Abstract
Cementless two-stage revision of infected total hip prostheses lacks the possibility of local antibiotic protection of the implant at the time of reimplantation, which leads to the concern that this protocol may not sufficiently eradicate periprosthetic infection. Moreover, early implant loosening as much as 18% and stem subsidence as much as 30% have been reported. To determine whether a cementless revision could eradicate infection and achieve sufficient implant stability, we prospectively followed 36 patients with two-stage revisions for septic hip prostheses. We used a uniform protocol of a 6-week spacer interval, specific local and systemic antibiotic therapies, and cementless modular revision stems. The minimum followup was 24 months (mean, 35 months; range, 24–60 months). In one patient, the spacer was changed when the C-reactive protein value failed to normalize after 6 weeks, and the reimplantation was performed after an additional 6 weeks. No infections recurred. There was no implant loosening and a 94% bone-ingrowth fixation of stems. Subsidence occurred in two patients. The Harris hip score increased from a preoperative mean of 41 to 90 at 12 months after reimplantation and later. Using cementless prostheses in two-stage revisions of periprosthetic infections of the hip in combination with a specific local and systemic antibiotic therapy seems to eradicate infection and provide implant stability.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic infections occur in less than 1% of patients but nevertheless are a serious complication of hip arthroplasty [22, 26]. In patients with early infection occurring within 4 weeks of implantation, the implant can be left in place with a high probability of success whereas late infections typically require prosthesis revision to eradicate the infection [9, 31]. In these patients, one can use either a one-stage revision, in which the new implant typically is fixed with antibiotic-loaded cement [60], or a two-stage revision, in which an antibiotic-loaded spacer usually is placed in position for a certain time before the final prosthesis is implanted [15, 25, 26, 43]. Garvin and Hanssen [26] reported an average survival rate of a one-stage revision to be 82% and of a two-stage revision to be 91% in their literature review. The fixation method chosen for the final prosthesis in the two-stage technique usually involves the use of cement because this allows the surgeon to add antibiotics to the cement to help prevent recurrent infection [12, 15, 25, 26, 43].
A disadvantage of the cemented revision technique relates to the fact that the osseous bed of the prosthesis has not only been enlarged by loosening of the primary prosthesis but also become thinner and sclerotic. This reduces the ability of the cement to adhere to the bone. Dohmae et al. [11] reported the resistance of the bone-cement interface to shear force-related failure was reduced by 79% when comparing a cemented revision implant with a cemented primary implant. Wirtz and Niethard [68] reported a higher rerevision rate associated with aseptic loosening of cemented revision prostheses compared with cementless components (ie, 15.1% versus 4.3% for the acetabular cup and 12.7% versus 5.5% for the stem). This advantage of cementless revisions also may exist for implant fixation in two-step septic revisions although exact data concerning middle- and long-term survival rates of cemented and cementless implants in septic revision are absent from the literature.
Promising results have been reported with two-stage revisions using cementless implants, particularly with eradication rates between 82% and 100% (Table 1) [16, 30, 40, 42, 45, 51, 67]. Nevertheless, because the use of cementless components at the second stage does not allow the surgeon to add local antibiotics to the cement to help prevent recurrent infection, there is concern that recurrent infection rates will be higher with cementless fixation [12, 67]. Moreover, early aseptic loosening as much as 18% and stem subsidence as much as 30% have been reported with the cementless technique [40, 51, 67].
Table 1.
Results of two-stage cementless revision of periprosthetic infection of the hip
| Study | Number | Study type | Followup | Spacer/beads | Local antibiotics | Duration of intravenous antibiotics | Interval until reimplantation | Antibiotics after implantation | Implants | Eradication rate | Aseptic loosening | HHS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wilson and Dorr [67] | 22/13* | Retrospective | ≥ 3 years, 48 months | Resection arthroplasty | No | 3 weeks parenteral | 6–12 weeks | 3 days parenteral | Different, nm stems | 91%/100% cementless | 7.6% stem loose | 75 |
| Nestor et al. [51] | 34 | Retrospective | 47 (24–72) mo | Resection arthroplasty | No | ≥ 4 weeks parenteral | 8 (3–19) months | Different | Different, nm stems | 82% | 18% stem loose | |
| Fehring et al. [16] | 25 | Retrospective | 41 (24–98) months | Beads | Tobramycin in 16 cases | 6 weeks parenteral | 4.8 months | Different, nm stems + modular with pf | 92% | 0% | 81 (30–100) | |
| Haddad et al. [30] | 50 | Retrospective | 5.8 (2–8.7) years | Beads + cement ball | Gentamicin | 5 days parenteral and then oral | 3 weeks | ≥ 3 months | Different, nm stems | 92% | 8% stem subsidence | 78 (54–92) |
| Koo et al. [40] | 22 | Prospective, includes acute and late periprosthetic infections | 41 (24–78) months | Spacer, beads | Vancomycin, gentamicin, cefotaxime | 6 weeks | 6–12 weeks | Different, nm stems | 95% | 5% cup loose, 30% stem subsidence | ||
| Hofmann et al. [35] | 27 | Retrospective | 76 (28–148) months | Old stem and new polyethylene cup | Tobramycin | 6 weeks parenteral, in 17 cases additional oral for 6 weeks | Different, nm stems | 94% | 0% | 53 (36–68) | ||
| Kraay et al. [41] | 33 | Retrospective | ≥ 2 years | Spacer in 16 cases | Tobramycin in 16 cases | ≥ 6 weeks parenteral | 7.4 (3–37) months | Different, nm + modular stems | 92% | 9% cup, 0% stem | ||
| Masri et al. [45] | 29 | Retrospective | ≥ 2 years | PROSTALAC® spacer | Tobramycin, vancomycin, cefuroxime, penicillin† | 6 weeks parenteral or in combination with oral | 12 weeks | 5 days intravenous | Different, nm stems, 2 strut grafts | 90% | 0% | 70 (42–100) |
| Current study | 36 | Prospective | ≥ 2 years 35 (24–60) months | Spacer | Specific: gentamicin, clindamycin, vancomycin, ampicillin, ofloxacin | 2 weeks parenteral, 4 weeks oral | 6 weeks | 2 weeks parenteral, 4 weeks oral | One modular stem with distal fixation | 100% | 6% stem subsidence, 0% loosening | 90 (60–100) |
* 13 of 22 reimplantations without cement; †combination of another local antibiotic with tobramycin; HHS = Harris hip score; nm = nonmodular; pf = proximal fixation.
