Abstract
Meniscus transplantation is indicated for persistent pain in a meniscectomized knee. Currently, grafts are prepared aseptically, which provides limited protection against donor-derived infection. The performance of a novel, sterilized meniscus was compared with an aseptically prepared one in an experimental model. Twenty-two sheep were divided into three groups: aseptic meniscal allograft, sterile meniscal allograft, and medial meniscectomy. Animals were euthanized 2 and 4 months after surgery. Meniscal assessments included cell viability, histology, and biomechanical testing. Articular cartilage was evaluated through histology and Outerbridge scoring. Aseptic and sterile allografts had cell viabilities of 59.7% and 58.7%, respectively, at 4 months, which was less than native controls. Grafts had decreased compressive strength at 4 months compared with their preimplantation moduli and were weaker than native menisci. In operated knees, the tibial plateau had more severe degenerative changes, although Outerbridge scores were similar between operated groups. Overall, the allografts were similar in their cellularity and biomechanical properties but were inferior to the native tissue at these end points. The severity of chondral damage in the allograft knees could not be distinguished from meniscectomized joints. The sterilization process does not appear to compromise tissue integrity and provides additional allograft safety.
Introduction
The menisci are paired fibrocartilaginous structures vital to the maintenance of knee health. Each meniscus transmits greater than 50% of load across its compartment, absorbs shock, and facilitates synovial fluid distribution [26]. Meniscal tears are common (61 per 100,000) and are treated by resection to create a stable rim of tissue. A majority of patients report functional improvement and symptomatic relief after meniscectomy. Despite the subjective success of the procedure, radiographic joint changes are documented as a sequelae of meniscectomy with moderate correlation to the quantity of tissue removed [9]. Symptom recurrence potentially signals progression of chondral degeneration and may occur within 6 months of surgery [3]. Meniscus transplantation remains the mainstay treatment option for the symptomatic postmeniscectomy knee, with good clinical outcomes reported beyond 5 years [11, 32, 34].
Structurally, fibrochondrocytes reside in lacunae in the extracellular matrix of the meniscus. This sequestered location decreases the likelihood of a graft-host reaction but creates a reservoir for pathogens. Although the risk of disease transmission from a musculoskeletal graft is estimated at 0.00015%, the high volume of transplants performed represents a large potential burden of disease. Specifically, unprocessed tissue (with donor screening and serologic testing only) is estimated to carry a one in one million risk of HIV and one in 421,000 risk of hepatitis C (HCV) [20]. Standard sterilization methods (ethylene oxide and γ-irradiation) are unusable on meniscal tissue as a result of an increased incidence of synovitis and decreased mechanical stability, respectively [14, 35]. Consequently, standard processing is limited to an aseptic, antibiotic soak with limited tissue penetration.
The BioCleanse® technique (RTI Biologics, Alachua, FL) is designed to access the potential disease reservoirs in musculoskeletal tissues. Chemical sterilants are applied during low-temperature vacuum-pressure cycling to facilitate pathogen and blood removal. With this, biologic implants are able to achieve a degree of sterility approaching the most stringent standards of synthetic implants (10−6, or a one in one million chance of a viable microbe being present after processing) [20]. In vitro testing of dirty (purposely contaminated) specimens has confirmed effective viral inactivation (including HIV and HCV) and bacterial spore killing [22]. Effective pathogen removal through the BioCleanse® process further reduces the risk of disease transmission from allograft tissue.
Like with any tissue processing, the ability of the treated graft to perform after implantation is a major concern. The performance of a sterilized graft was compared with a standard aseptic meniscal allograft, addressing the following questions: What effect does the surgical treatment (meniscus transplant or meniscectomy) have on the gross structure of the ovine knee? Does sterilization inhibit the cellular repopulation and viability of the meniscal allograft? Does sterilization compromise the compressive strength of the allograft? Is the normal histology of the articular cartilage and meniscus retained after transplantation?
Materials and Methods
All protocols and procedures were approved by the animal care and use committee (IACUC) at our institution. The 22 Dorset sheep (Ovis aries) were approximately 1 year old and weighed 50 to 60 kg at the time of surgery. Sheep were assigned randomly to one of three surgical groups and either left or right stifle operation. The first group (nine sheep) received an aseptically treated medial meniscus. The second group (nine sheep) received a meniscal allograft prepared using the sterile protocol. As an adjunct, the third group (four sheep) underwent medial meniscectomy. Eight sheep (three aseptic, three sterile, two meniscectomy) were euthanized at 2 months with the remainder (six aseptic, five sterile, two meniscectomy) evaluated 4 months after surgery. One sheep from the sterile treatment group was euthanized 5 weeks after surgery as a result of persistent poor health and was removed from the study. Necropsy results indicated no infection or gross pathologic features of the grafted joint.
