stage. (A) confocal image of embryos stained with antibodies against Erk2, showing apical distribution at 8 cell stage and diffuse staining of morula and blastocyst. (B) Distribution of E-cadherin and β-catenin in 8-cell stage embryo. Embryos were stained with primary antibodies against E-cadherin and β-catenin. The left panel shows an 8-cell stage embryo stained with E-cadherin alone. The right panel shows another embryo co-stained with β-catenin and Erk-2. Co-staining of E-cadherin and pErk2 is not shown due to cross-reactivity of the antibodies. (C) Standard epifluorescence images of embryos stained with anti-Cdx2 and anti-Nanog, counter-stained with Dapi-antifad gold. (D and E) Expression of Cdx2, Nanog and Erk2 in embryos exposed to the MapK inhibitor PD98095 (20μM) for 24 hours. (F) Percentage of Nanog and Cdx2 positive cells within morula-stage embryos with or without exposure to PD98095. 6 embryos were scored in non-treated experiments, 7 embryos were scored in PD98059 treated experiments. Total cell numbers and Nanog/Cdx2 positive cell numbers in two experiments are listed in supplemental table 1. Error bars reflect standard deviations. p=0.780917 for Nanog expression; p= 0.000201 for Cdx2 expression, by two-tailed Student test.
To test whether MapK signaling could be functionally linked to trophectoderm formation, we investigated whether blockade of Erk2 signaling might compromise Cdx2 expression and alter embryo development in vitro. Treatment of 8 cell stage embryos with the Map kinase inhibitor PD98059 caused a loss of expression of Erk2 and phospho-Erk1/2 (p44/p42) (Fig. 4D and supplementary figure 1), and markedly attenuated Cdx2 expression within the morula and blastocyst (Fig 4D, E, and F). The effects of Map kinase inhibition were specific to Cdx2 expression, as the expression of Nanog and Nanog-positive cells was unaffected (Fig. 4E,F). We observed that blockade of Ras-MapK signaling is associated with a marked developmental delay of the embryos, as determined by progression to blastocoele formation (Fig. 4G). Importantly, this effect requires exposure of the embryos at the 8 cell stage, when Erk2 signaling is asymmetrically localized. If the PD98059 compound is applied at the morula stage, no effect on Cdx2 expression or morula cavitation is observed (data not shown), suggesting a critical period for Erk2 action. The delay in blastocoele formation is likewise accompanied by a decrease in the outgrowth of trophoblastic giant cells when PD 908059-treated embryos were explanted into tissue culture (supplemental Fig. S3). These data implicate Ras-MapK signaling in maintaining the integrity and function of trophectoderm and in sustaining the outgrowth of trophoblastic elements in the developing murine embryo.
We failed to observe defects in blastocyst formation when we treated murine embryos with inhibitors of p38 JNK and PI3 kinase (not shown). These results disagree with reports that blastocoele formation is abrogated by JNK inhibition but not MapK inhibition 18. These apparent discrepancies may be due to our use of different embryo culture media (KSOM, vs. M2), different MapK inhibitors (PD98059, vs. U0126), or a different duration and timing of inhibitor administration. Moreover, prolonged treatment with U0126 can cause a compensatory induction of MAPK1 (Erk2) 19. Ras-MapK activation has been linked to Gata6 expression 20 and primitive endoderm (PE) specification in the murine embryo 21. By titrating doxycycline dose, we explored the effect of varying levels of Ras expression on the induction of markers of trophectoderm and primitive endoderm. At low levels of Ras, the iRas ES cells express both trophectoderm markers Cdx2 and Hand1 as well as the primitive endoderm marker Gata6. At higher levels of Ras, trophectoderm markers predominated (supplemental Fig. 2). Therefore, it is likely that Ras-MapK signaling plays a role in two sequential developmental steps within the early murine embryo: a position-dependent segregation of TE and ICM at the 8-cell to morula transition, and a position-independent segregation of epiblast and hypoblast.