The majority of the reports concerning cementless two-stage revisions have limited validity because they are retrospective studies with small numbers of patients and lack a unified protocol regarding spacer implantation, duration of spacer period, technique and implant device of the cementless reimplantation, and the surgeon concerned (Table 1) [35, 42, 45]. Therefore, it is not clear whether cementless two-stage septic revision can achieve a reproducible high eradication rate and implant stability. Nonetheless, because of these first encouraging reports and the advantages of cementless fixation in aseptic hip revisions, we changed our protocol for treating periprosthetic late infections at the end of 2002 from a two-stage cemented revision to a two-stage cementless revision that involved a standardized procedure with a 6-week spacer implantation, local and systemic antibiotics (2 weeks parenteral and 4 weeks oral administration) specific for the pathogen concerned, and the use of modular curved cementless revision stems.
Using this new protocol, we then addressed the following questions in a prospective study: (1) What is the rate of eradication of infection associated with this standardized protocol for two-stage septic hip prosthesis revision with local and systemic administration of pathogen-specific antibiotics? (2) What is the rate of early aseptic loosening of the prosthesis components and the rate of stem subsidence and bone-ingrowth fixation as described by Engh et al. [13] using modular cementless revision stems? (3) What level of Harris hip score [33] and rate of complications can be expected using this protocol?
Materials and Methods
We prospectively followed 44 patients with late periprosthetic infections of a hip prosthesis who underwent septic, two-stage revision surgery between November 2002 and April 2006. Owing to the high rates of failure in patients who have revision surgery for failure attributable to methicillin-resistant staphylococcus infections [39, 59], three patients who had such infections during the chosen period were immediately excluded from the protocol of two-stage cementless revision surgery. These three patients were treated successfully using two-stage cemented revisions with bacteria-specific local and systemic antibiotic therapies. Four additional patients were excluded from the final evaluation because their preoperative aspirate was falsely positive. One patient died from unrelated causes 18 months postoperative with an infection-free status, leaving 36 patients followed for at least 24 months (mean, 35 months; range, 24–60 months). All patients gave informed consent to participate in the study and the protocol was approved by the research ethics boards of the two institutions.
There were 20 women and 16 men with an average age of 69 ± 10 years. The average body mass index of the patients was 28.8 ± 6.3. The original diagnosis that led to the primary arthroplasty was osteoarthritis in 32 patients, femoral head fracture in three, and femoral head necrosis in one. The comorbidities included diabetes mellitus in six patients, chronic obstructive pulmonary disease in four, hypertonicity in 21, and hypothyroidism in four. The removed acetabular components were cemented in 13 patients and cementless in 23; the removed stems were cemented in 17 patients and cementless in 19. The average lifespan of the primary implant was 4.4 ± 4.0 years. In 10 patients, a primary implant was involved, but 11 patients already had one revision operation, eight had three operations, six had four operations, and one had seven operations. Most of these operations had been performed in other institutions to treat the periprosthetic infection. Three patients had fistulae in the hip region.
The periprosthetic infection was diagnosed by hip aspiration, which is a standard procedure in our clinic before any revision of a hip prosthesis is performed. According to our previously described procedure, the harvested fluid was immediately aspirated into vials containing BD BACTEC-PEDS-PLUS/F-Medium (Becton Dickinson, Heidelberg, Germany) and was cultivated for 14 days [21, 27, 38, 60]. The periprosthetic infection was confirmed by bacteriologic and histologic examinations of five samples of the periprosthetic tissue and loosening membrane taken during the revision surgery. According to the criteria of Virolainen et al. [63], Atkins et al. [2], and Pandey et al. [55], the diagnosis of infection was positive when at least one of the following conditions had been fulfilled: (1) observation of the same microorganism in at least two of the cultures and (2) observation of a microorganism in at least one sample and at least five neutrophilic polymorphonuclear leukocytes per high-power field (x400) in the associated histologic preparation as described by Mirra et al. [47, 48], Feldman et al. [17], Lonner et al. [44], and Pandey et al. [55]. In a previous study, we showed this method resulted in a sensitivity of 100%, a specificity of 98.1%, a positive predictive value of 95.2%, a negative predictive value of 100%, and an accuracy of 98.6% [21]. In the 36 patients of the current study, the identity of the microorganism in the aspirated fluid was confirmed in the intraoperative samples. We identified two organisms in 11 cases (Table 2). Once the antibiotic susceptibility profile of the microorganisms obtained from the preoperative aspiration fluid had been analyzed, our microbiologist (LF) prepared a specific mixture of antibiotics for use in the spacer cement and in the systemic treatment (Table 3).
Table 2.
Microorganisms identified as the cause of periprosthetic infections
| Microorganism | Number of cases |
|---|---|
| Staphylococcus epidermidis | 16 |
| Enterococcus faecalis | 7 |
| Staphylococcus aureus | 4 |
| Staphylococcus capitis | 4 |
| Streptococcus agalactiae | 3 |
| Propionibacterium acnes | 3 |
| Staphylococcus hominis | 2 |
| Staphylococcus warneri | 1 |
| Staphylococcus haemolyticus | 1 |
| Staphylococcus simulans | 1 |
| Peptostreptococcus micros | 1 |
| Peptostreptococcus magnus | 1 |
| Streptococcus mitis | 1 |
| Enterobacter sakazakii | 1 |
| Corynebacterium striatum | 1 |
Table 3.
