Abstract
Purpose
Enhancer of zeste homologue 2 (EZH2), a histone methyltransferase, plays a key role in transcriptional repression through chromatin remodeling. Our objective was to determine the expression pattern of EZH2 and assess the anticancer effect of EZH2 depletion in pancreatic cancer cells.
Experimental Design
Immunohistochemistry and cytosolic/nuclear fractionation were performed to determine the expression pattern of EZH2 in normal pancreas and human pancreatic tumors. We used RNA interference, Western blotting, RT-PCR, and chromatin immunoprecipitation to study the effect of EZH2 depletion on pancreatic cancer cell proliferation and survival.
Results
We detected nuclear overexpression of EZH2 in pancreatic cancer cell lines and in 71 of 104 (68%) cases of human pancreatic adenocarcinomas. EZH2 nuclear accumulation was more frequent in poorly differentiated pancreatic adenocarcinomas (in 31 of 34 cases, p<0.001). We found that genetic depletion of EZH2 results in re-expression of p27Kip1 and decreased pancreatic cancer cell proliferation. Moreover, we showed that EZH2 depletion sensitized pancreatic cancer cells to doxorubicin and gemcitabine leading to a significant induction of apoptosis suggesting that the combination of EZH2 inhibitors and standard chemotherapy could be a superior potential treatment for pancreatic cancer.
Conclusions
Our results demonstrate nuclear accumulation of EZH2 as a hallmark of poorly differentiated pancreatic adenocarcinoma, identify the tumor suppressor p27Kip1 as a new target gene of EZH2, show that EZH2 nuclear overexpression contributes to pancreatic cancer cell proliferation, and suggest EZH2 as a potential therapeutic target for the treatment of pancreatic cancer.
Introduction
Pancreatic cancer, the fourth leading cause of cancer deaths in the United States, kills more than 30,000 Americans every year. Not only is there no cure, but also there are no effective treatments for this disease. The five-year survival rate for people with pancreatic cancer is 3% (1).
Deciphering the cancer epigenetic code promises to dramatically change our understanding of pancreatic cancer leading to the discovery of new oncomarkers and targets to develop superior diagnostic and treatment strategies. Recent evidence suggests that epigenetic silencing of tumor suppressor genes plays a significant role in the tumor development (2). Epigenetic control of gene expression occurs in two main ways: either the DNA itself is chemically altered (usually methylation of cytosines by DNA methyltransferases), or histones, proteins that package DNA into chromatin (the main component of chromosomes), are modified (2). Posttranslational modification of histones determine whether the chromatin is tightly packed leading to gene repression, or relaxed, in which case gene expression is active (2). The Polycomb group (PcG) proteins repress gene expression through the formation of multiple, unique complexes, that ultimately lead to the methylation of both histones and DNA (2-4). Specifically, PcG complexes containing the histone methyltransferase EZH2 silence chromatin via methylation of histone H3-lysine 27 (H3-K27) (3). Thus, EZH2 is thought to have the potential to silence genes that could be involved in tumorigenesis. Indeed, EZH2 gene amplification was first reported in hematological malignancies (5-6) and EZH2 catalyzed methylation of H3-K27 is frequently associated with PcG-mediated silencing of tumor suppressor genes such as hMLH1, ARHI and RASSF1A in ovarian cancer (7) and E-cadherin in gastric cancer (8).
While recent studies suggest EZH2 overexpression as an important factor of prostate (9) and breast (10) carcinoma progression, the expression pattern of EZH2 in human pancreatic cancer and the role of EZH2 in the proliferation, survival and chemoresistance of pancreatic cancer cells remain unknown. Here, we demonstrate aberrant EZH2 nuclear overexpression in pancreatic cancer cell lines and most pancreatic adenocarcinomas. We show that depletion of EZH2 results in re-expression of the p27Kip1 tumor suppressor and decreased pancreatic cancer cell proliferation. Furthermore, for the first time, we show that EZH2 plays a role in pancreatic cancer chemoresistance suggesting that combination of EZH2 inhibitors with standard chemotherapy could be a superior potential therapy for pancreatic cancer.
Materials and Methods
Reagents, plasmids and cells
All chemicals were obtained from Sigma (St. Louis, MO). An EZH2-specific targeting short hairpin RNA vector was generated as previously described (11) using the target sequence 5’-GACTCTGAATGCAGTTGCT-3’. All cell lines were obtained from the ATCC.
Immunohistochemistry
The Institutional Review Board at the Mayo Clinic approved all studies carried out on human specimens. EZH2 antibody was obtained from BD Biosciences Pharmingen (San Diego, CA). EZH2 immunostaining was performed on 104 resected primary pancreatic adenocarcinoma specimens. Two pathologists (AVO and DDB) independently reviewed all cases and classified the tumors as well differentiated (n=20), moderately differentiated (n=50) or poorly differentiated (n=34). For each case, the most representative section reflecting the major features of the primary pancreatic tumor (i.e., histological type) was selected for immunohistochemical examination to determine the expression of EZH2. Immunohistochemical staining was performed as described (12). EZH2 nuclear accumulation was defined as positive staining of more than 10% of cancer cell nuclei throughout the tumor regardless of cytoplasmic staining.