The allografts were prepared as follows. Fifteen Dorset sheep were obtained as meniscus donors. After sacrifice using an IACUC-approved method, knees were shipped on wet ice to RTI-Biologics, where medial menisci were randomized to receive aseptic treatment or sterilization. Aseptic medial meniscal allografts were immersed in an antibiotic solution and packaged within 48 hours of donor sacrifice. Medial menisci in the sterile group went through the proprietary BioCleanse® sterilization protocol. BioCleanse® is a low-temperature chemical sterilization process that uses pressure-vacuum cycling to remove pathogens, blood, and lipids from tissue [15, 20, 28]. Individually packaged menisci were frozen to −70°C and transported to the test facility on dry ice.
Surgeries were performed with the sheep under general anesthesia using a method modified from Jackson et al. [12]. After anesthesia induction, a 6- to 8-cm anterior midline incision was made from midpatella to 2 cm below the joint line. The retinacular layer was opened and the medial collateral ligament released with a bone block to open the medial compartment. To complete the meniscectomy, the coronary ligament was detached at the capsular surface and the horns transected at their bony attachment. For sheep receiving allografts, transosseous tunnels were drilled at both insertions using an anterior cruciate ligament drill guide and 2-mm Kirschner wire. Concurrently, a side-specific medial meniscal allograft was thawed in room temperature saline. Allografts were detached from the bone block and the horns captured with Number 2 FiberWire® (Arthrex, Naples, FL) in a Mason-Allen configuration. The menisci were anatomically reduced and the transosseous sutures tied on the anteromedial tibia. Capsular tissue and the coronary ligament were reattached to the meniscus using 2–0 Ethibond® sutures (Ethicon, Somerville, NJ) with an all-inside technique. The medial collateral ligament origin was reduced with a small fragment screw (Synthes, Paoli, PA) and the incision closed in multiple layers with Vicryl® sutures (Ethicon). At the end of study, sheep were euthanized using 120 mg/kg Euthasol® (390 mg pentobarbital/50 mg phenytoin per milliliter; Virbac AH, Inc, Fort Worth, TX).
After euthanasia, both knees were examined for passive range of motion, crepitus, and incision healing. Joints were opened, photographed, and cultured for bacterial and fungal infection. The anterior and posterior horns were sharply released from their insertions and meniscal dimensions were obtained with calipers. The meniscus was divided into thirds, anterior, middle, and posterior, for biomechanics. Sections (5 mm) at the anterior-middle and middle-posterior junctions plus anterior and posterior horns were prepared for cell viability and histologic assays. Biomechanical sections were frozen to −80°C and cell viability/histology sections were immersed in 0.9% saline. The femoral and tibial articular cartilage was graded independently by two investigators using the Outerbridge criteria [24]. India ink was applied to both articular surfaces and then rinsed to remove excess stain on the surface. The inked femoral condyle and tibial plateau were photographed before fixation.
Sections of the meniscus were evaluated using a cell viability kit (Molecular Probes; Invitrogen, Carlsbad, CA). Tissue was rinsed twice in phosphate-buffered saline and incubated in 1.5 mL of 2 μmol/L calcein AM and 4 μmol/L ethidium homodimer for 30 minutes at 37°C in a covered dish. Images of the body, meniscal tip, capsular, tibial, and femoral surfaces were captured on a Nikon Eclipse TE200 laser-scanning confocal microscope (Nikon Instruments Inc, Melville, NY) using Metamorph® software (Molecular Devices, Sunnyvale, CA). Live and dead cells were counted digitally to determine percent viability.
For histology, tissue was fixed in 10% neutral buffered formalin for 7 days. Coronal sections of the central femoral condyle and apposing tibial plateau were decalcified in 20% formic acid/10% citric acid for 3 weeks. Specimens were dehydrated sequentially in ethyl alcohol and embedded in paraffin. Six-micrometer sections, stained with hematoxylin and eosin, safranin O/fast green, or picrosirius red, were imaged on a Nikon Eclipse 80i microscope using QImaging software (QImaging, Surrey, BC, Canada). Synovial membrane stained with hematoxylin and eosin was scored by two investigators (AGM and JMW) on a scale of 0 (no inflammation) to 3 (severe inflammation). Cartilage damage to the femoral condyle and tibial plateau was quantified with a modified Mankin scale [19]. Hematoxylin and eosin-stained sections of the meniscus were evaluated for cell penetration into the meniscus using a method described by Arnoczky et al. [5].