Local and systemic antibiotic therapies
| Antibiotic | Number local | Number intravenous | Number oral |
|---|---|---|---|
| Flucloxacillin | 10 | ||
| Rifampicin | 10 | 17 | |
| Vancomycin | 24 | 6 | |
| Ciprofloxacin | 6 | 10 | |
| Loracarbef | 6 | ||
| Imipenem + cilastatin | 6 | ||
| Ampicillin | 2 | ||
| Ampicillin + sulbactam | 4 | ||
| Amoxicillin + clavolanic acid | 9 | ||
| Gentamicin | 36 | 3 | |
| Gentamicin | 6 (add) | ||
| Cefuroxim | 2 | 3 | |
| Levofloxacin | 3 | ||
| Linezolid | 2 | ||
| Penicillin G | 2 | ||
| Clindamycin | 30 | 2 | |
| Ofloxacin | 3 | ||
| Cefaczolin | 1 | ||
| Cotrimoxazol | 1 | ||
| Fusidic acid | 1 |
add = additional.
In the first stage of revision, we removed all foreign materials and performed a radical débridement of all infected tissue, then inserted a mobile spacer with an acetabular and a femoral component. This was performed in 21 patients using a posterolateral approach (Fig. 1). The infected prosthetic stem was removed by the transfemoral approach with a modified extended trochanteric osteotomy in 15 patients, in five because of deformity of the femur and use of a corrective osteotomy, in six because of cement extending distally in the femoral canal, in three because of well-fixed cementless stems with a large-pore surface, in three because of thin bone with a risk of fracture, and in six because of concomitant plate osteosynthesis treatment of a periprosthetic fracture (Fig. 2) [18, 19]. Once the spacer implantation had been completed, the extended trochanteric osteotomy was fixed with double cerclage wires.
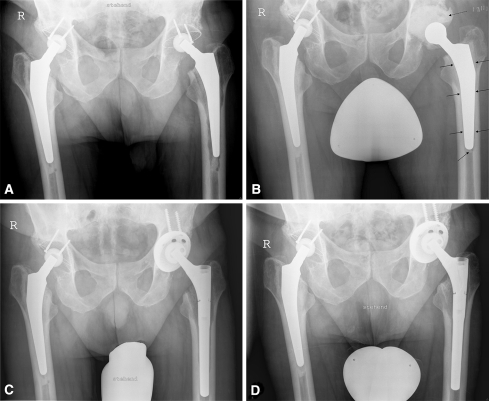
Fig. 1A–D.
The radiographs illustrate the case of a 73-year-old man with a periprosthetic infection caused by Staphylococcus epidermidis treated with two-stage revision of a cemented left hip prosthesis. (A) A preoperative radiograph shows osteolysis around the left stem attributable to periprosthetic infection. (B) A radiograph shows the hip 2 weeks after removal of the prosthesis and implantation of the spacer. The broken arrow marks the cement spacer of the cup and the unbroken arrows mark the cement layer of the stem spacer. (C) A radiograph taken 2 weeks after surgery shows the implanted cementless prosthesis (Allofit-S™ and Revitan®). (D) A radiograph shows the hip 2 years after surgery.
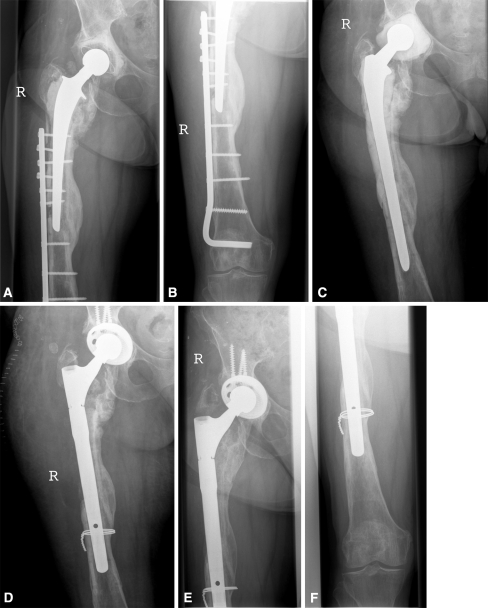
Fig. 2A–F.
The radiographs illustrate the case of an 83-year-old woman with plate osteosynthesis of a periprosthetic fracture with sequestering and an infection with Enterococcus faecalis treated with two-stage transfemoral revision of the cemented hip prosthesis. Preoperative radiographs show (A) the acetabular cup and proximal femur, and (B) the distal femur. (C) A radiograph shows the hip 2 weeks after removal of the prosthesis and placement of the spacer. (D) A radiograph taken 2 weeks after surgery shows the implanted cementless prosthesis (Allofit-S™ and Revitan®). (E) The acetabular cup, proximal femur, and (F) distal femur are seen in these radiographs obtained 2 years after surgery.
We hand-formed a cup-shaped acetabulum spacer from antibiotic-loaded cement (with a specific mixture of antibiotics recommended by the microbiologist) (Figs. 1, 2). For the stem component, the spacer was created by placing antibiotic-loaded cement around an old prosthesis stem model (monoblock devices, in most cases no longer used for primary implantations). After coating the cement with the patient’s blood, the spacer was inserted in the femur as polymerization of the cement was proceeding. This technique leads to a cement layer around the stem in all cases and stable fixation of the spacer component (Figs. 1, 2). The blood prevents a perfect cement interdigitation into the bone to facilitate easier cement removal at the second stage of revision. In the spacer cement, a maximum of 10% of the total cement powder amount was added as antibiotic using industrially prepared Palacos® R-G cement (six times) and COPAL® cement (30 times) (Heraeus, Darmstadt, Germany). Adding antibiotics to the spacer cement meant 13 patients were treated locally with two antibiotics, 17 patients with three antibiotics, and six patients with four antibiotics (Table 3).