Immunoblot analysis and antibodies
For immunoblots, cells were lysed as described previously (13). Nuclear/cytosolic fractionation was done by Dignam method (14). Protein sample concentration was quantified and equal amount (50 μg whole, nuclear, or cytosolic protein extract) of protein was loaded in each well of SDS-polyacrylamide (PAGE) gel. Cell or tissue extracts were separated by 10% SDS-PAGE, transferred to polyvinylidene diflouride membrane (PVDF), and probed as indicated. Antibodies for immunoblot analysis were obtained from the following suppliers: EZH2, PARP, p27Kip1 from BD Biosciences Pharmingen (San Diego, CA); IκBα from Santa Cruz Biotechnology (Santa Cruz, CA); trimethyl-K27 Histone H3 and Histone H3 from Upstate (Lake Placid, NY); Cu/Zn superoxide dismutase (SOD) from Stressgen (Victoria, BC, Canada); β-actin from Novus (Littleton, CO). Bound antibodies were detected as previously described.
Reverse transcription polymerase chain reaction (RT-PCR)
Expression of mRNA of p27Kip1 and MYT1 was determined by RT-PCR. Complementary DNA (cDNA) was generated from 2 μg of total RNA by reverse transcription using a Reverse Transcription System Kit (Promega, Madison, WI). To quantify the expression in each cDNA sample, the target was amplified by PCR in parallel with an internal control, GAPDH. Increased expression of MYT1, EZH2 target gene, was used as a positive control of EZH2 depletion. The following primers were used: p27Kip1 upstream 5’- AGGATGTC AGCGGGAGCCGG-3’ and downstream 5’-CTTCTTGGGCGTCTGCTCCA-3’; MYT1 upstream 5’-ATCCCAGTCCCAGCCTACTT-3’ and downstream 5’-GTCTCCCTCCTG GACCTCAC-3’. Primer pairs that detect GAPDH were employed as previously described (13). Amplification was performed by using a thermal cycling program with varying numbers of cycles for p27Kip1 (22 cycles) and MYT1 (22 cycles) in which each cycle consisted of 94°C for 45 sec, 58°C for 45 sec, and 72°C for 1min. Amplification for GAPDH (22 cycles) was performed following a cycling program, in which each cycle consisted of 94°C for 45 sec, 50°C for 45 sec, and 72°C for 1 min. All PCR products were subjected to electrophoresis through a native 8% polyacrylamide gel and were visualized by staining with ethidium bromide.
Chromatin immunoprecipitation assay (ChIP)
Panc04.03 cells were cross-linked with formaldehyde for 15 min at 25°C, harvested in SDS lysis buffer (Upstate Biotechnology, Lake Placid, NY, USA), and sheared to fragment DNA (500−1000 bp). Samples were then immunoprecipitated using an agarose-conjugated EZH2, trimethyl-Histone H3 (Lys27), acetyl-Histone H3 (Lys14) antibody, or rabbit control IgG at 4°C overnight. Antibodies for ChIP analysis were obtained from the following suppliers: trimethyl-H3-K27 and acetyl-H4-K20 from Upstate Biotechnology (Lake Placid, NY); EZH2 from BD PharMingen (San Diego, CA). Following immunoprecipitation, samples were washed and eluted using the Chromatin Immunoprecipitation Kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer's instructions. Cross-links were removed at 65°C for 6 hours and immunoprecipitated DNA was purified using phenol/chloroform extraction and ethanol precipitation. 212 bp of the p27Kip1 promoter and 154 bp of the p27Kip1 exon 1 were detected in immunoprecipitated samples by PCR. PCR products were separated on a native 10% polyacrylamide gel and visualized by staining with ethidium bromide.
Statistical analysis
Data were analyzed using the Prism software package (GraphPad, Inc., San Diego, CA). Associations between EZH2 nuclear accumulation and degree of tumor differentiation were analyzed using Fisher's exact test for 2 by 2 contingency tables or chi square test for larger tables. Two-sided tests were used. Chi square critical value for 0.05 probability level is 3.841, all chi square values exceeding 3.841 and Ps < 0.05 were considered to indicate statistical significance.