To perform biomechanical evaluation, samples were thawed in saline until they reached room temperature. A puck was created from the center of each section using a 3-mm dermal punch and custom cutter, resulting in a cylindrical sample 3 mm in diameter by 1.5 mm in height. Stress relaxation tests were conducted with a customized servo-motor-driven compression machine. Control of the actuator and data acquisition was done by LabVIEW™ (National Instruments, Austin, TX) with the load cell calibrated at the beginning of each test. For each specimen, the preload was determined by applying the smallest discernable load for 1200 seconds. Preload data were collected for the final 180 seconds at 5 Hz. The stress relaxation test was conducted at 15% of the original height loaded at 0.016 mm/second. The actuator position was held constant for 2400 seconds with data collected for the final 180 seconds at 5 Hz used for determining the stress-relaxed equilibrium. Young’s modulus was calculated for each sample.
Images captured at necropsy were imported into Metamorph® software to determine total area of the articular surfaces, the bare area of the tibial plateau, and area stained with India ink. Meniscal coverage and India ink staining are reported as a percentage of total articular area.
Mean and standard deviation are reported for all calculations. Except for meniscus dimensions (one-way ANOVA), nonparametric analyses, including Kruskal-Wallis (to reduce Type I error) with Mann-Whitney post hoc testing, were used to compare groups in this study. Statistical evaluations were performed using GraphPad InStat® software (GraphPad Software Inc, San Diego, CA) with a p = 0.05 level of significance.
Results
Postsurgical changes, including inflammation, altered meniscal geometry, and cartilage changes, were present in all operated knees regardless of treatment group (Fig. 1; Table 1). The mean inflammatory score of the allografted joints was greater than that of the contralateral, unoperated controls at 2 months (p = 0.04); however, the inflammation had subsided by 4 months (p = 0.81). Sterile allografts were shorter in their medial to lateral dimension than the aseptic (p < 0.01) or native (p = 0.01) menisci. The aseptic allografts were larger in their anterior to posterior dimension than either the sterile (p = 0.04) or control (p < 0.01) menisci (Table 1). In terms of area, allografts covered 10% to 15% less of the tibial plateau than the control menisci (p < 0.01). The contralateral, unoperated knees had pristine articular cartilage and did not stain with India ink. The percentage of India ink staining was greater in the operated knees than in controls (p = 0.03), although no statistical difference was noted between treatment groups or the tibia and femur (p = 0.61). The Outerbridge scores in knees receiving allografts were higher than those of contralateral controls (p = 0.04); however, no difference was observed between the sterile and aseptic graft (p = 0.51). The mean score increased between 2 and 4 months, although the trend was not significant (p = 0.45) (Table 1).
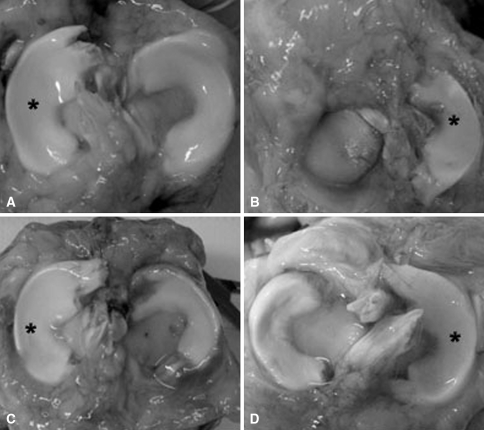
Fig. 1A–D.
Photographs show menisci at 4 months in (A) contralateral control, (B) meniscectomized, (C) aseptic allograft, and (D) sterile allograft. A synovial-derived connective tissue covers the periphery of the plateau in the meniscectomized joint. Both allografts show laxity in their attachment and displacement toward the periphery. *Lateral meniscus.
Table 1.