The parenteral antibiotic therapy was similarly designed individually by the microbiologist (LF). Intravenous antibiotic administration was initiated during surgery once the implant had been removed, the infected and ischemic tissues had been effectively débrided, and at least five samples of tissue had been obtained for bacteriologic assessment from the joint capsule, the membrane around the loosened region, and the purportedly infected tissues. We administered two antibiotics parenterally in 15 patients (Table 3). According to the recommendations of Zimmerli et al. [70, 72], systemic antibiotic therapy was given 2 weeks parenterally and after that was continued orally. The high bioavailability of the antibiotics rifampicin and ciprofloxacin allowed their oral administration from the third day after surgery, at which time both were given in combination to decrease the risk of resistance, as described by Zimmerli et al. [70–72]. Two antibiotics were administered orally in 17 patients (Table 3).
After a 6-week period with antibiotic treatment, spacers were removed and reimplantation of a cementless, press-fit acetabular component (Allofit-S™; Zimmer GmbH, Winterthur, Switzerland) and a modular cementless revision stem (Revitan® curved; Zimmer GmbH) was performed (except two patients who received a Burch/Schneider™ Reinforcement Cage [Zimmer GmbH]), followed by the same individual antibiotic regime for another 6 weeks as recommended by Zimmerli [71] and Trampuz and Zimmerli [62] (Figs. 1, 2).
Reimplantation was performed without osteotomy using a posterolateral approach in the 21 patients (Fig. 1) [19]. Care was taken to ensure a solid, three-surface fixation of the Revitan® stem [20], whereby the distal component first was fixed firmly in position and then the proximal component of the required length attached in situ. In the 15 patients with implant removal using the transfemoral approach, the osteotomy was opened 6 weeks later to remove the spacer. In all patients, we observed early healing of the osteotomy. After implantation of the cementless revision stem and cup, the osteotomy was closed again with double cerclage wires. The length of the fixation zone of the stems implanted endofemorally was determined from the two distal of the three fixation zones described by Fink et al. [20] and was 8.2 ± 2.4 cm (range, 4–15 cm). The prostheses implanted by the transfemoral approach had a circular fixation zone in the femoral isthmus of 4.1 ± 1.1 cm (range, 1.5–5.5 cm). In three patients in whom the distal circular fixation zone in the isthmus was less than 3 cm owing to destruction or widening of the isthmus, we used static distal interlocking of the stem to improve distal fixation [18]. This involved insertion of three locking screws after implantation of the distal Revitan® component using the implantation alignment guide. During the reimplantation, at least five samples of tissue near the spacer were removed for bacteriologic and histologic examinations. All operations were done by the senior author (BF) in two different institutions.
After the first operation, the patients were mobilized with partial weightbearing of 10 to 20 kg to minimize potential wear of the articulating spacer. After reimplantation, the treated leg was mobilized with a partial weightbearing of 10 kg for 6 weeks. Afterward, weightbearing was increased gradually at a rate of 10 kg per week. In patients who had reimplantation via the transfemoral approach, we advised against flexion beyond 70° for 6 weeks after the operation to avoid movement of the bony flap.
The patients were examined clinically and radiographically every 3 months for the first 2 years after surgery. Inflammatory parameters (C-reactive protein [CRP]) also were followed. According to Haddad et al. [30], Wilson and Dorr [67], Masri et al. [45], and Zimmerli et al. [72], to be judged infection-free at followup, a patient had to be free of clinical signs for infection (fever, local pain, redness, warmth, sinus tract infection) and have a CRP level less than 10 mg/dL and no radiographic sign of osteolysis. Clinical assessment was based on the Harris hip score [33].
All radiographic examinations were performed independently by two experienced examiners (AG, BF). Standardized radiographs of the hip with the femur in two planes (the anteroposterior view was performed in a standing position) were performed with a standard tube to cassette distance of 115 cm. All measurements in a sequence of radiographs were corrected for magnification using the prosthetic head diameter as a reference as described by Nunn et al. [53]. The bony defects of the acetabulum and the femur were classified according to the system described by Pak et al. and Paprosky et al. [54, 57] (Table 4). Radiolucent lines of the cups were determined in Zones I, II, and III on the anteroposterior films as described by DeLee and Charnley [10] and of the stems in both planes. We judged radiolucencies greater than 2 mm as indicative of loosening [7]. Subsidence of the stem components was analyzed using methods described by Callaghan et al. [6] and was judged when a change of stem position of at least 3 mm was seen. We assessed fixation of the femoral stem radiographically using the criteria of Engh et al. [13] (bone-ingrowth fixation, stable fibrous fixation, unstable fixation). Reliability for the radiographic examinations was high, with intrarater and interrater intraclass correlation coefficients of 0.99 and 0.98, respectively.
Table 4.
Distribution of the bony defects*
| Paprosky type | Number of bony defects | |
|---|---|---|
| Acetabulum | Femur | |
| 1 | 3 | |
| 2A | 13 | 11 |
| 2B | 4 | 6 |
| 2C | 14 | 5 |
| 3A | 3 | 2 |
| 3B | 2 | 3 |
| 3C | 6 | |
Descriptive statistics were computed with SPSS® for Windows® (SPSS Inc, Chicago, IL).
Results
There was no evidence of recurrence of infection in any of the patients for the whole of the observation period. None of the samples taken at the time of spacer removal and reimplantation were positive for bacterial infection. The inflammation parameter (CRP) decreased to less than 10 mg/dL within 4 weeks of explantation of the infected prostheses in all but one patient. In this patient, the CRP still had not normalized by 6 weeks after surgery, therefore repeat débridement was performed along with exchange of the spacer. Reimplantation of a cementless revision stem was performed 6 weeks later after normalization of the inflammation parameter. This patient remained free of infection during the following 4-year observation period, therefore he was not regarded as having failed treatment in the final assessment.