Results
EZH2 is accumulated in the nucleus of pancreatic cancer cells
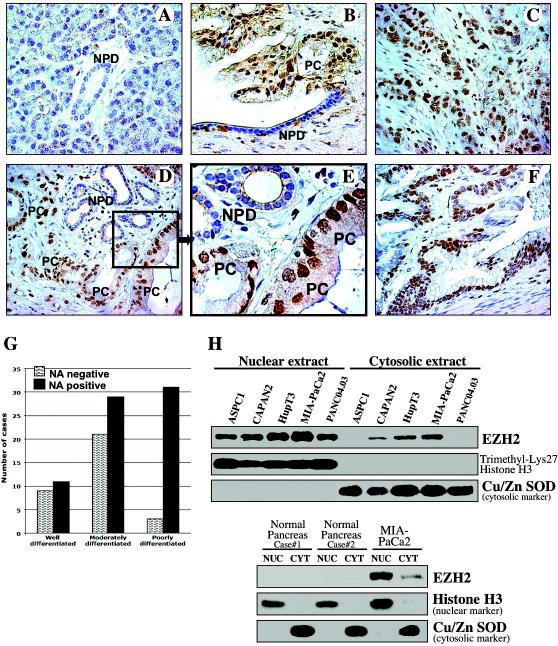
Using immunohistochemical staining for EZH2, we found weak cytoplasmic EZH2 expression in normal human pancreatic ductal and acinar cells (Fig. 1A). Nuclear accumulation of EZH2 was found in well, moderate and poorly differentiated adenocarcinomas in 11 of 20 (55%), 29 of 50 (58%) and 31 of 34 (91%) cases, respectively (Fig. 1B-F). EZH2 nuclear accumulation significantly associated with dedifferentiation of tumors (Chi-square test for independence 12.296, p=0.0021). It was significantly more frequent in poorly differentiated adenocarcinomas (Fig. 1G), p=0.0003, odds ratio 7.750 (CI 2.162 to 27.775), relative risk 1.614 (CI 1.280 to 2.034). Our results suggest that nuclear accumulation of EZH2 is associated with the loss of pancreatic cancer differentiation.
Figure 1. EZH2 is overexpressed and accumulated in the nucleus of pancreatic cancer cells.
(A-F) Immunohistochemical analysis of EZH2 expression and localization in (A) normal human pancreas and (B-F) pancreatic adenocarcinoma specimens. A, normal pancreatic duct (NPD) is indicated. B, Malignant pancreatic duct shows nuclear accumulation of EZH2, whereas adjacent normal pancreatic ductal cells show no nuclear EZH2 staining. Pancreatic cancer cells (PC). C, EZH2 nuclear accumulation in a poorly differentiated pancreatic adenocarcinoma. D, Nuclear accumulation of EZH2 found in cancer cells of moderately differentiated pancreatic adenocarcinoma but not in adjacent normal pancreatic ductal cells. E, Higher magnification of the delineated inset of (D) image. F, Nuclear accumulation of EZH2 in cancer cells of well differentiated pancreatic adenocarcinoma. G, Distribution of EZH2 staining patterns in pancreatic carcinomas. Nuclear accumulation (NA). H, Equivalent amounts (50 μg) of nuclear and cytosolic proteins isolated from the indicated pancreatic cancer cell lines and normal human pancreas tissue were separated by SDS-PAGE and immunoblotted.
Using nuclear/cytosolic fractionation, we found nuclear expression of EZH2 in pancreatic cancer cell lines ASPC1, CAPAN2, HupT3, MIA-PaCa2 and Panc04.03 (Fig. 1H, upper panel). In contrast to these findings, EZH2 expression was not detected in normal human pancreatic cells (Fig 1H, lower panel). Thus, nuclear overexpression of EZH2 appears to be a feature of pancreatic cancer cells.
Suppression of EZH2 inhibits pancreatic cancer cell proliferation
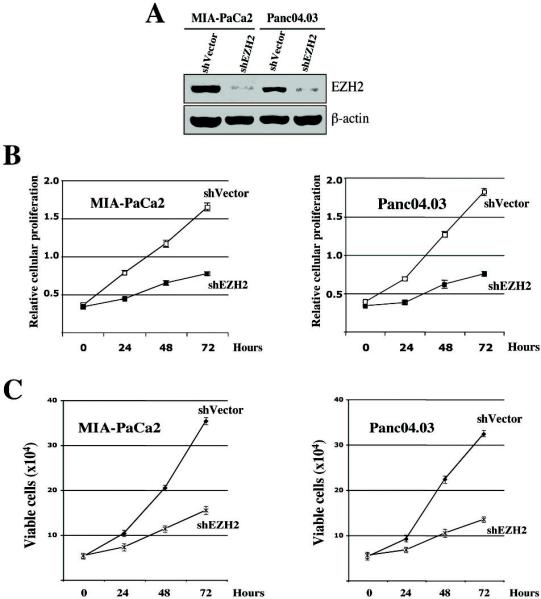
Our data identify overexpression of EZH2 in pancreatic cancer cells, but its role in regulating pancreatic cancer cell proliferation and survival is unknown. In order to address this question, we depleted EZH2 using short hairpin RNA (shRNA) interference (Fig. 2A) and assayed the ability of cells to proliferate using a well-established colorimetric technique (MTS assay) and trypan blue staining. We observe that transfection of an EZH2-targeting construct (shEZH2) into MIA-PaCa2 and Panc04.03 pancreatic cancer cells results in decreased proliferation in these pancreatic tumor cell lines as compared to vector control (shVector) transfected cells (Fig. 2B, C). We did not observe an increased apoptosis in EZH2-depleted cancer cells (data not shown). These results suggest that EZH2 participates in the regulation of signaling cascades that positively influence pancreatic cancer cell proliferation.