Our results demonstrate that Ras-MapK signaling leads to transcriptional upregulation of Cdx2 within an hour. The trophectoderm specification factor Cdx2 is a phosphoprotein, but we have found no evidence that activation of Ras-MapK signaling alters Cdx2 phosphorylation (data not shown). It is possible that Ras mediates Cdx2 upregulation through a cascade of transcription factors, such as Sp1/Sp3 and Ap2gamma. The Ap2 gamma deficient mouse embryo displays defects in trophectoderm and is completely devoid of Cdx2 expression 22. Protein and RNA levels of Ap2 gamma are rapidly elevated when Ras expression is induced in the iRas ES cells, supporting this hypothesis (supplemental figure S4).
In murine models, gene knock-outs of Erk2 23, Cdx2 12, and AP2 γ 22 have shown defects in ectoplacental cone formation and implantation, thereby implicating them as critical regulators of trophectoderm development. These genes are also expressed in the blastocyst, but their deficiency in these genetic knock-out models does not appear to compromise the specification of trophectoderm, perhaps because the early embryonic effects of these genes are mediated by maternal RNA. Analyzing these factors in complex maternal and zygotic gene knock-outs is required to define their precise role in specifying the trophectoderm lineage. The actions of these genes behave differently than that of TEAD4, an early onset zygotic transcript critical for TE formation 24.
While recent reports have suggested that developmental asymmetry may be initiated as early as the 4-cell stage of the cleaving mouse embryo 25, the classical model holds that distinct cell fates are not irreversibly established until the 8-16 cell transition, which entail cleavages that yield daughter cells differing in size and identity 26 27.
The opposing effect of Ras-MapK and E-cadherin/β-catenin signaling is a well-defined mechanism for determining polarity in cultured epithelial cells 28. The apical restriction of Erk2 in the 8-cell embryo in a pattern complementary to the basal-lateral distribution of E-cadherinβ-catenin (figure 4 and 29) is similar to that of Par3, Par6 and aPKC, components of an apical polarity complex that has also been shown to influence TE/ICM fate choice 30. Our data establish that activation of Ras-MapK signaling can divert the fate of ES cells towards trophectoderm, and that blockade of Erk2 function in the murine embryo compromises Cdx2 expression and blastocoele formation, and delays embryo development. We cannot conclude with certainty that the apical localization of Erk2 within the 8-cell embryo is directly responsible for establishing the trophectoderm lineage, but our data, taken together with genetic models that implicate Erk2 and Ap2γ in early placental function, further establishes a role for Ras-Map kinase signaling in promoting trophectoderm integrity and function in murine development.
Methods
Tumor pathology and animal care
Tumors and teratomas were obtained by subcutaneous injection of 1 million ESCs into Rag2(-/)γC(-/-) mice. Fixation, sectioning and staining were performed by the Rodent Pathology Core Facility of the Harvard Medical School. A protocol for animal handling and maintenance in this study has been approved by the ARCH committee of the Children's Hospital Boston.
Cell culture
Embryonic stem cells were cultured with 1000U/ml ESGRO, 15% fetal bovine serum (Hyclone), 2mM glutamine, penicillin and streptomycin, 0.1mM each of non-essential amino acids (Invitrogen). Blastocyst derived trophoblastic stem cells were provided by Janet Rossant and cultured in TS culturing media containing: RPMI 1640, 20% serum (Hyclone), Fgf4 (25ng/ml), Heparin (1ng/ml), 100μM β-mercaptoethanol, 1mM sodium pyruvate, 2mM glutamine, penicillin and streptomycin (Invitrogen).
Targeting constructs for generating inducible cell lines
HRasQ61L vector (Upstate) was subcloned into the pLox vector. The G12V mutation was introduced by the Quick-change mutagenesis kit (Stratagene) into the plox-HRasQ61L vector, and the 61L mutation was changed back to wild-type (61Q). ERas was amplified from cDNA of ESCs and cloned into the plox vector. All the plox constructs containing Ras mutants have been verified by sequencing, and subsequently transfected into Aniv15 cells by electroporation. Successfully targeted clones were selected in G418 (300mg/ml).