Two patients had stem subsidence. An 83-year-old patient with a fixation zone of 7.1 cm 6 months after surgery had 5-mm subsidence and a 44-year-old patient with a fixation zone of 3 cm had 3-mm subsidence 6 months after surgery. No additional subsidence was observed during the subsequent followup examinations in these two patients. These two patients had stable fibrous fixation of the stem at the 2-year followup according to the criteria of Engh et al. [13]. All others had bone-ingrowth fixation of the cementless stem (94%). We observed no patients with loosening of the prosthetic components. One patient had a spacer dislocation treated with closed reduction, and one patient had deep vein thrombosis treated with a double dose of low-molecular–weight heparin.
The Harris hip score increased from a preoperative level of 41 ± 15 points to 69 ± 14 points 3 months after surgery. Six months after surgery, the score was 84 ± 17 points, and 12 months afterward, 90 ± 12 points. This level was maintained for the remainder of the observation period, with 90 ± 13 points 18 months after surgery and 90 ± 14 points 24 months afterward.
Discussion
Cementless two-stage revisions of infected total hip prostheses lack the possibility of local antibiotic protection of the implant at the time of reimplantation. Although encouraging results concerning the eradication rate were reported in some retrospective studies with nonuniform protocols, there is concern that cementless two-stage revision surgery may not sufficiently eradicate periprosthetic infections [12, 16, 30, 35, 40, 41, 45, 51, 67]. Moreover, early implant loosening as much as 18% and stem subsidence as much as 30% have been reported [40, 51, 67]. Therefore, it is not clear whether cementless two-stage septic revision can achieve a reproducible high eradication rate and implant stability. We therefore asked the following questions: (1) What is the eradication rate of infections associated with a standardized protocol for two-stage septic hip prosthesis revisions with local and systemic administration of pathogen-specific antibiotics? (2) What are the rates of aseptic early loosening of the prosthesis components and of stem subsidence and bone-ingrowth fixation using modular cementless revision stems? (3) What level of Harris hip score and rate of complications can be expected using this protocol?
One limitation of our study is the relatively short followup. Although reinfections can occur up to 5 years after implantation, they usually are observed within the first postoperative year [41, 42, 45, 61]. Moreover, nearly all of the published reports on two-stage cementless revisions had a minimal followup of 2 years (Table 1). Subsidence of the stem and early loosening of the components also would be recognizable within this period [3–5, 28, 29, 34, 46, 56, 64, 65]. Mjöberg et al. [49] reported, however, this period is not sufficient to analyze cup migration, therefore we did not consider this parameter. Thus, the limited period of postoperative observation only substantiates conclusions with respect to the presence of recurrent infection, stem subsidence, biologic fixation of the stem, and clinical and radiographic evidence for cup loosening. The decision not to aspirate the joint again before reimplantation of the cementless prosthesis, as reported by Masri et al. [45], also might be questioned. A second aspiration would have required a delay in antibiotic therapy for at least 2 weeks, if not 4 weeks [50]. This would have been followed by a 2-week incubation period; therefore, the second operation would have been delayed between 4 and 6 weeks. Moreover, the local levels of antibiotic released by the spacer likely would influence the detection of viable bacteria [9]. For these reasons, we decided to forgo the second aspiration and rather to make a decision based on clinical findings and the CRP as described by Hsieh et al. [36]. The high rate of success regarding eradication of infection suggests our approach was appropriate. Finally, our outcomes in these 36 patients my not reflect those in large populations. It can be assumed that if the number of patients increased, recurrent infections would be observed eventually. However, all the published reports, which are mostly retrospective studies of treatment methods for periprosthetic infections, also consider similar numbers of patients (Table 1).
Our technique differs from previously published techniques with cementless two-stage revisions in four ways (Table 1). First, the antibiotic used in the antibiotic-loaded cement of the spacer and for systemic treatment was chosen based on the sensitivity of the bacterium causing the infection. Second, we chose a shorter period of 2 weeks of intravenous antibiotic treatment. Third, reimplantation was performed after a 6-week spacer interval. Fourth, we used only modular revision stems with distal fixation in the femoral diaphysis.
Some authors have described, mostly in retrospective studies, a high level of eradication after two-stage cementless revisions, although there are differences in the treatment regimen regarding duration of antibiotic therapy, use of spacers, and time to reimplantation (Table 1). The fact that there are differences in procedures not only between studies but also within studies means it cannot be decided which period of parenteral antibiotic treatment and which spacer period are the most suitable. The 2-week period of parenteral antibiotics we used seems short. However, it is consistent with the recommendations of Zimmerli et al. [70, 71] and Trampuz and Zimmerli [62] and has been used by others (eg, Hsieh et al. [37] with 95% eradication) [37, 72]. Also, the total duration of antibiotic treatment of 3 months for our patients was consistent with the recommendations of Zimmerli [71] and Trampuz and Zimmerli [62]. The 6-week spacer period we used also is short but has been used by others (Table 1) [25, 40, 49]. Moreover, the 100% rate of eradication suggests our protocol is adequate.
The survival rate of cementless implants in aseptic hip revisions is believed by some to be higher than that of cemented implants [16, 40, 41]. This is well demonstrated for aseptic revision in a review article by Wirtz and Niethard [68]. A few reports describe the stability of cementless fixation after septic revision surgery using mostly nonmodular implants: Fehring et al. [16] achieved stable bone-ingrowth fixation in 96% of their cases using nonmodular and modular cementless prostheses with proximal fixation, whereas Nestor et al. [51] reported an implant stability of 79% using nonmodular, proximal porous-coated stems. Wilson and Dorr [67], however, achieved only 38% bone-ingrowth fixation after 3 years in, admittedly, a small group of 13 patients using a cementless nonmodular stem with proximal fixation. Moreover, the rate of early loosening of cementless revisions stems varies from 0% to 18% (Table 1). In our opinion, the low rates of subsidence (6%) and loosening (0%) and high rate of bone-ingrowth fixation (94%) of the cementless modular revision stem system we used are attributable to the distal fixation procedures in viable bone and to the modularity of the stems. Thus, as described in an anatomic study [20], the in situ assembly of the components enabled effective distal fixation of the distal component in an adequate osseous bed before the proximal component was added and corrected for leg length and antetorsion.