Figure 2. Suppression of EZH2 inhibits pancreatic cancer cell proliferation.
MIA-PaCa2 and Panc04.03 pancreatic cancer cells were transfected with a control vector (shVector) or the shEZH2 silencing vector (shEZH2). A, 48 hours post-transfection, the cell pellet was collected and protein was obtained. Cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and probed with antibodies to indicated proteins. B, Relative cell viability was measured using colorimetric MTS assay at indicated times as described (13). C, Numbers of viable cells were counted by the trypan blue exclusion assay as described (30).
Depletion of EZH2 leads to re-expression of p27Kip1 tumor suppressor gene in pancreatic cancer cells
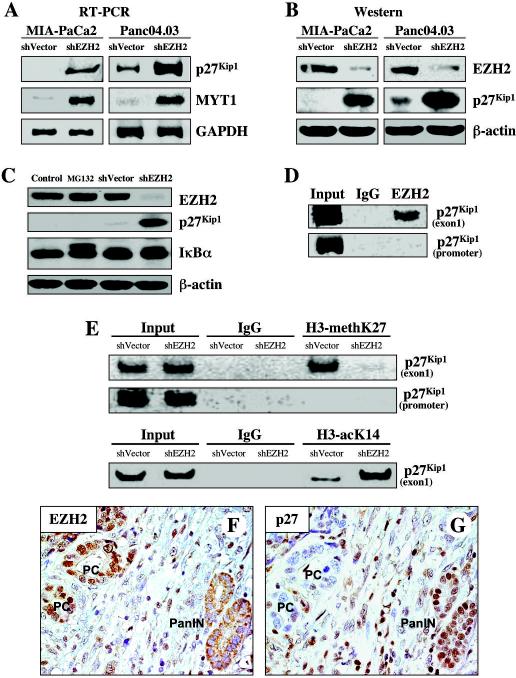
Using RT-PCR and immunoblotting, we performed screening of tumor suppressor genes, which might be re-expressed upon depletion of EZH2 in pancreatic cancer cells. Because EZH2 suppression leads to decreased pancreatic cancer cell proliferation, we analyzed the cyclin-dependent kinase (CDK) inhibitors p15, p16, p21, p27, p57 expression levels in pancreatic cancer cells, as well as the known EZH2-target gene MYT1 as a control for EZH2 suppression. Suppression of EZH2 in MIA-PaCa2 and Panc04.03 resulted in an increase in MYT1 gene expression consistent with the known role of EZH2 in the repression of MYT1 gene transcription. However, although EZH2 depletion resulted in the re-expression of multiple CDK inhibitor genes in several pancreatic cancer cell lines tested (data not shown), only p27Kip1 was uniformly and significantly re-expressed at both the transcriptional and protein levels after depletion of EZH2 in all of the pancreatic cancer cell lines examined (Fig. 3A, B, and data not shown). While it has been suggested that loss of p27Kip1 protein in human cancer is not related to its gene alterations but mainly resulting from increased degradation of the p27Kip1 protein (15, 16), we found that treatment of pancreatic cancer cells with the proteasome inhibitor MG132 did not increase the level of p27Kip1 protein expression (Fig. 3C). Our results identify the tumor suppressor p27Kip1 as a new target gene of EZH2 in human pancreatic cancer.
Figure 3. Depletion of EZH2 leads to re-expression of p27Kip1 in pancreatic cancer cells.
MIA-PaCa2 and Panc04.03 pancreatic cancer cells were transfected with a control vector (shVector) or the shEZH2 silencing vector (shEZH2). A, 48 hours post-transfection, the cell pellet was collected, mRNA was obtained and RT-PCR was performed as described in Materials and Methods. Increased expression of MYT1, a known EZH2 target gene, was used as a positive control. B, 48 hours post-transfection, the cell pellet was collected, protein was obtained. Cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and probed with antibodies to indicated proteins. C, 48 hours post-transfection, Panc04.03 cell pellet was collected. Panc04.03 cancer cells were treated with DMSO (Control) or MG132 (10 μmol/L) for 6 hours. Cell lysates were prepared, separated by SDS-PAGE (50μg/well), transferred to PVDF membrane, and immunoblotted as indicated. An increase of IκBα expression was used as a positive marker of MG132 treatment. D, Binding of EZH2 to the promoter or exon 1 of p27Kip1 gene was assayed in Panc04.03 cancer cells by ChIP. E, Panc04.03 cancer cells were transfected with a control vector (shVector) or the shEZH2 silencing vector (shEZH2). 48 hours post-transfection, genomic chromatin fragments were immunoprecipitated with trimethyl-H3-K27 and acetyl-H3-K14 at the p27Kip1 promoter or exon 1. PCR analysis on input chromatin (first 2 lanes) confirmed that equal chromatin amounts were used for ChIP. (F, G) Immunohistochemical staining of a pair of serial sections from the same pancreatic tumor demonstrates nuclear localization of EZH2 (F) and loss of p27Kip1 expression (G) in the same cancer cells, whereas absence of EZH2 nuclear staining, but p27Kip1 nuclear accumulation has been observed in benign pancreatic lesions (G, F). Pancreatic intraepithelial neoplasia (PanIN).