Comparison of aseptic and sterile allografts at 4 months
| Parameter | Aseptic allograft | Sterile allograft | p Value* |
|---|---|---|---|
| Anterior-posterior (mm) | 35.2 (1.1)§ | 31.0 (1.3) | < 0.05 |
| Medial-lateral (mm) | 20.2 (0.6) | 17.4 (0.6)† | < 0.01 |
| Height (mm) | 6.4 (1.5) | 5.9 (0.9) | NS |
| Width (mm) | 10.5 (1.5) | 9.1 (2.3) | NS |
| Plateau coverage (%) | 69.5 (6.0)‡ | 65.8 (1.2)‡ | NS |
| Cell viability (%) | 59.7 (3.7)‡ | 58.7 (5.2)‡ | NS |
| Penetration (%) | |||
| Femoral surface | 40.7 (6.5)† | 42.2 (2.6)† | NS |
| Tibial surface | 30.3 (1.3)† | 31.3 (2.2)† | NS |
| Compressive modulus (kPa) | 37.4 (11.7)‡ | 27.5 (10.5)‡ | NS |
| Outerbridge score | |||
| Femur | 1.8 (1.2)† | 1.8 (1.1)† | NS |
| Tibia | 2.5 (0.5)† | 2.2 (1.1)† | NS |
| India ink (%) | |||
| Femur | 4.4 (1.2)† | 8.3 (2.1)‡ | NS |
| Tibia | 9.9 (1.2)† | 12.7 (4.0)† | NS |
| Mankin score | |||
| Femur | 6.3 (2.5)† | 6.4 (2.0)† | NS |
| Tibia | 7.8 (1.3)† | 6.8 (2.1)† | NS |
Values are expressed as means, with standard deviations in parentheses; * comparison between sterile and aseptic allografts; †p < 0.05, ‡p < 0.01, §p < 0.001 in comparison to native controls; NS = not significant.
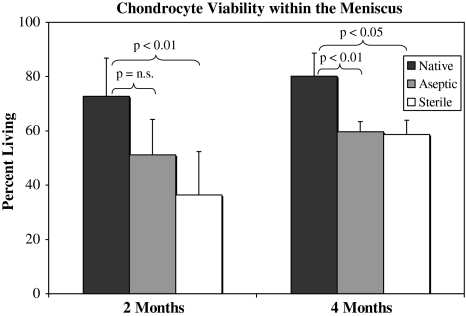
Meniscal allografts underwent repopulation with increased cell viability, although the control level was not attained at 4 months (Fig. 2; Table 1) Medial menisci in control knees had greater than 70% cell viability at both end points (p = 0.40) with an even distribution of living cells throughout the surface and meniscal body. Dead cells predominantly were localized to the tibial and femoral surfaces. At implantation, all meniscal allografts were devoid of living cells as a result of deep-freezing. Graft viability had increased at 4 months, although no difference was observed between grafts (p = 0.86) nor were control levels attained by 4 months (p < 0.01) (Fig. 2). Living and dead cells were concentrated in the perisurface regions with a persistently acellular meniscal body. Histologically, native menisci had 100% fibrochondrocyte depth from the femoral and tibial surfaces. Allografts had approximately 40% penetration from the femoral and 30% penetration from the tibial surface. Differences between femoral and tibial penetration were significant (p = 0.01) as were differences between allografts and control (p = 0.01) (Table 1).
Fig. 2.
A comparison of the cell viability in the allografted and control menisci is shown. Differences between the grafts were not significant at either time. n.s. = not significant; error bars = standard deviation.
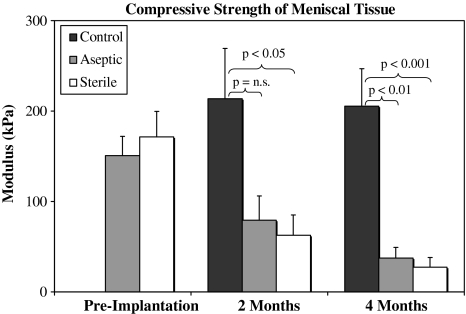
The compressive strength of the meniscal allografts after implantation was inferior to contralateral, native menisci (Fig. 3). Allografts had comparable compressive strength to each other at preimplantation at 2 and 4 months (p = 0.73, p = 0.68, p = 0.53, respectively). For aseptic and sterile allografts, a temporal decrease in Young’s modulus was observed, although the trend was significant only when comparing preimplantation with 4 months (p < 0.01 and p < 0.01, respectively). The modulus of the native menisci was significantly higher than the allografts at 2 (p = 0.01) and 4 months (p < 0.01).
Fig. 3.
The compressive modulus of native and allografted menisci across the study is shown. The temporal trend of decreased moduli in the allografts was significant when comparing the preimplantation with 4-month values. n.s. = not significant; error bars = standard deviation.