The Harris hip scores for our patients reflect those reported by Hofmann et al. [35] with 92 points after a 2-year followup with their cementless, two-stage revision technique for infected prostheses. Our patients’ scores are somewhat better than those reported by Fehring et al. [16] with 81 points or Masri et al. [45] with 70 points after cementless revision.
Our data lend support to the supposition that two-stage cementless revisions of infected hip endoprostheses combined with specifically designed local and systemic antibiotic therapy regimens can lead to high levels of eradication of infection comparable to the rates (90%–95%) achieved by two-stage cemented revisions with antibiotic-loaded cement and can achieve low rates of stem subsidence and aseptic loosening [25, 26, 43]. We believe three factors contributed to its success. First, the nature of the infecting microorganism and its antibiotic susceptibility were known. We performed preoperative aspiration of the joint in every patient with prosthetic loosening and incubated the aspirate for 14 days before assessment [21, 27, 38, 60]. This long incubation period is necessary because the bacteria causing the periprosthetic infection usually occur in very small numbers in the form of a biofilm and also often are in a sessile state characterized by a slow rate of reproduction [8, 24, 27, 52, 58]. If the incubation period is of sufficient duration, an accuracy of approximately 90% can be achieved with the aspiration method [1, 66]. We believe lack of sufficient incubation led to the reported low sensitivity (46.1%) of the preoperative aspiration [35]. Second, we believe rigorous removal of all foreign material and radical débridement of inflamed and necrotic tissues during the operation are essential for the success of any form of septic prosthesis revision [26, 45, 60]. Third, a specific systemic therapy with an antibiotic of high bioavailability to which the bacteria are highly sensitive coupled with a high dose of locally applied specific antibiotic in the antibiotic-loaded cement seems decisive for effective treatment of periprosthetic infections [9]. Although there are a limited number of studies concerned with the local release of antibiotics contained in the cement, this is a procedure during one-stage and two-stage septic prosthesis revisions that has become well accepted [23, 32, 60, 61]. In one-stage revisions of prostheses with bacteria-specific antibiotics added to the cement, eradication rates of 88% and 91% were reported by Steinbrink and Frommelt [60] and Wroblewski [69], respectively. Masri et al. [45] also reported a success rate of 89.7% in their retrospective study involving bacteria-specific antibiotic mixed into the cement of a PROSTALAC® spacer (DePuy Orthopaedics, Inc, Warsaw, IN). As the use of several antibiotics seems to result in synergistic effects for local release patterns, we always used at least two antibiotics in the cement and preferred COPAL® cement to Palacos® R-G cement whenever possible because the former had better release of gentamicin [14, 32]. Some have used vancomycin and tobramycin as local antibiotics on a regular basis because they have a broad spectrum of activity [16, 41]. However, not all bacteria can be treated successfully with these agents (eg, some gram-negative organisms), therefore, this is an argument for investigating the antibiotic resistance pattern of the isolated bacteria and selecting a specific antibiotic for treatment. Our results support the use of this procedure.
We decided to exclude methicillin-resistant staphylococcus infections from this study protocol for cementless reimplantation without addition of antibiotic to the implant because of the poor results of revisions in infections induced by resistant bacteria [39, 59]. The 100% eradiation rate with our study protocol suggests this protocol also may be effective for infections with resistant bacteria. Because of the increasing numbers of periprosthetic infections with resistant bacteria, we will extend this concept of treatment to periprosthetic infections induced by resistant bacteria.
In this prospective study using a standardized protocol for two-stage cementless revisions of periprosthetic infections of hip prostheses using specific local and systemic antibiotic therapies and modular revision stems, we achieved 100% eradication of infections. Implant stability with no early aseptic loosening, bone-ingrowth fixation in 94% of the stems, absence of stem subsidence in 94%, and Harris hip scores of 90 points were achieved, suggesting our protocol is appropriate for treating late periprosthetic hip infections.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Ali F, Wilkinson JM, Cooper JR, Kerry RM, Hamer AJ, Norman P, Stockley I. Accuracy of joint aspiration for the preoperative diagnosis of infection in total hip arthroplasty. J Arthroplasty. 2006;21:221–226. [DOI] [PubMed]
- 2.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J Clin Microbiol. 1998;36:2932–2939. [DOI] [PMC free article] [PubMed]
- 3.Böhm P, Bischel O. Femoral revision with the Wagner SL revision stem: evaluation of one hundred and twenty-nine revisions followed for a mean of 4.8 years. J Bone Joint Surg Am. 2001;83:1023–1031. [DOI] [PubMed]
- 4.Böhm P, Bischel O. The uncemented diaphyseal fixation of femoral revision stems in case of large bone defects: analysis of twelve years experience with the Wagner SL revision stem [in German]. Z Orthop Ihre Grenzgeb. 2001;139:229–239. [DOI] [PubMed]