EZH2 depletion affects repressive chromatin status leading to re-expression of p27Kip1 in pancreatic cancer cells
EZH2-mediated recruitment of DNA methyltransferases to target gene promoters is one mechanism by which EZH2 can repress gene transcription (4). To investigate whether p27Kip1 may be inactivated by DNA hypermethylation through EZH2-mediated recruitment of DNA methyltransferase to p27Kip1 gene in pancreatic cancer cells, we examined CpG islands located in both the promoter and exon 1 of p27Kip1 gene, for hypermethylation by methylation-specific PCR. We did not detect hypermethylation at p27Kip1 promoter or exon 1 in Panc04.03 cancer cells (data not shown) suggesting that re-expression of p27Kip1 in EZH2-depleted pancreatic cancer cells is not dependent on the DNA methylation status of the p27Kip1 promoter or exon 1.
Opening of chromatin to allow transcription factors to gain access to gene promoters and regulate gene transcription is one of the major functions of histone modifications (i.e., phosphorylation, acetylation and methylation) (2). PcG complexes containing the histone methyltransferase EZH2 silence chromatin via methylation of histone H3-lysine 27 (H3-K27) (3). Methylation H3-K27 is a known feature associated with repressive chromatin status and epigenetic gene silencing (3). To evaluate the possibility that suppression of EZH2 alters its ability to silence p27Kip1 gene through transcriptional repression, we utilized ChIP to assess EZH2 binding and H3-K27 methylation at the p27Kip1 promoter and exon 1 in Panc04.03 cancer cells transfected with the shEZH2 or control vector. We found that EZH2 is bound to p27Kip1 at exon 1, but not within the p27Kip1 promoter region (Fig. 3D). Consistently, EZH2-depleted cancer cells showed a significant decrease in methylation of H3-K27 bound to exon 1 of p27Kip1, but not to its promoter (Fig. 3E, upper panel). It has been also shown that PcG (EED/EZH2) mediated repression of gene activity by interacting with histone deacetylases (17). Acetylation of H3-K14 is associated with open, actively transcribed genomic regions (18). We found that EZH2-depleted cancer cells demonstrate an increased acetylation of H3-K14, a marker of active transcription, at the exon 1 of p27Kip1 (Fig. 3E, lower panel). These results suggest that EZH2 regulates p27Kip1 expression through the maintenance of inactive chromatin at the p27Kip1 gene.
Nuclear overexpression of EZH2 is significantly associated with loss of p27 expression in human pancreatic adenocarcinomas
To test the hypothesis whether EZH2 nuclear overexpression leads to loss of p27Kip1 expression in human pancreatic tumors in vivo, we have performed immunohistochemical analysis of EZH2 and p27Kip1 expression in a series of human pancreatic adenocarcinomas. Using a pair of serial sections, we detected nuclear accumulation of EZH2 and loss of p27Kip1 expression in pancreatic cancer cells, whereas nuclear accumulation of p27Kip1 but not EZH2 was observed in benign pancreatic lesions (Fig. 3F, G). We found aberrant EZH2 nuclear accumulation and loss of p27Kip1 expression in 61% and 67% of pancreatic carcinomas, respectively (Fig. 3F, G). Loss of p27Kip1 expression was more frequently found in tumors with nuclear accumulation of EZH2 (p<0.001; Fig. 3F, G) than in tumors without nuclear accumulation of EZH2. Moreover, simultaneous EZH2 nuclear accumulation and loss of p27Kip1 expression are associated with the loss of pancreatic cancer differentiation (p<0.001). Consistent with our in vitro data, our in vivo results demonstrate that aberrant nuclear overexpression of EZH2 is significantly associated with loss of p27Kip1 expression in human pancreatic cancer cells.