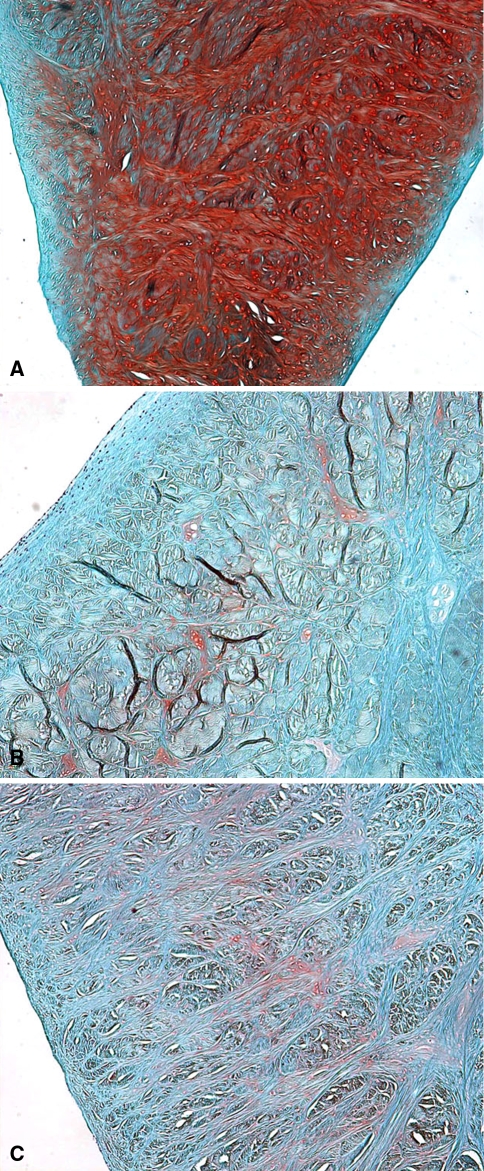
Sterile and aseptic meniscal allografts had similar histologic properties, although they did not provide significant chondroprotection over meniscectomy (Figs. 4, 5; Table 1). The safranin O staining of the allografted menisci was decreased 2 and 4 months after implantation when compared with native menisci (Fig. 4). Staining for collagen fiber orientation showed no qualitative difference between the allograft and native menisci. Native femoral condyles and tibial plateaus had normal-looking articular cartilage with consistent safranin O staining from medial to lateral and from surface to deep zones. Operated knees had a loss of articular curvature accompanied by medial ridge formation and reactive cartilage formation. Surface damage (fragmentation, fissuring, fibrillation) was concentrated in the central habitually loaded region of the femoral condyles and tibial plateau bare area (Fig. 5). The Mankin scores of the allograft treatment groups did not differ for the femoral condyle or tibial plateau at 2 and 4 months (p = 0.49) (Table 1). Grades in surgically treated knees were nonzero compared with the pristine controls, which was significant for both types of allografts (p = 0.03). The subcategories of the Mankin score, cellularity, proteoglycan content (as revealed by safranin O staining), structural changes, and tidemark integrity did not show consistent trends or differences between groups.
Fig. 4A–C.
Photomicrographs show sections of the (A) control, (B) aseptic allograft, and (C) sterile allograft that were stained with safranin O for proteoglycans (original magnification, ×40). Both types of allografts had a qualitative decrease in staining for proteoglycans with some cloning at 4 months.
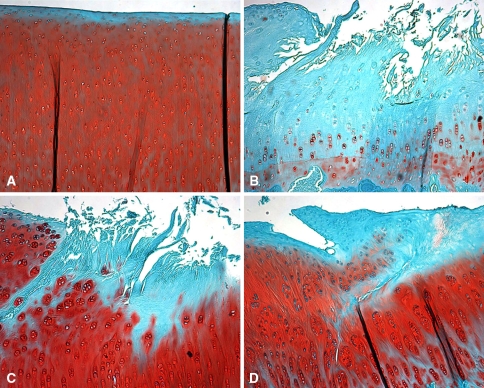
Fig. 5A–D.
Histologic features of the tibial plateau are shown in these photomicrographs of sections of tibia from (A) control, (B) meniscectomized, (C) aseptic allografted, and (D) sterile allografted knees that were stained with safranin O for proteoglycans (original magnification, ×40). Surgically treated knees had fissuring and fibrillation of the surface accompanied by a decrease in proteoglycan staining. Allografted knees had chondrocyte cloning with intense proteoglycan staining in regions adjacent to damage.