- 5.Böhm P, Bischel O. The use of tapered stems for femoral revision surgery. Clin Orthop Relat Res. 2004;420:148–159. [DOI] [PubMed]
- 6.Callaghan JJ, Salvati EA, Pellicci PM, Wilson PD, Ranawat CS. Results of revision for mechanical failure after cemented total hip replacement, 1979 to 1982: a two to five-year follow-up. J Bone Joint Surg Am. 1985;67:1074–1085. [PubMed]
- 7.Cordero-Ampuero J, Garcia-Cimbrelo E, Munuera L. Fixation of cementless acetabular cups: a radiographic 4-8-year study of 102 porous-coated components. Acta Orthop Scand. 1994;65:263–266. [DOI] [PubMed]
- 8.Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop Relat Res. 2005;437:7–11. [DOI] [PubMed]
- 9.Cui Q, Mihalko WM, Shields JS, Ries M, Saleh HJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871–882. [DOI] [PubMed]
- 10.DeLee JG, Charnley J. Radiological demarcation of cementless sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed]
- 11.Dohmae Y, Bechthold JE, Sherman RE, Puno RM, Gustilo RB. Reduction in cement-bone interface shear strength between primary and revision arthroplasty. Clin Orthop Relat Res. 1988;236:214–220. [PubMed]
- 12.Duncan CP, Masri BA. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. Instr Course Lect. 1995;44:305–313. [PubMed]
- 13.Engh CA, Glassman AH, Suthers KE. The case of porous-coated hip implants: the femoral side. Clin Orthop Relat Res. 1990;261:63–81. [PubMed]
- 14.Ensing GT, van Horn JR, van der Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin Orthop Relat Res. 2008;466:1492–1498. [DOI] [PMC free article] [PubMed]
- 15.Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res. 2004;427:37–46. [DOI] [PubMed]
- 16.Fehring TK, Calton TF, Griffin WL. Cementless fixation in 2-stage reimplantation for periprosthetic sepsis. J Arthroplasty. 1999;14:175–181. [DOI] [PubMed]
- 17.Feldman DS, Lonner JH, Desai P, Zuckerman JD. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–1813. [DOI] [PubMed]
- 18.Fink B, Grossmann A, Schubring S, Schulz MS, Fuerst M. A modified transfemoral approach using modular cementless revision stems. Clin Orthop Relat Res. 2007;462:105–114. [DOI] [PubMed]
- 19.Fink B, Grossmann A, Schubring S, Schulz MS, Fuerst M. Short-term results of hip revisions with a curved cementless modular stem in association with the surgical approach. Arch Orthop Trauma Surg. 2008 April 4. [Epub ahead of print]. [DOI] [PubMed]
- 20.Fink B, Hahn M, Fuerst M, Thybaut L, Delling G. Principle of fixation of the cementless modular revision stem Revitan. Unfallchirurg. 2005;108:1029–1037. [DOI] [PubMed]
- 21.Fink B, Makowiak C, Fuerst M, Berger I, Schäfer P, Frommelt L. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J Bone Joint Surg Br. 2008;90:874–878. [DOI] [PubMed]
- 22.Fitzgerald RH Jr. Infected total hip arthroplasty: diagnosis and treatment. J Am Acad Orthop Surg. 1995;3:249–262. [DOI] [PubMed]
- 23.Frommelt L, Kühn KD. Properties of bone cement: antibiotic-loaded cement. In: Breusch SJ, Malchau H, eds. The Well-Cemented Total Hip Arthroplasty: Theory and Practice. Heidelberg, Germany: Springer-Verlag; 2005:86–92.
- 24.Gallo J, Kolár M, Novotný R, Riháková P, Tichá V. Pathogenesis of prosthesis-related infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:27–35. [DOI] [PubMed]
- 25.Garvin KL, Evans BG, Salvati EA, Brause BD. Palacos gentamicin for the treatment of deep periprosthetic hip infections. Clin Orthop Relat Res. 1994;298:97–105. [PubMed]
- 26.Garvin KL, Hanssen AD. Current concepts review: infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77:1576–1588. [DOI] [PubMed]
- 27.Gollwitzer H, Diehl P, Gerdesmeyer L, Mittelmeier W. Diagnostic strategies in cases of suspected periprosthetic infection of the knee: a review of the literature and current recommendations. Orthopäde. 2006;35:904–916. [DOI] [PubMed]
- 28.Grünig R, Morscher E, Ochsner PE. Three- to 7-year results with the uncemented SL femoral revision prosthesis. Arch Orthop Trauma Surg. 1997;116:187–197. [DOI] [PubMed]
- 29.Gustilo RB, Pasternak HS. Revision total hip arthroplasty with titanium ingrowth prosthesis and bone grafting for failed cemented femoral component loosening. Clin Orthop Relat Res. 1988;235:111–119. [PubMed]
- 30.Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br. 2000;82:689–694. [DOI] [PubMed]
- 31.Hanssen AD, Osmon DR. Evaluation of a staging system for infected hip arthroplasty. Clin Orthop Relat Res. 2002;403:16–22. [DOI] [PubMed]
- 32.Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85. [DOI] [PubMed]
- 33.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 34.Hedley AK, Gruen TA, Ruoff DP. Revision of failed total hip arthroplasties with uncemented porous-coated anatomic components. Clin Orthop Relat Res. 1988;235:75–90. [PubMed]
- 35.Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J Arthroplasty. 2005;20:874–879. [DOI] [PubMed]
- 36.Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma. 2004;56:1247–1252. [DOI] [PubMed]
- 37.Hsieh PH, Shih CH, Chang YH, Lee MD, Shih HN, Yang WE. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg Am. 2004;86:1989–1997. [PubMed]
- 38.Ince A, Rupp J, Frommelt L, Katzer A, Gille J, Löhr JF. Is “aseptic” loosening of the prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in patients with low-grade infection? Clin Infect Dis. 2004;39:1599–1603. [DOI] [PubMed]
- 39.Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Relat Res. 2002;404:116–124. [DOI] [PubMed]
- 40.Koo KH, Yang JW, Cho SH, Song HR, Park HB, Ha YC, Chang JD, Kim SY, Kim YH. Impregnation of vancomycin, gentamicin, and cefotaxime in the cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty. 2001;16:882–892. [DOI] [PubMed]