Embryo chimera
Host embryos were obtained from C57BL/6 mice and were collected at the two cell stage. To observe chimerization at the blastocyst stage, ~15 ES cells or ES-TS cells were labeled with pKH26 (Sigma) and injected into the 4-8 cell stage embryo. After injection, the embryos were cultured in vitro in KSOM+AA (Specialty media). For observation of chimerism in fetuses, 15 ES or ES-TS cells labeled with GFP by lentiviral vectors were injected into blastocyst stage embryos, followed by uterus transfer into pseudopregnant CD1 females.
RT-PCR for cell lineages analysis
Tumor samples were dissociated with 0.5% collagenase and processed in RNA-STAT. Total RNA was used to generate cDNA by amplification with random hexamers and RTIII reverse transcriptase (Invitrogen). cDNA was amplified for 28 cycles, with annealing temperature of 48C.
Embryo collection, culture and staining
Mouse embryos were collected from CD1 mice (Charles River) that were superovulated by PMSG and HCG (7.5IU each, Sigma). Embryos from C57/B6 mice were also tested but no apparent differences were observed. After removing cumulus cells (Hyaluronidase 0.03%, Sigma), embryos were collected at 1.5 dpc and incubated in KSOM+AA (Specialty media) until different developmental stages. For MapK inhibitor experiments, embryos were incubated in KSOM+AA containing 20μM of PD98095 (Calbiochem) for 24 hours prior to fixation and staining. 8-cell stage pre-compaction embryos (identified 24 hours after collection of 2-cell embryos from oviducts) were assessed for the kinetics of blastocoele formation by incubation in KSOM+AA with or without 20μM PD98095. Embryos were observed after an additional 24 hours, and number of embryos progressing to a particular stage were noted.
Whole mount immunostaining procedure is as follows: embryos were fixed in 4% paraformaldehyde (20 min), permeabilized with 0.2%Triton X-100 (30 min), and blocked with 3% BSA/PBS (2 hours), followed by binding of primary (overnight) and secondary antibodies (2 hours). The primary antibodies used were: anti-Cdx2 (Biogenex Mu392-UC, 1:50); anti-Nanog (Abcam Ab21603-100, 1:200); anti-pErk-2 (Cell-signaling technology 9108, 1:1000); anti-phosphor-Erk1/2 (Cell Signaling Technology, 9106, 1:1000), anti-β-catenin (BD Pharmingen #610153, 1:1000) and anti-E-cadherin (BD pharmingen#610182, 1:1000). Alexa flour 488 anti-mouse antibody and Alexa flour 596 anti-rabbit antibody (Molecular probe, A11029 and A11037). Epifluoresence microscopy was performed on an inverted Leica microscope. The confocal images were captured using a Zeiss LSM510 Meta NLO Laser Scanning Confocal Microscope, consisting of a Zeiss Axioplan 2 upright microscope with 10x (0.2NA) dry objective, 25x (0.75NA) multi-imersion, 40x (1.3NA) oil, 63X (1.4NA) oil immersion objective, a Melles Griot 50mW 488/568/647 nm Kr/Ar multi-line laser, HE/NE lasers (543 and 633 nm), and a water-cooled two-photon laser (Chameleon, Coherent). A Fujitsu Seimens Scaleo 600 workstation was used for computer control of the system.
Western immunoblot analysis
iRasES cells were plated on gelatin-treated 6-well plates (at 106 cells/well) in media containing Lif or Fgf4/heparin, as indicated in the figure. At time points after plating and induction, the cells were harvested, boiled with SDS sample buffer and resolved by 12.5% SDS-PAGE (Bio-Rad), followed by transfer onto a PVDF membrane. Antibodies used for detections were anti-Cdx2 (Biogenex, Mu392-UC), anti-Nanog (Bethyl Laboratories, A300-397A), anti-Ras (Upstate, 05-516), anti-Oct4 (Santa Cruz, sc-5279) and anti-Actin (Abcam, ab8266), Western blotting for phosphorylated forms of ERK1/2, p38, and Akt was performed with antibodies purchased from Cell Signaling. The antibodies used were: Phospho-p44/42 MAP Kinase (#9101), Phospho-p38 MAP Kinase (#9211) and Phospho-Akt (#9271). The p44/42 doublet for Erk1/2 is poorly resolved on some gels, giving the appearance of only a single band (e.g., Fig. 3C).