Discussion
The goal of this study was to evaluate the performance of a novel, sterilized meniscal allograft in comparison to a standard aseptically prepared graft. Our question was whether this novel sterile-prepared meniscus would perform as well as the current standard aseptically prepared meniscus when transplanted. At 4 months, both grafts had similar cellular repopulation, histologic appearance, and biomechanical properties, suggesting in vivo remodeling and repopulation occurred in parallel. This study was unable to conclusively show the chondroprotective effect of meniscal allograft transplantation in comparison to meniscectomy.
This study was limited by the low number of groups and animals. The high variability of in vivo models demands a large number of subjects to permit meaningful evaluation of small-scale differences. Additional times would be useful to track healing of the allograft and accumulation of chondral damage. Although compressive evaluation is relevant, the tensile modulus should be determined to evaluate the integrity of the circumferential collagen fibers. The assessment of chondral damage was semiquantitative using established scoring methods; however, analysis of actual local moduli would permit better characterization of the pressure distribution. Replication of this type of study also should include fixation methods relative to the human technique.
Human and animal studies have linked proper allograft sizing and fixation to functional outcome and chondroprotection [7, 12, 26, 27]. The grafts fit well despite not being size-matched to the recipient and a 10% donor-recipient weight difference. The sterile grafts did not shrink in vivo, a secondary effect of other preservation methods (lyophilization). Second-look arthroscopy has revealed substantial shrinkage and near resorption of lyophilized grafts as early as 6 to 8 months after implantation [21, 34]. Thus, if shrinkage were to occur in the sterilized grafts, some alteration of geometry would likely have been present at 4 months. The aseptic and sterile allografts were similar in appearance, although they lacked normal insertional anatomy and positioning. The fixation method, transosseous and capsular suturing, is a potential contributing factor to the differences in overall dimension and decreased plateau coverage in allografted knees. In addition, it is hypothesized, without primary bony fixation of the anterior and posterior horns at the time of implantation, meniscal allograft transplantation does not lead to substantial chondroprotection when evaluated in time zero biomechanical studies [25]. Early cartilage wear, resulting from graft extrusion, is a known sequelae of posterior traction suture failure [29]. Szomor et al. [30] reported increased tibial plateau exposure in sheep with a similar fixation method. Conversely, in transosseous-anchored allografts without peripheral suturing, plateau coverage was similar to native menisci [1]. Studies of anterior cruciate ligament graft healing have reported weaker tunnel healing in soft tissue grafts than in bone-blocked constructs [31]. At 4 months, the majority of allografts had laxity or failure in one or both insertions yet were firmly healed to the coronary ligament. Motion at the graft-bone interface and decreased strength of the tissue to hold the traction sutures could be contributing etiologic factors. Additional investigation using a bone bridge technique could address fixation effects on allograft geometry and the effect of sterilization on bone healing.
Deep-frozen allografts have a nonviable population of fibrochondrocytes and function as a scaffold for implantation [5]. The acellular nature of the implant does not negatively affect outcomes, because frozen grafts have performed as well as cryopreserved ones in human and animal studies [21]. Data indicate the graft is repopulated by the host as early as 4 weeks after implantation with cells derived from the synovium [13]. This study found cells at the capsular and femoral surfaces with fibroblast-like morphologic features, suggesting a similar source for repopulation. Both types of allografts had decreased viability and incomplete cell penetration into the meniscal body at 4 months. This is not unexpected, because previous canine and ovine models have established an outside-in progression of allograft repopulation that is incomplete at 6 months [5, 30].
Qualitatively, a decrease in meniscal proteoglycan content persisted at 4 months. Previous studies have confirmed a reduction in glycosaminoglycan content as early as 1 week postimplantation and continuing to 1 year [8]. This could be indicative of incomplete cellular migration into the scaffold and repopulation by cells that are not chondrogenic. Additional investigation of the synthetic capabilities and phenotype of cells in the graft is merited to elucidate the imbalance in metabolism. A sequela of the proteoglycan loss is decreased compressive strength of the meniscal allografts [10]. The anionic sulfate groups repulse one another and attract water into the meniscal substance, which provides resistance to compressive force.