EZH2 depletion affects pancreatic cancer chemoresistance
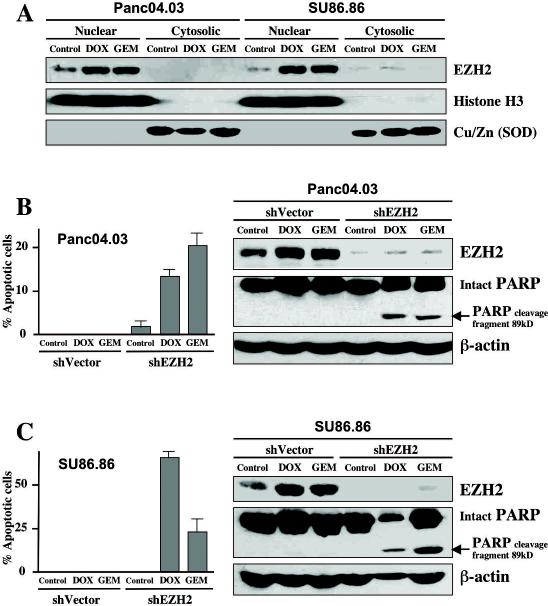
Pancreatic cancer is less chemosensitive than other common solid malignancies, with partial response to chemotherapy of less than 10% (19). The role of chemotherapy for pancreatic cancer is limited because pancreatic cancer rapidly develops a chemoresistance leading to fatal patient outcome (19, 20). With any chemotherapy regimen, average survival rates are between three and six months (20). Thus, a better understanding of tumor chemoresistance enables discovery of novel targets in order to develop a superior pancreatic cancer therapy. Gemcitabine, a deoxycytidine nucleoside analog, is currently the standard chemotherapeutic agent used in treatment for metastatic pancreatic cancer (20). Doxorubicin, an anthracycline antibiotic, has been used as a chemotherapeutic agent in a variety of carcinomas including pancreatic cancer. Interestingly, we found that treatment of Panc04.03 and SU86.86 cells with either doxorubicin or gemcitabine induces the nuclear accumulation of EZH2 in pancreatic cancer cells (Fig. 4A). Moreover, although treatment of pancreatic cancer cells with doxorubicin (1 μmol/L) or gemcitabine (100 nmol/L) did not induce apoptosis in control transfected cells, EZH2 depletion sensitized cancer cells to doxorubicin and gemcitabine, leading to a significant increase in apoptosis of pancreatic cancer cells (Fig. 4B, C). Our results suggest that nuclear overexpression of EZH2 might participate in pancreatic cancer chemoresistance and that the combination of EZH2 inhibitors with gemcitabine or doxorubicin could be superior to conventional therapy for pancreatic cancer.
Figure 4. EZH2 depletion affects pancreatic cancer chemoresistance.
A, Panc04.03 and SU86.86 pancreatic cancer cells were treated with DW (control), 1 μmol/L Doxorubicin (DOX) or 100 nmol/L Gemcitabine (GEM) for 24 hours. Nuclear and cytosolic fractions were prepared, separated by SDS-PAGE (50μg/well), transferred to PVDF membrane, and probed with the indicated antibodies. (B-C), Panc04.03 (B) and SU86.86 (C) pancreatic cancer cells were treated with DW (control), Doxorubicin (1 μmol/L) or Gemcitabine (100 nmol/L) for 48 hours. Whole cell lysates were prepared, separated by SDS-PAGE (50μg/well), transferred to PVDF membrane, and immunoblotted as indicated. The percentage of apoptotic cells was determined by Hoechst staining as previously described (31). Columns, mean; bars, SD.
Discussion
Epigenetic silencing of tumor suppressor genes can prevent programmed cell death and lead to uncontrolled proliferation in human tumors (2, 18). Recent studies implicate EZH2, a member of the PcG regulators of gene activity, in the pathogenesis of human cancer, including prostate (9), breast (10), gastric (21) and bladder (22) carcinomas, whereas the role of EZH2 in pancreatic cancer remains unknown.
In the present study, we demonstrate aberrant nuclear overexpression of EZH2 in pancreatic cancer cell lines and in 71 of 104 (68%) cases of human pancreatic adenocarcinomas, thus identifying EZH2 as a potential oncomarker in pancreatic cancer. We find that EZH2 nuclear accumulation is strongly associated with poorly differentiated pancreatic adenocarcinoma suggesting EZH2 as one of molecular determinants of pancreatic tumor dedifferentiation. Our findings are supported by other studies showing overexpression of EZH2 in advanced prostate (9) and breast (10) cancers, whereas nuclear accumulation of EZH2 was more frequently found in poorly differentiated breast carcinomas (23).
We identify EZH2 as a positive regulator of pancreatic cancer cell proliferation. We find that EZH2 depletion inhibits the proliferation but not survival of pancreatic cancer cells. Our data are in agreement with another study demonstrating that EZH2 knockdown in prostate cancer cells results in decreased proliferation with no effect on cancer cell survival (9). A previous study has also shown that EZH2 contributes to the proliferation of breast cancer cells (23). Our findings of EZH2 nuclear accumulation in most (in 31 of 34 cases) of poorly differentiated pancreatic adenocarcinoma, highly proliferating tumor fraction, support the hypothesis that EZH2 could play an important role in pancreatic cancer proliferation.