Bound primary antibodies were recognized by horseradish peroxidase-linked secondary antibodies (Amersham), and visualized with ECL substrate (Amersham) and Kodak BioMax light film.
Supplementary Material
Table 1.
The effect of MapK inhibitor on blastocoele formation
| 8-cell | morula | blastocyst | fragmented | |
|---|---|---|---|---|
| -PD | 2/66(3.03%) | 11/66(16.67%) | 47/66(71.12%) | 6/66(9.90%) |
| +PD | 12/63(19.05%) | 38/63(60.32%) | 4/63(6.35%) | 9/63(14.29%) |
The effect of MapK inhibition on the kinetics of blastocoele formation is shown as the percentage and actual numbers of embryos (over total embryos in each culture condition) at different developmental stages after 24 hours of in vitro culturing. Mouse embryos were treated from 8-cell stage and observed for the presence of blastocoels after 24 hours
Acknowledgements
This study was supported by grants from the NIH and the NIH Director's Pioneer Award of the NIH Roadmap for Medical Research. G.Q.D. is a recipient of the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research. We are grateful to Dr. Janet Rossant of the Hospital for Sick Kid for providing TS cells, experimental advice, and critical reading of this manuscript.
Reference
- 1.Nagy A, et al. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–21. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–5. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 3.Kunath T, et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–61. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- 4.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 5.Hahn WC, et al. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 6.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoidmyeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 7.Klucher KM, Lopez DV, Daley GQ. Secondary mutation maintains the transformed state in BaF3 cells with inducible BCR/ABL expression. Blood. 1998;91:3927–34. [PubMed] [Google Scholar]
- 8.Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Katsuki M, et al. Embryonal tumors from transgenic mouse zygotes carrying human activated c-Ha-ras genes. Mol Biol Med. 1989;6:567–72. [PubMed] [Google Scholar]
- 10.Zaehres H, et al. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305. doi: 10.1634/stemcells.2004-0252. [DOI] [PubMed] [Google Scholar]
- 11.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 12.Strumpf D, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 13.Mitsui K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]; Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 14.Niwa H, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–29. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–5. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- 16.Burdon T, Chambers I, Stracey C, Niwa H, Smith A. Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs. 1999;165:131–43. doi: 10.1159/000016693. [DOI] [PubMed] [Google Scholar]
- 17.Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6:117–31. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- 18.Maekawa M, et al. Requirement of the MAP kinase signaling pathways for mouse preimplantation development. Development. 2005;132:1773–83. doi: 10.1242/dev.01729. [DOI] [PubMed] [Google Scholar]
- 19.Maekawa M, Yamamoto T, Kohno M, Takeichi M, Nishida E. Requirement for ERK MAP kinase in mouse preimplantation development. Development. 2007;134:2751–9. doi: 10.1242/dev.003756. [DOI] [PubMed] [Google Scholar]
- 20.Cheng AM, et al. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 21.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early Lineage Segregation between Epiblast and Primitive Endoderm in Mouse Blastocysts through the Grb2-MAPK Pathway. Dev Cell. 2006;10:615–24. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Auman HJ, et al. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–47. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- 23.Saba-El-Leil MK, et al. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4:964–8. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagi R, et al. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134:3827–36. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- 25.Piotrowska-Nitsche K, Perea-Gomez A, Haraguchi S, Zernicka-Goetz M. Four-cell stage mouse blastomeres have different developmental properties. Development. 2005;132:479–90. doi: 10.1242/dev.01602. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MH, Ziomek CA. Induction of polarity in mouse 8-cell blastomeres: specificity, geometry, and stability. J Cell Biol. 1981;91:303–8. doi: 10.1083/jcb.91.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland AE, Speed TP, Calarco PG. Inner cell allocation in the mouse morula: the role of oriented division during fourth cleavage. Dev Biol. 1990;137:13–25. doi: 10.1016/0012-1606(90)90003-2. [DOI] [PubMed] [Google Scholar]
- 28.Rajalingam K, et al. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol. 2005;7:837–43. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- 29.De Vries WN, et al. Maternal beta-catenin and E-cadherin in mouse development. Development. 2004;131:4435–45. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- 30.Plusa B, et al. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J Cell Sci. 2005;118:505–15. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.