Meniscal allograft transplantation functions to decelerate the progression of chondral damage in meniscectomized knees. Meniscectomy is known to alter the contact mechanics of the tibiofemoral joint and results in radiographic cartilage degeneration [4, 33]. Removal of greater than 1/3 of the width alters contact area and pressure similar to total meniscectomy [18]. Articular cartilage undergoes biochemical changes and decreases in tensile modulus as early as 2 months after meniscus removal in animal models [6, 16]. Meniscal allograft transplantation provides chondroprotection, although it does not altogether eliminate progressive damage [2, 23]. Animal models had mild cartilage changes, more than intact or sham operations, but less than meniscectomy, delayed transplantation, and transplantation with alternative or incongruous tissue [1, 2, 17, 23]. Kelly et al. [16] reported an average Mankin score less than 1 at 4 months after a fresh allograft. More commonly, large animal studies have shown moderate chondral damage with modified Mankin scores ranging from 5 to 10 in meniscectomized and allografted knees [2, 23, 30]. Damage is localized to the central tibial plateau with relative preservation of the covered regions, suggesting a focal increase of contact forces. The allograft protected the peripheral surface from shear damage but not completely from increased loading, resulting in osteophyte formation.
The novel, sterilized meniscus had similar properties to the standard allograft throughout the duration of this study. No negative reactions to the sterile-processed menisci were observed, suggesting it may be applicable for human use. It was reported the performance of meniscal allografts in animal models correlated well with their behavior in human transplantation [21]. Application of this protocol to human grafts will provide an added safety measure against donor-derived infections and processing contamination. If consistent, reliable sterility is proven, it potentially could increase the availability of meniscal grafts for transplantation. With the increasing number of meniscectomies and younger population, it is likely the need for meniscus transplantation will increase as well. Although further investigation is needed to evaluate the long-term effects of sterilization on the tissue, this technology appears to be a safe and promising development in the field.
Acknowledgments
We thank David Karwo, Adam Yanke, Predrag Bursac, and Lauren Brown for technical assistance.
Footnotes
One or more of the authors (VMW, BJC, JMW) has received funding from RTI-Biologics (Alachua, FL).
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aagaard H, Jorgensen U, Bojsen-Moller F. Reduced degenerative articular cartilage changes after meniscal allograft transplantation in sheep. Knee Surg Sports Traumatol Arthrosc. 1999;7:184–191. [DOI] [PubMed]
- 2.Aagaard H, Jorgensen U, Bojsen-Moller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406:218–227. [DOI] [PubMed]
- 3.Alford JW, Lewis P, Kang RW, Cole BJ. Rapid progression of chondral disease in the lateral compartment of the knee following meniscectomy. Arthroscopy. 2005;21:1505–1509. [DOI] [PubMed]
- 4.Alhalki MM, Howell SM, Hull ML. How three methods for fixing a medial meniscal autograft affect tibial contact mechanics. Am J Sports Med. 1999;27:320–328. [DOI] [PubMed]
- 5.Arnoczky SP, DiCarlo EF, O’Brien SJ, Warren RF. Cellular repopulation of deep-frozen meniscal autografts: an experimental study in the dog. Arthroscopy. 1992;8:428–436. [DOI] [PubMed]
- 6.Elliott DM, Guilak F, Vail TP, Wang JY, Setton LA. Tensile properties of articular cartilage are altered by meniscectomy in a canine model of osteoarthritis. J Orthop Res. 1999;17:503–508. [DOI] [PubMed]
- 7.Elliott DM, Jones R 3rd, Setton LA, Scully SP, Vail TP, Guilak F. Joint degeneration following meniscal allograft transplantation in a canine model: mechanical properties and semiquantitative histology of articular cartilage. Knee Surg Sports Traumatol Arthrosc. 2002;10:109–118. [DOI] [PubMed]
- 8.Fabbriciani C, Lucania L, Milano G, Schiavone Panni A, Evangelisti M. Meniscal allografts: cryopreservation vs deep-frozen technique: an experimental study in goats. Knee Surg Sports Traumatol Arthrosc. 1997;5:124–134. [DOI] [PubMed]
- 9.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30:664–670. [PubMed]
- 10.Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990;252:19–31. [PubMed]