However, it is still unclear how EZH2 controls cancer cell proliferation. To address this question, we performed screening of numerous tumor suppressor genes, which might be re-expressed upon depletion of EZH2 in pancreatic cancer cells. We identified the p27Kip1 tumor suppressor as a novel EZH2 regulated gene. We are unaware of any previous report that has assessed the role of EZH2 in the regulation of p27Kip1 expression. The cyclin-dependent kinase inhibitor p27Kip1 is a tumor suppressor that regulates cell cycle by binding to cyclin-dependent kinases (24). Reduced p27Kip1 expression is correlated with poor disease outcome in cases of lung, colon, prostate, ovary and breast carcinomas (25). A decrease or loss of p27Kip1 expression is frequently observed in human pancreatic cancer (26). Although p27Kip1 expression appears to be an important prognostic factor in numerous human malignancies, it has been suggested that loss of p27Kip1 protein is not related to its gene alterations but mainly resulting from increased degradation of the p27Kip1 protein (15, 16). However, we found that treatment of pancreatic cancer cells with the proteasome inhibitor MG132 did not increase the level of p27Kip1 protein expression, whereas p27Kip1 expression was significantly upregulated in EZH2-depleted pancreatic cancer cells at both the transcriptional and protein levels. Our findings suggest that EZH2 nuclear overexpression contributes to epigenetic silencing of p27Kip1 gene leading to downregulation of p27Kip1 mRNA and protein levels in pancreatic cancer cells. To confirm our in vitro results showing re-expression of p27Kip1 in EZH2 depleted cancer cells, we demonstrate in vivo that EZH2 nuclear accumulation is significantly associated with loss of p27Kip1 expression in human pancreatic adenocarcinomas. These results support a speculation that EZH2 might contribute to pancreatic cancer cell proliferation by epigenetic silencing of tumor suppressor genes, in part through suppression of p27Kip1. Whether EZH2 could suppress p27Kip1 expression in a similar manner in other human malignancies remains to be determined.
Pancreatic cancer is one of the most drug-resistant tumors (19, 20). Our results identify EZH2 as an important factor of pancreatic cancer cell chemoresistance. We found that EZH2 depletion sensitized cancer cells to doxorubicin and gemcitabine leading to a significant decrease in survival of pancreatic cancer cells. Our findings are supported by another study showing that loss of EZH2-mediated methylation of H3-K27 re-sensitize ovarian cancer cells to cisplatin (7). It has also been demonstrated that trimethylation of H3-K27 is an important prognostic indicator for clinical outcome in patients with pancreatic cancer (27). Increased sensitivity of EZH2 depleted pancreatic cancer cells to doxorubicin and gemcitabine seems to be mediated, in part, by changes in gene expression. We found that EZH2 down-regulates the expression of p27Kip1 tumor suppressor in pancreatic cancer cells. Although the speculative role of 27Kip1 in pancreatic cancer chemoresistance remains to be investigated, reduced p27Kip1 correlates with poor survival after platinum based chemotherapy for non-small cell lung and ovarian carcinomas (28, 29). Because EZH2 mediated H3-K27 methylation is a mark of heterochromatin, it is also possible that loss of chromatin compaction could allow increased DNA damage at lower doxorubicin and gemcitabine doses leading to a decreased survival in EZH2 depleted pancreatic cancer cells.
In summary, our study provides novel information on the mechanisms underlying the growth and chemoresistance of pancreatic cancer, and emerges a demand for development of pharmacological inhibitor of EZH2 as a potential anticancer agent for the chemotherapy of pancreatic cancer.
Acknowledgments
We thank Dr. Jann Sarkaria and Dr. Gasper Kitange for their help with methylation analysis and Darren Riehle for tissue microarray data acquisition.
Grant support: This work was supported in part by the Mayo Foundation, and a Specialized Program of Research Excellence (SPORE) grant in pancreatic cancer (P50 CA102701) to D.D.B. D.D.B. is a recipient of a Scholar Award from the Leukemia and Lymphoma Society of America.
Footnotes
Statement of Clinical Relevance
Our study provides novel insight into the mechanism by which the histone methyltransferase EZH2 contributes to both pancreatic tumor cell proliferation and chemoresistance. We have found EZH2 nuclear accumulation in most pancreatic adenocarcinomas, whereas EZH2 expression is undetectable in normal pancreatic tissue, thus identifying EZH2 as a potential diagnostic oncomarker of pancreatic cancer. Immunohistochemical detection of EZH2 nuclear accumulation in biopsy specimens obtained by pancreatic needle or brush biopsy might be a useful method for pathological diagnosis of pancreatic cancer. We identify EZH2 as a positive regulator of pancreatic cancer proliferation. Moreover, we show for the first time that RNAi-mediated gene silencing of EZH2 sensitizes pancreatic cancer cells to doxorubicin and gemcitabine resulting in a significant induction of apoptosis, thus identifying EZH2 as a protein that contributes to pancreatic cancer chemoresistance. Taken together, our findings suggest that the combination of EZH2 inhibitors and standard chemotherapy could be a superior treatment for human pancreatic cancer.