- 41.Kraay MJ, Goldberg VM, Fitzgerald SJ, Salata MJ. Cementless two-staged total hip arthroplasty for deep periprosthetic infection. Clin Orthop Relat Res. 2005;441:243–249. [DOI] [PubMed]
- 42.Lai KA, Shen WJ, Yang CY, Lin RM, Lin CJ, Jou IM. Two-stage cementless revision THR after infection: 5 recurrences in 40 cases followed 2.5–7 years. Acta Orthop Scand. 1996;67:325–328. [DOI] [PubMed]
- 43.Lieberman JR, Callaway GH, Salvati EA, Pellici PM, Brause BD. Treatment of the infected total hip arthroplasty with a two staged reimplantation protocol. Clin Orthop Relat Res. 1994;301:205–212. [PubMed]
- 44.Lonner JH, Desai P, Dicesare PE, Steiner G, Zuckerman JD. The reliability of analysis of intraoperative frozen sections for identifying active infection during revision hip or knee arthroplasty. J Bone Joint Surg Am. 1996;78:1553–1558. [DOI] [PubMed]
- 45.Masri BA, Panagiotopoulos KP, Greidanus NV, Garbuz DS, Duncan CP. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty. 2007;22:72–78. [DOI] [PubMed]
- 46.McInnis DP, Horne G, Devane PA. Femoral revision with a fluted, tapered, modular stem: seventy patients followed for a mean of 3.9 years. J Arthroplasty. 2006;21:372–380. [DOI] [PubMed]
- 47.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthetic failure. Clin Orthop Relat Res. 1976;117:221–240. [PubMed]
- 48.Mirra JM, Marder RA, Amstutz HC. The pathology of failed total joint arthroplasty. Clin Orthop Relat Res. 1982;170:175–183. [PubMed]
- 49.Mjöberg B, Selvik G, Hansson LI, Rosenqvist R, Önnerfält R. Mechanical loosening of total hip prostheses: a radiographic and roentgen stereogrammatic study. J Bone Joint Surg Br. 1986;68:770–774. [DOI] [PubMed]
- 50.Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection: a comparison-group study. J Bone Joint Surg Am. 2000;82:1552–1557. [DOI] [PubMed]
- 51.Nestor BJ, Hanssen AD, Ferrer-Bonzalez R, Fitzgerald RH. The use of porous prostheses in delayed reconstruction of total hip replacements that have failed because of infection. J Bone Joint Surg Am. 1994;76:349–359. [DOI] [PubMed]
- 52.Neut D, van Horn JR, van Kooten TG, van der Mei HC, Busscher HJ. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin Orthop Relat Res. 2003;413:261–268. [DOI] [PubMed]
- 53.Nunn D, Freeman MA, Hil PF, Evans SJ. The measurement of migration of the acetabular component of hip prostheses. J Bone Joint Surg Br. 1989;71:629–631. [DOI] [PubMed]
- 54.Pak JH, Paprosky WG, Jablonsky WS, Lawrence JM. Femoral strut allografts in cementless revision total hip arthroplasty. Clin Orthop Relat Res. 1993;295:172–178. [PubMed]
- 55.Pandey R, Drakouilakis E, Athanasou NA. An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol. 1999;52:118–123. [DOI] [PMC free article] [PubMed]
- 56.Paprosky WG, Greidanus NV, Antoniou J. Minimum 10-year results of extensively porous-coated stems in revision hip arthroplasty. Clin Orthop Relat Res. 1999;369:230–242. [DOI] [PubMed]
- 57.Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty: a 6-year follow-up evaluation. J Arthroplasty. 1994;9:33–44. [DOI] [PubMed]
- 58.Peters G, Hermann M, von Eiff C. The changing pattern of coagulase-negative staphylococci as infectious pathogens. Curr Opin Infect Dis. 1995;8(suppl):S12–S19. [DOI]
- 59.Salgado CD, Dash S, Cantey R, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. [DOI] [PubMed]
- 60.Steinbrink K, Frommelt L. Treatment of periprosthetic infection of the hip using one-stage exchange surgery. Orthopäde. 1995;24:335–343. [PubMed]
- 61.Stockley I, Mockford BJ, Hoad-Reddick, Norman P. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J Bone Joint Surg Br. 2008;90:145–148. [DOI] [PubMed]
- 62.Trampuz A, Zimmerli W. New strategies for the treatment of infectious associated with prosthetic joints. Curr Opin Investig Drugs. 2005;6:185–190. [PubMed]
- 63.Virolainen P, Lahteenmaki H, Hiltunen A, Sipola E, Meurman O, Nelimarkka O. The reliability of diagnosis of infection during revision arthroplasties. Scand J Surg. 2002;91:178–181. [DOI] [PubMed]
- 64.Warren PJ, Thompson P, Flechter MD. Transfemoral implantation of the Wagner SL stem: the abolition of subsidence and enhancement of osteotomy union rate using Dall-Miles cables. Arch Orthop Trauma Surg. 2002;122:557–560. [DOI] [PubMed]
- 65.Weber M, Hempfing A, Orler R. Femoral revision using the Wagener stem: results at 2–9 years. Int Orthop. 2002;26:36–39. [DOI] [PMC free article] [PubMed]
- 66.Williams JL, Norman P, Stockley I. The value of hip aspiration versus tissue biopsy in diagnosing infection before exchange hip arthroplasty surgery. J Arthroplasty. 2004;19:582–586. [DOI] [PubMed]
- 67.Wilson MG, Dorr LD. Reimplantation of infected total hip arthroplasties in the absence of antibiotic cement. J Arthroplasty. 1989;4:263–269. [DOI] [PubMed]
- 68.Wirtz DC, Niethard FU. Etiology, diagnosis and therapy of aseptic hip prosthesis loosening: a status assessment. Z Orthop Ihre Grenzgeb. 1997;135:270–280. [DOI] [PubMed]
- 69.Wroblewski BM. One-stage revision of infected cemented total hip arthroplasty. Clin Orthop Relat Res. 1986;211:103–107. [PubMed]
- 70.Zimmerli W. The role of antimicrobial agents in the management of infected arthroplasties. Orthopäde. 1995;24:308–313. 7478490
- 71.Zimmerli W. Infection and musculoskeletal conditions: prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol. 2006;20:1045–1063. [DOI] [PubMed]
- 72.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampicin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537–1541. [DOI] [PubMed]