- 11.Graf KW Jr, Sekiya JK, Wojtys EM. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20:129–140. [DOI] [PubMed]
- 12.Jackson DW, McDevitt CA, Simon TM, Arnoczky SP, Atwell EA, Silvino NJ. Meniscal transplantation using fresh and cryopreserved allografts: an experimental study in goats. Am J Sports Med. 1992;20:644–656. [DOI] [PubMed]
- 13.Jackson DW, Whelan J, Simon TM. Cell survival after transplantation of fresh meniscal allografts: DNA probe analysis in a goat model. Am J Sports Med. 1993;21:540–550. [DOI] [PubMed]
- 14.Jackson DW, Windler GE, Simon TM. Intraarticular reaction associated with the use of freeze-dried, ethylene oxide-sterilized bone-patella tendon-bone allografts in the reconstruction of the anterior cruciate ligament. Am J Sports Med. 1990;18:1–10; discussion 10–11. [DOI] [PubMed]
- 15.Jones DB, Huddleston PM, Zobitz ME, Stuart MJ. Mechanical properties of patellar tendon allografts subjected to chemical sterilization. Arthroscopy. 2007;23:400–404. [DOI] [PubMed]
- 16.Kelly BT, Potter HG, Deng XH, Pearle AD, Turner AS, Warren RF, Rodeo SA. Meniscal allograft transplantation in the sheep knee: evaluation of chondroprotective effects. Am J Sports Med. 2006;34:1464–1477. [DOI] [PubMed]
- 17.Lazovic D, Wirth CJ, Knosel T, Gosse F, Maschek HG. [Meniscus replacement using incongruent transplants: an experimental study][in German]. Z Orthop Ihre Grenzgeb. 1997;135:131–137. [DOI] [PubMed]
- 18.Lee SJ, Aadalen KJ, Malaviya P, Lorenz EP, Hayden JK, Farr J, Kang RW, Cole BJ. Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med. 2006;34:1334–1344. [DOI] [PubMed]
- 19.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips: II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed]
- 20.McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35:2148–2158. [DOI] [PubMed]
- 21.Milachowski KA, Weismeier K, Wirth CJ. Homologous meniscus transplantation: experimental and clinical results. Int Orthop. 1989;13:1–11. [DOI] [PubMed]
- 22.Mills CR, Roberts MR. BioCleanse tissue processing system: biological safety [technical monograph]. Alachua, FL: Regeneration Technologies, Inc; March 30, 2000. Available at: www.endoplus.nl/userpdf/BioCleanseTissueProcessingSystem.pdf. Accessed July 27, 2008.
- 23.Mora G, Alvarez E, Ripalda P, Forriol F. Articular cartilage degeneration after frozen meniscus and Achilles tendon allograft transplantation: experimental study in sheep. Arthroscopy. 2003;19:833–841. [DOI] [PubMed]
- 24.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43:752–757. [DOI] [PubMed]
- 25.Paletta GA Jr, Manning T, Snell E, Parker R, Bergfeld J. The effect of allograft meniscal replacement on intraarticular contact area and pressures in the human knee: a biomechanical study. Am J Sports Med. 1997;25:692–698. [DOI] [PubMed]
- 26.Rijk PC. Meniscal allograft transplantation. Part I: background, results, graft selection and preservation, and surgical considerations. Arthroscopy. 2004;20:728–743. [DOI] [PubMed]
- 27.Rodeo SA. Meniscal allografts: where do we stand? Am J Sports Med. 2001;29:246–261. [DOI] [PubMed]
- 28.Schimizzi A, Wedemeyer M, Odell T, Thomas W, Mahar AT, Pedowitz R. Effects of a novel sterilization process on soft tissue mechanical properties for anterior cruciate ligament allografts. Am J Sports Med. 2007;35:612–616. [DOI] [PubMed]
- 29.Shibuya S. Meniscus transplantation using a cryopreserved allograft. Histological and ultrastructural study of the transplanted meniscus. J Orthop Sci. 1999;4:135–141. [DOI] [PubMed]
- 30.Szomor ZL, Martin TE, Bonar F, Murrell GA. The protective effects of meniscal transplantation on cartilage: an experimental study in sheep. J Bone Joint Surg Am. 2000;82:80–88. [DOI] [PubMed]
- 31.Tomita F, Yasuda K, Mikami S, Sakai T, Yamazaki S, Tohyama H. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:461–476. [DOI] [PubMed]
- 32.van Arkel ER, de Boer HH. Survival analysis of human meniscal transplantations. J Bone Joint Surg Br. 2002;84:227–231. [DOI] [PubMed]
- 33.Verma NN, Kolb E, Cole BJ, Berkson MB, Garretson R, Farr J, Fregly B. The effects of medial meniscal transplantation techniques on intra-articular contact pressures. J Knee Surg. 2008;21:20–26. [DOI] [PubMed]
- 34.Wirth CJ, Peters G, Milachowski KA, Weismeier KG, Kohn D. Long-term results of meniscal allograft transplantation. Am J Sports Med. 2002;30:174–181. [DOI] [PubMed]
- 35.Yahia L, Zukor D. Irradiated meniscal allotransplants of rabbits: study of the mechanical properties at six months postoperation. Acta Orthop Belg. 1994;60:210–215. [PubMed]