References
- 1.Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2:25–8. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 2.Santos-Rosa H, Caldas C. Chromatin modifier enzymes, the histone code and cancer. Eur J Cancer. 2005;41:2381–402. doi: 10.1016/j.ejca.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 4.Viré E, Brenner C, Deplus R, Blanchon L, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;16(439):871–74. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso C, Mignon C, Hetet G, Grandchamps B, Fontes M, Colleaux L. The human EZH2 gene: genomic organisation and revised mapping in 7q35 within the critical region for malignant myeloid disorders. Eur J Hum Genet. 2000;8:174–80. doi: 10.1038/sj.ejhg.5200439. [DOI] [PubMed] [Google Scholar]
- 6.Visser HP, Gunster MJ, Kluin-Nelemans HC, et al. The Polycomb group protein EZH2 is upregulated in proliferating, cultured human mantle cell lymphoma. Br J Haematol. 2001;112:950–8. doi: 10.1046/j.1365-2141.2001.02641.x. [DOI] [PubMed] [Google Scholar]
- 7.Abbosh PH, Montgomery JS, Starkey JA, et al. Dominant-negative histone H3 lysine 27 mutant derepresses silenced tumor suppressor genes and reverses the drug-resistant phenotype in cancer cells. Cancer Res. 2006;66:5582–91. doi: 10.1158/0008-5472.CAN-05-3575. [DOI] [PubMed] [Google Scholar]
- 8.Fujii S, Ochiai A. Enhancer of zeste homolog 2 downregulates E-cadherin by mediating histone H3 methylation in gastric cancer cells. Cancer Sci. 2008;99:738–46. doi: 10.1111/j.1349-7006.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 10.Kleer CG, Cao Q, Varambally S, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakaria S, Gomes TS, Savoy DN, et al. Differential regulation of TCR-mediated gene transcription by Vav family members. J Exp Med. 2004;199:429–34. doi: 10.1084/jem.20031228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ougolkov A, Zhang B, Yamashita K, et al. Associations among beta-TrCP, an E3 ubiquitin ligase receptor, beta-catenin, and NF-kappaB in colorectal cancer. J Natl Cancer Inst. 2004;96:1161–70. doi: 10.1093/jnci/djh219. [DOI] [PubMed] [Google Scholar]
- 13.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3□eta participates in nuclear factor-□appaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65:2076–81. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 14.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–89. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loda M, Cukor B, Tam S, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–4. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 16.Pagano M, Tam SW, Theodoras AM, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 17.van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23:474–8. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- 18.Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–56. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- 19.Kollmannsberger C, Peters HD, Fink U. Chemotherapy in advanced pancreatic adenocarcinoma. Cancer Treat Rev. 1998;24:133–56. doi: 10.1016/s0305-7372(98)90079-2. [DOI] [PubMed] [Google Scholar]
- 20.Casper ES, Kelsen DP. Adenocarcinoma of the pancreas: overview of workup and management. Adv Oncology. 1996;11:17–22. [Google Scholar]
- 21.Matsukawa Y, Semba S, Kato H, Ito A, Yanagihara K, Yokozaki H. Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci. 2006;97:484–91. doi: 10.1111/j.1349-7006.2006.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raman JD, Mongan NP, Tickoo SK, Boorjian SA, Scherr DS, Gudas LJ. Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res. 2005;11:8570–6. doi: 10.1158/1078-0432.CCR-05-1047. [DOI] [PubMed] [Google Scholar]
- 23.Raaphorst FM, Meijer CJ, Fieret E, et al. Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia. 2003;5(6):481–8. doi: 10.1016/s1476-5586(03)80032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 25.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 26.Hu YX, Watanabe H, Li P, et al. An immunohistochemical analysis of p27 expression in human pancreatic carcinomas. Pancreas. 2000;21:226–30. doi: 10.1097/00006676-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Wei Y, Xia W, Zhang Z, et al. Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Mol Carcinog. 2008 doi: 10.1002/mc.20413. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oshita F, Kameda Y, Nishio K, et al. Increased expression levels of cyclin-dependent kinase inhibitor p27 correlate with good responses to platinum-based chemotherapy in non-small cell lung cancer. Oncol Rep. 2000;7:491–5. doi: 10.3892/or.7.3.491. [DOI] [PubMed] [Google Scholar]
- 29.Masciullo V, Ferrandina G, Pucci B, et al. p27Kip1 expression is associated with clinical outcome in advanced epithelial ovarian cancer: multivariate analysis. Clin Cancer Res. 2000;6:4816–22. [PubMed] [Google Scholar]
- 30.Axanova L, Morré DJ, Morré DM. Growth of LNCaP cells in monoculture and coculture with osteoblasts and response to tNOX inhibitors. Cancer Letters. 2005;225:35. doi: 10.1016/j.canlet.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong DK, Kaufmann SH, Ottaviano YL, et al. Epidermal growth factor-mediated apoptosis of MDA-MB-468 human breast cancer cells. Cancer Res. 1994;54:5280–3. [PubMed] [Google Scholar]